Electrical Bioimpedance in Milk Adulterated with Water: Measurement Methodology for Quantification, Influence of Temperature, and Mathematical Modelling
Abstract
1. Introduction
2. Materials and Methods
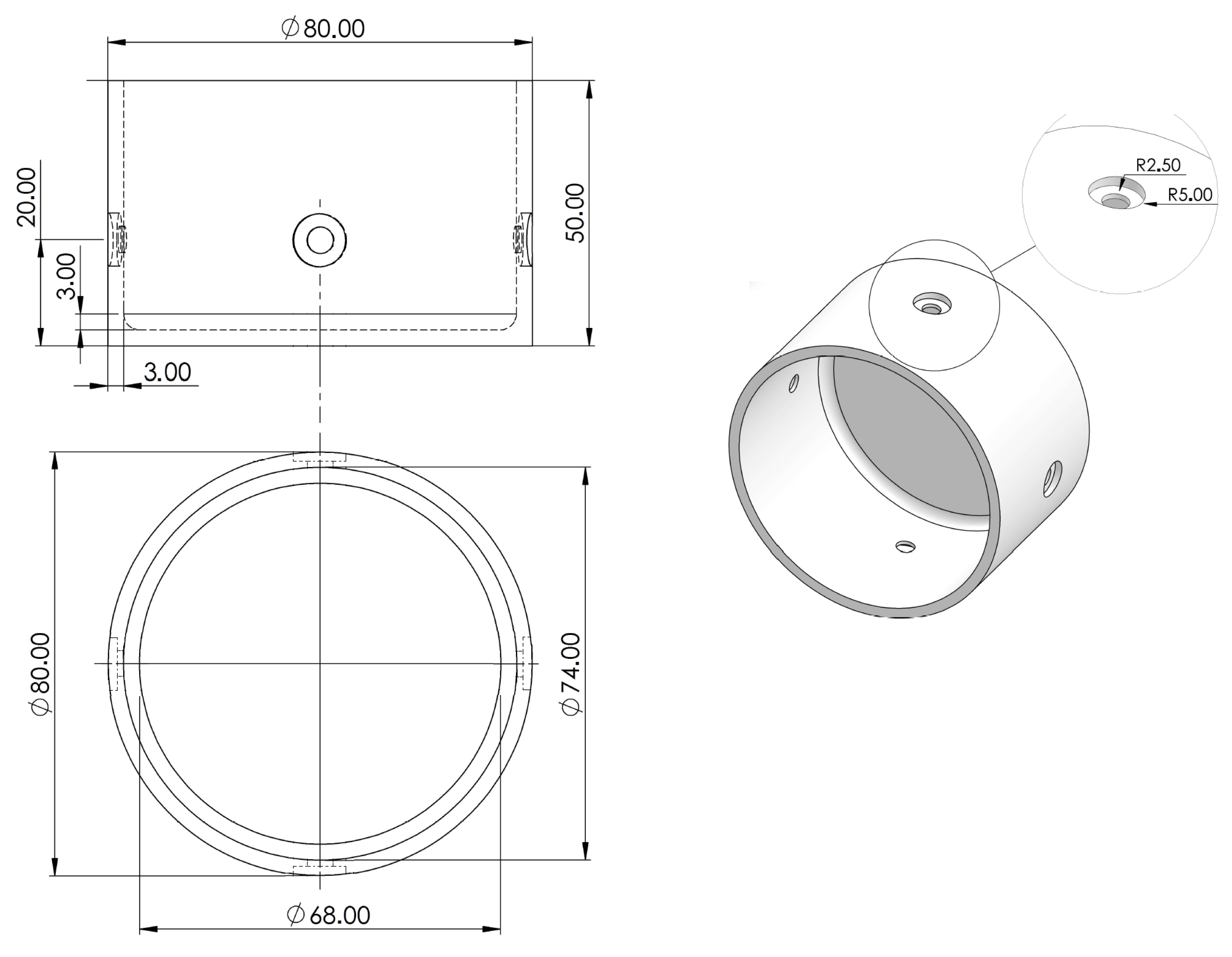
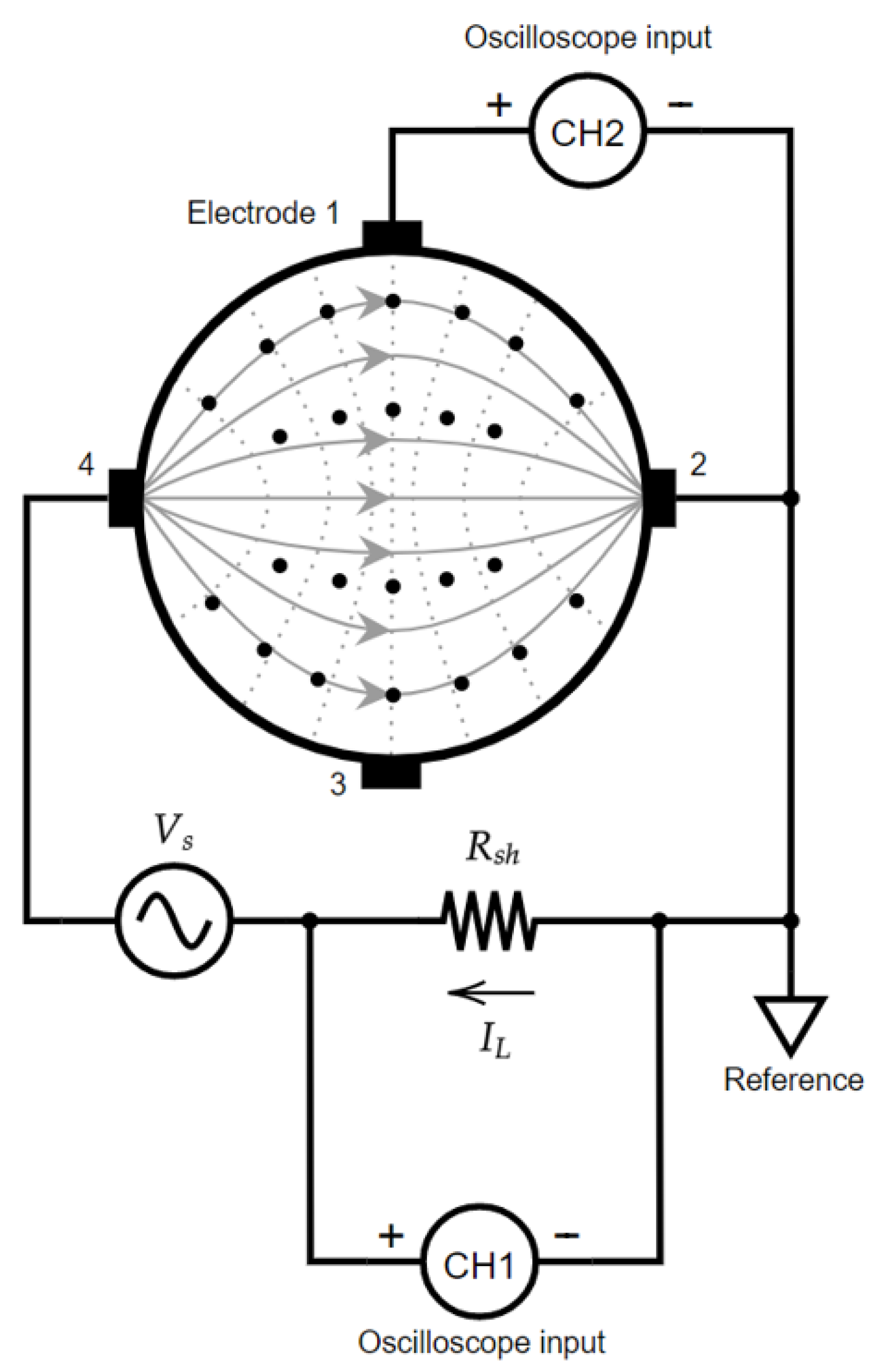
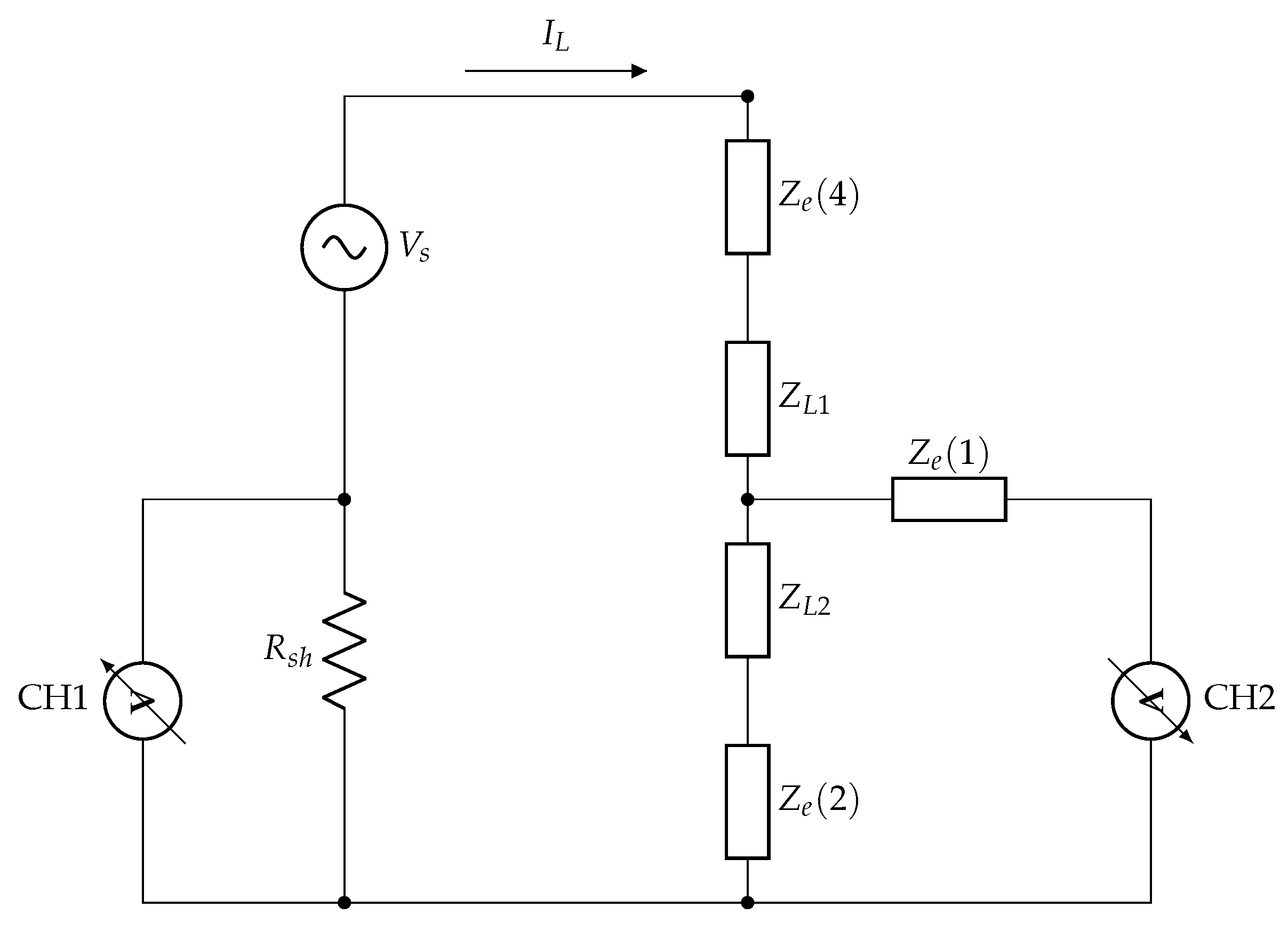
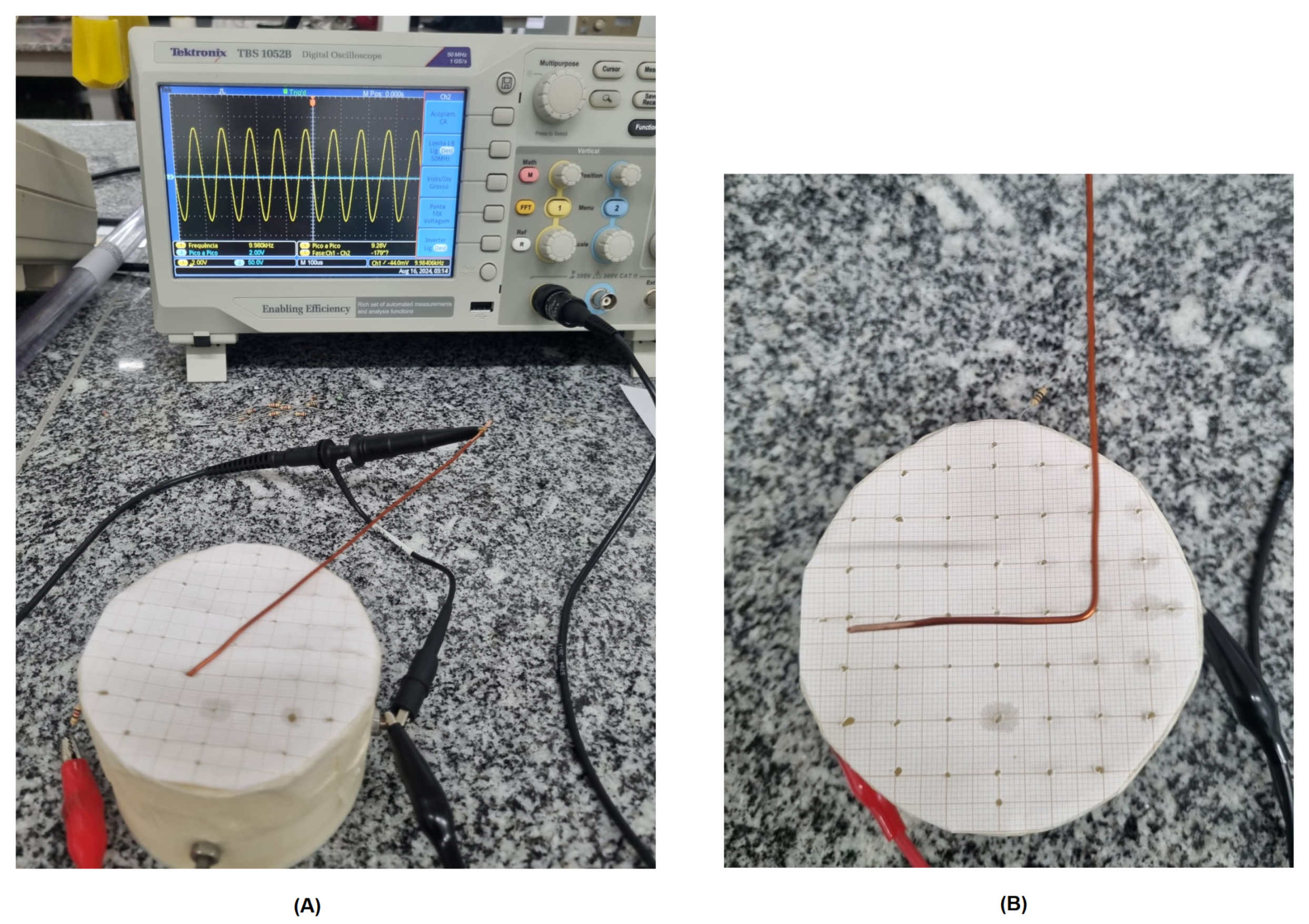
2.1. Validation of the Prototype
2.2. UHT Milk Analysis
2.3. Statistical Analysis and Mathematical Validation Model
3. Results and Discussion
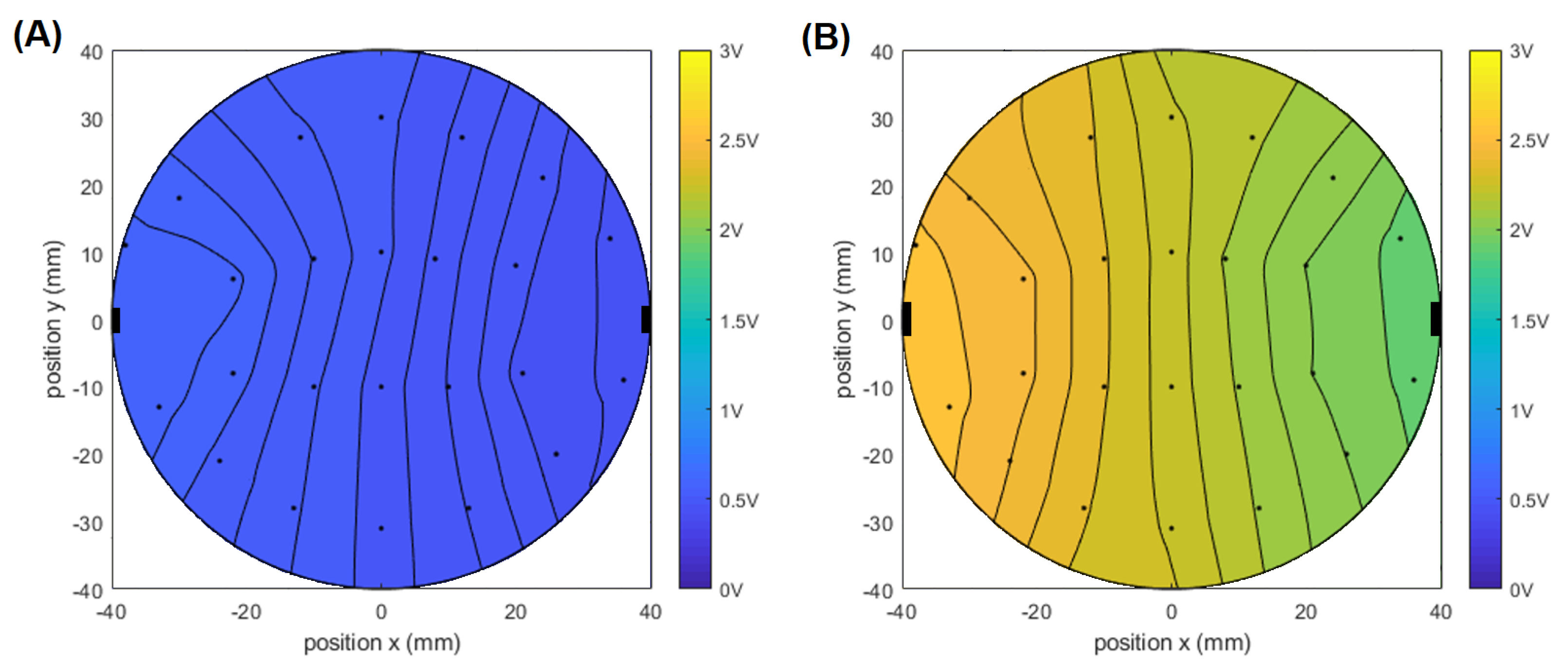
3.1. Bioimpedance Measurement Prototype Validation
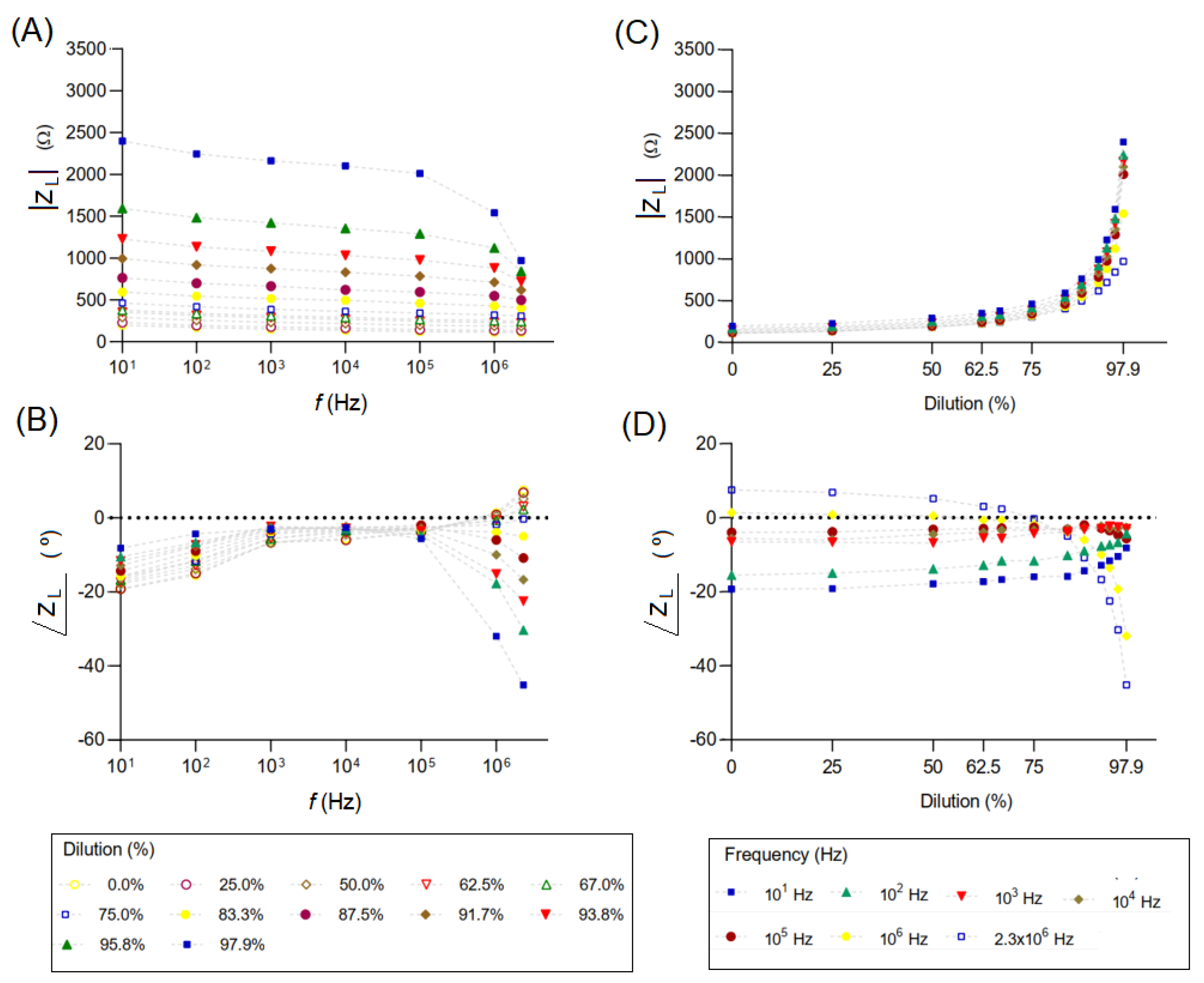
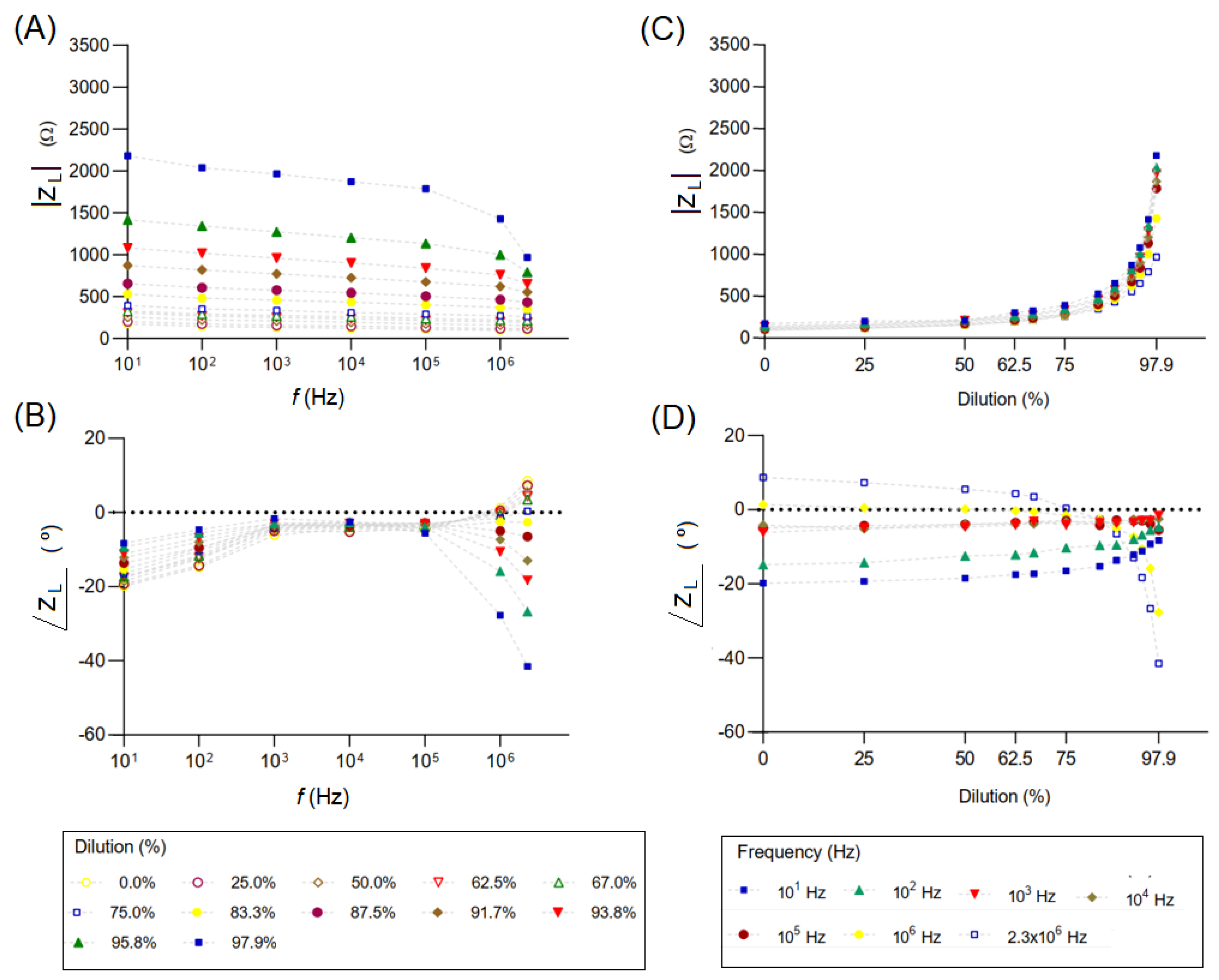
3.2. Analysis of Adulterated Milk and the Influence of Temperature
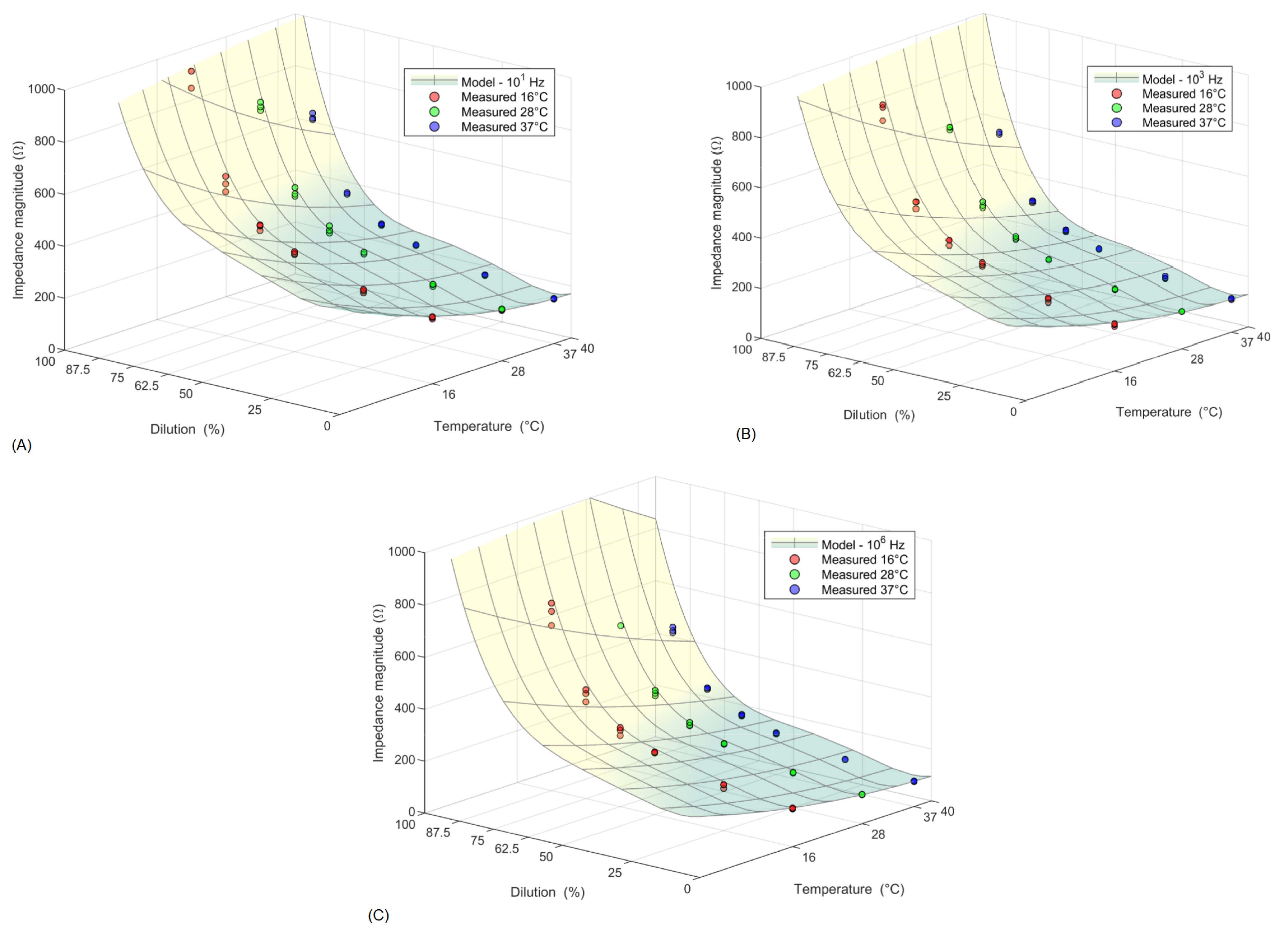
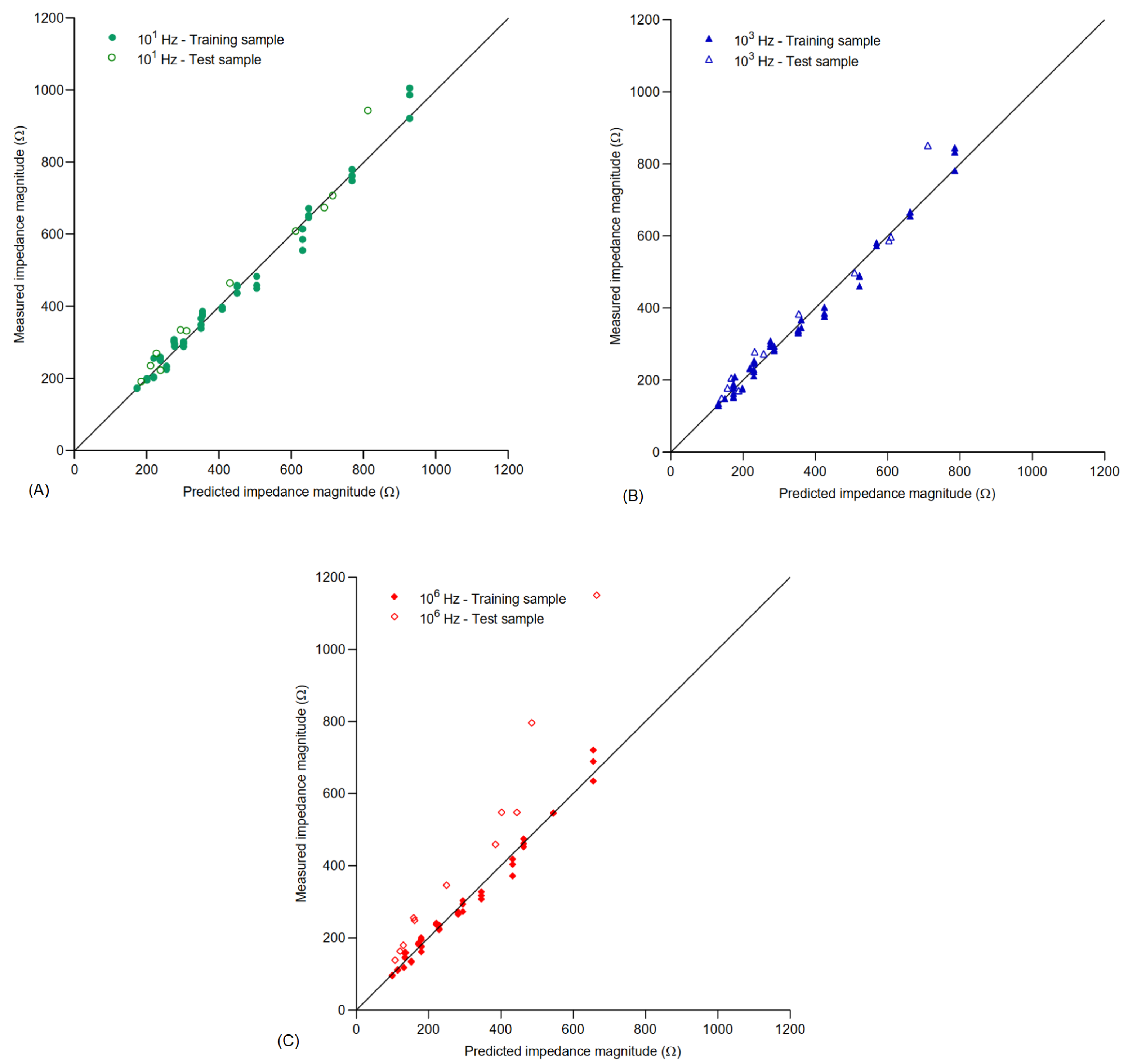
3.3. Mathematical Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IBGE. IBGE Indicators: Livestock Production Statistics; Technical report; IBGE: Rio de Janeiro, Brazil, 2020.
- EMBRAPA. Milk Yearbook 2018: Indicators, Trends and Opportunities for Those Living in the Dairy Sector; Technical Report; EMBRAPA: São Paulo, Brazil, 2018. [Google Scholar]
- Bertolini, A.B.; Rossi, G.A.M. Physical-chemical analysis and fraud detection in heat-treated milk processed by ultra-high temperature (UAT) sold in the Midwest Region of the State of São Paulo. J. Hyg. Anim. Sanity 2017, 11, 374–381. [Google Scholar] [CrossRef][Green Version]
- Oliveira, K.B.; Kobori, C.N.; Ubaldo, J.C.S.R. Assessment of physico-chemical quality, labelling and occurrence of adulteration in UHT milk samples. Dairy Inst. Mag. Cândido Tostes 2019, 74, 195–206. [Google Scholar]
