Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate
Abstract
:1. Introduction
2. Cu-Based Catalyst-Activated PMS Processes
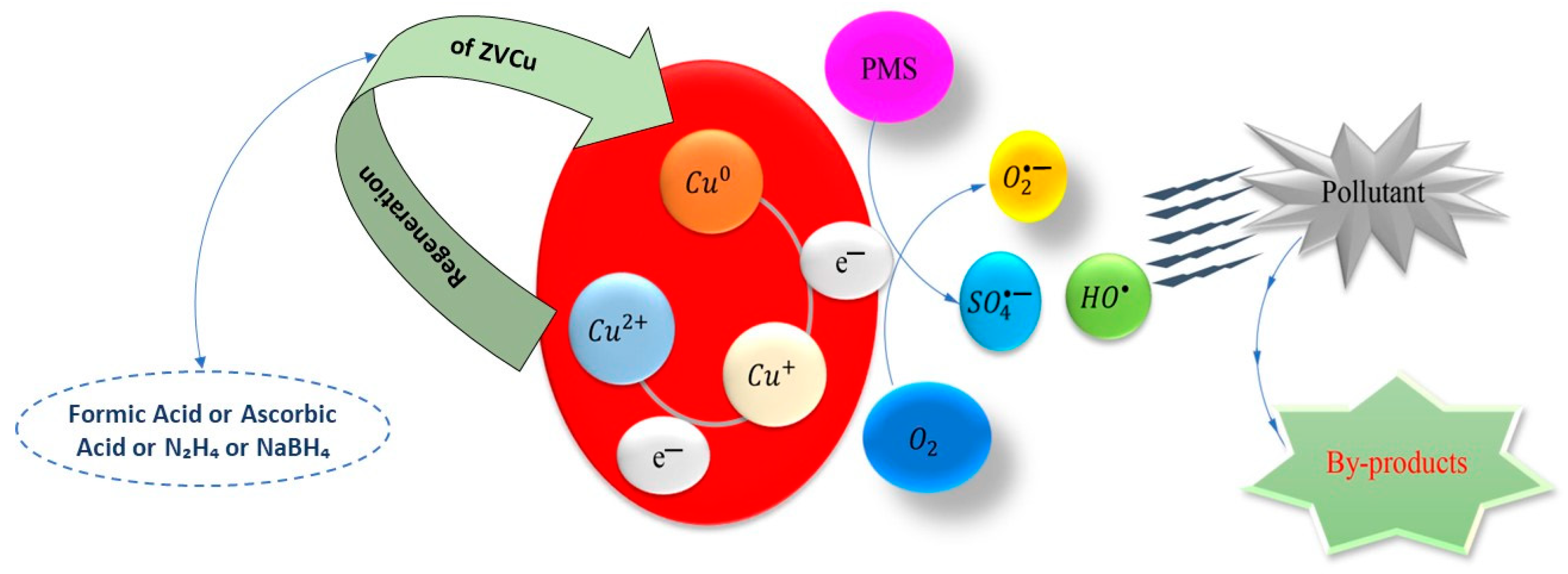
2.1. Zero-Valent Copper
2.2. Copper Oxide
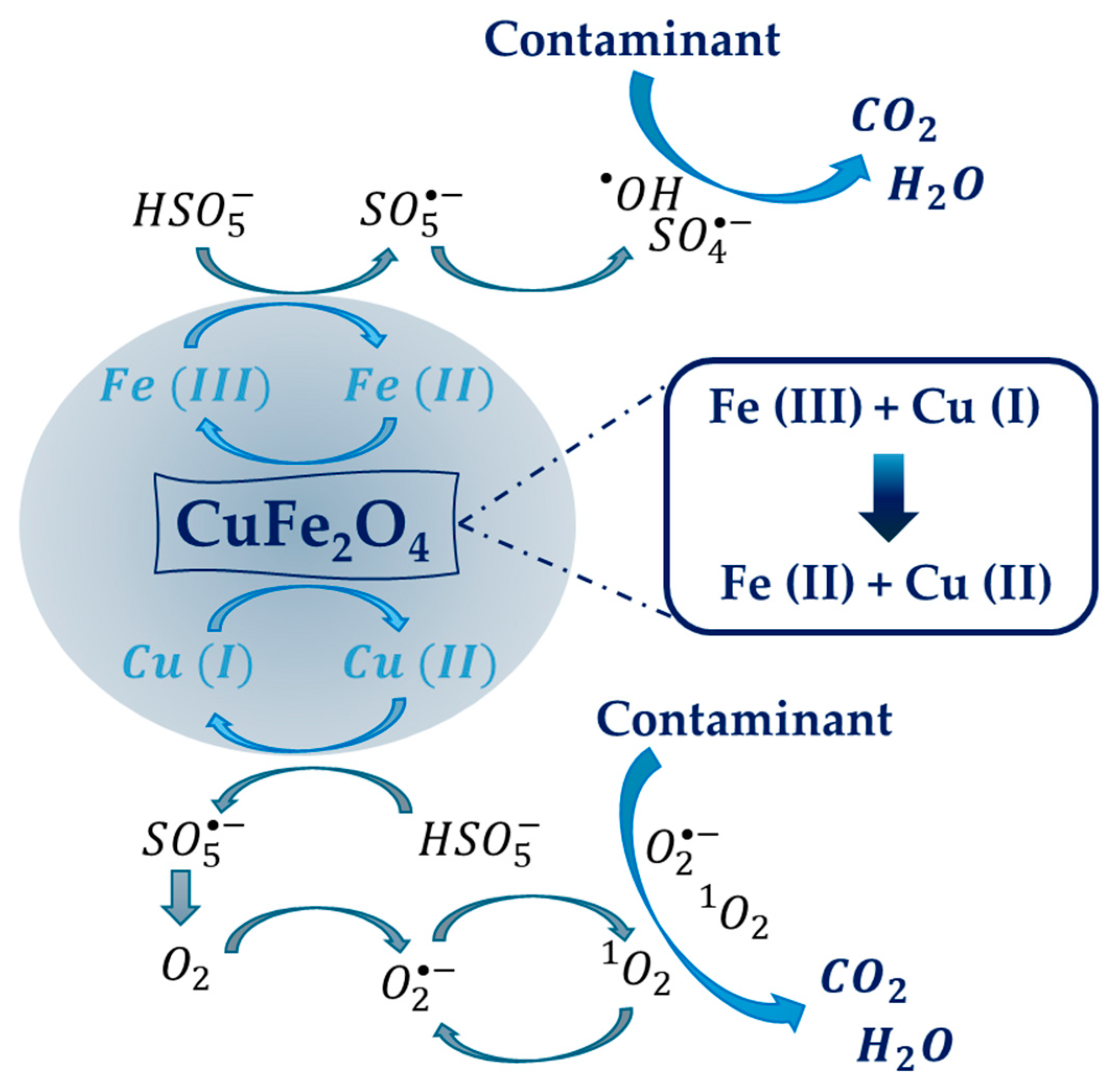
2.3. Magnetic Copper Ferrite
2.4. Cu-Based Designed Catalyst Materials
2.4.1. Cu-Based MOFs
- -
- Solvothermal synthesis: This is the most widely used method to produce Cu-MOFs. In this technique, the copper salts and organic linkers are dissolved in a solvent and heated in a sealed container (often an autoclave) at elevated temperatures, typically in the range of 80° C to 220° C. The solvothermal conditions facilitate the self-assembly of the metal ions and organic linkers into the desired structural structure [106]. This method allows for precise control over the size, morphology, and crystallinity of the MOFs by adjusting parameters such as temperature, reaction time, and solvent type.
- -
- MW-assisted synthesis: This offers a fast and energy-efficient approach to producing Cu-MOFs. This method involves the use of MW radiation to heat the reaction mixture, significantly reducing the synthesis time compared to conventional solvothermal methods. MW-assisted synthesis can improve the nucleation and growth rates of MOFs, leading to high-quality crystalline materials with uniform particle sizes [107]. The rapid heating and cooling cycles provided by MW irradiation also help to minimize the formation of defects within the MOF structure.
- -
- Electrochemical synthesis: This is an emerging technique for producing Cu-MOFs, where an electric current is applied to drive the assembly of the framework. In this method, a copper anode is dissolved to release copper ions in an electrolyte solution containing organic linkers. The electric field promotes the interaction between the copper ions and the linkers, which results in the formation of the MOF on the electrode surface [108]. Electrochemical synthesis is advantageous for producing thin films of MOF directly on conductive substrates, which makes it suitable for applications in sensors and electronic devices.
- -
- Sonochemical synthesis: This uses ultrasonic waves to induce chemical reactions and facilitate the formation of copper MOFs. The ultrasonic waves generate high temperatures and localized pressures within the reaction mixture, improving the reaction kinetics and promoting the formation of the MOF structure [109]. This method is known for its simplicity, fast reaction times, and ability to produce MOFs with unique morphologies and improved surface areas.
- -
- Mechanochemical synthesis: This involves the use of mechanical force to induce chemical reactions between copper salts and organic linkers. This technique typically employs grinding or ball milling to physically mix and activate the reactants, leading to the formation of the MOF structure without the need for solvents or high temperatures [110]. Mechanochemical synthesis is environmentally friendly and scalable, which makes it a promising approach for the large-scale production of Cu-MOFs.
