Effect of Dietary Enrichment with Hempseed (Cannabis sativa L.) on Blood Pressure Changes in Growing Mice between Ages of 5 and 30 Weeks
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Diets
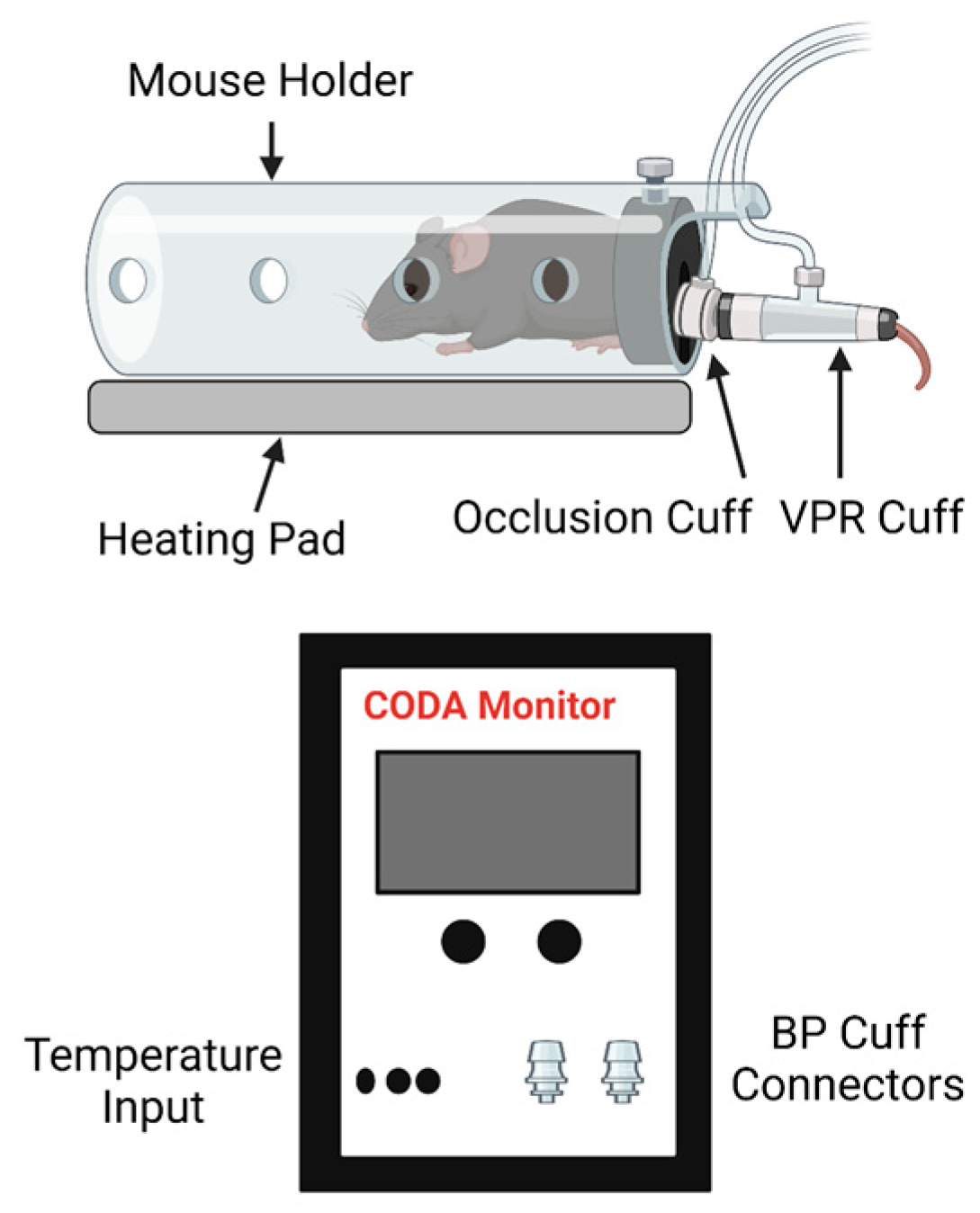
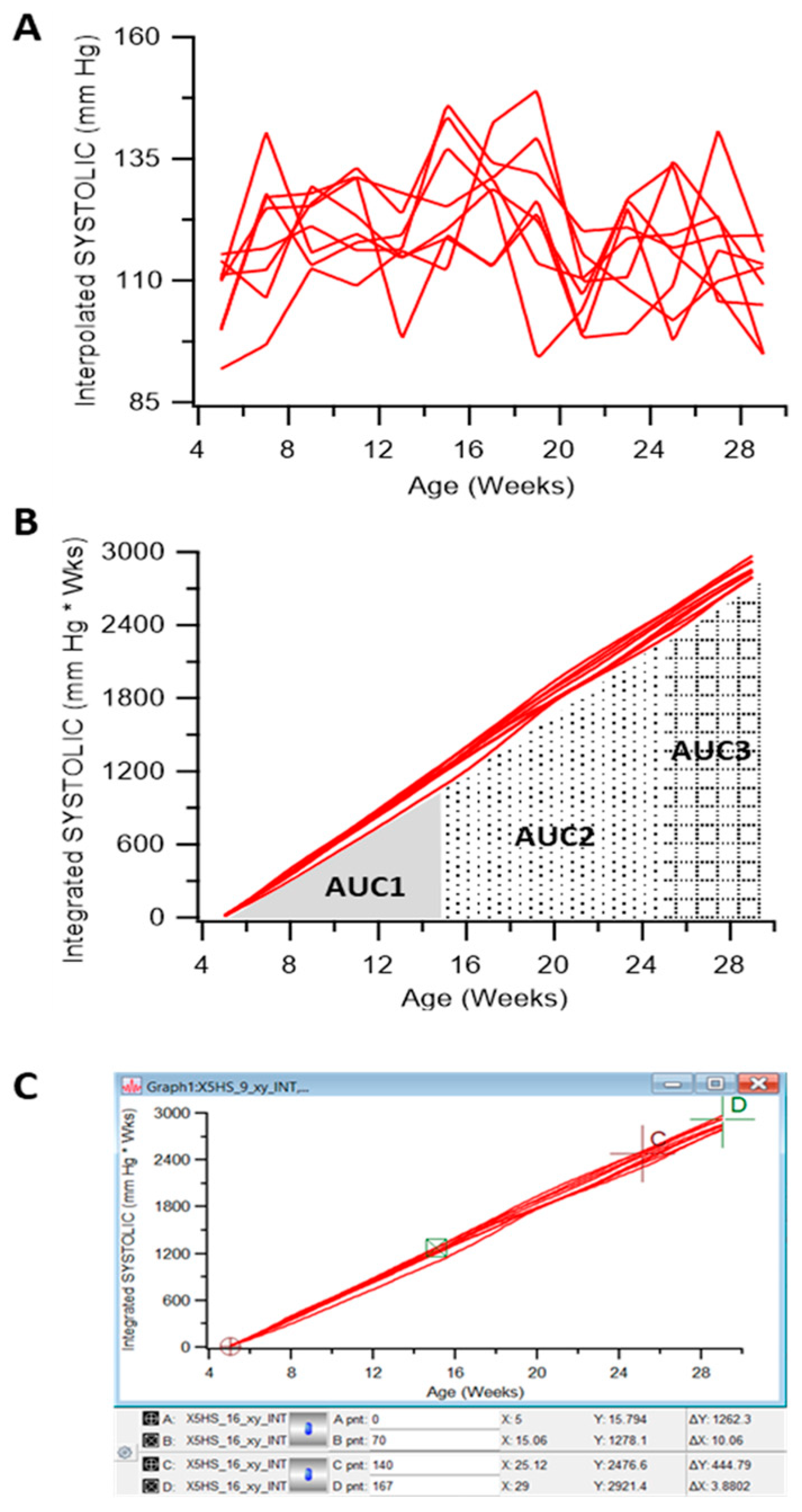
2.3. Measurement of Blood Pressure and Area under the Curve (AUC) Analysis
2.4. Measurement of Body Composition (DXA Scan)
2.5. Calculations of BS, BMI, LMI, and FMI
- (i)
- Body surface area (BS, cm2) = k Mb2/3, where Mb = body mass (g), and k = constant.
- (i)
- Body mass index (BMI, g/cm2): Body weight (g)/Body surface area (cm2).
- (ii)
- Lean mass index (LMI, g/cm2): Lean (g)/Body surface area (cm2).
