Exploring Facial Somatosensory Distortion in Chronic Migraine: The Role of Laterality and Emotion Recognition—A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Recruitment
2.3. Ethics
2.4. Study Locations
2.5. Course of the Study
2.5.1. Questionnaires
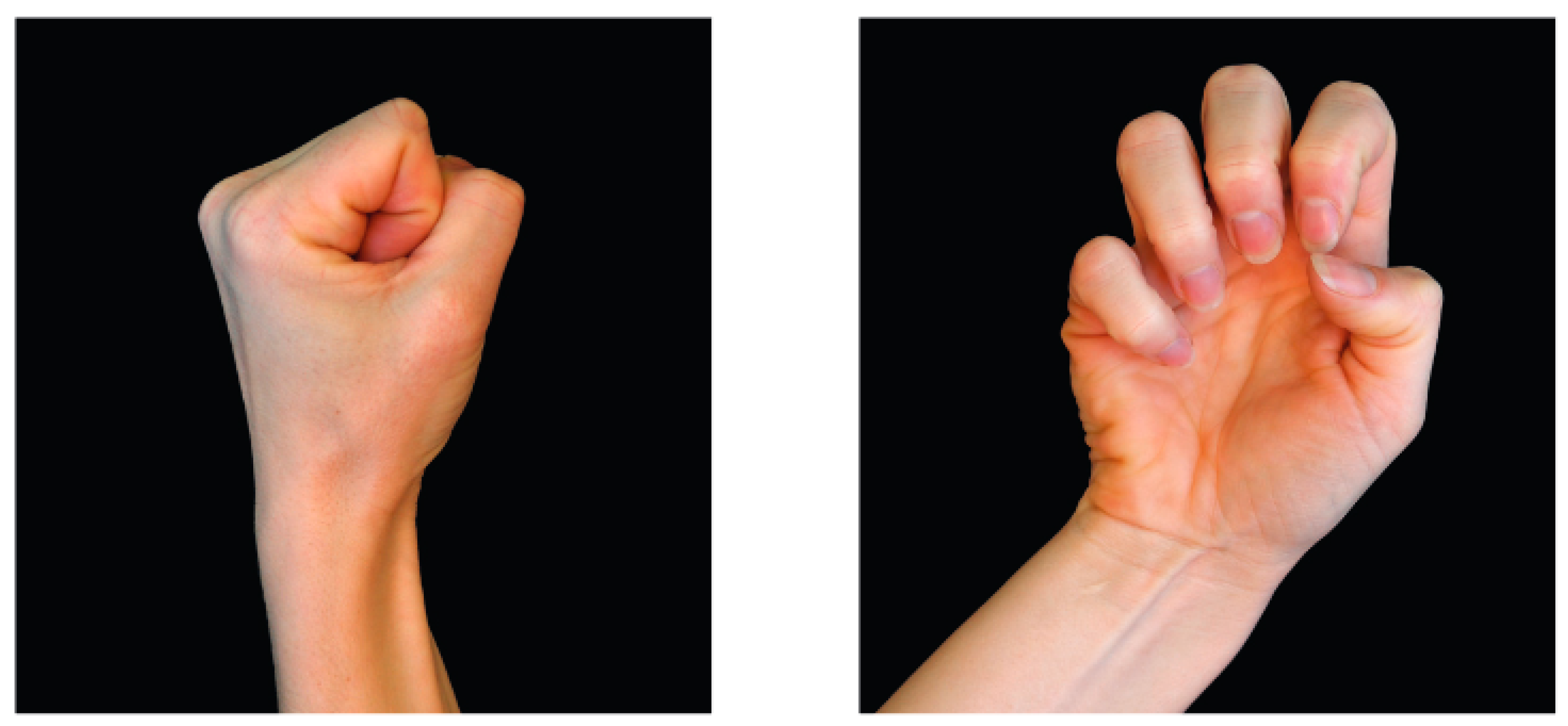
2.5.2. Laterality Recognition
“Judge intuitively, as quickly but also as accurately as possible, the pictures of hands, whether it is a right or a left hand. Carry out the task calmly”.
“Judge intuitively, as quickly but also as accurately as possible, the images of neck movements, whether it is a movement of the neck to the right or the left. Keep in mind the first-person perspective. Carry out the task calmly”.
