Acute Effects of Soft Tissue Modalities on Muscular Ultrasound Characteristics and Isometric Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Modalities
2.3.1. Static Stretching
2.3.2. Foam Rolling
2.3.3. Percussion Massage
2.4. Ultrasound Assessment
2.5. Isometric Testing and Surface EMG
2.6. Statistical Analysis
3. Results
3.1. Ultrasound Measurements
3.2. Isometric and EMG Measruments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shellock, F.G.; Prentice, W.E. Warming-up and stretching for improved physical performance and prevention of sports-related injuries. Sports Med. 1985, 2, 267–278. [Google Scholar] [CrossRef]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Rattray, B. Warm-up strategies for sport and exercise: Mechanisms and applications. Sports Med. 2015, 45, 1523–1546. [Google Scholar] [CrossRef]
- Shrier, I. Does stretching improve performance? a systematic and critical review of the literature. Clin. J. Sport Med. 2004, 14, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Behm, D.G.; Blazevich, A.J.; Kay, A.D.; McHugh, M. Acute effects of muscle stretching on physical performance, range of motion, and injury incidence in healthy active individuals: A systematic review. Appl. Physiol. Nutr. Metab. 2016, 41, 1–11. [Google Scholar] [CrossRef]
- Amako, M.; Oda, T.; Masuoka, K.; Yokoi, H.; Campisi, P. Effect of static stretching on prevention of injuries for military recruits. Mil. Med. 2003, 168, 442–446. [Google Scholar] [CrossRef]
- Behm, D.G.; Kay, A.D.; Trajano, G.S.; Alizadeh, S.; Blazevich, A.J. Effects of stretching on injury risk reduction and balance. J. Clin. Exerc. Physiol. 2021, 10, 106–116. [Google Scholar] [CrossRef]
- Herrera, E.; Osorio-Fuentealba, C. Impact of warm-up methods on strength-speed for sprinters in athletics: A mini review. Front. Sports Act. Living 2024, 6, 1360414. [Google Scholar] [CrossRef]
- Kerautret, Y.; Di Rienzo, F.; Eyssautier, C.; Guillot, A. Selective effects of manual massage and foam rolling on perceived recovery and performance: Current knowledge and future directions toward robotic massages. Front. Physiol. 2020, 11, 598898. [Google Scholar] [CrossRef] [PubMed]
- Davis, H.L.; Alabed, S.; Chico, T.J.A. Effect of sports massage on performance and recovery: A systematic review and meta-analysis. BMJ Open Sport Exerc. Med. 2020, 6, e000614. [Google Scholar] [CrossRef]
- Cole, G. The evidence behind foam rolling: A review. Sport Olymp. Paralympic Stud J. 2018, 3, 194–206. [Google Scholar]