2.4.2. Cu-Based LDHs
| Catalyst | Contaminant | Reactive Species | Efficiency and Reusability | Ref. |
|---|---|---|---|---|
| HKUST-1 | Saccharomyces cerevisiae (>105 CFU·mL−1) Geotrichum candidum (>105 CFU·mL−1) | - | 100% in 25 h >79% in 25 h | [123] |
| HKUST-1 | E. coli (1010 CFU·mL−1) | , | 100% in 30 min 4 cycles, 100% | [124] |
| FeCu-MOF | Methylene blue (0.2 mM) | 100% in 30 min 3 cycles, 87.1% | [125] | |
| CuCo-MOF | Nimesulide (20 mg·L−1) | , | 100% in 25 min | [126] |
| CuCo-MOF-74 | Methylene blue (0.2 mM) | 100% in 30 min 5 cycles, 76.4% | [116] | |
| Co1Cu1-MOF | Tetracycline (20 mg·L−1) | , 1, | 98.17% in 30 min 4 cycles, 71.26% | [101] |
| NH2–Fe2.4Cu1-MOF in polyacrylonitrile spheres | RhB (10 mg·L−1) | , | 80.92% in 90 min 5 cycles, >77% | [127] |
| Co2Cu1-LDH | Lomefloxacin (10 mg·L−1) | , | 96.2% in 30 min 10 cycles, >95% | [102] |
| MgCuFe-LDH | RhB (5 mg·L−1) Acetaminophen (5 mg·L−1) | , | 97.6% in 25 min 93% in 20 min | [128] |
| Cu-Co-Fe-LDH | Nitrobenzene (2 mg·L−1) | at pH < 7 at pH > 9 | 100% in 6 min 5 cycles, >87% | [129] |
| CuMn-LDH | BPA (5 mg·L−1) | 1, | 100% 90 min 4 cycles, >95% | [130] |
| CoCu-LDH | Sulfamethoxazole (10 mg·L−1) | , | 86.6% in 60 min | [131] |
| CoCu-LDH@polyvinylidene fluoride (PVDF) | Sulfamethoxazole Sulfacetamide Lomefloxacin Carbamazepine (each 10 mg·L−1) | , | 92.8% in 60 min 89.6% in 60 min 97.1% in 60 min 91.8% in 60 min | [131] |
| CuCoFe-LDH coated on biochar | Phenanthrene (1 mg·L−1) | , | 96.5% in 15 min 4 cycles, >80% | [132] |
| CuCoFe-LDH | Glyphosate (100 mg·L−1) | 99.54% in 5 min 5 cycles, 90.34% | [133] | |
| CuO nanoparticles–DES (DL-menthol/Fenchyl alcohol) | RhB (20 mg·L−1) | , | 98% in 18 min | [134] |
| Cu-BDC * MOF-DES (Choline chloride/urea) | AO7 (30 mg·L−1) | - | 99% in 120 min | [135] |
2.4.3. Cu-Based DESs
3. Investigative Methodologies for Assessing PMS Activation with Copper-Based Catalysts
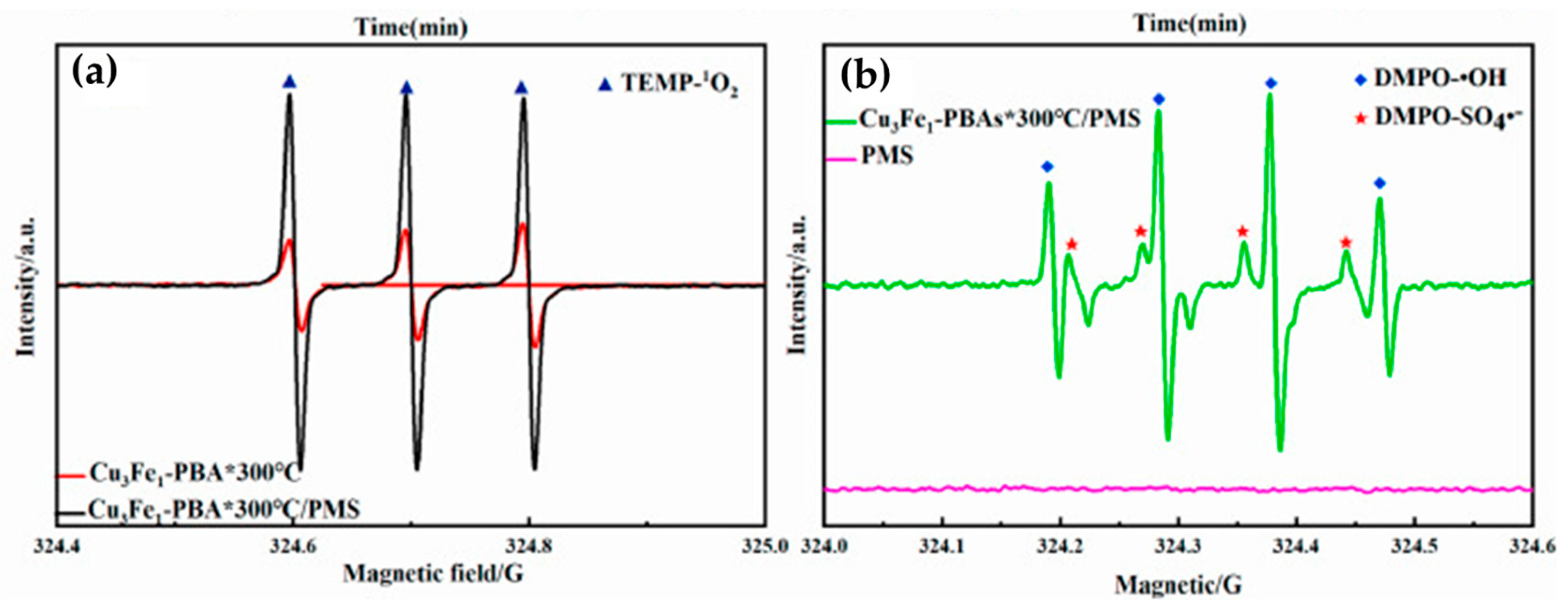
3.1. EPR and ESR Methods
3.2. Radical Quenching
4. Factors Influencing Reactivity
4.1. Impact of pH on PMS Activation
4.2. Impact of PMS and Catalyst Concentrations
4.3. Anions
4.4. Natural Organic Matter
5. Coupling Copper-Based Catalyst/PMS Systems with Other Advanced Oxidation Techniques
6. Mechanisms for PMS Activation by Copper-Based Catalysts
6.1. Radical Pathways
6.2. Non-Radical Pathways
7. Toxicity Assessment after PMS-Based Processes
8. Conclusions and Outlooks
- -
- Future research directions: Continued research should focus on enhancing the stability and reusability of Cu-based catalysts, particularly through innovative catalyst design and the exploration of new materials such as bimetallic MOFs and advanced LDHs. Further investigation into the mechanisms of PMS activation, especially under real-world conditions, will be critical for optimizing these systems.
- -
- Real applications: The translation of laboratory findings into large-scale industrial applications remains a key objective. This includes scaling up the synthesis of Cu-based catalysts and integrating them into existing wastewater treatment infrastructure. The potential for coupling Cu-based catalysts with other AOPs offers exciting opportunities for developing more efficient and comprehensive treatment systems.
- -
- Sustainability considerations: As environmental regulations become more stringent, the demand for sustainable water treatment technologies will continue to grow. Cu-based catalysts, with their low toxicity and high efficiency, are well-positioned to meet this demand. Future developments should prioritize minimizing the environmental footprint of these catalysts, ensuring they contribute to a circular economy by enabling the recovery and reuse of resources.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manzoor, M.H.; Naz, N.; Naqvi, S.M.G.; Ashraf, S.; Ashiq, M.Z.; Verpoort, F. Wastewater Treatment Using Metal-Organic Frameworks (MOFs). Appl. Mater. Today 2024, 40, 102358. [Google Scholar] [CrossRef]
- Alayande, A.B.; Qi, W.; Karthikeyan, R.; Popat, S.C.; Ladner, D.A.; Amy, G. Use of Reclaimed Municipal Wastewater in Agriculture: Comparison of Present Practice versus an Emerging Paradigm of Anaerobic Membrane Bioreactor Treatment Coupled with Hydroponic Controlled Environment Agriculture. Water Res. 2024, 265, 122197. [Google Scholar] [CrossRef]
- Lincho, J.; Martins, R.C.; Gomes, J. Paraben Compounds—Part I: An Overview of Their Characteristics, Detection, and Impacts. Appl. Sci. 2021, 11, 2307. [Google Scholar] [CrossRef]
- Kumar, R.; Qureshi, M.; Vishwakarma, D.K.; Al-Ansari, N.; Kuriqi, A.; Elbeltagi, A.; Saraswat, A. A Review on Emerging Water Contaminants and the Application of Sustainable Removal Technologies. Case Stud. Chem. Environ. Eng. 2022, 6, 100219. [Google Scholar] [CrossRef]
- Silva, S.; Cardoso, V.V.; Duarte, L.; Carneiro, R.N.; Almeida, C.M.M. Characterization of Five Portuguese Wastewater Treatment Plants: Removal Efficiency of Pharmaceutical Active Compounds through Conventional Treatment Processes and Environmental Risk. Appl. Sci. 2021, 11, 7388. [Google Scholar] [CrossRef]
- Pandey, P.K.; Kass, P.H.; Soupir, M.L.; Biswas, S.; Singh, V.P. Contamination of Water Resources by Pathogenic Bacteria. AMB Express 2014, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Hazra, M.; Joshi, H.; Williams, J.B.; Watts, J.E.M. Antibiotics and Antibiotic Resistant Bacteria/Genes in Urban Wastewater: A Comparison of Their Fate in Conventional Treatment Systems and Constructed Wetlands. Chemosphere 2022, 303, 135148. [Google Scholar] [CrossRef]
- Mary Ealias, A.; Meda, G.; Tanzil, K. Recent Progress in Sustainable Treatment Technologies for the Removal of Emerging Contaminants from Wastewater: A Review on Occurrence, Global Status and Impact on Biota. Rev. Environ. Contam. Toxicol. 2024, 262, 16. [Google Scholar] [CrossRef]
- Hassani, A.; Scaria, J.; Ghanbari, F.; Nidheesh, P. V Sulfate Radicals-Based Advanced Oxidation Processes for the Degradation of Pharmaceuticals and Personal Care Products: A Review on Relevant Activation Mechanisms, Performance, and Perspectives. Environ. Res. 2023, 217, 114789. [Google Scholar] [CrossRef]
- Pisharody, L.; Gopinath, A.; Malhotra, M.; Nidheesh, P.V.; Kumar, M.S. Occurrence of Organic Micropollutants in Municipal Landfill Leachate and Its Effective Treatment by Advanced Oxidation Processes. Chemosphere 2022, 287, 132216. [Google Scholar] [CrossRef]
- Sandoval, M.A.; Vidal, J.; Calzadilla, W.; Salazar, R. Solar (Electrochemical) Advanced Oxidation Processes as Efficient Treatments for Degradation of Pesticides. Curr. Opin. Electrochem. 2022, 36, 101125. [Google Scholar] [CrossRef]
- Ike, I.A.; Linden, K.G.; Orbell, J.D.; Duke, M. Critical Review of the Science and Sustainability of Persulphate Advanced Oxidation Processes. Chem. Eng. J. 2018, 338, 651–669. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of Persulfate (PS) and Peroxymonosulfate (PMS) and Application for the Degradation of Emerging Contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Liu, G.; Li, C.; Stewart, B.A.; Liu, L.; Zhang, M.; Yang, M.; Lin, K. Enhanced Thermal Activation of Peroxymonosulfate by Activated Carbon for Efficient Removal of Perfluorooctanoic Acid. Chem. Eng. J. 2020, 399, 125722. [Google Scholar] [CrossRef]
- Mahdi-Ahmed, M.; Chiron, S. Ciprofloxacin Oxidation by UV-C Activated Peroxymonosulfate in Wastewater. J. Hazard. Mater. 2014, 265, 41–46. [Google Scholar] [CrossRef]
- Long, M.; Li, D.; Li, H.; Ma, X.; Zhao, Q.; Wen, Q.; Song, F. Synergetic Effect of Photocatalysis and Peroxymonosulfate Activated by MFe2O4 (M = Co, Mn, or Zn) for Enhanced Photocatalytic Activity under Visible Light Irradiation. RSC Adv. 2022, 12, 20946–20955. [Google Scholar] [CrossRef]
- Fu, J.; Feng, L.; Liu, Y.; Zhang, L.; Li, S. Electrochemical Activation of Peroxymonosulfate (PMS) by Carbon Cloth Anode for Sulfamethoxazole Degradation. Chemosphere 2022, 287, 132094. [Google Scholar] [CrossRef] [PubMed]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Zhou, X.; Wu, Q.; Zheng, H.; Chang, J.; Ren, N. Enhanced Peroxymonosulfate Activation for Sulfamethazine Degradation by Ultrasound Irradiation: Performances and Mechanisms. Chem. Eng. J. 2018, 335, 145–153. [Google Scholar] [CrossRef]
- Bouzayani, B.; Rosales, E.; Pazos, M.; Elaoud, S.C.; Sanromán, M.A. Homogeneous and Heterogeneous Peroxymonosulfate Activation by Transition Metals for the Degradation of Industrial Leather Dye. J. Clean. Prod. 2019, 228, 222–230. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of Sulfate Radical through Heterogeneous Catalysis for Organic Contaminants Removal: Current Development, Challenges and Prospects. Appl. Catal. B 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Hu, J.; Zou, Y.; Li, Y.; Xiao, Y.; Li, M.; Lin, L.; Li, B.; Li, X. Efficacy and Mechanism of Peroxymonosulfate Activation by Single-Atom Transition Metal Catalysts for the Oxidation of Organic Pollutants: Experimental Validation and Theoretical Calculation. J. Colloid Interface Sci. 2023, 645, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yin, K.; Li, N.; Liu, H.; Chen, J.; Zhou, X.; Zhang, Y. Efficient Activation of Peroxymonosulfate by Copper Supported on Polyurethane Foam for Contaminant Degradation: Synergistic Effect and Mechanism. Chem. Eng. J. 2022, 427, 131741. [Google Scholar] [CrossRef]