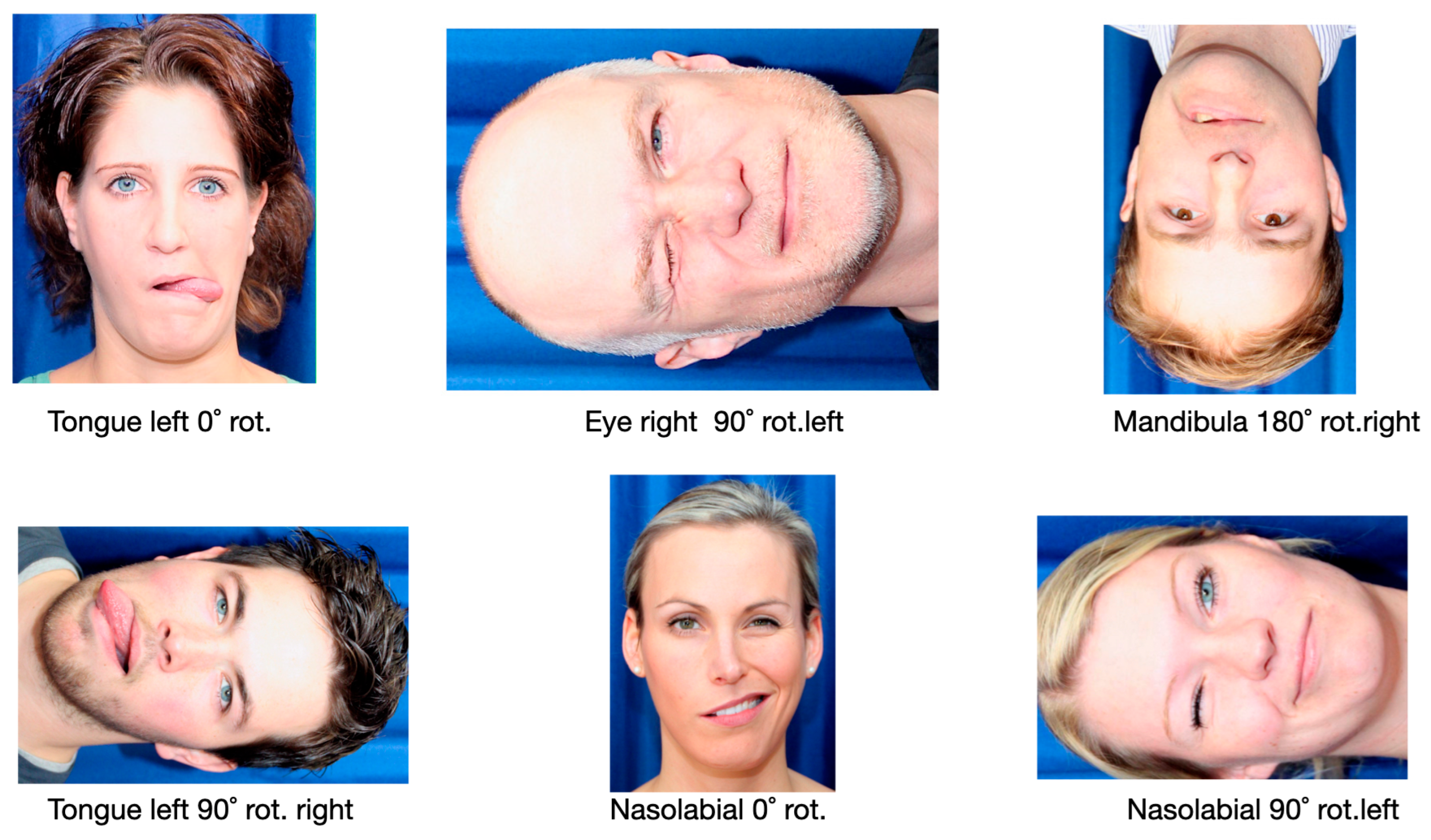
“Judge intuitively, as quickly but also as accurately as possible the images of faces, whether the activity in the face is on the left or right side. The first-person perspective must be considered. Carry out the task calmly”.
2.5.3. Facial Basic Emotion Recognition
“Judge intuitively, as quickly but also as accurately as possible, the images of faces now shown and the emotions visible in them, happy, surprised, frightened, disgusted, angry, and sad. Carry out the task calmly and don’t be irritated by the picture jumping to the next picture. Just keep doing the task”.
3. Statistical Analysis
4. Results
4.1. Demographic Characteristics of the Compared Groups
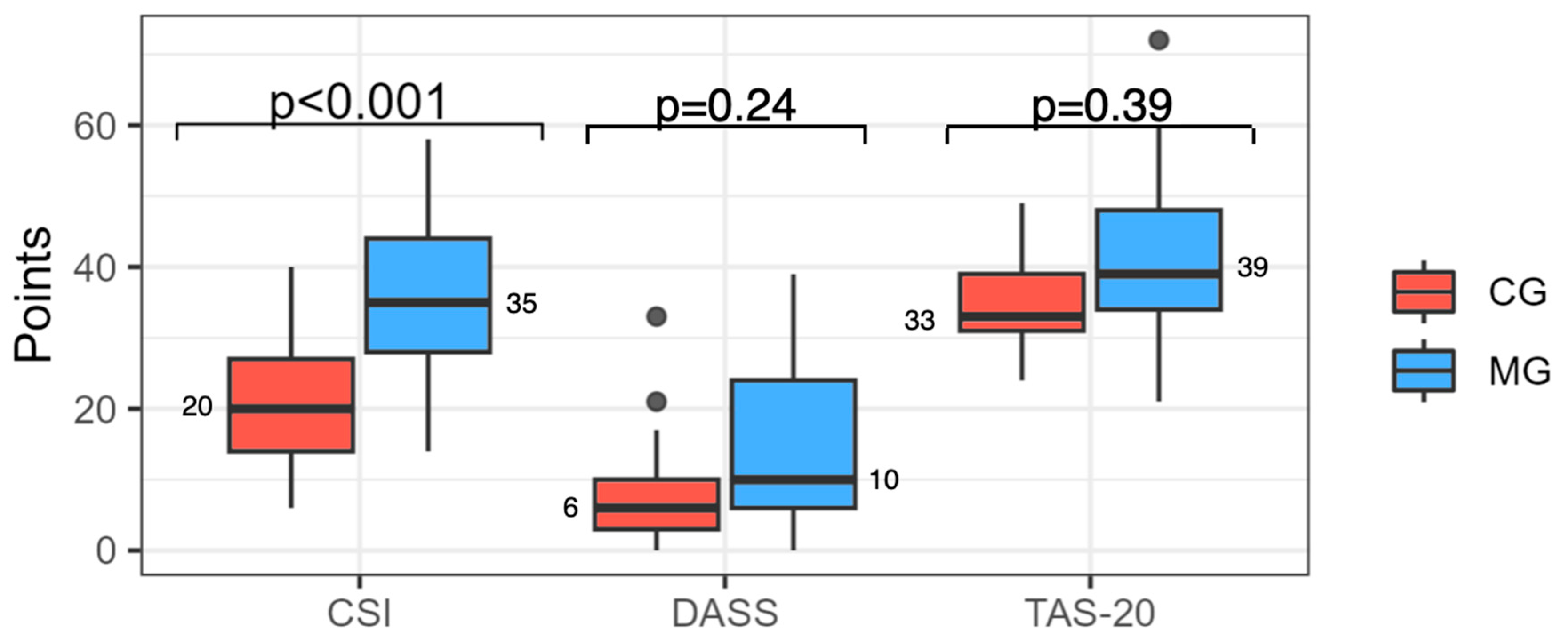
4.2. Questionnaires
4.3. Laterality Recognition
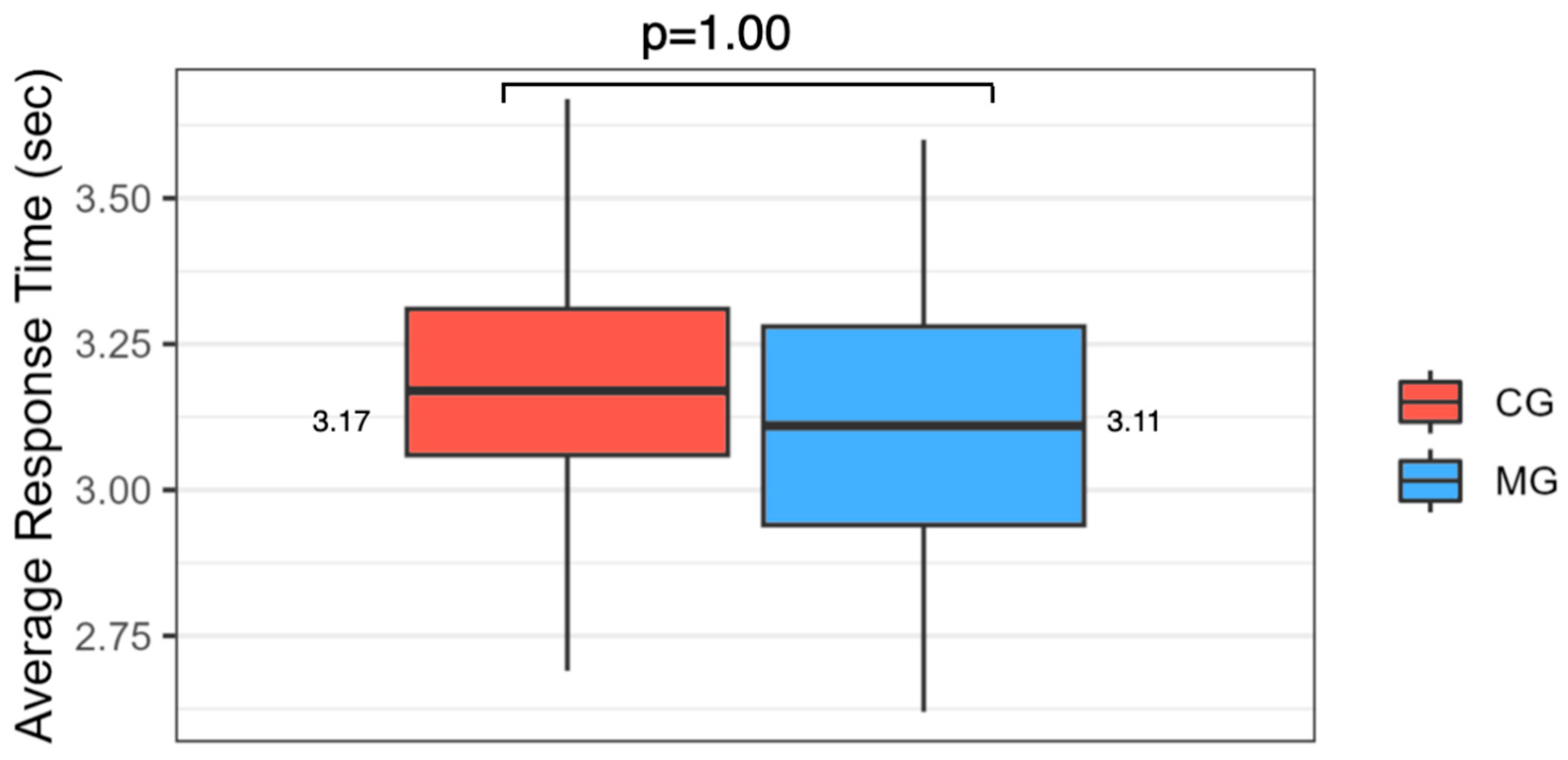
4.4. Facial Emotion Recognition
4.5. Correlation of Alexithymia and Facial Emotion Recognition
5. Discussion
5.1. Methodology
5.2. Comparison of the Results with Existing Literature
5.3. Interpretation of the Results and Outlook
5.4. Clinical Implementation
6. Conclusions
- The chronic migraine group is characterized by clear central sensitization but not by a significant difference in alexithymia compared with the control group.
- There is a weak to moderate correlation between alexithymia in the migraine group and emotion recognition for all basic emotions, except disgust.
- In the control group, there is a moderate correlation between alexithymia and the emotions of fear, disgust, and surprise.
- Particularly high scores on the Central Sensitization Inventory (CSI) and the Toronto Alexithymia Scale-20 (TAS-20) may suggest facial somatosensory distortion, which is expressed in association reduced facial emotion recognition.