- (iii)
- Fat mass index (FMI, g/cm2): Fat (g)/Body surface area (cm2).
2.6. Statistical Analysis
3. Results
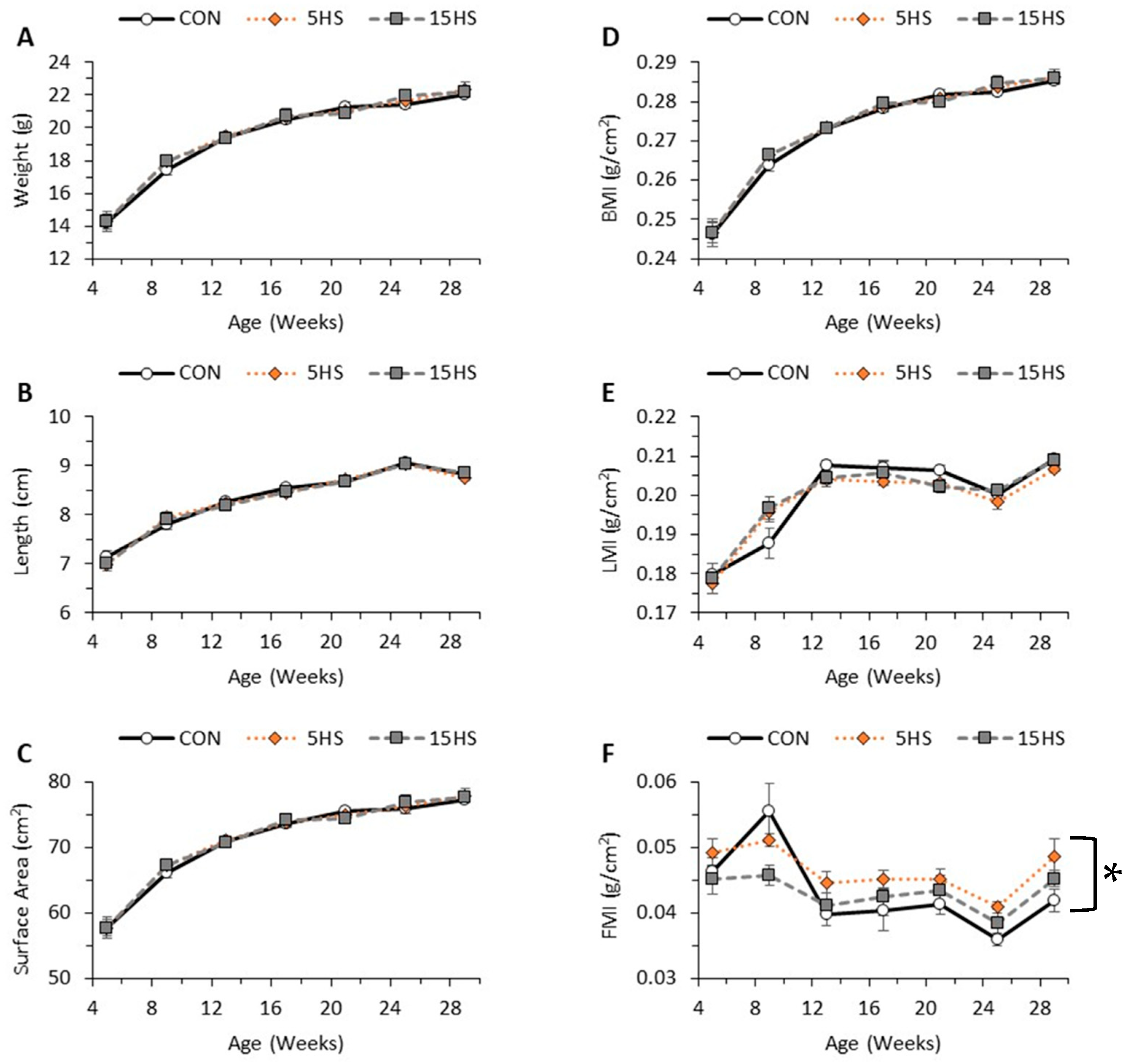
3.1. Effect of Diet and Age on Body Weight, Length, Surface Area, BMI, LMI, and FMI
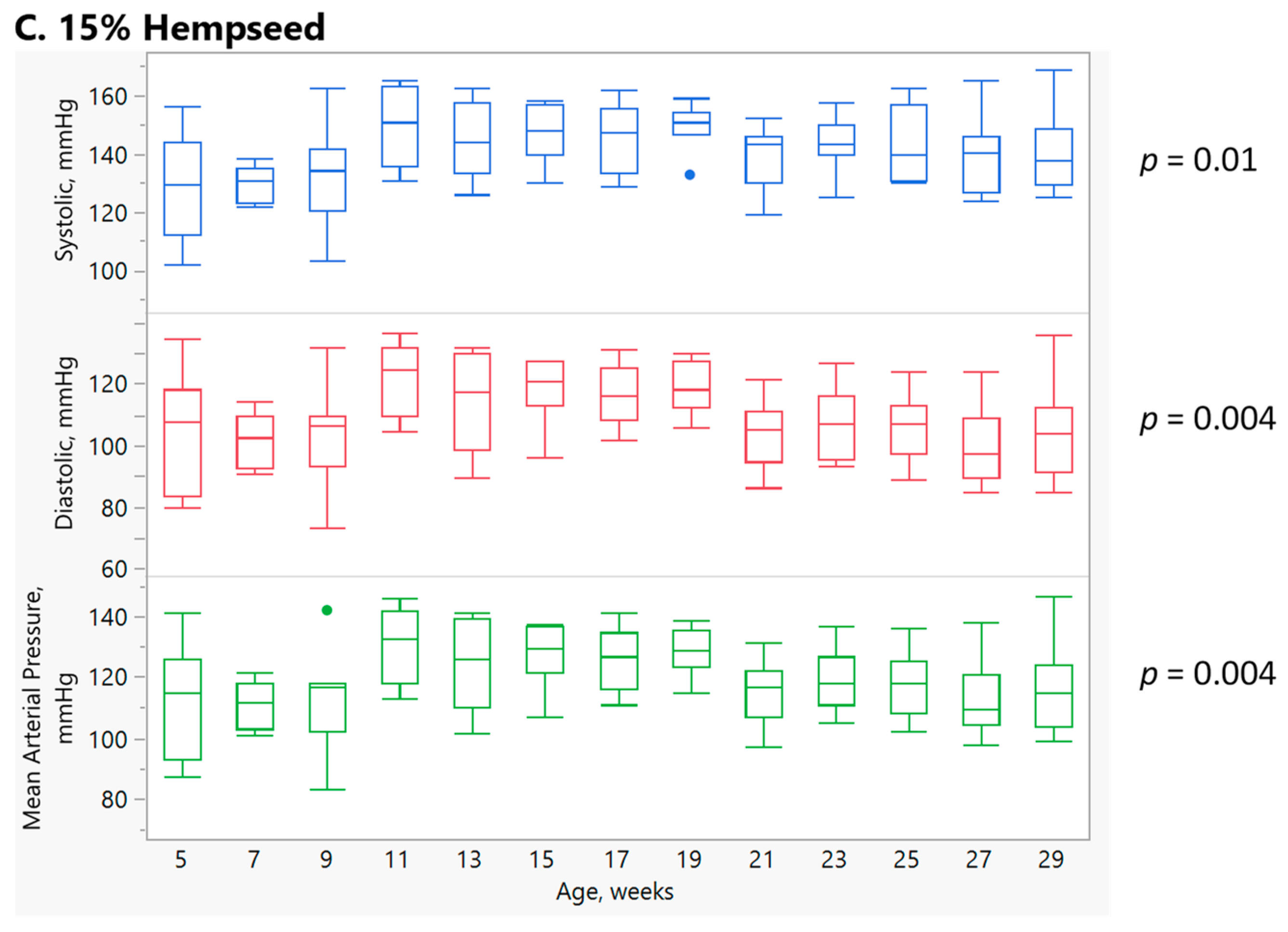
3.2. Effect of Diet and Age on Systolic, Diastolic, and Mean Arterial Blood Pressure
3.3. Relationship between Body Mass and Body Composition and Systolic, Diastolic, and Mean Arterial Blood Pressure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp Seeds (Cannabis sativa L.) as a Valuable Source of Natural Ingredients for Functional Foods—A Review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef] [PubMed]
- Farinon, B.; Molinari, R.; Costantini, L.; Merendino, N. The Seed of Industrial Hemp (Cannabis sativa L.): Nutritional Quality and Potential Functionality for Human Health and Nutrition. Nutrients 2020, 12, 1935. [Google Scholar] [CrossRef]
- Montero, L.; Ballesteros-Vivas, D.; Gonzalez-Barrios, A.F.; Sánchez-Camargo, A.D.P. Hemp Seeds: Nutritional Value, Associated Bioactivities and the Potential Food Applications in the Colombian Context. Front. Nutr. 2022, 9, 1039180. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Leyva, D.; Pierce, G.N. The Cardiac and Haemostatic Effects of Dietary Hempseed. Nutr. Metab. 2010, 7, 32. [Google Scholar] [CrossRef]
- Kakkar, S.; Tandon, R.; Tandon, N. The Rising Status of Edible Seeds in Lifestyle Related Diseases: A Review. Food Chem. 2023, 402, 134220. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, R.; Abe-Inge, V.; Kwofie, E.M.; Baum, J.I.; Kubow, S. Sustainable Healthy Diet Modeling for a Plant-Based Dietary Transitioning in the United States. npj Sci. Food 2023, 7, 61. [Google Scholar] [CrossRef]
- Mylan, J.; Andrews, J.; Maye, D. The Big Business of Sustainable Food Production and Consumption: Exploring the Transition to Alternative Proteins. Proc. Natl. Acad. Sci. USA 2023, 120, e2207782120. [Google Scholar] [CrossRef]
- Rizzo, G.; Storz, M.A.; Calapai, G. The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition. Foods 2023, 12, 3505. [Google Scholar] [CrossRef]
- PR Newswire. Global $544.93 Million Hemp Seeds Markets to 2027. PR Newswire US. 2021. Available online: https://www.prnewswire.com/news-releases/global-544-93-million-hemp-seeds-markets-to-2027--301284430.html (accessed on 28 August 2024).