- Cunha, M.F. Review: UHT Milk and the Gelatinization Phenomenon. Food Process. Res. Cent. Bull. 2001, 19, 341–352. [Google Scholar]
- Gennari, A.; Grave, E.; Wagner, E.; Mallmann, G.; Souza, C.F.V. Physical-Chemical and Microbiological Evaluation of Milk During Processing. Mag. Acad. Highlights 2014, 6, 91–95. [Google Scholar]
- Behmer, M.L.A. Milk Technology: Production, Industrialization and Analysis.; Nobel: São Paulo, Brazil, 1984. [Google Scholar]
- Brito, M.A.; Brito, J.R.; Arcuri, E.; Lange, C.; Silva, M.; Souza, G. Composition; Technical report; Brazilian Agricultural Research Corporation: Brasilia, Brazil, 2007. [Google Scholar]
- Abrantes, M.R.; Campêlo, C.S.; Silva, J.B.A. Milk fraud: Methods of detection and implications for the consumer. Adolfo Lutz Inst. Mag. 2014, 73, 244–251. [Google Scholar]
- MAPA. Normative Instruction No. 30. Officializes the Manual of Official Methods for Analysis of Food of Animal Origin; Technical report; MAPA: Brasília, Brazil, 2018. [Google Scholar]
- Pinheiro, L.A.F. Detection of Fraud in Milk with Water by Volumetric Thermal Capacity. Master’s Thesis, Faculty of Pharmacy and Biochemistry of Federal University of Juiz de Fora, Juiz de Fora, MG, Brazil, 2015. [Google Scholar]
- Wanderley, C.H.; Silva, A.C.O.; Silva, F.E.R.; Márcio, E.T.; Junior, C.A.C. Evaluation of the sensitivity of analytical methods for checking fluid milk fraud. Life Sci. J. 2012, 32, 34–42. [Google Scholar]
- BRASIL. Normativa nº 68, de 12 de Dezembro de 2006. Oficializa os Métodos Analíticos Oficiais Físico-Químicos, Para Controle de Leite e Produtos Lácteos; Techreport 68; Diario Oficial da Uniao: Brasilia, Brazil, 2006. [Google Scholar]