- These results should be interpreted with caution but offer innovative ideas for further clinical research.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Laterality Recognition
Appendix B. Facial Emotion Recognition
References
- Edmeads, J.; Mackell, J.A. The Economic Impact of Migraine: An Analysis of Direct and Indirect Costs. Headache J. Head Face Pain 2002, 42, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Saylor, D.; Steiner, T. The Global Burden of Headache. Semin. Neurol. 2018, 38, 182–190. [Google Scholar] [CrossRef]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 310 Diseases and Injuries, 1990–2015: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden, and Comorbidity. Neurol. Clin. 2019, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Fuensalida-Novo, S.; Palacios-Ceña, M.; Fernández-Muñoz, J.J.; Castaldo, M.; Wang, K.; Catena, A.; Arendt-Nielsen, L.; Fernández-de-las-Peñas, C. The Burden of Headache Is Associated to Pain Interference, Depression and Headache Duration in Chronic Tension Type Headache: A 1-Year Longitudinal Study. J. Headache Pain 2017, 18, 119. [Google Scholar] [CrossRef]
- Steiner, T.J.; Stovner, L.J.; Jensen, R.; Uluduz, D.; Katsarava, Z. Migraine Remains Second among the World’s Causes of Disability, and First among Young Women: Findings from GBD2019. J. Headache Pain 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Grande, R.B.; Aaseth, K.; Gulbrandsen, P.; Lundqvist, C.; Russell, M.B. Prevalence of Primary Chronic Headache in a Population-Based Sample of 30- to 44-Year-Old Persons. Neuroepidemiology 2008, 30, 76–83. [Google Scholar] [CrossRef]
- Jull, G.; Sterling, M.; Falla, D.; Treleaven, J.; O’Leary, S. Whiplash, Headache and Neck Pain—Research Based Directions for Physical Therapies; Chruchill Livingstone Elsevier: Amsterdam, The Netherlands, 2008; ISBN 978-0-443-10047-5. [Google Scholar]
- Ashina, S.; Buse, D.C.; Bjorner, J.B.; Bendtsen, L.; Lyngberg, A.C.; Jensen, R.H.; Lipton, R.B. Health-Related Quality of Life in Tension-Type Headache: A Population-Based Study. Scand. J. Pain 2021, 21, 778–787. [Google Scholar] [CrossRef]
- Malmberg-Ceder, K.; Haanpää, M.; Korhonen, P.E.; Kautiainen, H.; Veromaa, V.; Soinila, S. The Role of Psychosocial Risk Factors in the Burden of Headache. J. Pain Res. 2019, 12, 1733–1741. [Google Scholar] [CrossRef]
- von Piekartz, H.; Wallwork, S.B.; Mohr, G.; Butler, D.S.; Moseley, G.L. People with Chronic Facial Pain Perform Worse than Controls at a Facial Emotion Recognition Task, but It Is Not All about the Emotion. J. Oral. Rehabil. 2015, 42, 243–250. [Google Scholar] [CrossRef]
- von Piekartz, H.; Mohr, G. Reduction of Head and Face Pain by Challenging Lateralization and Basic Emotions: A Proposal for Future Assessment and Rehabilitation Strategies. J. Man. Manip. Ther. 2014, 22, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Ziegeler, C.; Schulte, L.H.; May, A. Altered Trigeminal Pain Processing on Brainstem Level in Persistent Idiopathic Facial Pain. Pain 2021, 162, 1374. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.E.; Schrepf, A.; Clauw, D.J. Pain Mechanisms and Centralized Pain in Temporomandibular Disorders. J. Dent. Res. 2016, 95, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Lin, C. Brain Signature of Chronic Orofacial Pain: A Systematic Review and Meta-Analysis on Neuroimaging Research of Trigeminal Neuropathic Pain and Temporomandibular Joint Disorders. PLoS ONE 2014, 9, e94300. [Google Scholar] [CrossRef] [PubMed]
- La Touche, R.; Paris-Alemany, A.; Hidalgo-Pérez, A.; López-de-Uralde-Villanueva, I.; Angulo-Diaz-Parreño, S.; Muñoz-García, D. Evidence for Central Sensitization in Patients with Temporomandibular Disorders: A Systematic Review and Meta-Analysis of Observational Studies. Pain Pract. 2018, 18, 388–409. [Google Scholar] [CrossRef]
- Woolf, C.J. Central Sensitization: Implications for the Diagnosis and Treatment of Pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- van Griensven, H.; Schmid, A.; Trendafilova, T.; Low, M. Central Sensitization in Musculoskeletal Pain: Lost in Translation? J. Orthop. Sports Phys. Ther. 2020, 50, 592–596. [Google Scholar] [CrossRef]
- Nijs, J.; George, S.Z.; Clauw, D.J.; Fernández-de-las-Peñas, C.; Kosek, E.; Ickmans, K.; Fernández-Carnero, J.; Polli, A.; Kapreli, E.; Huysmans, E.; et al. Central Sensitisation in Chronic Pain Conditions: Latest Discoveries and Their Potential for Precision Medicine. Lancet Rheumatol. 2021, 3, e383–e392. [Google Scholar] [CrossRef]
- Linder, M.; Michaelson, P.; Röijezon, U. Laterality Judgments in People with Low Back Pain—A Cross-Sectional Observational and Test–Retest Reliability Study. Man. Ther. 2016, 21, 128–133. [Google Scholar] [CrossRef]
- Moseley, G.L. Graded Motor Imagery for Pathologic Pain: A Randomized Controlled Trial. Neurology 2006, 67, 2129–2134. [Google Scholar] [CrossRef]