- Guo, S.; Chen, M.; You, L.; Wei, Y.; Cai, C.; Wei, Q.; Zhang, H.; Zhou, K. 3D Printed Hierarchically Porous Zero-Valent Copper for Efficient Pollutant Degradation through Peroxymonosulfate Activation. Sep. Purif. Technol. 2023, 305, 122437. [Google Scholar] [CrossRef]
- Singh, S.; Patidar, R.; Srivastava, V.C.; Kumar, P.; Singh, A.; Lo, S.-L. Ellipsoid-Shaped Copper Oxide as an Effective Peroxymonosulfate Activator for Perfluorooctanoic Acid Decomposition. Mater. Today Commun. 2023, 34, 105107. [Google Scholar] [CrossRef]
- Zuo, X.; Jiang, A.; Zou, S.; Wu, J.; Ding, B. Copper Oxides Activate Peroxymonosulfate for Degradation of Methylene Blue via Radical and Nonradical Pathways: Surface Structure and Mechanism. Environ. Sci. Pollut. Res. 2023, 30, 13023–13038. [Google Scholar] [CrossRef]
- Wang, C.; Dai, H.; Liang, L.; Li, N.; Cui, X.; Yan, B.; Chen, G. Enhanced Mechanism of Copper Doping in Magnetic Biochar for Peroxymonosulfate Activation and Sulfamethoxazole Degradation. J. Hazard. Mater. 2023, 458, 132002. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Liu, B.; Zhang, J.; Zhang, Y.; Zhang, G.; Wei, C.; Liang, J.; Liu, Y.; Zhang, W. Radicals Induced from Peroxomonosulfate by Nanoscale Zero-Valent Copper in the Acidic Solution. Water Sci. Technol. 2016, 74, 1946–1952. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Zhang, J.; Zhang, Y.; Zhang, G.; Li, W.; Wei, C.; Liang, J.; Liu, Y.; Shu, S. Degradation of 2,4-Dichlorophenol by Activating Persulfate and Peroxomonosulfate Using Micron or Nanoscale Zero-Valent Copper. J. Hazard. Mater. 2018, 344, 1209–1219. [Google Scholar] [CrossRef]
- Chi, H.; Wang, Z.; He, X.; Zhang, J.; Wang, D.; Ma, J. Activation of Peroxymonosulfate System by Copper-Based Catalyst for Degradation of Naproxen: Mechanisms and Pathways. Chemosphere 2019, 228, 54–64. [Google Scholar] [CrossRef]
- Hu, J.; Dong, H.; Qu, J.; Qiang, Z. Enhanced Degradation of Iopamidol by Peroxymonosulfate Catalyzed by Two Pipe Corrosion Products (CuO and δ-MnO2). Water Res. 2017, 112, 1–8. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Y.; Si, F.; Yao, C.; Du, M.; Hussain, I.; Kim, H.; Huang, S.; Lin, Z.; Hayat, W. Persulfate Non-Radical Activation by Nano-CuO for Efficient Removal of Chlorinated Organic Compounds: Reduced Graphene Oxide-Assisted and CuO (0 0 1) Facet-Dependent. Chem. Eng. J. 2019, 356, 178–189. [Google Scholar] [CrossRef]
- Wang, S.; Gao, S.; Tian, J.; Wang, Q.; Wang, T.; Hao, X.; Cui, F. A Stable and Easily Prepared Copper Oxide Catalyst for Degradation of Organic Pollutants by Peroxymonosulfate Activation. J. Hazard. Mater. 2020, 387, 121995. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tian, J.; Xiao, F.; Huang, R.; Gao, S.; Cui, F.; Wang, S.; Duan, X. Structure-Dependent Catalysis of Cuprous Oxides in Peroxymonosulfate Activation via Nonradical Pathway with a High Oxidation Capacity. J. Hazard. Mater. 2020, 385, 121518. [Google Scholar] [CrossRef]
- Li, G.; Zhong, Z.; Yang, C.; He, Q.; Peng, G. Degradation of Acid Orange 7 by Peroxymonosulfate Activated by Cupric Oxide. J. Water Supply Res. Technol.—AQUA 2019, 68, 29–38. [Google Scholar] [CrossRef]
- Qin, Q.; Qiao, N.; Liu, Y.; Wu, X. Spongelike Porous CuO as an Efficient Peroxymonosulfate Activator for Degradation of Acid Orange 7. Appl. Surf. Sci. 2020, 521, 146479. [Google Scholar] [CrossRef]
- Xu, Y.; Ai, J.; Zhang, H. The Mechanism of Degradation of Bisphenol A Using the Magnetically Separable CuFe2O4/Peroxymonosulfate Heterogeneous Oxidation Process. J. Hazard. Mater. 2016, 309, 87–96. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate Radicals Induced Degradation of Tetrabromobisphenol A with Nanoscaled Magnetic CuFe2O4 as a Heterogeneous Catalyst of Peroxymonosulfate. Appl. Catal. B 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Selvan, R.K.; Augustin, C.O.; Berchmans, L.J.; Saraswathi, R. Combustion Synthesis of CuFe2O4. Mater. Res. Bull. 2003, 38, 41–54. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, D.; Chu, W.; Li, M.; Lu, X. Nanoscaled Magnetic CuFe2O4 as an Activator of Peroxymonosulfate for the Degradation of Antibiotics Norfloxacin. Sep. Purif. Technol. 2019, 212, 536–544. [Google Scholar] [CrossRef]
- Kiani, R.; Mirzaei, F.; Ghanbari, F.; Feizi, R.; Mehdipour, F. Real Textile Wastewater Treatment by a Sulfate Radicals-Advanced Oxidation Process: Peroxydisulfate Decomposition Using Copper Oxide (CuO) Supported onto Activated Carbon. J. Water Process Eng. 2020, 38, 101623. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, L.; Ma, J.; Yang, J.; Feng, J.; Fan, Z. Sulfate Radicals Induced from Peroxymonosulfate by Magnetic Ferrospinel MFe2O4 (M = Co, Cu, Mn, and Zn) as Heterogeneous Catalysts in the Water. Appl. Catal. B 2015, 165, 572–578. [Google Scholar] [CrossRef]
- Wang, R.; An, H.; Zhang, H.; Zhang, X.; Feng, J.; Wei, T.; Ren, Y. High Active Radicals Induced from Peroxymonosulfate by Mixed Crystal Types of CuFeO2 as Catalysts in the Water. Appl. Surf. Sci. 2019, 484, 1118–1127. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y. Copper-Based Non-Precious Metal Heterogeneous Catalysts for Environmental Remediation. Chin. J. Catal. 2018, 39, 566–582. [Google Scholar] [CrossRef]
- Aflak, N.; Ben El Ayouchia, H.; Bahsis, L.; Anane, H.; Julve, M.; Stiriba, S.-E. Recent Advances in Copper-Based Solid Heterogeneous Catalysts for Azide–Alkyne Cycloaddition Reactions. Int. J. Mol. Sci. 2022, 23, 2383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-Based Catalysts for Persulfate and Peroxymonosulfate Activation in Heterogeneous Ways: A Review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Yang, L.; Ren, X.; Zhang, Y.; Chen, Z. One-Pot Preparation of Poly (Triazine Imide) with Intercalation of Cu Ions: A Heterogeneous Catalyst for Peroxymonosulfate Activation to Degradate Organic Pollutants under Sunlight. Inorg. Chem. Commun. 2022, 145, 109965. [Google Scholar] [CrossRef]
- Fu, C.; Yan, M.; Ma, H.; Zhang, S.; Yang, G.; Tian, H.; Yang, J.; Wang, Z.; Zhu, S.; Bhatt, K.; et al. Synthetic Organic Compounds Degradation by Dual Radical/Non-Radical Peroxymonosulfate with Copper Oxide as Efficient Heterogeneous Activator. J. Taiwan Inst. Chem. Eng. 2023, 146, 104839. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, M.; Ma, Q. Copper-Based Foliar Fertilizer and Controlled Release Urea Improved Soil Chemical Properties, Plant Growth and Yield of Tomato. Sci. Hortic. 2012, 143, 109–114. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.-P.; Wu, X.S. Review of Methanol Reforming-Cu-Based Catalysts, Surface Reaction Mechanisms, and Reaction Schemes. Int. J. Hydrogen Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, an Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar]
- Khalaj, M.; Kamali, M.; Khodaparast, Z.; Jahanshahi, A. Copper-Based Nanomaterials for Environmental Decontamination—An Overview on Technical and Toxicological Aspects. Ecotoxicol. Environ. Saf. 2018, 148, 813–824. [Google Scholar] [CrossRef]
- Fu, F.; Dionysiou, D.D.; Liu, H. The Use of Zero-Valent Iron for Groundwater Remediation and Wastewater Treatment: A Review. J. Hazard. Mater. 2014, 267, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, J.; Yang, B.; Huang, W.; Ma, L. Copper–Catalyzed Activation of Molecular Oxygen for Oxidative Destruction of Acetaminophen: The Mechanism and Superoxide-Mediated Cycling of Copper Species. Chemosphere 2017, 166, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Nidheesh, P.V.; Khatri, J.; Singh, T.S.A.; Gandhimathi, R.; Ramesh, S.T. Review of Zero-Valent Aluminium Based Water and Wastewater Treatment Methods. Chemosphere 2018, 200, 621–631. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale Zero Valent Iron and Bimetallic Particles for Contaminated Site Remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Kurosu, S.; Suzuki, M.; Kawase, Y. Hydroxyl Radical Generation by Zero-Valent Iron/Cu (ZVI/Cu) Bimetallic Catalyst in Wastewater Treatment: Heterogeneous Fenton/Fenton-like Reactions by Fenton Reagents Formed in-Situ under Oxic Conditions. Chem. Eng. J. 2018, 334, 1537–1549. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A Review of the Innovations in Metal-and Carbon-Based Catalysts Explored for Heterogeneous Peroxymonosulfate (PMS) Activation, with Focus on Radical vs. Non-Radical Degradation Pathways of Organic Contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Oh, W.-D.; Zaeni, J.R.J.; Lisak, G.; Lin, K.-Y.A.; Leong, K.-H.; Choong, Z.-Y. Accelerated Organics Degradation by Peroxymonosulfate Activated with Biochar Co-Doped with Nitrogen and Sulfur. Chemosphere 2021, 277, 130313. [Google Scholar] [CrossRef]