- Andrade, J.; Pereira, C.G.; de Almeida Junior, J.C.; Viana, C.C.R.; de Oliveira Neves, L.N.; da Silva, P.H.F.; Bell, M.J.V.; de Carvalho dos Anjos, V. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Fagnani, R. The Main Frauds in Milk; Techreport; Milkpoint: Piracicaba, Brazil, 2016. [Google Scholar]
- Lopes, A.M.; Machado, J.A.T.; Ramalho, E.; Silva, V. Milk characterization using electrical impedance spectroscopy and fractional models. Food Anal. Methods 2018, 11, 901–912. [Google Scholar] [CrossRef]
- Schumacher, L.L.; Viégas, J.; Naetzold, S.; Tonin, T.J.; Rocha, L.; Cauduro, L.; Moro, A.B.; Robalo, S.S. Use of electrical bioimpedance analysis to evaluate the quality of bovine raw milk. S. Afr. J. Anim. Sci. 2019, 49, 727–734. [Google Scholar] [CrossRef]
- Nascimento, W.L.; Anjos, L.; Bell, V.; José, M. Mathematical models for correlating electrical parameters and milk adulterants. QUARKS Braz. Electron. J. Phys. Chem. Mater. Sci. 2020, 2, 27–33. [Google Scholar] [CrossRef][Green Version]
- Santos, M.M., Jr. Identification of Bovine Milk Adulteration by the Wave Propagation Method in Medium Material Using Piezoelectric Diaphragms. Master’s Thesis, Faculty of Electrical Engineering of Paulista State University, Bauru, SP, Brazil, 2019. [Google Scholar]
- Bakr, A.A.; Radwan, A.G.; Madian, A.H.; Elwakil, A.S. Aging effect on apples bio-impedance using AD5933. In Proceedings of the 3rd International Conference on Advances in Computational Tools for Engineering Applications (ACTEA), Beirut, Lebanon, 3–5 July 2019; pp. 158–161. [Google Scholar]
- Freeborn, T.J.; Elwakil, A.S.; Maundy, B. Variability of Cole-model bioimpedance parameters using magnitude-only measurements of apples from a two-electrode configuration. Int. J. Food Prop. 2017, 20, 507–509. [Google Scholar] [CrossRef]