- Reinersmann, A.; Haarmeyer, G.S.; Blankenburg, M.; Frettlöh, J.; Krumova, E.K.; Ocklenburg, S.; Maier, C. Left Is Where the L Is Right. Significantly Delayed Reaction Time in Limb Laterality Recognition in Both CRPS and Phantom Limb Pain Patients. Neurosci. Lett. 2010, 486, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Walz, A.D.; Usichenko, T.; Moseley, G.L.; Lotze, M. Graded Motor Imagery and the Impact on Pain Processing in a Case of CRPS. Clin. J. Pain 2013, 29, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Breckenridge, J.D.; McAuley, J.H.; Butler, D.S.; Stewart, H.; Moseley, G.L.; Ginn, K.A. The Development of a Shoulder Specific Left/Right Judgement Task: Validity & Reliability. Musculoskelet. Sci. Pract. 2017, 28, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.O.; Röijezon, U.; Björklund, M.; Djupsjöbacka, M. Long-Term Adaptation to Neck/Shoulder Pain and Perceptual Performance in a Hand Laterality Motor Imagery Test. Perception 2010, 39, 119–130. [Google Scholar] [CrossRef]
- Luedtke, K.; Edlhaimb, J. Laterality Judgements in Patients with Frequent Episodic Migraine. Musculoskelet. Sci. Pract. 2021, 51, 102316. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Butler, D.S.; Beames, T. (Eds.) The Graded Motor Imagery Handbook; Noigroup Publ.: Adelaide, Australia, 2012; ISBN 978-0-9872467-5-2. [Google Scholar]
- Aaron, R.V.; Fisher, E.A.; de la Vega, R.; Lumley, M.A.; Palermo, T.M. Alexithymia in Individuals with Chronic Pain and Its Relation to Pain Intensity, Physical Interference, Depression, and Anxiety: A Systematic Review and Meta-Analysis. Pain 2019, 160, 994. [Google Scholar] [CrossRef]
- Montebarocci, O.; Surcinelli, P.; Rossi, N.; Baldaro, B. Alexithymia, Verbal Ability and Emotion Recognition. Psychiatr. Q. 2011, 82, 245–252. [Google Scholar] [CrossRef]
- Alazmi, L.; Gadsby, G.E.; Heneghan, N.R.; Punt, T.D. Do Trunk-Based Left/Right Judgment Tasks Elicit Motor Imagery? Musculoskelet. Sci. Pract. 2018, 35, 55–60. [Google Scholar] [CrossRef]
- Schmid, A.B.; Coppieters, M.W. Left/Right Judgment of Body Parts Is Selectively Impaired in Patients with Unilateral Carpal Tunnel Syndrome. Clin. J. Pain 2012, 28, 615–622. [Google Scholar] [CrossRef]
- Kano, M.; Fukudo, S. The Alexithymic Brain: The Neural Pathways Linking Alexithymia to Physical Disorders. Biopsychosoc. Med. 2013, 7, 1. [Google Scholar] [CrossRef]
- Moseley, G.L.; Butler, D.S. Explain Pain Supercharged—The Clinician’s Handbook. Noigroup Publications: Adelaide, Australia, 2017; ISBN 978-0-6480227. [Google Scholar]
- Moseley, G.L.; Zalucki, N.; Birklein, F.; Marinus, J.; van Hilten, J.J.; Luomajoki, H. Thinking about Movement Hurts: The Effect of Motor Imagery on Pain and Swelling in People with Chronic Arm Pain. Arthritis Rheum. 2008, 59, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Moseley, G.L.; Sim, D.F.; Henry, M.L.; Souvlis, T. Experimental Hand Pain Delays Recognition of the Contralateral Hand—Evidence That Acute and Chronic Pain Have Opposite Effects on Information Processing? Cogn. Brain Res. 2005, 25, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L.G. Graded Motor Imagery Is Effective for Long-Standing Complex Regional Pain Syndrome: A Randomised Controlled Trial. Pain 2004, 108, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Moseley, L.G. Is Successful Rehabilitation of Complex Regional Pain Syndrome Due to Sustained Attention to the Affected Limb? A Randomised Clinical Trial: Pain 2005, 114, 54–61. [Google Scholar] [CrossRef] [PubMed]
- International Headache Society International Classification of Headache Disorders, 3rd Edition. Cephalalgia 2018, 38, 1–211. [CrossRef]
- Taxer, B.; De Castro-Carletti, E.M.; Von Piekartz, H.; Leis, S.; Christova, M.; Armijo-Olivo, S. Facial Recognition, Laterality Judgement, Alexithymia and Resulting Central Nervous System Adaptations in Chronic Primary Headache and Facial Pain—A Systematic Review and Meta-analysis. J. Oral. Rehabil. 2024, 51, 1881–1897. [Google Scholar] [CrossRef]
- Bagby, R.M.; Taylor, G.J.; Parker, J.D. The Twenty-Item Toronto Alexithymia Scale—II. Convergent, Discriminant, and Concurrent Validity. J. Psychosom. Res. 1994, 38, 33–40. [Google Scholar] [CrossRef]
- Bagby, R.M.; Parker, J.D.; Taylor, G.J. The Twenty-Item Toronto Alexithymia Scale—I. Item Selection and Cross-Validation of the Factor Structure. J. Psychosom. Res. 1994, 38, 23–32. [Google Scholar] [CrossRef]
- Bach, M.; Bach, D.; de Zwaan, M.; Serim, M. Validierung Der Deutschen Version Der 20-Item Toronto-Alexithymie-Skala Bei Normalpersonen Und Psychiatrischen Patienten. [Validation of the German Version of the 20-Item Toronto Alexithymia Scale in Normal Adults and Psychiatric Inpatients.]. PPmP Psychother. Psychosom. Med. Psychol. 1996, 46, 23–28. [Google Scholar]
- Bagby, R.M.; Parker, J.D.A.; Taylor, G.J. Twenty-Five Years with the 20-Item Toronto Alexithymia Scale. J. Psychosom. Res. 2020, 131, 109940. [Google Scholar] [CrossRef]