- de Sousa, P.V.F.; de Oliveira, A.F.; da Silva, A.A.; Lopes, R.P. Environmental Remediation Processes by Zero Valence Copper: Reaction Mechanisms. Environ. Sci. Pollut. Res. 2019, 26, 14883–14903. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, Y.; Li, X.; Yu, H.; Wang, N.; Li, H.; Du, P.; Jiang, Y.; Fan, X.; Zhou, Z. Degradation of Sulfamethazine by Persulfate Activated with Nanosized Zero-Valent Copper in Combination with Ultrasonic Irradiation. Sep. Purif. Technol. 2020, 239, 116537. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Zhou, P.; Zhang, J.; Liu, J.; Zhang, Y.; Liang, J.; Liu, Y.; Liu, B.; Zhang, W. Degradation of Organic Contaminants by Activated Persulfate Using Zero Valent Copper in Acidic Aqueous Conditions. RSC Adv. 2016, 6, 99532–99539. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Liao, C.; Deng, Y.; Zhang, T.; Shih, K. Red Mud Powders as Low-Cost and Efficient Catalysts for Persulfate Activation: Pathways and Reusability of Mineralizing Sulfadiazine. Sep. Purif. Technol. 2016, 167, 136–145. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated Persulfate for Organic Chemical Degradation: A Review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M.; Manshouri, M. Textile Wastewater Decolorization by Zero Valent Iron Activated Peroxymonosulfate: Compared with Zero Valent Copper. J. Environ. Chem. Eng. 2014, 2, 1846–1851. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Liu, Y.; Liu, P. Heterogeneous Activation of Peroxymonosulfate by Cu/ZSM5 for Decolorization of Rhodamine B. Sep. Purif. Technol. 2014, 135, 1–6. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO Nanostructures: Synthesis, Characterization, Growth Mechanisms, Fundamental Properties, and Applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- Ghanbari, F.; Jaafarzadeh, N. Graphite-Supported CuO Catalyst for Heterogeneous Peroxymonosulfate Activation to Oxidize Direct Orange 26: The Effect of Influential Parameters. Res. Chem. Intermed. 2017, 43, 4623–4637. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Li, G. Synthesis of CuO Nanorods and Their Catalytic Activity in the Thermal Decomposition of Ammonium Perchlorate. J. Alloys Compd. 2008, 464, 532–536. [Google Scholar] [CrossRef]
- Oha, W.-D.; Luaa, S.-K.; Donga, Z.; Lima, T.-T. A Novel Three-Dimensional Spherical CuBi2O4 Nanocolumn Arrays with Persulfate and Peroxymonosulfate Activation Functionalities for 1H-Benzotriazole Removal. Nanoscale 2015, 7, 8149–8158. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, J.; Wu, D.; Zhou, Z.; Deng, Y.; Zhang, T.; Shih, K. Efficient Degradation of Sulfamethazine with CuCo2O4 Spinel Nanocatalysts for Peroxymonosulfate Activation. Chem. Eng. J. 2015, 280, 514–524. [Google Scholar] [CrossRef]
- Ji, F.; Li, C.; Deng, L. Performance of CuO/Oxone System: Heterogeneous Catalytic Oxidation of Phenol at Ambient Conditions. Chem. Eng. J. 2011, 178, 239–243. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croue, J.-P. Production of Sulfate Radical from Peroxymonosulfate Induced by a Magnetically Separable CuFe2O4 Spinel in Water: Efficiency, Stability, and Mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Kušić, H.; Koprivanac, N.; Selanec, I. Fe-Exchanged Zeolite as the Effective Heterogeneous Fenton-Type Catalyst for the Organic Pollutant Minimization: UV Irradiation Assistance. Chemosphere 2006, 65, 65–73. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, G.; Liu, Y.; Di, J.; Wang, Y.; Zhao, Z.; Sun, Q.; Xu, C.; Gao, J.; Duan, A.; et al. Hierarchical Macro-Meso-Microporous ZSM-5 Zeolite Hollow Fibers with Highly Efficient Catalytic Cracking Capability. Sci. Rep. 2014, 4, 7276. [Google Scholar] [CrossRef]
- Yang, Z.; Dai, D.; Yao, Y.; Chen, L.; Liu, Q.; Luo, L. Extremely Enhanced Generation of Reactive Oxygen Species for Oxidation of Pollutants from Peroxymonosulfate Induced by a Supported Copper Oxide Catalyst. Chem. Eng. J. 2017, 322, 546–555. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Yan, W.; Yang, S.; Wu, K.; Wang, G.; Jin, P.; Wei, J. Peroxymonosulfate Activation by Mesoporous CuO Nanocage for Organic Pollutants Degradation via a Singlet Oxygen-Dominated Pathway. J. Environ. Chem. Eng. 2021, 9, 106757. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Huang, W.; Wei, X.; Huang, W. Biochar Supported CuO Composites Used as an Efficient Peroxymonosulfate Activator for Highly Saline Organic Wastewater Treatment. Sci. Total Environ. 2020, 721, 137764. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Xu, H.; Wen, Q. Efficient Heterogeneous Activation of Peroxymonosulfate by Modified CuFe2O4 for Degradation of Tetrabromobisphenol A. Chem. Eng. J. 2020, 389, 124345. [Google Scholar] [CrossRef]
- Rodríguez-Chueca, J.; Barahona-García, E.; Blanco-Gutiérrez, V.; Isidoro-García, L.; Dos santos-García, A.J. Magnetic CoFe2O4 Ferrite for Peroxymonosulfate Activation for Disinfection of Wastewater. Chem. Eng. J. 2020, 398, 125606. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, H.; Yao, B.; Gao, X.; Yang, X.; Zhou, Y. Activation of Peroxymonosulfate (PMS) by Spinel Ferrite and Their Composites in Degradation of Organic Pollutants: A Review. Chem. Eng. J. 2021, 414, 128800. [Google Scholar] [CrossRef]
- Cai, C.; Liu, Y.; Xu, R.; Zhou, J.; Zhang, J.; Chen, Y.; Liu, L.; Zhang, L.; Kang, S.; Xie, X. Bicarbonate Enhanced Heterogeneous Activation of Peroxymonosulfate by Copper Ferrite Nanoparticles for the Efficient Degradation of Refractory Organic Contaminants in Water. Chemosphere 2023, 312, 137285. [Google Scholar] [CrossRef]
- Chandel, M.; Ghosh, B.K.; Moitra, D.; Patra, M.K.; Vadera, S.R.; Ghosh, N.N. Synthesis of Various Ferrite (MFe2O4) Nanoparticles and Their Application as Efficient and Magnetically Separable Catalyst for Biginelli Reaction. J. Nanosci. Nanotechnol. 2018, 18, 2481–2492. [Google Scholar] [CrossRef]
- Golshan, M.; Tian, N.; Mamba, G.; Kakavandi, B. Synergetic Photocatalytic Peroxymonosulfate Oxidation of Benzotriazole by Copper Ferrite Spinel: Factors and Mechanism Analysis. Toxics 2023, 11, 429. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, G.; Xian, G.; Ren, Z.; Wei, T.; Li, Q.; Zhang, Y.; Zou, Z. Tetracycline Degradation by Persulfate Activated with Magnetic γ-Fe2O3/CeO2 Catalyst: Performance, Activation Mechanism and Degradation Pathway. Sep. Purif. Technol. 2021, 259, 118156. [Google Scholar] [CrossRef]
- Deraz, N.M. Production and Characterization of Pure and Doped Copper Ferrite Nanoparticles. J. Anal. Appl. Pyrolysis 2008, 82, 212–222. [Google Scholar] [CrossRef]
- Subha, A.; Shalini, M.G.; Sahu, B.N.; Rout, S.; Sahoo, S.C. Role of Surface Defects and Anisotropy Variation on Magnetic Properties of Copper Ferrite Nanoparticles Prepared by Co-Precipitation Method. Mater. Chem. Phys. 2022, 286, 126212. [Google Scholar] [CrossRef]
- Ismael, M.; Wark, M. A Simple Sol–Gel Method for the Synthesis of Pt Co-Catalyzed Spinel-Type CuFe2O4 for Hydrogen Production; the Role of Crystallinity and Band Gap Energy. Fuel 2024, 359, 130429. [Google Scholar] [CrossRef]
- Amulya, M.A.S.; Nagaswarupa, H.P.; Kumar, M.R.A.; Ravikumar, C.R.; Kusuma, K.B.; Prashantha, S.C. Evaluation of Bifunctional Applications of CuFe2O4 Nanoparticles Synthesized by a Sonochemical Method. J. Phys. Chem. Solids 2021, 148, 109756. [Google Scholar] [CrossRef]
- Karakaş, Z.K. A Comprehensive Study on the Production and Photocatalytic Activity of Copper Ferrite Nanoparticles Synthesized by Microwave-Assisted Combustion Method as an Effective Photocatalyst. J. Phys. Chem. Solids 2022, 170, 110927. [Google Scholar] [CrossRef]
- Cao, X.; Xiao, F.; Lyu, Z.; Xie, X.; Zhang, Z.; Dong, X.; Wang, J.; Lyu, X.; Zhang, Y.; Liang, Y. CuFe2O4 Supported on Montmorillonite to Activate Peroxymonosulfate for Efficient Ofloxacin Degradation. J. Water Process Eng. 2021, 44, 102359. [Google Scholar] [CrossRef]
- Guan, Y.-H.; Ma, J.; Ren, Y.-M.; Liu, Y.-L.; Xiao, J.-Y.; Lin, L.; Zhang, C. Efficient Degradation of Atrazine by Magnetic Porous Copper Ferrite Catalyzed Peroxymonosulfate Oxidation via the Formation of Hydroxyl and Sulfate Radicals. Water Res. 2013, 47, 5431–5438. [Google Scholar] [CrossRef]
- Zhao, J.; Xiao, P.; Han, S.; Zulhumar, M.; Wu, D. Preparation of Magnetic Copper Ferrite Nanoparticle as Peroxymonosulfate Activating Catalyst for Effective Degradation of Levofloxacin. Water Sci. Technol. 2022, 85, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhang, Q.; Sun, X.; Chen, S.; Tang, J.; Zhu, J.-J.; Dang, Y. Doping Sb into CuFe2O4 Improved the Catalytic Performance in the Electrochemically Enhanced Homogeneous Peroxymonosulfate-Heterogeneous Catalytic System for the Degradation of Ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 108335. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, D.; Deng, Y.; Zhang, T.; Shih, K. Sulfate Radical-Mediated Degradation of Sulfadiazine by CuFeO2 Rhombohedral Crystal-Catalyzed Peroxymonosulfate: Synergistic Effects and Mechanisms. Environ. Sci. Technol. 2016, 50, 3119–3127. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.-D.; Dong, Z.; Hu, Z.-T.; Lim, T.-T. A Novel Quasi-Cubic CuFe2O4–Fe2O3 Catalyst Prepared at Low Temperature for Enhanced Oxidation of Bisphenol A via Peroxymonosulfate Activation. J. Mater. Chem. A Mater. 2015, 3, 22208–22217. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, W.; Jing, G.; Zhou, Z. Activation of Sulfite Autoxidation with CuFe2O4 Prepared by MOF-Templated Method for Abatement of Organic Contaminants. Environ. Pollut. 2020, 260, 114038. [Google Scholar] [CrossRef]
- Johnson, D.A. Some Thermodynamic Aspects of Inorganic Chemistry; Cambridge University Press: Cambridge, UK, 1982; ISBN 0521242045. [Google Scholar]