- Khaled, D.E.; Castellano, N.N.; Gazquez, J.A.; Garcia, S.R.M.; Manzano-Agugliaro, F. Cleaner quality control system using bioimpedance methods: A review for fruits and vegetables. J. Clean. Prod. 2017, 140, 1749–1762. [Google Scholar] [CrossRef]
- Al-Ali, A.A.; Elwakil, A.S.; Maundy, B.J. Bio-impedance Measurements with Phase Extraction using the Kramers-Kronig transform: Application to Strawberry Aging. In Proceedings of the IEEE 61st International Midwest Symposium on Circuits and Systems (MWSCAS), Windsor, ON, Canada, 5–8 August 2018; pp. 468–471. [Google Scholar]
- BRASIL. Manual de Métodos Oficiais Para Análise de Alimentos de Origem Animal; Techreport; MAPA: Brasilia, Brazil, 2007. [Google Scholar]
- Geller, A.M.; Ayres, L.; Carvalho, C.W.; Neuenfeld, R.H.; Oliveira, W.C. Ciência, Tecnologia e Inovação: Do Campo à Mesa; Chapter Escaneamento por Bioimpedância em Leite: Explorando a Técnica; IIDV: Recife, Brazil, 2020; Volume 1, pp. 653–674. [Google Scholar]
- Hui, G.; Ying, Y. Quantitative rapid analysis method for ofloxacin in raw milk based on molecule-specific recognition and electrochemical impedance spectrum. Trans. ASABE 2017, 60, 1439–1443. [Google Scholar] [CrossRef]
- Moro, L.C.; Porto, R.W. Single frequency electrical impedance tomography system with offline reconstruction algorithm. In Proceedings of the IEEE 6th Latin American Symposium on Circuits and Systems (LASCAS), Montevideo, Uruguay, 25–27 February 2015; pp. 1–4. [Google Scholar]
- Bertemes-Filho, P.; Valicheski, R.; Pereira, R.M.; Paterno, A.S. Bioelectrical impedance analysis for bovine milk: Preliminary results. In Proceedings of the International Conference on Electrical Bioimpedance, Gainesville, FL, USA, 4–8 April 2018; pp. 1–4. [Google Scholar]