- Konrad, A.; Glashüttner, C.; Reiner, M.M.; Bernsteiner, D.; Tilp, M. The acute effects of a percussive massage treatment with a hypervolt device on plantar flexor muscles’ range of motion and performance. J. Sports Sci. Med. 2020, 19, 690. [Google Scholar] [PubMed]
- Power, K.; Behm, D.; Cahill, F.; Carroll, M.; Young, W. An acute bout of static stretching: Effects on force and jumping performance. Med. Sci. Sports Exerc. 2004, 36, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.M.; Tilp, M.; Guilhem, G.; Morales-Artacho, A.; Konrad, A. Comparison of a single vibration foam rolling and static stretching exercise on the muscle function and mechanical properties of the hamstring muscles. J. Sports Sci. Med. 2022, 21, 287. [Google Scholar] [CrossRef] [PubMed]
- Škarabot, J.; Beardsley, C.; Štirn, I. Comparing the effects of self-myofascial release with static stretching on ankle range-of-motion in adolescent athletes. Int. J. Sports Phys. Ther. 2015, 10, 203. [Google Scholar] [PubMed]
- Beckett, J.R.; Schneiker, K.T.; Wallman, K.E.; Dawson, B.T.; Guelfi, K.J. Effects of static stretching on repeated sprint and change of direction performance. Med. Sci. Sports Exerc. 2009, 41, 444–450. [Google Scholar] [CrossRef]
- Young, W.B.; Behm, D.G. Effects of running, static stretching and practice jumps on explosive force production and jumping performance. J. Sports Med. Phys. Fit. 2003, 43, 21–27. [Google Scholar]
- Yildiz, S.; Gelen, E.; Çilli, M.; Karaca, H.; Kayihan, G.; Ozkan, A.; Sayaca, C. Acute effects of static stretching and massage on flexibility and jumping performance. J. Musculoskelet. Neuron. Interact. 2020, 20, 498. [Google Scholar]
- Janot, J.; Malin, B.; Cook, R.; Hagenbucher, J.; Draeger, A.; Jordan, M.; Quinn, E. Effects of self-myofascial release and static stretching on anaerobic power output. J. Fit. Res. 2013, 2, 41–54. [Google Scholar]
- Panidi, I.; Donti, O.; Konrad, A.; Dinas, P.C.; Terzis, G.; Mouratidis, A.; Gaspari, V.; Donti, A.; Bogdanis, G.C. Muscle architecture adaptations to static stretching training: A systematic review with meta-analysis. Sports Med. Open 2023, 9, 47. [Google Scholar] [CrossRef]
- Dennis, L.G.; Blais, E.A.; Wagstaff, K.E.; Li, L.; Biber, D. The Effect of Static Stretching on Proprioception, Pennation Angle, and Muscle Power Production. Int. J. Biotechnol. Bioeng. 2020, 6, 37. [Google Scholar]
- Young, H.; Jenkins, N.T.; Zhao, Q.; Mccully, K.K. Measurement of intramuscular fat by muscle echo intensity. Muscle Nerve 2015, 52, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Sobolewski, E.J.; Hall, A.B.; Rodriguez, G.C.; Richard, M.O. The Effect of Aerobic Exercise on Quadriceps Echo Intensity and Cross-Sectional Area. Int. J. Phys. Educ. Exerc. Sports 2020, 2, 6–9. [Google Scholar]