- Moser, J.; Punchihewa, S.; Infelta, P.P.; Graetzel, M. Surface Complexation of Colloidal Semiconductors Strongly Enhances Interfacial Electron-Transfer Rates. Langmuir 1991, 7, 3012–3018. [Google Scholar] [CrossRef]
- Popova, T.V.; Aksenova, N. V Complexes of Copper in Unstable Oxidation States. Russ. J. Coord. Chem. 2003, 29, 743–765. [Google Scholar] [CrossRef]
- Mo, Q.; Zheng, H.; Sheng, G. A Heterogeneously Activated Peroxymonosulfate with a Co and Cu Codoped Bimetallic Metal-Organic Framework Efficiently Degrades Tetracycline in Water. Mol. Catal. 2024, 553, 113817. [Google Scholar] [CrossRef]
- Guo, R.; Zhu, Y.; Cheng, X.; Li, J.; Crittenden, J.C. Efficient Degradation of Lomefloxacin by Co-Cu-LDH Activating Peroxymonosulfate Process: Optimization, Dynamics, Degradation Pathway and Mechanism. J. Hazard. Mater. 2020, 399, 122966. [Google Scholar] [CrossRef] [PubMed]
- Sheikh Asadi, A.M.; Cichocki, Ł.; Atamaleki, A.; Hashemi, M.; Lutze, H.; Imran, M.; Kong, L.; Wang, C.; Boczkaj, G. Catalysts for Advanced Oxidation Processes: Deep Eutectic Solvents-Assisted Synthesis—A Review. Water Resour. Ind. 2024, 31, 100251. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanroman, A. Metal–Organic Frameworks as Powerful Heterogeneous Catalysts in Advanced Oxidation Processes for Wastewater Treatment. Appl. Sci. 2022, 12, 8240. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Huang, Q.; Lin, X.; Zeb, A.; Wu, Y.; Xu, Z.; Xu, X. Recent Advances in Cu-Based Metal–Organic Frameworks and Their Derivatives for Battery Applications. ACS Appl. Energy Mater. 2022, 5, 7842–7873. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; AlMohisen, H.M.; Almehizia, A.A.; Naglah, A.M.; Tarek, M.; Said, G.E.; Khatab, T.K. Cu-Vit B3 MOF Solvothermal Preparation, Characterization and Evaluation as HIV-1 RNA Replication Inhibitor. J. Mol. Struct. 2024, 1317, 139120. [Google Scholar] [CrossRef]
- Seo, Y.-K.; Hundal, G.; Jang, I.T.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S. Microwave Synthesis of Hybrid Inorganic–Organic Materials Including Porous Cu3(BTC)2 from Cu(II)-Trimesate Mixture. Microporous Mesoporous Mater. 2009, 119, 331–337. [Google Scholar] [CrossRef]
- Campagnol, N.; Van Assche, T.R.C.; Li, M.; Stappers, L.; Dinca, M.; Denayer, J.F.M.; Binnemans, K.; De Vos, D.E.; Fransaer, J. On the Electrochemical Deposition of Metal-Organic Frameworks. J. Mater. Chem. A Mater. 2016, 4, 3914–3925. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Qiu, L.-G.; Xu, T.; Wu, Y.; Wang, W.; Wu, Z.-Y.; Jiang, X. Ultrasonic Synthesis of the Microporous Metal–Organic Framework Cu3(BTC)2 at Ambient Temperature and Pressure: An Efficient and Environmentally Friendly Method. Mater. Lett. 2009, 63, 78–80. [Google Scholar] [CrossRef]
- Klimakow, M.; Klobes, P.; Rademann, K.; Emmerling, F. Characterization of Mechanochemically Synthesized MOFs. Microporous Mesoporous Mater. 2012, 154, 113–118. [Google Scholar] [CrossRef]
- Xiang, Z.; Cao, D.; Shao, X.; Wang, W.; Zhang, J.; Wu, W. Facile Preparation of High-Capacity Hydrogen Storage Metal-Organic Frameworks: A Combination of Microwave-Assisted Solvothermal Synthesis and Supercritical Activation. Chem. Eng. Sci. 2010, 65, 3140–3146. [Google Scholar] [CrossRef]
- Bai, Y.; Nie, G.; He, Y.; Li, C.; Wang, X.; Ye, L. Cu-MOF for Effectively Organic Pollutants Degradation and E. coli Inactivation via Catalytic Activation of Peroxymonosulfate. J. Taiwan Inst. Chem. Eng. 2022, 132, 104154. [Google Scholar] [CrossRef]
- Quan, X.; Zhang, J.; Yin, L.; Tian, Y. Enhanced PMS Activation and NSAID Degradation Selectivity by Hydrophobic Microenvironment Regulation on Fe3+@Cu-MOF. Colloids Surf. A Physicochem. Eng. Asp. 2024, 695, 134156. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, Y.; Wang, D.; Zhu, M.; Sun, X.; Jiang, S.; Fan, Y.; Zhang, D.; Zhang, L. Surface-Functionalized PVDF Membranes by Facile Synthetic Cu-MOF-74 for Enhanced Contaminant Degradation and Antifouling Performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129640. [Google Scholar] [CrossRef]
- Wu, Y.; Liang, G.; Li, W.-B.; Zhong, X.-F.; Zhang, Y.-Y.; Ye, J.-W.; Yang, T.; Mo, Z.-W.; Chen, X.-M. Boosting the Degradation of Antibiotics via Peroxymonosulfate Activation with a Cu-Based Metal–Organic Framework. Chem. Sci. 2024, 15, 9733–9741. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Lu, S.; Su, L.; Wang, C.; Huang, J.; Zhou, J.; Tang, J.; Huang, M. Nano-Porous Bimetallic CuCo-MOF-74 with Coordinatively Unsaturated Metal Sites for Peroxymonosulfate Activation to Eliminate Organic Pollutants: Performance and Mechanism. Chemosphere 2021, 273, 129643. [Google Scholar] [CrossRef]
- Wang, H.; Dai, Y.; Wang, Y.; Yin, L. One-Pot Solvothermal Synthesis of Cu–Fe-MOF for Efficiently Activating Peroxymonosulfate to Degrade Organic Pollutants in Water:Effect of Electron Shuttle. Chemosphere 2024, 352, 141333. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Lomba-Fernández, B.; Pazos, M.; Rosales, E.; Sanromán, A. Peroxymonosulfate Activation by Different Synthesized CuFe-MOFs: Application for Dye, Drugs, and Pathogen Removal. Catalysts 2023, 13, 820. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, L.; Zhu, T.; Gao, Z.; Wang, C.; Geng, R.; Bai, W.; Cao, Y.; Zhu, J. Contribution of 1O2 in the Efficient Degradation of Organic Pollutants with Cu0/Cu2O/CuO@N–C Activated Peroxymonosulfate: A Case Study with Tetracycline. Environ. Pollut. 2024, 342, 123064. [Google Scholar] [CrossRef]
- Ji, Y.; Li, W.; Cao, W.; Yang, Z.; Zheng, C.; Zhang, W. Effective Degradation of Organic Pollutants by CuFe@C Composite Derived from Bimetallic MOFs Synthesized through Stepwise Feeding Method. J. Environ. Chem. Eng. 2024, 12, 112792. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered Double Hydroxide (LDH)-Based Materials: A Mini-Review on Strategies to Improve the Performance for Photocatalytic Water Splitting. J. Energy Chem. 2022, 64, 406–431. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Zhu, M.; Cheng, X.; Zhang, X.; Meng, X.; Huang, X.; Hao, H. Co–Cu–Al Layered Double Oxides as Heterogeneous Catalyst for Enhanced Degradation of Organic Pollutants in Wastewater by Activating Peroxymonosulfate: Performance and Synergistic Effect. Ind. Eng. Chem. Res. 2019, 58, 8699–8711. [Google Scholar] [CrossRef]
- Chiericatti, C.; Basilico, J.C.; Zapata Basilico, M.L.; Zamaro, J.M. Novel Application of HKUST-1 Metal–Organic Framework as Antifungal: Biological Tests and Physicochemical Characterizations. Microporous Mesoporous Mater. 2012, 162, 60–63. [Google Scholar] [CrossRef]
- Giráldez, A.; Fdez-Sanromán, A.; Terrón, D.; Sanromán, M.A.; Pazos, M. Nanostructured Copper-Organic Frameworks for the Generation of Sulphate Radicals: Application in Wastewater Disinfection. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef]
- Li, H.; Xu, C.; Li, N.; Rao, T.; Zhou, Z.; Zhou, Q.; Wang, C.; Xu, S.; Tang, J. Synthesis of Bimetallic FeCu-MOF and Its Performance as Catalyst of Peroxymonosulfate for Degradation of Methylene Blue. Materials 2022, 15, 7252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Liu, D.; Liu, S. Co Isomorphic Substitution for Cu-Based Metal Organic Framework Based on Electronic Structure Modulation Boosts Fenton-like Process. Sep. Purif. Technol. 2023, 306, 122526. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanromán, A. One-Pot Synthesis of Bimetallic Fe–Cu Metal–Organic Frameworks Composite for the Elimination of Organic Pollutants via Peroxymonosulphate Activation. Environ. Sci. Pollut. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhu, Z.; Zhang, H.; Lu, H.; Qiu, Y. Efficient Degradation of Organic Pollutants by Peroxymonosulfate Activated with MgCuFe-Layered Double Hydroxide. RSC Adv. 2019, 9, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sui, M.; Yuan, B.; Wang, J.; Lv, Y. Efficient Degradation of Nitrobenzene by Cu-Co-Fe-LDH Catalyzed Peroxymonosulfate to Produce Hydroxyl Radicals. Chem. Eng. J. 2019, 357, 140–149. [Google Scholar] [CrossRef]
- Xie, M.; Liang, M.; Liu, C.; Xu, Z.; Yu, Y.; Xu, J.; You, S.; Wang, D.; Rad, S. Peroxymonosulfate Activation by CuMn-LDH for the Degradation of Bisphenol A: Effect, Mechanism, and Pathway. Ecotoxicol. Environ. Saf. 2024, 270, 115929. [Google Scholar] [CrossRef]
- Guo, R.; Li, Y.; Chen, Y.; Liu, Y.; Niu, B.; Gou, J.; Cheng, X. Efficient Degradation of Sulfamethoxazole by CoCu LDH Composite Membrane Activating Peroxymonosulfate with Decreased Metal Ion Leaching. Chem. Eng. J. 2021, 417, 127887. [Google Scholar] [CrossRef]
- Wu, L.; Jin, T.; Li, D.; Wang, L.; Sun, Y. Heterogeneous Activation of Permonosulfate by Biochar Supporting CuCoFe Layered Double Hydroxide for Rapid Degradation of Phenanthrene. J. Environ. Chem. Eng. 2023, 11, 110718. [Google Scholar] [CrossRef]
- Dung, N.T.; Thao, V.D.; Huy, N.N. Decomposition of Glyphosate in Water by Peroxymonosulfate Activated with CuCoFe-LDH Material. Vietnam. J. Chem. 2021, 59, 813–822. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Hu, W.; Fan, Y. Mesoporous CuO Prepared in a Natural Deep Eutectic Solvent Medium for Effective Photodegradation of Rhodamine B. Molecules 2023, 28, 5554. [Google Scholar] [CrossRef]
- Patil, Y.A.; Shankarling, G.S. Deep Eutectic Solvent-Mediated, Energy-Efficient Synthesis of Copper Terephthalate Metal-Organic Framework and Its Application in Degradation of an Azo Dye. Chem. Eng. J. Adv. 2020, 3, 100032. [Google Scholar] [CrossRef]