- Veiga, E.A.; Bertemes-Filho, P. Bioelectrical impedance analysis of bovine milk fat. In Proceedings of the First Latin-American Conference on Bioimpedance (CLABIO), Joinville, Brazil, 6–9 November 2012; pp. 1–5. [Google Scholar]
- Mabrook, M.F.; Petty, M.C. Effect of composition on the electrical conductance of milk. J. Food Eng. 2003, 60, 321–325. [Google Scholar] [CrossRef]
- Doebelin, E.O. Measurement Systems: Application and Design; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Hua, P.; Webster, J.G.; Tompkins, W.J. Effect of the measurement method on noise handling and image quality of EIT imaging. In Proceedings of the 9th International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 4–7 November 1987; pp. 1429–1430. [Google Scholar]
- Bera, T.K.; Nagaraju, J. Studying the resistivity imaging of chicken tissue phantoms with different current patterns in Electrical Impedance Tomography (EIT). Measurement 2012, 45, 663–682. [Google Scholar] [CrossRef]
- Tarabi, N.; Mousazadeh, H.; Jafari, A.; Taghizadeh-Tameh, J.; Kiapey, A. Experimental evaluation of some current injection-voltage reading patterns in electrical impedance tomography (EIT) and comparison to simulation results - Case study: Large scales. Flow Meas. Instrum. 2022, 83, 102087. [Google Scholar] [CrossRef]
- Martinsen, O.G.; Grimnes, S. Bioimpedance and Bioelectricity Basics; Academic Press: London, UK, 2008. [Google Scholar]
- Geddes, L.A.; Baker, L.E. Principles of Applied Biomedical Instrumentation; Wiley-Interscience: New York, NY, USA, 1991. [Google Scholar]