- Databridge Market Research. Global Hemp Seed Market—Industry Trends and Forecast to 2030. 2023. Available online: https://www.databridgemarketresearch.com/reports/global-hemp-seed-market (accessed on 28 August 2024).
- Opyd, P.M.; Jurgoński, A.; Fotschki, B.; Juśkiewicz, J. Dietary Hemp Seeds More Effectively Attenuate Disorders in Genetically Obese Rats than Their Lipid Fraction. J. Nutr. 2020, 150, 1425–1433. [Google Scholar] [CrossRef]
- Majewski, M.; Jurgoński, A. The Effect of Hemp (Cannabis sativa L.) Seeds and Hemp Seed Oil on Vascular Dysfunction in Obese Male Zucker Rats. Nutrients 2021, 13, 2575. [Google Scholar] [CrossRef]
- Kaushal, N.; Dhadwal, S.; Kaur, P. Ameliorative Effects of Hempseed (Cannabis Sativa) against Hypercholesterolemia Associated Cardiovascular Changes. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Prociuk, M.A.; Edel, A.L.; Richard, M.N.; Gavel, N.T.; Ander, B.P.; Dupasquier, C.M.C.; Pierce, G.N. Cholesterol-Induced Stimulation of Platelet Aggregation Is Prevented by a Hempseed-Enriched Diet. Can. J. Physiol. Pharmacol. 2008, 86, 153–159. [Google Scholar] [CrossRef]
- Girgih, A.T.; Alashi, A.; He, R.; Malomo, S.; Aluko, R.E. Preventive and Treatment Effects of a Hemp Seed (Cannabis sativa L.) Meal Protein Hydrolysate against High Blood Pressure in Spontaneously Hypertensive Rats. Eur. J. Nutr. 2014, 53, 1237–1246. [Google Scholar] [CrossRef]
- Al-Khalifa, A.; Maddaford, T.G.; Chahine, M.N.; Austria, J.A.; Edel, A.L.; Richard, M.N.; Ander, B.P.; Gavel, N.; Kopilas, M.; Ganguly, R.; et al. Effect of Dietary Hempseed Intake on Cardiac Ischemia-Reperfusion Injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1198–R1203. [Google Scholar] [CrossRef]
- Saberivand, A.; Karimi, I.; Becker, L.A.; Moghaddam, A.; Azizi-Mahmoodjigh, S.; Yousefi, M.; Zavareh, S. The Effects of Cannabis sativa L. Seed (Hempseed) in the Ovariectomized Rat Model of Menopause. Methods Find. Exp. Clin. Pharmacol. 2010, 32, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Mihai, M.; Dulf, F.; Ona, A.; Muntean, L.; Ranga, F.; Urdă, C.; Pop, D.; Mihaiescu, T.; Mârza, S.M.; et al. Biochemical Changes Induced by the Administration of Cannabis Sativa Seeds in Diabetic Wistar Rats. Nutrients 2023, 15, 2944. [Google Scholar] [CrossRef] [PubMed]