- Yitzchaki, N.; Kuehne, T.E.; Mouser, J.G.; Buckner, S.L. Can changes in echo intensity be used to detect the presence of acute muscle swelling? Physiol. Meas. 2019, 40, 045002. [Google Scholar] [CrossRef] [PubMed]
- Şekir, U.; Arslan, G.; İlhan, O.; Akova, B. Effects of static and dynamic stretching on muscle architecture. Turk. J. Sports Med. 2019, 54, 158–168. [Google Scholar] [CrossRef]
- Nakao, S.; Ikezoe, T.; Yagi, M.; Umehara, J.; Nojiri, S. Changes in echo intensity of the gastrocnemius muscle with passive ankle dorsiflexion: Can echo intensity be used to assess muscle elongation? Front. Physiol. 2023, 14, 1197503. [Google Scholar] [CrossRef]
- Nakamura, M.; Ikezoe, T.; Nishishita, S.; Umehara, J.; Kimura, M.; Ichihashi, N. Acute effects of static stretching on the shear elastic moduli of the medial and lateral gastrocnemius muscles in young and elderly women. Musculoskelet. Sci. Pract. 2017, 32, 98–103. [Google Scholar] [CrossRef]
- Matsuo, H.; Kubota, M.; Shimada, S.; Kitade, I.; Matsumura, M.; Nonoyama, T.; Koie, Y.; Naruse, H.; Takahashi, A.; Oki, H. The Effect of Static Stretching Duration on Muscle Blood Volume and Oxygenation. J. Strength Cond. Res. 2022, 36, 379–385. [Google Scholar] [CrossRef]
- MacDonald, G.Z.; Penney, M.D.; Mullaley, M.E.; Cuconato, A.L.; Drake, C.D.; Behm, D.G.; Button, D.C. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J. Strength Cond. Res. 2013, 27, 812–821. [Google Scholar] [CrossRef]
- Ajimsha, M.S.; Al-Mudahka, N.R.; Al-Madzhar, J.A. Effectiveness of myofascial release: Systematic review of randomized controlled trials. J. Bodyw. Mov. Ther. 2015, 19, 102–112. [Google Scholar] [CrossRef]
- Macdonald, G.Z.; Button, D.C.; Drinkwater, E.J.; Behm, D.G. Foam Rolling as a Recovery Tool after an Intense Bout of Physical Activity. Med. Sci. Sports Exerc. 2014, 46, 131–142. [Google Scholar] [CrossRef]
- Roylance, D.S.; George, J.D.; Hammer, A.M.; Rencher, N.; Fellingham, G.W.; Hager, R.L.; Myrer, W.J. Evaluating acute changes in joint range-of-motion using self-myofascial release, postural alignment exercises, and static stretches. Int. J. Exerc. Sci. 2013, 6, 6. [Google Scholar]
- Healey, K.C.; Hatfield, D.L.; Blanpied, P.; Dorfman, L.R.; Riebe, D. The effects of myofascial release with foam rolling on performance. J. Strength Cond. Res. 2014, 28, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Wang, T.; Chang, C.; Chen, L.; Chu, H.; Lin, S.; Chang, S. Short-term effects of self-massage combined with home exercise on pain, daily activity, and autonomic function in patients with myofascial pain dysfunction syndrome. J. Phys. Ther. Sci. 2015, 27, 217–221. [Google Scholar] [CrossRef]