- Koch, A.S.; Kleiman, A.; Wegener, I.; Zur, B.; Imbierowicz, K.; Geiser, F.; Conrad, R. Factorial Structure of the 20-Item Toronto Alexithymia Scale in a Large Sample of Somatoform Patients. Psychiatry Res. 2015, 225, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Antony, M.M.; Bieling, P.J.; Cox, B.J.; Enns, M.W.; Swinson, R.P. Psychometric Properties of the 42-Item and 21-Item Versions of the Depression Anxiety Stress Scales in Clinical Groups and a Community Sample. Psychol. Assess. 1998, 10, 176–181. [Google Scholar] [CrossRef]
- Nilges, P.; Essau, C. Die Depressions-Angst-Stress-Skalen: Der DASS—Ein Screeningverfahren nicht nur für Schmerzpatienten. Der Schmerz 2015, 29, 649–657. [Google Scholar] [CrossRef]
- Schumacher, S.; Waschescio, H.J. Validierung einer deutschen Version des „Central Sensitization Inventory“ zur Identifizierung zentralnervöser Schmerzentwicklungen. Manuelletherapie 2019, 23, 129–133. [Google Scholar] [CrossRef]
- Dey, A.; Barnsley, N.; Mohan, R.; McCormick, M.; McAuley, J.H.; Moseley, G.L. Are Children Who Play a Sport or a Musical Instrument Better at Motor Imagery than Children Who Do Not? Br. J. Sports Med. 2012, 46, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Bray, H.; Moseley, G.L. Disrupted Working Body Schema of the Trunk in People with Back Pain. Br. J. Sports Med. 2011, 45, 168–173. [Google Scholar] [CrossRef]
- Kuttenreich, A.-M.; Volk, G.F.; Guntinas-Lichius, O.; von Piekartz, H.; Heim, S. Facial Emotion Recognition in Patients with Post-Paralytic Facial Synkinesis—A Present Competence. Diagnostics 2022, 12, 1138. [Google Scholar] [CrossRef]
- Kuttenreich, A.-M.; von Piekartz, H.; Heim, S. Is There a Difference in Facial Emotion Recognition after Stroke with vs. without Central Facial Paresis? Diagnostics 2022, 12, 1721. [Google Scholar] [CrossRef]
- Bowering, K.J.; O’Connell, N.E.; Tabor, A.; Catley, M.J.; Leake, H.B.; Moseley, G.L.; Stanton, T.R. The Effects of Graded Motor Imagery and Its Components on Chronic Pain: A Systematic Review and Meta-Analysis. J. Pain 2013, 14, 3–13. [Google Scholar] [CrossRef]
- Brunner, E.; Bathke, A.C.; Konietschke, F. Rank and Pseudo-Rank Procedures for Independent Observations in Factorial Designs: Using R and SAS; Springer Series in Statistics; Springer International Publishing: Cham, 2018; ISBN 978-3-030-02912-8. [Google Scholar]
- R Core Team. European Environment Agency. 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006 (accessed on 17 January 2023).
- Adams, G.R.; Gandhi, W.; Harrison, R.; Van Reekum, C.M.; Wood-Anderson, D.; Gilron, I.; Salomons, T.V. Do “Central Sensitization” Questionnaires Reflect Measures of Nociceptive Sensitization or Psychological Constructs? A Systematic Review and Meta-Analyses. Pain 2023, 164, 1222–1239. [Google Scholar] [CrossRef]
- Shahid, A.; Wilkinson, K.; Marcu, S.; Shapiro, C.M. Perceived Stress Questionnaire (PSQ). In STOP, THAT and One Hundred Other Sleep Scales; Shahid, A., Wilkinson, K., Marcu, S., Shapiro, C.M., Eds.; Springer: New York, NY, USA, 2011; pp. 273–274. ISBN 978-1-4419-9892-7. [Google Scholar]
- Piekartz, H.V.; Mohr, G.; Limbrecht, K.; Traue, H.; Kessler, H. Recognition of Emotional Facial Expressions and Alexithymia in Patients with Chronic Facial Pain. Ann. Psychiatry Ment. Health 2018, 6, 1134. [Google Scholar]
- Pedrosa Gil, F.; Ridout, N.; Kessler, H.; Neuffer, M.; Schoechlin, C.; Traue, H.C.; Nickel, M. Facial Emotion Recognition and Alexithymia in Adults with Somatoform Disorders. Depress. Anxiety 2008, 25, E133–E141. [Google Scholar] [CrossRef] [PubMed]
- von Korn, K.; Richter, M.; von Piekartz, H. [Changes in basic emotion recognition in patients with chronic low back pain. A cross-sectional study analyzing emotion recognition and alexithymia]. Schmerz 2014, 28, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kessler, H.; Schwarze, M.; Filipic, S.; Traue, H.C.; von Wietersheim, J. Alexithymia and Facial Emotion Recognition in Patients with Eating Disorders. Int. J. Eat. Disord. 2006, 39, 245–251. [Google Scholar] [CrossRef]
- Kessler, H.; Roth, J.; von Wietersheim, J.; Deighton, R.M.; Traue, H.C. Emotion Recognition Patterns in Patients with Panic Disorder. Depress. Anxiety 2007, 24, 223–226. [Google Scholar] [CrossRef]
- Braun, M.; Traue, H.C.; Frisch, S.; Deighton, R.M.; Kessler, H. Emotion Recognition in Stroke Patients with Left and Right Hemispheric Lesion: Results with a New Instrument—The FEEL Test. Brain Cogn. 2005, 58, 193–201. [Google Scholar] [CrossRef]
- Lesser, M.L. Design and Interpretation of Observational Studies: Cohort, Case–Control, and Cross-Sectional Designs. In Principles of Research Methodology; Supino, P.G., Borer, J.S., Eds.; Springer: New York, NY, USA, 2012; pp. 55–77. ISBN 978-1-4614-3359-0. [Google Scholar]
- Amiri, S.; Behnezhad, S.; Azad, E. Migraine Headache and Depression in Adults: A Systematic Review and Meta-Analysis. Neuropsychiatrie 2019, 33, 131–140. [Google Scholar] [CrossRef]
- Cologno, D.; Buzzi, M.G.; Carlesimo, G.A.; Cicinelli, P.; Costa, A.; Fadda, L.; Formisano, R.; Marconi, B.; Pero, S.; Caltagirone, C. Psychiatric Disorders and Pain Location in Unilateral Migraineurs. J. Headache Pain 2005, 6, 227–230. [Google Scholar] [CrossRef]