- Samsamikor, M.; Mackay, D.S.; Mollard, R.C.; Alashi, A.M.; Aluko, R.E. Hemp Seed Protein and Its Hydrolysate Compared with Casein Protein Consumption in Adults with Hypertension: A Double-Blind Crossover Study. Am. J. Clin. Nutr. 2024, 120, 56–65. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Tiemann, K.; Weyer, D.; Djoufack, P.C.; Ghanem, A.; Lewalter, T.; Dreiner, U.; Meyer, R.; Grohe, C.; Fink, K.B. Increasing Myocardial Contraction and Blood Pressure in C57BL/6 Mice during Early Postnatal Development. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H464–H474. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and Mice: Relating Their Ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef]
- Reeves, P.G. Components of the AIN-93 Diets as Improvements in the AIN-76A Diet. J. Nutr. 1997, 127, 838S–841S. [Google Scholar] [CrossRef]
- Wang, Y.; Thatcher, S.E.; Cassis, L.A. Measuring Blood Pressure Using a Noninvasive Tail Cuff Method in Mice. Methods Mol. Biol. 2017, 1614, 69–73. [Google Scholar] [CrossRef]
- Daugherty, A.; Rateri, D.; Hong, L.; Balakrishnan, A. Measuring Blood Pressure in Mice Using Volume Pressure Recording, a Tail-Cuff Method. J. Vis. 2009, 27, e1291. [Google Scholar] [CrossRef]
- Feng, M.; DiPetrillo, K. Non-Invasive Blood Pressure Measurement in Mice. Methods Mol. Biol. 2009, 573, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.R.; Rosner, B.A.; Chen, W.; Srinivasan, S.R.; Berenson, G.S. Using the Area under the Curve to Reduce Measurement Error in Predicting Young Adult Blood Pressure from Childhood Measures. Stat. Med. 2004, 23, 3421–3435. [Google Scholar] [CrossRef] [PubMed]
- Burgess, K.; Jovanović, S.; Sudhir, R.; Jovanović, A. Area under the Curve Analysis of Blood Pressure Reveals Increased Spontaneous Locomotor Activity in SPAK Knock-in Mice: Relevance for Hypotension Induced by SPAK Inhibition? Physiol. Rep. 2019, 7, e13997. [Google Scholar] [CrossRef] [PubMed]
- Jungers, W.L. Scaling. Why Is Animal Size so Important? By K. Schmidt-Nielsen. New York: Cambridge University Press. 1984. Xi + 241 pp., Figures, Tables, Appendices, References, Index. $29.95 (Cloth), $9.95 (Paper). Am. J. Phys. Anthropol. 1986, 69, 129–130. [Google Scholar] [CrossRef]
- Cheung, M.C.; Spalding, P.B.; Gutierrez, J.C.; Balkan, W.; Namias, N.; Koniaris, L.G.; Zimmers, T.A. Body Surface Area Prediction in Normal, Hypermuscular, and Obese Mice. J. Surg. Res. 2009, 153, 326–331. [Google Scholar] [CrossRef]