- Chaitow, L. Research in the water and fascia: Micro-tornadoes, hydrogenated diamonds & nanocrystals. Massage Today 2009, 9, 17–21. [Google Scholar]
- Schroeder, A.N.; Best, T.M. Is self-myofascial release an effective preexercise and recovery strategy? A literature review. Curr. Sports Med. Rep. 2015, 14, 200–208. [Google Scholar] [CrossRef]
- Yektaei, M.; Akkoç, O.; Devran, S.; Kurtdere, I.; Kirandi, Ö.; Bayraktar, B. Effect of Acute Foam Roller and Percussion Therapy on Muscle Architecture and Muscle Stiffness. SPORMETRE: J. Phys. Educ. Sport Sci. 2023, 21, 21–34. [Google Scholar]
- Brigatto, F.A.; Soares, E.G.; Braz, T.V.; DE CAMARGO, J.B.; Hartz, C.S.; Batista, D.R.; Col, L.O.; Marchetti, P.H.; Aoki, M.S.; Lopes, C.R. Acute Effect of Different Duration of Foam Rolling Protocols on Muscle Thickness, Pain Pressure Threshold, and Volume Load on Multiple Sets of Knee Extension. Int. J. Exerc. Sci. 2021, 14, 742. [Google Scholar]
- Torrente, Q.M.; Killingback, A.; Adds, P.J.; Robertson, C. The effect of self-myofascial release on the pennation angle of the vastus medialis oblique and the vastus lateralis in athletic male individuals: An ultrasound investigation. Int. J. Sports Phys. Ther. 2022, 17, 636. [Google Scholar] [CrossRef]
- Martin, J. A critical evaluation of percussion massage gun devices as a rehabilitation tool focusing on lower limb mobility: A literature review. SportRxiv 2021. [Google Scholar] [CrossRef]
- Lee, C.; Chu, I.; Lyu, B.; Chang, W.; Chang, N. Comparison of vibration rolling, nonvibration rolling, and static stretching as a warm-up exercise on flexibility, joint proprioception, muscle strength, and balance in young adults. J. Sports Sci. 2018, 36, 2575–2582. [Google Scholar] [CrossRef]
- Menek, M.Y.; Menek, B. Effects of percussion massage therapy, dynamic stretching, and static stretching on physical performance and balance. J. Back Musculoskelet. Rehabil. 2023, 37, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Sams, L.; Langdown, B.L.; Simons, J.; Vseteckova, J. The Effect Of Percussive Therapy On Musculoskeletal Performance And Experiences Of Pain: A Systematic Literature Review. Int. J. Sports Phys. Ther. 2023, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Silva, R.; Vigário, P.; Martins, P.N.; Casanova, F.; Fernandes, R.J.; Sampaio, A.R. The effects of massage guns on performance and recovery: A systematic review. J. Funct. Morphol. Kinesiol. 2023, 8, 138. [Google Scholar] [CrossRef]