- Shahzad, A.; Jawad, A.; Ifthikar, J.; Chen, Z.; Chen, Z. The Hetero-Assembly of Reduced Graphene Oxide and Hydroxide Nanosheets as Superlattice Materials in PMS Activation. Carbon 2019, 155, 740–755. [Google Scholar] [CrossRef]
- Shahzad, A.; Ali, J.; Ifthikar, J.; Aregay, G.G.; Zhu, J.; Chen, Z.; Chen, Z. Non-Radical PMS Activation by the Nanohybrid Material with Periodic Confinement of Reduced Graphene Oxide (RGO) and Cu Hydroxides. J. Hazard. Mater. 2020, 392, 122316. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.S.; Longo, M.A.; Rodríguez, A.; Deive, F.J. The Role of Deep Eutectic Solvents in Catalysis. A Vision on Their Contribution to Homogeneous, Heterogeneous and Electrocatalytic Processes. J. Ind. Eng. Chem. 2024, 132, 36–49. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Munro, H.L.; Rasheed, R.K.; Tambyrajah, V. Preparation of Novel, Moisture-Stable, Lewis-Acidic Ionic Liquids Containing Quaternary Ammonium Salts with Functional Side Chains. Chem. Commun. 2001, 1, 2010–2011. [Google Scholar] [CrossRef]
- Peng, Q.; Ye, L.; Wen, N.; Chen, H.; Zhu, Y.; Niu, H.; Tian, H.; Huang, D.; Huang, Y. Nitrogen Vacancy-Modified g-C3N4 Nanosheets Controlled by Deep Eutectic Solvents for Highly Efficient Photocatalytic Atrazine Degradation: Non-Radical Dominated Holes Oxidation. Sep. Purif. Technol. 2025, 354, 128879. [Google Scholar] [CrossRef]
- Plaza-Mayoral, E.; Dalby, K.N.; Falsig, H.; Chorkendorff, I.; Sebastián-Pascual, P.; Escudero-Escribano, M. Preparation of Tunable Cu−Ag Nanostructures by Electrodeposition in a Deep Eutectic Solvent. ChemElectroChem 2024, 11, e202400094. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.-F.; Mohanty, N.; Kurakalva, R.M. A Rapid Spectrophotometric Determination of Persulfate Anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef] [PubMed]
- Araújo, K.C.; Nóbrega, E.T.D.; Moreira, A.J.; Lemos, S.G.; Fragoso, W.D.; Pereira, E.C. Fast and Efficient Processes for Oxidation and Monitoring of Polycyclic Aromatic Hydrocarbons in Environmental Matrices. Catal. Commun. 2024, 187, 1068340. [Google Scholar] [CrossRef]
- Moreira, A.J.; Borges, A.C.; De Souza, B.B.; Barbosa, L.R.; De Mendonça, V.R.; Freschi, C.D.; Freschi, G.P.G. Microwave Discharge Electrodeless Mercury Lamp (Hg-MDEL): An Energetic, Mechanistic and Kinetic Approach to the Degradation of Prozac. J. Environ. Chem. Eng. 2019, 7, 102916. [Google Scholar] [CrossRef]
- Wang, L.; Lan, X.; Peng, W.; Wang, Z. Uncertainty and Misinterpretation over Identification, Quantification and Transformation of Reactive Species Generated in Catalytic Oxidation Processes: A Review. J. Hazard. Mater. 2021, 408, 124436. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jia, Y.; Zhou, M.; Su, X.; Sun, J. High-Efficiency Degradation of Organic Pollutants with Fe, N Co-Doped Biochar Catalysts via Persulfate Activation. J. Hazard. Mater. 2020, 397, 122764. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, C.; Zeng, Y.; Luo, Y.; Xu, J.; Wang, C. Peroxymonosulfate Activation by CuFe-Prussian Blue Analogues for the Degradation of Bisphenol S: Effect, Mechanism, and Pathway. Chemosphere 2023, 331, 138748. [Google Scholar] [CrossRef]
- Xu, X.; Qin, J.; Wei, Y.; Ye, S.; Shen, J.; Yao, Y.; Ding, B.; Shu, Y.; He, G.; Chen, H. Heterogeneous Activation of Persulfate by NiFe2−XCoxO4-RGO for Oxidative Degradation of Bisphenol A in Water. Chem. Eng. J. 2019, 365, 259–269. [Google Scholar] [CrossRef]
- Liu, B.; Song, W.; Zhang, W.; Zhang, X.; Pan, S.; Wu, H.; Sun, Y.; Xu, Y. Fe3O4@CNT as a High-Effective and Steady Chainmail Catalyst for Tetracycline Degradation with Peroxydisulfate Activation: Performance and Mechanism. Sep. Purif. Technol. 2021, 273, 118705. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Facile Synthesis of Hierarchically Structured Magnetic MnO2/ZnFe2O4 Hybrid Materials and Their Performance in Heterogeneous Activation of Peroxymonosulfate. ACS Appl. Mater. Interfaces 2014, 6, 19914–19923. [Google Scholar] [CrossRef]
- Nardi, G.; Manet, I.; Monti, S.; Miranda, M.A.; Lhiaubet-Vallet, V. Scope and Limitations of the TEMPO/EPR Method for Singlet Oxygen Detection: The Misleading Role of Electron Transfer. Free Radic. Biol. Med. 2014, 77, 64–70. [Google Scholar] [CrossRef]
- Yun, E.-T.; Lee, J.H.; Kim, J.; Park, H.-D.; Lee, J. Identifying the Nonradical Mechanism in the Peroxymonosulfate Activation Process: Singlet Oxygenation versus Mediated Electron Transfer. Environ. Sci. Technol. 2018, 52, 7032–7042. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, H.; Lian, C.; Wei, F.; Zhang, D.; Wu, G.; Chen, B.; Wang, S. Fe, Co, Ni Nanocrystals Encapsulated in Nitrogen-Doped Carbon Nanotubes as Fenton-like Catalysts for Organic Pollutant Removal. J. Hazard. Mater. 2016, 314, 129–139. [Google Scholar] [CrossRef]
- Shao, P.; Yin, X.; Yu, C.; Han, S.; Zhao, B.; Li, K.; Li, X.; Yang, Z.; Yuan, Z.; Shi, Q. Enhanced Activation of Peroxymonosulfate via Sulfate Radicals and Singlet Oxygen by SrCoxMn1−xO3 Perovskites for the Degradation of Rhodamine B. Processes 2023, 11, 1279. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of Peroxymonosulfate and Its Activation Methods for Degradation of Environmental Organic Pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Zou, J.; Ma, J.; Zhang, J. Comment on Electrolytic Manipulation of Persulfate Reactivity by Iron Electrodes for TCE Degradation in Groundwater. Environ. Sci. Technol. 2014, 48, 4630–4631. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miao, J.; Duan, X.; Guan, D.; Zhong, Y.; Wang, S.; Zhou, W.; Shao, Z. Postsynthesis Growth of CoOOH Nanostructure on SrCo0.6Ti0.4O3−δ Perovskite Surface for Enhanced Degradation of Aqueous Organic Contaminants. ACS Sustain. Chem. Eng. 2018, 6, 15737–15748. [Google Scholar] [CrossRef]
- Liang, P.; Zhang, C.; Duan, X.; Sun, H.; Liu, S.; Tade, M.O.; Wang, S. N-Doped Graphene from Metal–Organic Frameworks for Catalytic Oxidation of p-Hydroxylbenzoic Acid: N-Functionality and Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 2693–2701. [Google Scholar] [CrossRef]
- Bokare, A.D.; Wonyong, C. Singlet-Oxygen Generation in Alkaline Periodate Solution. Environ. Sci. Technol. 2015, 49, 14392–14400. [Google Scholar] [CrossRef]
- Haag, W.R.; Gassman, E. Singlet Oxygen in Surface Waters—Part I: Furfuryl Alcohol as a Trapping Agent. Chemosphere 1984, 13, 631–640. [Google Scholar] [CrossRef]
- Yin, J.J.; Xia, Q.; Fu, P.P. UVA Photoirradiation of Anhydroretinol–Formation of Singlet Oxygen and Superoxide. Toxicol. Ind. Health 2007, 23, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Bao, Y.; Sheng, T.; Yi, Q.; Zhu, Q.; Shen, B.; Xing, M.; Lo, I.M.C.; Zhang, J. Singlet Oxygen Triggered by Robust Bimetallic MoFe/TiO2 Nanospheres of Highly Efficacy in Solar-Light-Driven Peroxymonosulfate Activation for Organic Pollutants Removal. Appl. Catal. B 2021, 286, 119930. [Google Scholar] [CrossRef]
- Yang, P.; Li, S.; Xiaofu, L.; Xiaojing, A.; Liu, D.; Huang, W. Singlet Oxygen-Dominated Activation of Peroxymonosulfate by CuO/MXene Nanocomposites for Efficient Decontamination of Carbamazepine under High Salinity Conditions: Performance and Singlet Oxygen Evolution Mechanism. Sep. Purif. Technol. 2022, 285, 120288. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.-W. Identification of Sulfate and Hydroxyl Radicals in Thermally Activated Persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, T.; Hou, C.; Huang, B.; Du, J.; Liu, N.; Zhou, X.; Zhang, Y. Efficient Activation of Peroxymonosulfate by Biochar-Loaded Zero-Valent Copper for Enrofloxacin Degradation: Singlet Oxygen-Dominated Oxidation Process. Nanomaterials 2022, 12, 2842. [Google Scholar] [CrossRef]
- Teel, A.L.; Ahmad, M.; Watts, R.J. Persulfate Activation by Naturally Occurring Trace Minerals. J. Hazard. Mater. 2011, 196, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-H.; Huang, Y.-F.; Huang, C.; Chen, C.-Y. Efficient Decolorization of Azo Dye Reactive Black B Involving Aromatic Fragment Degradation in Buffered Co2+/PMS Oxidative Processes with a Ppb Level Dosage of Co2+-Catalyst. J. Hazard. Mater. 2009, 170, 1110–1118. [Google Scholar] [CrossRef]
- Sun, J.; Song, M.; Feng, J.; Pi, Y. Highly Efficient Degradation of Ofloxacin by UV/Oxone/Co2+ Oxidation Process. Environ. Sci. Pollut. Res. 2012, 19, 1536–1543. [Google Scholar] [CrossRef]
- Mo, Y.; Xu, W.; Zhang, X.; Zhou, S. Enhanced Degradation of Rhodamine B through Peroxymonosulfate Activated by a Metal Oxide/Carbon Nitride Composite. Water 2022, 14, 2054. [Google Scholar] [CrossRef]
- Shah, N.S.; Khan, J.A.; Sayed, M.; Khan, Z.U.H.; Ali, H.S.; Murtaza, B.; Khan, H.M.; Imran, M.; Muhammad, N. Hydroxyl and Sulfate Radical Mediated Degradation of Ciprofloxacin Using Nano Zerovalent Manganese Catalyzed S2O82−. Chem. Eng. J. 2019, 356, 199–209. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2018, 53, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Jing, K.; Li, R.; Wang, F.; Ping, C.; Lv, W. Sulfate Radical-Induced Transformation of Trimethoprim with CuFe2O4/MWCNTs as a Heterogeneous Catalyst of Peroxymonosulfate: Mechanisms and Reaction Pathways. RSC Adv. 2018, 8, 24787–24795. [Google Scholar]