- Montgomery, D.C.; Peck, E.A.; Vinning, G.G. Introduction to Linear Regression Analysis; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Caldeira, L.A.; Rocha, V.R.; Fonseca, C.M.; Melo, L.M.; Cruz, A.G.; Oliveira, L.L.S. Characterization of milk commercialized in Janaúba-MG. Aliment. E Nutr. 2010, 21, 191–195. [Google Scholar]
- Kartheek, M.; Smith, A.A.; Muthu, A.K.; Manavalan, R. Determination of adulterants in food: A review. J. Chem. Pharm. Res. 2011, 3, 629–636. [Google Scholar]
- Mareze, J.; Marioto, L.R.M.; Gonzaga, N.; Tamanini, G.C.D.R.; Beloti, V. Detection of adulteration of pasteurised milk by official tests. Semin. Biol. Health Sci. 2015, 36, 283–290. [Google Scholar]
- Rosa, L.S.; Garbin, C.M.; Zamboni, L.; Bonacina, S.M. Evaluation of the physicochemical quality of ultra pasteurised milk marketed in the municipality of Erechim-RS. Health Surveill. Discuss. 2015, 3, 99–107. [Google Scholar]
- Oshima, M. Empirical formula for correcting electrical conductivity values of milk in relation to temperature. Jpn. J. Zootech. Sci. 1978, 49, 180–188. [Google Scholar]
- Das, S.; Sivaramakrishna, M.; Biswas, K.; Goswami, B. A low cost instrumentation system to analyze different types of milk adulteration. ISA Trans. 2015, 56, 268–275. [Google Scholar] [CrossRef]
- Tripathy, S.; Ghole, A.R.; Deep, K.; Vanjari, S.R.K.; Singh, S.G. A comprehensive approach for milk adulteration detection using inherent bio-physical properties as ‘Universal Markers’: Towards a miniaturized adulteration detection platform. Food Chem. 2017, 217, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Durante, G.; Becari, W.; Lima, F.A.S.; Peres, E.M. Electrical impedance sensor for real-time detection of bovine milk adulteration. IEEE Sens. J. 2016, 16, 861–865. [Google Scholar] [CrossRef]