- Leabeater, A.; Clarke, A.; James, L.; Huynh, M.; Driller, M. Under the Gun: The effect of percussive massage therapy on physical and perceptual recovery in active adults. J. Athl. Train. 2023, 59, 310–316. [Google Scholar] [CrossRef]
- Trainer, J.H.; Pascarella, M.; Paul, R.W.; Thomas, S.J. Acute effects of percussive therapy on the posterior shoulder muscles differ based on the athlete’s soreness response. Int. J. Sports Phys. Ther. 2022, 17, 887. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.S.; Oranchuk, D.J.; Burton, A.M.; Phan, D.C. Age-, sex-, and region-specific differences in skeletal muscle size and quality. Appl. Physiol. Nutr. Metab. 2020, 45, 1253–1260. [Google Scholar] [CrossRef]
- Nuzzo, J.L. Narrative review of sex differences in muscle strength, endurance, activation, size, fiber type, and strength training participation rates, preferences, motivations, injuries, and neuromuscular adaptations. J. Strength Cond. Res. 2023, 37, 494–536. [Google Scholar] [CrossRef]
- Behm, D.G.; Alizadeh, S.; Daneshjoo, A.; Anvar, S.H.; Graham, A.; Zahiri, A.; Goudini, R.; Edwards, C.; Culleton, R.; Scharf, C. Acute effects of various stretching techniques on range of motion: A systematic review with meta-analysis. Sports Med. -Open 2023, 9, 107. [Google Scholar] [CrossRef]
- Cornell, D.J.; Ebersole, K.T. Influence of an acute bout of self-myofascial release on knee extension force output and electro-mechanical activation of the quadriceps. Int. J. Sports Phys. Ther. 2020, 15, 732. [Google Scholar] [CrossRef]
- Place, N.; Blum, Y.; Armand, S.; Maffiuletti, N.A.; Behm, D.G. Effects of a short proprioceptive neuromuscular facilitation stretching bout on quadriceps neuromuscular function, flexibility, and vertical jump performance. J. Strength Cond. Res. 2013, 27, 463–470. [Google Scholar] [CrossRef]
- Sobolewski, E.J.; Wein, L.D.; Crow, J.M.; Carpenter, K.M. Intra-rater and inter-rater reliability of the process of obtaining cross-sectional area and echo intensity measurements of muscles from ultrasound images. J. Ultrason. 2021, 21, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Ruple, B.A.; Mesquita, P.H.; Godwin, J.S.; Sexton, C.L.; Osburn, S.C.; McIntosh, M.C.; Kavazis, A.N.; Libardi, C.A.; Young, K.C.; Roberts, M.D. Changes in vastus lateralis fibre cross-sectional area, pennation angle and fascicle length do not predict changes in muscle cross-sectional area. Exp. Physiol. 2022, 107, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Stock, M.S.; Olinghouse, K.D.; Drusch, A.S.; Mota, J.A.; Hernandez, J.M.; Akalonu, C.C.; Thompson, B.J. Evidence of muscular adaptations within four weeks of barbell training in women. Hum. Mov. Sci. 2016, 45, 7–22. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Fauth, M.L.; Petushek, E.J.; Feldmann, C.R.; Hsu, B.E.; Garceau, L.R.; Lutsch, B.N.; Ebben, W.P. Reliability of surface electromyography during maximal voluntary isometric contractions, jump landings, and cutting. J. Strength Cond. Res. 2010, 24, 1131–1137. [Google Scholar] [CrossRef]