- Gouma, E.; Simos, Y.; Verginadis, I.; Lykoudis, E.; Evangelou, A.; Karkabounas, S. A Simple Procedure for Estimation of Total Body Surface Area and Determination of a New Value of Meeh’s Constant in Rats. Lab. Anim. 2012, 46, 40–45. [Google Scholar] [CrossRef]
- Gargiulo, S.; Gramanzini, M.; Megna, R.; Greco, A.; Albanese, S.; Manfredi, C.; Brunetti, A. Evaluation of Growth Patterns and Body Composition in C57Bl/6J Mice Using Dual Energy X-Ray Absorptiometry. Biomed. Res. Int. 2014, 2014, 253067. [Google Scholar] [CrossRef]
- Gros, R.; Van Wert, R.; You, X.; Thorin, E.; Husain, M. Effects of Age, Gender, and Blood Pressure on Myogenic Responses of Mesenteric Arteries from C57BL/6 Mice. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H380–H388. [Google Scholar] [CrossRef] [PubMed]
- Wiesmann, F.; Ruff, J.; Hiller, K.H.; Rommel, E.; Haase, A.; Neubauer, S. Developmental Changes of Cardiac Function and Mass Assessed with MRI in Neonatal, Juvenile, and Adult Mice. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H652–H657. [Google Scholar] [CrossRef] [PubMed]
- Urbina, E.M.; Mendizábal, B.; Becker, R.C.; Daniels, S.R.; Falkner, B.E.; Hamdani, G.; Hanevold, C.; Hooper, S.R.; Ingelfinger, J.R.; Lanade, M.; et al. Association of Blood Pressure Level With Left Ventricular Mass in Adolescents. Hypertension 2019, 74, 590–596. [Google Scholar] [CrossRef]
- Frazzini, S.; Torresani, M.C.; Roda, G.; Dell’Anno, M.; Ruffo, G.; Rossi, L. Chemical and Functional Characterization of the Main Bioactive Molecules Contained in Hulled Cannabis sativa L. Seeds for Use as Functional Ingredients. J. Agric. Food Res. 2024, 16, 101084. [Google Scholar] [CrossRef]
- Senila, L.; Neag, E.; Cadar, O.; Kovacs, M.H.; Becze, A.; Senila, M. Chemical, Nutritional and Antioxidant Characteristics of Different Food Seeds. Appl. Sci. 2020, 10, 1589. [Google Scholar] [CrossRef]
- Malomo, S.A.; Onuh, J.O.; Girgih, A.T.; Aluko, R.E. Structural and Antihypertensive Properties of Enzymatic Hemp Seed Protein Hydrolysates. Nutrients 2015, 7, 7616–7632. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Lammi, C.; Laura Capriotti, A.; Bollati, C.; Cavaliere, C.; Maria Montone, C.; Bartolomei, M.; Boschin, G.; Li, J.; Piovesana, S.; et al. Isolation and Functional Characterization of Hemp Seed Protein-Derived Short- and Medium-Chain Peptide Mixtures with Multifunctional Properties for Metabolic Syndrome Prevention. Food Res. Int. 2023, 163, 112219. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sen, U. More than Just an Enzyme: Dipeptidyl Peptidase-4 (DPP-4) and Its Association with Diabetic Kidney Remodelling. Pharmacol. Res. 2019, 147, 104391. [Google Scholar] [CrossRef]