- Olmez-Hanci, T.; Arslan-Alaton, I. Comparison of Sulfate and Hydroxyl Radical Based Advanced Oxidation of Phenol. Chem. Eng. J. 2013, 224, 10–16. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of Peroxodisulfate and Peroxymonosulfate by Ultrasound with Different Frequencies: Impact on Ibuprofen Removal Efficient, Cost Estimation and Energy Analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, C.; Chen, L.; Duan, J.; Li, F.; Liu, W. Different Reaction Mechanisms of SO4•− And•OH with Organic Compound Interpreted at Molecular Orbital Level in Co (II)/Peroxymonosulfate Catalytic Activation System. Water Res. 2023, 229, 119392. [Google Scholar] [CrossRef]
- Avetta, P.; Pensato, A.; Minella, M.; Malandrino, M.; Maurino, V.; Minero, C.; Hanna, K.; Vione, D. Activation of Persulfate by Irradiated Magnetite: Implications for the Degradation of Phenol under Heterogeneous Photo-Fenton-like Conditions. Environ. Sci. Technol. 2015, 49, 1043–1050. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, H.; Zhang, X.; Li, B.; Guo, R.; Cheng, Q.; Cheng, X. Synthesis of Magnetic CuO/MnFe2O4 Nanocompisite and Its High Activity for Degradation of Levofloxacin by Activation of Persulfate. Chem. Eng. J. 2019, 360, 848–860. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Dionysiou, D.D.; Liu, C.; Tong, Y.; Gao, J.; Fang, G.; Zhou, D. Efficient Activation of Peroxymonosulfate by Copper Sulfide for Diethyl Phthalate Degradation: Performance, Radical Generation and Mechanism. Sci. Total Environ. 2020, 749, 142387. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Fan, Y.; Tong, T.; Han, X.; Du, Y. Non-Radical-Dominated Catalytic Degradation of Bisphenol A by ZIF-67 Derived Nitrogen-Doped Carbon Nanotubes Frameworks in the Presence of Peroxymonosulfate. Chem. Eng. J. 2018, 336, 721–731. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. Magnetic Fe3O4/Carbon Sphere/Cobalt Composites for Catalytic Oxidation of Phenol Solutions with Sulfate Radicals. Chem. Eng. J. 2014, 245, 1–9. [Google Scholar] [CrossRef]
- Huang, B.-C.; Jiang, J.; Huang, G.-X.; Yu, H.-Q. Sludge Biochar-Based Catalysts for Improved Pollutant Degradation by Activating Peroxymonosulfate. J. Mater. Chem. A Mater. 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, W.; He, J.; Du, Y. CoMoO4 as a Novel Heterogeneous Catalyst of Peroxymonosulfate Activation for the Degradation of Organic Dyes. RSC Adv. 2017, 7, 36193–36200. [Google Scholar] [CrossRef]
- Yu, B.; Li, Z.; Zhang, S. Zero-Valent Copper-Mediated Peroxymonosulfate Activation for Efficient Degradation of Azo Dye Orange G. Catalysts 2022, 12, 700. [Google Scholar] [CrossRef]
- Oh, W.-D.; Veksha, A.; Chen, X.; Adnan, R.; Lim, J.-W.; Leong, K.-H.; Lim, T.-T. Catalytically Active Nitrogen-Doped Porous Carbon Derived from Biowastes for Organics Removal via Peroxymonosulfate Activation. Chem. Eng. J. 2019, 374, 947–957. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, R.; Guo, Y.; Xu, L.; Liu, J. Effects of Chloride Ions on Bleaching of Azo Dyes by Co2+/Oxone Regent: Kinetic Analysis. J. Hazard. Mater. 2011, 190, 1083–1087. [Google Scholar] [CrossRef]
- Wang, P.; Yang, S.; Shan, L.; Niu, R.; Shao, X. Involvements of Chloride Ion in Decolorization of Acid Orange 7 by Activated Peroxydisulfate or Peroxymonosulfate Oxidation. J. Environ. Sci. 2011, 23, 1799–1807. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of Chloride Ion on Degradation of Acid Orange 7 by Sulfate Radical-Based Advanced Oxidation Process: Implications for Formation of Chlorinated Aromatic Compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef]
- Chan, K.H.; Chu, W. Degradation of Atrazine by Cobalt-Mediated Activation of Peroxymonosulfate: Different Cobalt Counteranions in Homogenous Process and Cobalt Oxide Catalysts in Photolytic Heterogeneous Process. Water Res. 2009, 43, 2513–2521. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Huang, X.; Ma, F.; Wang, L.; Duan, X.; Wang, S. Catalytic Removal of Aqueous Contaminants on N-Doped Graphitic Biochars: Inherent Roles of Adsorption and Nonradical Mechanisms. Environ. Sci. Technol. 2018, 52, 8649–8658. [Google Scholar] [CrossRef]
- Faheem; Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of Biochar in Advanced Oxidation Processes: Supportive, Adsorptive, and Catalytic Role. Environ. Sci. Pollut. Res. 2020, 27, 37286–37312. [Google Scholar] [CrossRef]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-Mediated Activation of Persulfate and Peroxymonosulfate in Both Homogeneous and Heterogeneous Ways: A Review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Duan, X.; Ao, Z.; Zhou, L.; Sun, H.; Wang, G.; Wang, S. Occurrence of Radical and Nonradical Pathways from Carbocatalysts for Aqueous and Nonaqueous Catalytic Oxidation. Appl. Catal. B 2016, 188, 98–105. [Google Scholar] [CrossRef]
- Jiang, M.; Lu, J.; Ji, Y.; Kong, D. Bicarbonate-Activated Persulfate Oxidation of Acetaminophen. Water Res. 2017, 116, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Zhang, Q.; Shang, Y.; Wang, W.; Li, Q.; Yue, Q.; Gao, B.; Xu, X. Sulfate Saturated Biosorbent-Derived Co-S@ NC Nanoarchitecture as an Efficient Catalyst for Peroxymonosulfate Activation. Appl. Catal. B 2020, 262, 118302. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-Doped Biochar Fiber with Graphitization from Boehmeria Nivea for Promoted Peroxymonosulfate Activation and Non-Radical Degradation Pathways with Enhancing Electron Transfer. Appl. Catal. B 2020, 269, 118850. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Lu, J. New Insights into Atrazine Degradation by Cobalt Catalyzed Peroxymonosulfate Oxidation: Kinetics, Reaction Products and Transformation Mechanisms. J. Hazard. Mater. 2015, 285, 491–500. [Google Scholar] [CrossRef]
- Drosos, M.; Ren, M.; Frimmel, F.H. The Effect of NOM to TiO2: Interactions and Photocatalytic Behavior. Appl. Catal. B 2015, 165, 328–334. [Google Scholar] [CrossRef]
- He, X.; de la Cruz, A.A.; O’Shea, K.E.; Dionysiou, D.D. Kinetics and Mechanisms of Cylindrospermopsin Destruction by Sulfate Radical-Based Advanced Oxidation Processes. Water Res. 2014, 63, 168–178. [Google Scholar] [CrossRef]
- Nagar, N.; Devra, V. Activation of Peroxodisulfate and Peroxomonosulfate by Green Synthesized Copper Nanoparticles for Methyl Orange Degradation: A Kinetic Study. J. Environ. Chem. Eng. 2017, 5, 5793–5800. [Google Scholar] [CrossRef]
- Guo, R.; Chen, Y.; Nengzi, L.; Meng, L.; Song, Q.; Gou, J.; Cheng, X. In Situ Preparation of Carbon-Based Cu-Fe Oxide Nanoparticles from CuFe Prussian Blue Analogues for the Photo-Assisted Heterogeneous Peroxymonosulfate Activation Process to Remove Lomefloxacin. Chem. Eng. J. 2020, 398, 125556. [Google Scholar] [CrossRef]
- Chen, J.; Rasool, R.T.; Ashraf, G.A.; Guo, H. The Stimulation of Peroxymonosulfate via Novel Co0.5Cu0.5Fe2O4 Heterogeneous Photocatalyst in Aqueous Solution for Organic Contaminants Removal. Mater. Sci. Semicond. Process 2023, 157, 107321. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ghanbari, F.; Ahmadi, M. Efficient Degradation of 2,4-Dichlorophenoxyacetic Acid by Peroxymonosulfate/Magnetic Copper Ferrite Nanoparticles/Ozone: A Novel Combination of Advanced Oxidation Processes. Chem. Eng. J. 2017, 320, 436–447. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Yang, Z.; Shih, K.; Ying, G.-G.; Feng, Y. Activation of Ozone by Peroxymonosulfate for Selective Degradation of 1,4-Dioxane: Limited Water Matrices Effects. J. Hazard. Mater. 2022, 436, 129223. [Google Scholar] [CrossRef]
- Feizi, R.; Ahmad, M.; Jorfi, S.; Ghanbari, F. Sunset Yellow Degradation by Ultrasound/Peroxymonosulfate/CuFe2O4: Influential Factors and Degradation Processes. Korean J. Chem. Eng. 2019, 36, 886–893. [Google Scholar] [CrossRef]
- Liu, X.; Huang, F.; Yu, Y.; Zhao, P.; Zhou, Y.; He, Y.; Xu, Y.; Zhang, Y. Ofloxacin Degradation over Cu–Ce Tyre Carbon Catalysts by the Microwave Assisted Persulfate Process. Appl. Catal. B 2019, 253, 149–159. [Google Scholar] [CrossRef]
- Mahamuni, N.N.; Adewuyi, Y.G. Advanced Oxidation Processes (AOPs) Involving Ultrasound for Waste Water Treatment: A Review with Emphasis on Cost Estimation. Ultrason. Sonochem 2010, 17, 990–1003. [Google Scholar] [CrossRef] [PubMed]
- Guan, Y.-H.; Ma, J.; Li, X.-C.; Fang, J.-Y.; Chen, L.-W. Influence of PH on the Formation of Sulfate and Hydroxyl Radicals in the UV/Peroxymonosulfate System. Environ. Sci. Technol. 2011, 45, 9308–9314. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, D.; Zhao, Y.; Li, S.; Wei, X.; Meng, F.; Huang, W.; Lei, Z. Singlet Oxygen Dominated Peroxymonosulfate Activation by CuO-CeO2 for Organic Pollutants Degradation: Performance and Mechanism. Chemosphere 2019, 233, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Nie, Z.; Liu, H.; Boczkaj, G.; Liu, W.; Kong, L.; Zhan, J. Singlet Oxygen-Dominated Peroxymonosulfate Activation by Layered Crednerite for Organic Pollutants Degradation in High Salinity Wastewater. J. Environ. Sci. 2024, 135, 86–96. [Google Scholar] [CrossRef]
- Wei, Y.; Miao, J.; Ge, J.; Lang, J.; Yu, C.; Zhang, L.; Alvarez, P.J.J.; Long, M. Ultrahigh Peroxymonosulfate Utilization Efficiency over CuO Nanosheets via Heterogeneous Cu (III) Formation and Preferential Electron Transfer during Degradation of Phenols. Environ. Sci. Technol. 2022, 56, 8984–8992. [Google Scholar] [CrossRef]