- Yang, J.F.; Winter, D.A. Electromyography reliability in maximal and submaximal isometric contractions. Arch.Phys.Med.Rehabil. 1983, 64, 417–420. [Google Scholar] [PubMed]
- Holm, S. A simple sequentially rejective multiple test procedure. JSTOR 1979, 2, 65–70. [Google Scholar]
- Weir, J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Cond. Res. 2005, 19, 231–240. [Google Scholar]
- Cheatham, S.W.; Kolber, M.J.; Cain, M.; Lee, M. The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: A systematic review. Int. J. Sports Phys. Ther. 2015, 10, 827. [Google Scholar]
- Comfort, P.; McMahon, J.J.; Lake, J.P.; Ripley, N.J.; Triplett, N.T.; Haff, G.G. Relative strength explains the differences in multi-joint rapid force production between sexes. PLoS ONE 2024, 19, e0296877. [Google Scholar] [CrossRef]
- Nimphius, S.; McBride, J.M.; Rice, P.E.; Goodman-Capps, C.L.; Capps, C.R. Comparison of quadriceps and hamstring muscle activity during an isometric squat between strength-matched men and women. J. Sports Sci. Med. 2019, 18, 101. [Google Scholar] [PubMed]
- Chino, K.; Takahashi, H. Measurement of gastrocnemius muscle elasticity by shear wave elastography: Association with passive ankle joint stiffness and sex differences. Eur. J. Appl. Physiol. 2016, 116, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Martín-Santana, E.; Hernández-Sánchez, S.; Herrero-Alonso, A.J.; García-López, D. Effects of static-stretching and whole-body-vibration during warm-ups on bench-press kinematics in males and females college-aged. RICYDE. Rev. Int. De Cienc. Del 2015, 11, 348–359. [Google Scholar]
- Dick, T.J.; Hug, F. Advances in imaging for assessing the design and mechanics of skeletal muscle in vivo. J. Biomech. 2023, 155, 111640. [Google Scholar] [CrossRef]
- Kwah, L.K.; Pinto, R.Z.; Diong, J.; Herbert, R.D. Reliability and validity of ultrasound measurements of muscle fascicle length and pennation in humans: A systematic review. J. Appl. Physiol. 2013, 114, 761–769. [Google Scholar] [CrossRef]
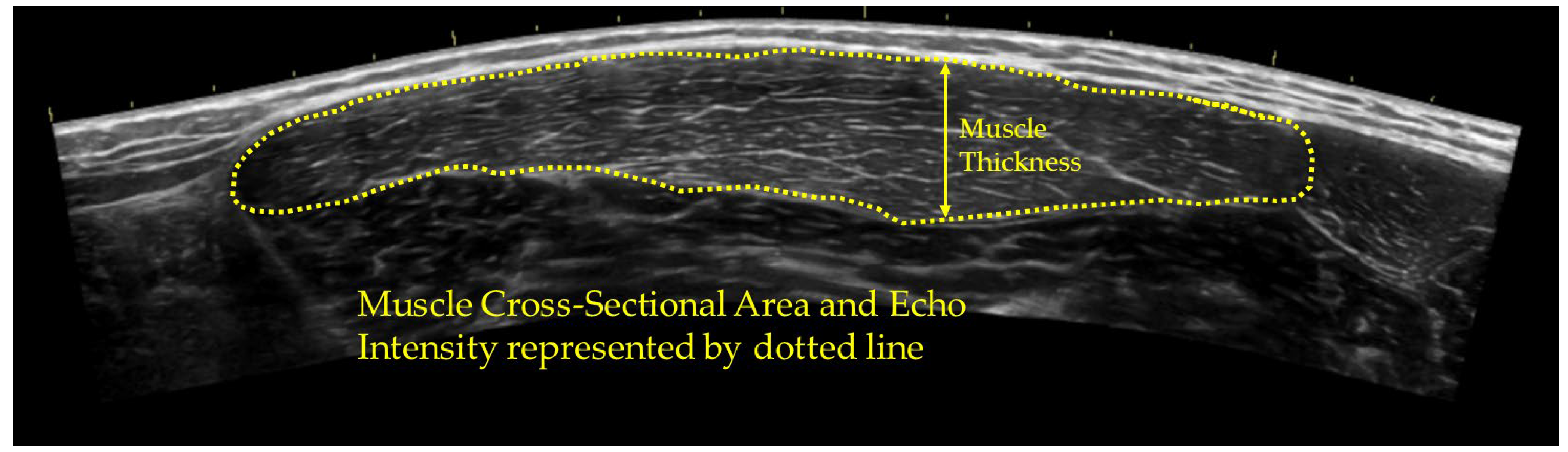
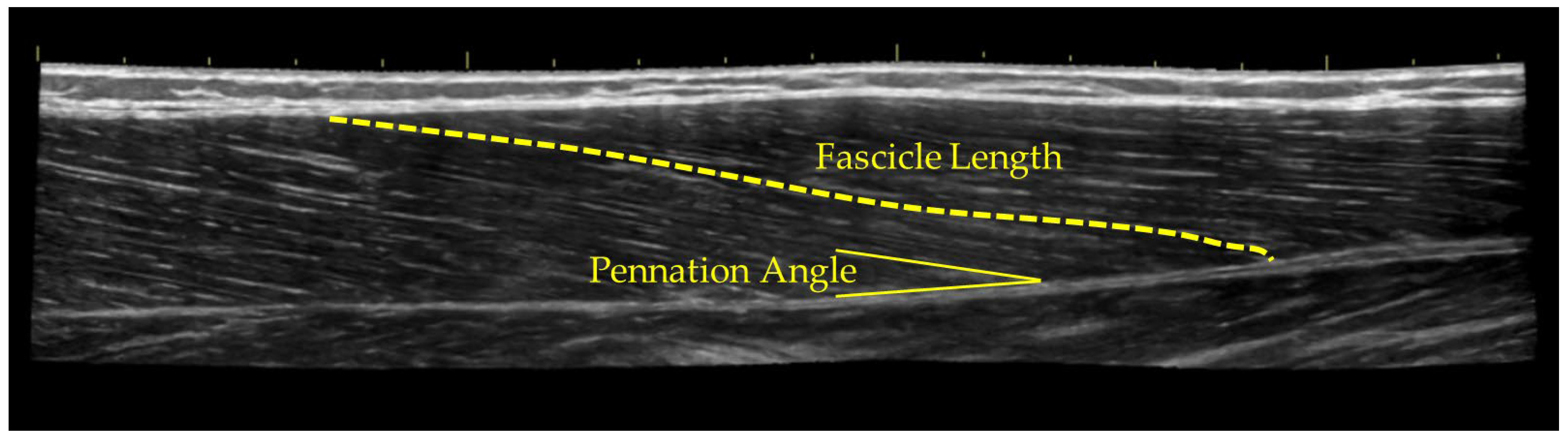
| Control | Stretch | Foam Roll | Percussion Massage | |||||