- Sullivan, A.; Cousins, S.; Ridsdale, L. Psychological Interventions for Migraine: A Systematic Review. J. Neurol. 2016, 263, 2369–2377. [Google Scholar] [CrossRef]
- Lin, I.; Wiles, L.; Waller, R.; Goucke, R.; Nagree, Y.; Gibberd, M.; Straker, L.; Maher, C.G.; O’Sullivan, P.P.B. What Does Best Practice Care for Musculoskeletal Pain Look like? Eleven Consistent Recommendations from High-Quality Clinical Practice Guidelines: Systematic Review. Br. J. Sports Med. 2020, 54, 79–86. [Google Scholar] [CrossRef]
- Veirman, E.; Van Ryckeghem, D.M.L.; De Paepe, A.; Kirtley, O.J.; Crombez, G. Multidimensional Screening for Predicting Pain Problems in Adults: A Systematic Review of Screening Tools and Validation Studies. Pain Rep. 2019, 4, e775. [Google Scholar] [CrossRef] [PubMed]
- Butt, M.N.; Maryum, M.; Amjad, I.; Khan, O.J.; Awan, L. Effects of Aerobic Exercise and Progressive Muscle Relaxation on Migraine. J. Pak. Med. Assoc. 2022, 72, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Suzuki, S.; Haruyama, Y.; Okamura, M.; Shiina, T.; Fujita, H.; Kobashi, G.; Sairenchi, T.; Uchiyama, K.; Hirata, K. Central Sensitization in Migraine Is Related to Restless Legs Syndrome. J. Neurol. 2021, 268, 1395–1401. [Google Scholar] [CrossRef]
- Danno, D.; Wolf, J.; Ishizaki, K.; Kikui, S.; Hirata, K.; Takeshima, T. Cranial Autonomic Symptoms in Migraine Are Related to Central Sensitization: A Prospective Study of 164 Migraine Patients at a Tertiary Headache Center. BMC Neurol. 2022, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Do Vale Braido, G.V.; Svensson, P.; Dos Santos Proença, J.; Mercante, F.G.; Fernandes, G.; de Godoi Gonçalves, D.A. Are Central Sensitization Symptoms and Psychosocial Alterations Interfering in the Association between Painful TMD, Migraine, and Headache Attributed to TMD? Clin. Oral. Investig. 2022, 27, 681–690. [Google Scholar] [CrossRef]
- Haas, J.; Eichhammer, P.; Traue, H.C.; Hoffmann, H.; Behr, M.; Crönlein, T.; Pieh, C.; Busch, V. Alexithymic and Somatisation Scores in Patients with Temporomandibular Pain Disorder Correlate with Deficits in Facial Emotion Recognition. J. Oral. Rehabil. 2013, 40, 81–90. [Google Scholar] [CrossRef]
- Grynberg, D.; Chang, B.; Corneille, O.; Maurage, P.; Vermeulen, N.; Berthoz, S.; Luminet, O. Alexithymia and the Processing of Emotional Facial Expressions (EFEs): Systematic Review, Unanswered Questions and Further Perspectives. PLoS ONE 2012, 7, e42429. [Google Scholar] [CrossRef]
- Wabnegger, A.; Ille, R.; Schwingenschuh, P.; Katschnig-Winter, P.; Kögl-Wallner, M.; Wenzel, K.; Schienle, A. Facial Emotion Recognition in Parkinson’s Disease: An fMRI Investigation. PLoS ONE 2015, 10, e0136110. [Google Scholar] [CrossRef]
- Nijs, J.; Goubert, D.; Ickmans, K. Recognition and Treatment of Central Sensitization in Chronic Pain Patients: Not Limited to Specialized Care. J. Orthop. Sports Phys. Ther. 2016, 46, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Ceña, M.; Lima Florencio, L.; Natália Ferracini, G.; Barón, J.; Guerrero, Á.L.; Ordás-Bandera, C.; Arendt-Nielsen, L.; Fernández-de-Las-Peñas, C. Women with Chronic and Episodic Migraine Exhibit Similar Widespread Pressure Pain Sensitivity. Pain Med. 2016, 17, 2127–2133. [Google Scholar] [CrossRef]
- IASP. IASP Terminology—IASP. Available online: https://www.iasp-pain.org/terminology?navItemNumber=576#Pain (accessed on 9 March 2021).
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP Classification of Chronic Pain for ICD-11: Chronic Primary Pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Galea, O.; Thomas, L.; Jull, G.; Treleaven, J. Cervical Musculoskeletal Impairments in Migraine and Tension Type Headache: A Systematic Review and Meta-Analysis. Musculoskelet. Sci. Pract. 2019, 42, 67–83. [Google Scholar] [CrossRef]
- Luedtke, K.; Boissonnault, W.; Caspersen, N.; Castien, R.; Chaibi, A.; Falla, D.; Fernández-de-las-Peñas, C.; Hall, T.; Hirsvang, J.R.; Horre, T.; et al. International Consensus on the Most Useful Physical Examination Tests Used by Physiotherapists for Patients with Headache: A Delphi Study. Man. Ther. 2016, 23, 17–24. [Google Scholar] [CrossRef]
- Lüdtke, K. Evidenzorientierte Untersuchungs- und Behandlungsmethoden für Migränepatienten. Der Schmerzpatient 2018, 1, 76–81. [Google Scholar] [CrossRef][Green Version]
- Castien, R.; De Hertogh, W. A Neuroscience Perspective of Physical Treatment of Headache and Neck Pain. Front. Neurol. 2019, 10, 276. [Google Scholar] [CrossRef]
- Adams, L.M.; Turk, D.C. Central Sensitization and the Biopsychosocial Approach to Understanding Pain. J. Appl. Biobehav. Res. 2018, 0, e12125. [Google Scholar] [CrossRef]
- Stewart, W.F.; Roy, J.; Lipton, R.B. Migraine Prevalence, Socioeconomic Status, and Social Causation. Neurology 2013, 81, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Probyn, K.; Bowers, H.; Caldwell, F.; Mistry, D.; Underwood, M.; Matharu, M.; Pincus, T. Prognostic Factors for Chronic Headache. Neurology 2017, 89, 291–301. [Google Scholar] [CrossRef]