- Chen, H.-H.; Li, W.; Wang, Y.; Xu, B.; Hu, X.; Li, X.-B.; Liu, J.-Y.; Zhang, C.; Zhang, C.-Y.; Xing, X.-H. Mining and Validation of Novel Hemp Seed-Derived DPP-IV-Inhibiting Peptides Using a Combination of Multi-Omics and Molecular Docking. J. Agric. Food Chem. 2023, 71, 9164–9174. [Google Scholar] [CrossRef]
- Aita, S.E.; Montone, C.M.; Taglioni, E.; Capriotti, A.L. Chapter Six—Hempseed Protein-Derived Short- and Medium-Chain Peptides and Their Multifunctional Properties. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2024; Volume 110, pp. 275–325. ISBN 1043-4526. [Google Scholar]
- Chen, H.; Xu, B.; Wang, Y.; Li, W.; He, D.; Zhang, Y.; Zhang, X.; Xing, X. Emerging Natural Hemp Seed Proteins and Their Functions for Nutraceutical Applications. Food Sci. Hum. Wellness 2023, 12, 929–941. [Google Scholar] [CrossRef]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of Potentially Bioactive Peptides Generated from the Enzymatic Hydrolysis of Hempseed Proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef] [PubMed]
- Amponsah-Offeh, M.; Diaba-Nuhoho, P.; Speier, S.; Morawietz, H. Oxidative Stress, Antioxidants and Hypertension. Antioxidants 2023, 12, 281. [Google Scholar] [CrossRef] [PubMed]
- Griendling, K.K.; Camargo, L.L.; Rios, F.J.; Alves-Lopes, R.; Montezano, A.C.; Touyz, R.M. Oxidative Stress and Hypertension. Circ. Res. 2021, 128, 993–1020. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, C.-Y.; Li, H.-B.; Wu, S.-H.; Li, G.-L. Prophylactic Effects of Hemp Seed Oil on Perimenopausal Depression: A Role of HPA Axis. J. Oleo Sci. 2023, 72, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Seely, E.W.; Walsh, B.W.; Gerhard, M.D.; Williams, G.H. Estradiol With or Without Progesterone and Ambulatory Blood Pressure in Postmenopausal Women. Hypertension 1999, 33, 1190–1194. [Google Scholar] [CrossRef]
- Wojcik, M.; Krawczyk, M.; Wojcik, P.; Cypryk, K.; Wozniak, L.A. Molecular Mechanisms Underlying Curcumin-Mediated Therapeutic Effects in Type 2 Diabetes and Cancer. Oxid. Med. Cell. Longev. 2018, 2018, 9698258. [Google Scholar] [CrossRef]