| Cross-Sectional Area (cm2) | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| Male * | 16.8 ± 4.0 | 18.5 ± 5.6 | 16.7 ± 4.4 | 16.7 ± 4.3 | 18.9 ± 4.4 | 18.6 ± 5.2 | 17.7 ± 4.3 | 18.5 ± 6.0 |
| Female | 10.5 ± 3.1 | 11.2 ± 2.5 | 13.7 ± 2.8 | 14.9 ± 3.3 | 12.5 ± 3.5 | 13.2 ± 3.2 | 11.0 ± 2.4 | 10.8 ± 2.0 |
| Echo Intensity (a.u.) | ||||||||
| Male | 48.2 ± 18.7 | 52.7 ± 13.5 | 60.8 ± 13.7 | 61.5 ± 10.4 | 61.8 ± 13.7 | 56.0 ± 14.7 | 57.6 ± 15.4 | 60.4 ± 14.9 |
| Female | 55.9 ± 11.7 | 59.5 ± 10.0 | 58.7 ± 5.7 | 57.8 ± 6.8 | 66.1 ± 11.6 | 65.0 ± 7.6 | 62.5 ± 9.7 | 58.0 ± 10.1 |
| Pennation Angle (°) | ||||||||
| Male * | 15.3 ± 4.8 | 16.1 ± 3.3 | 14.3 ± 3.8 | 14.0 ± 4.3 | 16.0 ± 3.7 | 16.1 ± 4.2 | 15.0 ± 3.6 | 14.2 ± 2.4 |
| Female | 13.5 ± 3.6 | 14.7 ± 3.3 | 11.7 ± 3.2 | 12.1 ± 3.8 | 14.5 ± 3.5 | 14.8 ± 2.8 | 12.7 ± 2.4 | 12.9 ± 2.7 |
| Fascicle Length (cm) | ||||||||
| Male * | 10.7 ± 1.6 | 10.7 ± 1.3 | 11.2 ± 2.4 | 11.5 ± 2.8 | 11.0 ± 1.7 | 10.5 ± 2.1 | 10.0 ± 1.6 | 10.4 ± 2.3 |
| Female | 9.8 ± 0.9 | 9.7 ± 1.3 | 10.3 ± 1.5 | 10.5 ± 1.7 | 9.7 ± 0.9 | 9.9 ± 1.2 | 9.1 ± 1.3 | 9.5 ± 1.3 |
| Muscle Thickness (cm) | ||||||||
| Male * | 2.5 ± 0.5 | 2.6 ± 0.4 | 2.2 ± 0.5 | 2.3 ± 0.5 | 2.5 ± 0.4 | 2.3 ± 0.5 | 2.3 ± 0.3 | 2.2 ± 0.4 |
| Female | 2.1 ± 0.5 | 2.2 ± 0.4 | 2.0 ± 0.5 | 2.0 ± 0.5 | 2.0 ± 0.4 | 2.0 ± 0.3 | 2.0 ± 0.8 | 1.9 ± 0.5 |
| Peak Torque (Nm) | ||||||||
| Male * | 224.1 ± 58.2 | 228.9 ± 55.9 | 227.9 ± 60.2 | 226.1 ± 71.1 | 224.8 ± 75.3 | 241.7 ± 75.1 | 227.2 ± 56.9 | 235.8 ± 74.7 |
| Female | 122.3 ± 54.9 | 125.5 ± 56.3 | 118.4 ± 48.6 | 116.2 ± 56.6 | 120.5 ± 70.2 | 121.2 ± 69.5 | 121.4 ± 51.4 | 119.2 ± 47.3 |
| Peak EMG (mV) | ||||||||
| Male | 2.6 ± 1.6 | 1.4 ± 0.9 | 1.6 ± 0.6 | 1.8 ± 0.9 | 1.6 ± 0.8 | 1.8 ± 1.0 | 2.1 ± 1.2 | 1.7 ± 0.8 |
| Female | 1.9 ± 0.7 | 1.4 ± 0.5 | 0.9 ± 0.5 | 1.5 ± 0.6 | 1.2 ± 0.8 | 1.0 ± 0.7 | 1.0 ± 0.3 | 0.9 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobolewski, E.; Topham, W.; Hosey, R.; Waheeba, N.; Rett, T. Acute Effects of Soft Tissue Modalities on Muscular Ultrasound Characteristics and Isometric Performance. Appl. Sci. 2024, 14, 7994. https://doi.org/10.3390/app14177994
Sobolewski E, Topham W, Hosey R, Waheeba N, Rett T. Acute Effects of Soft Tissue Modalities on Muscular Ultrasound Characteristics and Isometric Performance. Applied Sciences. 2024; 14(17):7994. https://doi.org/10.3390/app14177994
Chicago/Turabian StyleSobolewski, Eric, William Topham, Ryan Hosey, Nora Waheeba, and Thelen Rett. 2024. "Acute Effects of Soft Tissue Modalities on Muscular Ultrasound Characteristics and Isometric Performance" Applied Sciences 14, no. 17: 7994. https://doi.org/10.3390/app14177994