- Pan, J.; Gao, B.; Duan, P.; Guo, K.; Akram, M.; Xu, X.; Yue, Q.; Gao, Y. Improving Peroxymonosulfate Activation by Copper Ion-Saturated Adsorbent-Based Single Atom Catalysts for the Degradation of Organic Contaminants: Electron-Transfer Mechanism and the Key Role of Cu Single Atoms. J. Mater. Chem. A Mater. 2021, 9, 11604–11613. [Google Scholar] [CrossRef]
- Shang, Y.; Chen, C.; Zhang, P.; Yue, Q.; Li, Y.; Gao, B.; Xu, X. Removal of Sulfamethoxazole from Water via Activation of Persulfate by Fe3C@NCNTs Including Mechanism of Radical and Nonradical Process. Chem. Eng. J. 2019, 375, 122004. [Google Scholar] [CrossRef]
- Liu, T.; Wu, K.; Wang, M.; Jing, C.; Chen, Y.; Yang, S.; Jin, P. Performance and Mechanisms of Sulfadiazine Removal Using Persulfate Activated by Fe3O4@CuOx Hollow Spheres. Chemosphere 2021, 262, 127845. [Google Scholar] [CrossRef] [PubMed]
- Dung, N.T.; Trang, T.T.; Thao, V.D.; Thu, T.V.; Tung, N.Q.; Huy, N.N. Enhanced Degradation of Organic Dyes by Peroxymonosulfate with Fe3O4-CoCO3/RGO Hybrid Activation: A Comprehensive Study. J. Taiwan Inst. Chem. Eng. 2022, 133, 104279. [Google Scholar] [CrossRef]
- Tian, W.; Zhang, H.; Qian, Z.; Ouyang, T.; Sun, H.; Qin, J.; Tadé, M.O.; Wang, S. Bread-Making Synthesis of Hierarchically Co@C Nanoarchitecture in Heteroatom Doped Porous Carbons for Oxidative Degradation of Emerging Contaminants. Appl. Catal. B 2018, 225, 76–83. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Shao, Z.; Wang, S. Nonradical Reactions in Environmental Remediation Processes: Uncertainty and Challenges. Appl. Catal. B 2018, 224, 973–982. [Google Scholar] [CrossRef]
- Song, H.; Liu, Z.; Guan, Z.; Yang, F.; Xia, D.; Li, D. Efficient Persulfate Non-Radical Activation of Electron-Rich Copper Active Sites Induced by Oxygen on Graphitic Carbon Nitride. Sci. Total Environ. 2021, 762, 143127. [Google Scholar] [CrossRef]
- Pang, Y.; Luo, K.; Tang, L.; Li, X.; Yu, J.; Guo, J.; Liu, Y.; Zhang, Z.; Yue, R.; Li, L. Carbon-Based Magnetic Nanocomposite as Catalyst for Persulfate Activation: A Critical Review. Environ. Sci. Pollut. Res. 2019, 26, 32764–32776. [Google Scholar] [CrossRef]
- Yun, E.-T.; Yoo, H.-Y.; Bae, H.; Kim, H.-I.; Lee, J. Exploring the Role of Persulfate in the Activation Process: Radical Precursor versus Electron Acceptor. Environ. Sci. Technol. 2017, 51, 10090–10099. [Google Scholar] [CrossRef]
- Wang, G.; An, W.; Zhang, Y.; Liu, Z.; Yang, S.; Jin, P.; Ding, D. Mesoporous Carbon Framework Supported Cu-Fe Oxides as Efficient Peroxymonosulfate Catalyst for Sustained Water Remediation. Chem. Eng. J. 2022, 430, 133060. [Google Scholar] [CrossRef]
- Tian, M.; Ren, X.; Ding, S.; Fu, N.; Wei, Y.; Yang, Z.; Yao, X. Effective Degradation of Phenol by Activating PMS with Bimetallic Mo and Ni Co-Doped g C3N4 Composite Catalyst: A Fenton-like Degradation Process Promoted by Non-Free Radical 1O2. Environ. Res. 2024, 243, 117848. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Ren, X.; Ding, S.; Fan, X.; Lu, Z.; Fu, N.; Tian, M. Modulation of Morphology and Phase of Magnetically Separable 1T-WS2/CuFe2O4 Heterojunctions for Acceleration of Peroxymonosulfate Decomposition for Rapid Degradation of Phenol. Sep. Purif. Technol. 2024, 348, 127635. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Liu, Y.; Wang, J. Visible Light-Enhanced Interface Interaction for PMS Activation towards the Removal of Emerging Organic Pollutants: Performance, Mechanism and Toxicity. Sep. Purif. Technol. 2025, 354, 128741. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, T.; Chen, R.; Zheng, S.; Yin, J.; Qi, G.; Li, X.; Zheng, H.; Sun, Y. Cobalt Oxides Grown In-Situ on Carbon Nitride Nanosheets for Efficient Peroxymonosulfate Activation and Organic Contaminants Degradation: Performance, Mechanism, and Application Study. Sep. Purif. Technol. 2025, 353, 128646. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Wang, H.; Liu, H.; Lin, Y.; Zhang, J.; Dong, X.; Sheng, J. Interfacial Engineering and Vacancy Design of Quasi-2D NiCoAl-LDH/Kaolin Hybrid for Activating Peroxymonosulfate to Boost Degradation of Antibiotics. Sep. Purif. Technol. 2025, 354, 128674. [Google Scholar] [CrossRef]
- Ma, Y.; Meng, Y.; Wang, Z.; Xin, Y.; Lv, X.; Li, Q.; Wang, H.; Xie, H.; Zhang, Z. In-Situ Construction of Mesoporous CuCo2O4 Decorated CNTs Networks as a Long-Lasting Peroxymonosulfate Activator for Rapid Removal of Aqueous Micropollutants. Chem. Eng. J. 2023, 476, 146694. [Google Scholar] [CrossRef]
- Brillas, E.; Peralta-Hernández, J.M. Antibiotic Removal from Synthetic and Real Aqueous Matrices by Peroxymonosulfate-Based Advanced Oxidation Processes. A Review of Recent Development. Chemosphere 2024, 351, 141153. [Google Scholar] [CrossRef] [PubMed]
- Derbalah, A.; Sakugawa, H. Sulfate Radical-Based Advanced Oxidation Technology to Remove Pesticides from Water A Review of the Most Recent Technologies. Int. J. Environ. Res. 2024, 18, 11. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, L.; Xu, W.; Hu, Z.; Jia, M.; Liu, L. A Novel Way of Activating Peroxysulfate by Zero-Valent Copper and Ferroferric Oxide Co-Modified Biochar to Remove Bisphenol A in Aqueous Solution: Performance, Mechanism and Potential Toxicity. Appl. Catal. A Gen. 2022, 636, 118575. [Google Scholar] [CrossRef]
- Li, H.; Su, L.; Zheng, J.; Lu, S.; Yang, Z.; Wang, C.; Xu, S.; Zhou, Q.; Tang, J.; Huang, M.; et al. MOFs Derived Carbon Supporting CuCo Nanospheres as Efficient Catalysts of Peroxymonosulfate for Rapid Removal of Organic Pollutant. Chem. Eng. J. 2023, 451, 139114. [Google Scholar] [CrossRef]
- Liu, X.; Pei, Y.; Cao, M.; Yang, H.; Li, Y. Magnetic CuFe2O4 Nanoparticles Anchored on N-Doped Carbon for Activated Peroxymonosulfate Removal of Oxytetracycline from Water: Radical and Non-Radical Pathways. Chemosphere 2023, 334, 139025. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cheng, X.; Zhang, H.; Gou, J.; Zhang, X.; Wu, D.; Dionysiou, D.D. Insights into Performance and Mechanism of ZnO/CuCo2O4 Composite as Heterogeneous Photoactivator of Peroxymonosulfate for Enrofloxacin Degradation. J. Hazard. Mater. 2023, 448, 130946. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, J.; Peng, J.; Zhang, H.; Zhang, Y.; Lai, B. Efficient Degradation of Sulfamethoxazole by the CuO@Al2O3 (EPC) Coupled PMS System: Optimization, Degradation Pathways and Toxicity Evaluation. Chem. Eng. J. 2019, 359, 1097–1110. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Zhang, C.; Dai, M.; Zhang, Z.; Xi, Y.; Quan, B.; Lu, S.; Liu, Y. Degrading Arsanilic Acid and Adsorbing the Released Inorganic Arsenic Simultaneously in Aqueous Media with CuFe2O4 Activating Peroxymonosulfate System: Factors, Performance, and Mechanism. Chem. Eng. J. 2021, 424, 128537. [Google Scholar] [CrossRef]


| Catalyst | Contaminant | Reactive Species | Efficiency | Ref. |
|---|---|---|---|---|
| ZVCu | ||||
| Nanoscale ZVCu | Benzoic acid (BA) | , | 100% in 10 min | [27] |
| ZVCu | 2,4-DCP | , | 56.7% of TOC in 120 min. | [28] |
| ZVCu | NPX | , | 91.0% in 30 min | [29] |
| Copper oxide | ||||
| CuO | Iopamidol | , | 100% in 15 min | [30] |
| CuO | Phenol | , | 65% in 60 min | [31] |
| CuO | BPA | 1, | 100% in 20 min | [32] |
| BPA | Surface-activated PMS | 100% in 120 min | [33] | |
| CuO | AO7 | , | 95.38% in 15 min | [34] |
| Spongelike porous CuO | AO7 | 85% in 60 min | [35] | |
| Copper ferrite | ||||
| TBBPA | , | 99% in 30 min | [36] | |
| ATZ | , | >98% in 15 min | [37] | |
| BPA | , | 95.2% in 60 min | [38] | |
| Norfloxacin | , | >90% in 120 min | [39] | |
| Iopromide | , | ~100% in 10 min | [40] | |
| Sulfadiazine (SDZ) | , | 86% in 12 min | [41] | |
| Orange I | , | 77.8–79.3% in 30 min | [42] |
| Scavenging Agents | Specific ROS | Kinetic Constants (M−1s−1) | Determination of ROS | Ref. |
|---|---|---|---|---|
| MeOH | 3.2 × 106 | , | [36] | |
| 9.7 × 108 | ||||
| EtOH | 1.6–7.7 × 107 | , | [155] | |
| 1.2–2.8 × 108 | ||||
| TBA | 4–9.1 × 105 | [158] | ||
| 3.8–7.6 × 108 | ||||
| p-BQ | 1.0 × 109 | [166] | ||
| FFA | 1 | 3.2 × 107 | 1 | [160] |
| NaN3 | 1 | 1.2 × 108 | 1 | [162] |
| Phenol | 8.8 × 109 | , | [165] | |
| 6.6 × 109 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzayani, B.; Lomba-Fernández, B.; Fdez-Sanromán, A.; Elaoud, S.C.; Sanromán, M.Á. Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate. Appl. Sci. 2024, 14, 8075. https://doi.org/10.3390/app14178075
Bouzayani B, Lomba-Fernández B, Fdez-Sanromán A, Elaoud SC, Sanromán MÁ. Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate. Applied Sciences. 2024; 14(17):8075. https://doi.org/10.3390/app14178075
Chicago/Turabian StyleBouzayani, Bakhta, Bárbara Lomba-Fernández, Antía Fdez-Sanromán, Sourour Chaâbane Elaoud, and Maria Ángeles Sanromán. 2024. "Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate" Applied Sciences 14, no. 17: 8075. https://doi.org/10.3390/app14178075
APA StyleBouzayani, B., Lomba-Fernández, B., Fdez-Sanromán, A., Elaoud, S. C., & Sanromán, M. Á. (2024). Advancements in Copper-Based Catalysts for Efficient Generation of Reactive Oxygen Species from Peroxymonosulfate. Applied Sciences, 14(17), 8075. https://doi.org/10.3390/app14178075