- Sparks, C.A.; Streff, H.M.; Williams, D.W.; Blanton, C.A.; Gabaldón, A.M. Dietary Hempseed Decreases Femur Maximum Load in a Young Female C57BL/6 Mouse Model but Does Not Influence Bone Mineral Density or Micro-Architecture. Nutrients 2022, 14, 4224. [Google Scholar] [CrossRef]
- Blanton, C.A.; Barrott, J.J.; Kunz, K.; Bunde, E.; Streff, H.M.; Sparks, C.A.; Williams, D.W.; Gabaldόn, A.M. The Impact of Hempseed Consumption on Bone Parameters and Body Composition in Growing Female C57BL/6 Mice. Int. J. Environ. Res. Public Health 2022, 19, 5839. [Google Scholar] [CrossRef]

| Ingredient, g per kg Diet | Control Diet a | 5% Hempseed | 15% Hempseed |
|---|---|---|---|
| Casein, High Nitrogen | 200 | 185 | 155 |
| L-Cystine | 3 | 3 | 3 |
| Sucrose b | 100 | 100 | 100 |
| Cornstarch | 397.486 | 395.99 | 392.997 |
| Dyetrose | 132 | 132 | 132 |
| Soybean Oil | 70 | 52 | 16 |
| t-Butylhydroquinone | 0.014 | 0.01 | 0.003 |
| Cellulose | 50 | 34.5 | 3.5 |
| Mineral Mix #210025 c | 35 | 35 | 35 |
| Vitamin Mix # 310025 d | 10 | 10 | 10 |
| Choline Bitartrate | 2.5 | 2.5 | 2.5 |
| Hempseed e | 0 | 50 | 150 |
| Total | 1000 | 1000 | 1000 |
| Kilocalories per kg | 3760 | 3814 | 3922 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanton, C.A.; Streff, H.M.; Gabaldón, A.M. Effect of Dietary Enrichment with Hempseed (Cannabis sativa L.) on Blood Pressure Changes in Growing Mice between Ages of 5 and 30 Weeks. Appl. Sci. 2024, 14, 8006. https://doi.org/10.3390/app14178006
Blanton CA, Streff HM, Gabaldón AM. Effect of Dietary Enrichment with Hempseed (Cannabis sativa L.) on Blood Pressure Changes in Growing Mice between Ages of 5 and 30 Weeks. Applied Sciences. 2024; 14(17):8006. https://doi.org/10.3390/app14178006
Chicago/Turabian StyleBlanton, Cynthia A., Hailey M. Streff, and Annette M. Gabaldón. 2024. "Effect of Dietary Enrichment with Hempseed (Cannabis sativa L.) on Blood Pressure Changes in Growing Mice between Ages of 5 and 30 Weeks" Applied Sciences 14, no. 17: 8006. https://doi.org/10.3390/app14178006
APA StyleBlanton, C. A., Streff, H. M., & Gabaldón, A. M. (2024). Effect of Dietary Enrichment with Hempseed (Cannabis sativa L.) on Blood Pressure Changes in Growing Mice between Ages of 5 and 30 Weeks. Applied Sciences, 14(17), 8006. https://doi.org/10.3390/app14178006