- Sude, M.; Ghodinde, K. Electrical impedance sensor for real-time detection of urea and starch in milk. In Proceedings of the 3rd International Conference on Trends in Electronics and Informatics (ICOEI), Tirunelveli, India, 23–25 April 2019; pp. 431–434. [Google Scholar]
- Bahçeci, K.S.; Acar, J. Modeling the combined effects of pH, temperature and ascorbic acid concentration on the heat resistance of Alicyclobacillus acidoterrestis. Int. J. Food Microbiol. 2007, 120, 266–273. [Google Scholar] [CrossRef] [PubMed]



| Percentage of Water in Milk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 25% | 50% | 75% | 97.9% | ||||||
| (Hz) | ||||||||||
| Percentage of Water in Milk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 25% | 50% | 75% | 97.9% | ||||||
| (Hz) | ||||||||||
| Percentage of Water in Milk | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.0% | 25% | 50% | 75% | 97.9% | ||||||
| (Hz) | ||||||||||
| Dilution | Temperature | |||||
|---|---|---|---|---|---|---|
| (%V/V) | 16 | 28 | 37 | |||
| AC | AC | AC | ||||
| 00.0 | −0.025 | 0.32 | −0.023 | 0.39 | −0.020 | 0.42 |
| 25.0 | −0.031 | 0.33 | −0.024 | 0.38 | −0.022 | 0.42 |
| 50.0 | −0.036 | 0.35 | −0.029 | 0.41 | −0.022 | 0.58 |
| 62.5 | −0.043 | 0.38 | −0.034 | 0.43 | −0.030 | 0.47 |
| 67.0 | −0.047 | 0.40 | −0.036 | 0.44 | −0.032 | 0.50 |
| 75.0 | −0.054 | 0.42 | −0.042 | 0.47 | −0.034 | 0.36 |
| 83.3 | −0.079 | 0.51 | −0.057 | 0.58 | −0.053 | 0.57 |
| 87.5 | −0.112 | 0.60 | −0.080 | 0.62 | −0.070 | 0.62 |
| 91.7 | −0.186 | 0.74 | −0.121 | 0.73 | −0.102 | 0.68 |
| 93.8 | −0.250 | 0.80 | −0.194 | 0.83 | −0.142 | 0.73 |
| 95.8 | −0.411 | 0.88 | −0.224 | 0.83 | −0.217 | 0.83 |
| 97.9 | −0.815 | 0.95 | −0.540 | 0.94 | −0.446 | 0.92 |
| Frequency | Adjus. | RMSE | |
|---|---|---|---|
| 10 Hz | 0.9957 | 0.9950 | 15.0282 |
| 1 kHz | 0.9969 | 0.9965 | 11.2313 |
| 1 MHz | 0.9949 | 0.9941 | 12.2881 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, W.d.C.; Geller, A.M.; Neuenfeld, R.H.; Carvalho, C.W.; Húngaro, H.M.; Ayres, L.C.; Oliveira, M.B.P.P.; Porto, R.W. Electrical Bioimpedance in Milk Adulterated with Water: Measurement Methodology for Quantification, Influence of Temperature, and Mathematical Modelling. Appl. Sci. 2024, 14, 8026. https://doi.org/10.3390/app14178026
Oliveira WdC, Geller AM, Neuenfeld RH, Carvalho CW, Húngaro HM, Ayres LC, Oliveira MBPP, Porto RW. Electrical Bioimpedance in Milk Adulterated with Water: Measurement Methodology for Quantification, Influence of Temperature, and Mathematical Modelling. Applied Sciences. 2024; 14(17):8026. https://doi.org/10.3390/app14178026
Chicago/Turabian StyleOliveira, Wemerson de Castro, Ana Maria Geller, Renato Hartwig Neuenfeld, Claudia Wollmann Carvalho, Humberto Moreira Húngaro, Luciano Carvalho Ayres, Maria Beatriz Prior Pinto Oliveira, and Rodrigo Wolff Porto. 2024. "Electrical Bioimpedance in Milk Adulterated with Water: Measurement Methodology for Quantification, Influence of Temperature, and Mathematical Modelling" Applied Sciences 14, no. 17: 8026. https://doi.org/10.3390/app14178026
APA StyleOliveira, W. d. C., Geller, A. M., Neuenfeld, R. H., Carvalho, C. W., Húngaro, H. M., Ayres, L. C., Oliveira, M. B. P. P., & Porto, R. W. (2024). Electrical Bioimpedance in Milk Adulterated with Water: Measurement Methodology for Quantification, Influence of Temperature, and Mathematical Modelling. Applied Sciences, 14(17), 8026. https://doi.org/10.3390/app14178026