- Bottiroli, S.; Galli, F.; Viana, M.; Sances, G.; Tassorelli, C. Traumatic Experiences, Stressful Events, and Alexithymia in Chronic Migraine With Medication Overuse. Front. Psychol. 2018, 9, 704. [Google Scholar] [CrossRef]
- Yalınay Dikmen, P.; Onur Aysevener, E.; Kosak, S.; Ilgaz Aydınlar, E.; Sağduyu Kocaman, A. Relationship between MIDAS, Depression, Anxiety and Alexithymia in Migraine Patients. Acta Neurol. Belg. 2020, 120, 837–844. [Google Scholar] [CrossRef]
- Matamala-Gomez, M.; Bottiroli, S.; Sances, G.; Allena, M.; De Icco, R.; Ghiotto, N.; Guaschino, E.; Sandrini, G.; Tassorelli, C. Facial Expressions Modulate Pain Perception in Patients with Chronic Migraine. Cephalalgia 2022, 42, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Von Piekartz, H.; Paris-Alemany, A. Assessment and Brain Training of Patients Experiencing Head and Facial Pain with a Distortion of Orofacial Somatorepresentation: A Narrative Review. Appl. Sci. 2021, 11, 6857. [Google Scholar] [CrossRef]
- Meise, R.; Carvalho, G.F.; Thiel, C.; Luedtke, K. Additional Effects of Pain Neuroscience Education Combined with Physiotherapy on the Headache Frequency of Adult Patients with Migraine: A Randomized Controlled Trial. Cephalalgia 2023, 43, 3331024221144781. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L.M. Integrating Cognitive Psychology, Neurology and Neuroimaging. Acta Psychol. 2001, 107, 155–181. [Google Scholar] [CrossRef]
- Osuagwu, B.A.; Vuckovic, A. Similarities between Explicit and Implicit Motor Imagery in Mental Rotation of Hands: An EEG Study. Neuropsychologia 2014, 65, 197–210. [Google Scholar] [CrossRef]
- Moseley, L.G. I Can’t Find It! Distorted Body Image and Tactile Dysfunction in Patients with Chronic Back Pain. Pain 2008, 140, 239–243. [Google Scholar] [CrossRef]
- Limakatso, K.; Corten, L.; Parker, R. The Effects of Graded Motor Imagery and Its Components on Phantom Limb Pain and Disability in Upper and Lower Limb Amputees: A Systematic Review Protocol. Syst. Rev. 2016, 5, 145. [Google Scholar] [CrossRef]
- Lagueux, E.; Charest, J.; Lefrançois-Caron, E.; Mauger, M.-E.; Mercier, E.; Savard, K.; Tousignant-Laflamme, Y. Modified Graded Motor Imagery for Complex Regional Pain Syndrome Type 1 of the Upper Extremity in the Acute Phase: A Patient Series. Int. J. Rehabil. Res. 2012, 35, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Birklein, F.; Humm, A.; Maier, C.; Maihöfner, C.; Middeldorf, S.; Quasthoff, S.; Siemers, F.; Sommer, C. Diagnostik und Therapie komplexer regionaler Schmerzsyndrome (CRPS), S1-Leitlinie 2018; in Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie. Mainz, Deutschland. Available online: https://www.dgn.org/leitlinien (accessed on 24 April 2024).
- Breckenridge, J.D.; McAuley, J.H.; Butler, D.S.; Stewart, H.; Moseley, G.L.; Ginn, K.A. Shoulder Left/Right Judgement Task: Development and Establishment of a Normative Dataset. Physiotherapy 2015, 101, e169–e170. [Google Scholar] [CrossRef]
- Wallwork, S.B.; Butler, D.S.; Fulton, I.; Stewart, H.; Darmawan, I.; Moseley, G.L. Left/Right Neck Rotation Judgments Are Affected by Age, Gender, Handedness and Image Rotation. Man. Ther. 2013, 18, 225–230. [Google Scholar] [CrossRef]
- Magni, N.E.; McNair, P.J.; Rice, D.A. Sensorimotor Performance and Function in People with Osteoarthritis of the Hand: A Case–Control Comparison. Semin. Arthritis Rheum. 2018, 47, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Stanton, T.R.; Lin, C.-W.C.; Bray, H.; Smeets, R.J.E.M.; Taylor, D.; Law, R.Y.W.; Moseley, G.L. Tactile Acuity Is Disrupted in Osteoarthritis but Is Unrelated to Disruptions in Motor Imagery Performance. Rheumatology 2013, 52, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- von Piekartz, H.; Lüers, J.; Daumeyer, H.; Mohr, G. Is kinesiophobia associated with changes in left/right judgment and emotion recognition during a persisting pain condition?: A cross-sectional study. Schmerz 2017, 31, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Wallwork, S.B.; Butler, D.S.; Moseley, G.L. Dizzy People Perform No Worse at a Motor Imagery Task Requiring Whole Body Mental Rotation; A Case-Control Comparison. Front. Hum. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef]
- Ekman, P. Basic Emotions. In Handbook of Cognition and Emotion; John Wiley & Sons Inc.: Hoboken, NJ, USA, 1999. [Google Scholar]
- Jongen, S.; Axmacher, N.; Kremers, N.A.W.; Hoffmann, H.; Limbrecht-Ecklundt, K.; Traue, H.C.; Kessler, H. An Investigation of Facial Emotion Recognition Impairments in Alexithymia and Its Neural Correlates. Behav. Brain Res. 2014, 271, 129–139. [Google Scholar] [CrossRef]
- Galderisi, S.; Mancuso, F.; Mucci, A.; Garramone, S.; Zamboli, R.; Maj, M. Alexithymia and Cognitive Dysfunctions in Patients with Panic Disorder. Psychother. Psychosom. 2008, 77, 182–188. [Google Scholar] [CrossRef]
- Kätsyri, J.; Saalasti, S.; Tiippana, K.; von Wendt, L.; Sams, M. Impaired Recognition of Facial Emotions from Low-Spatial Frequencies in Asperger Syndrome. Neuropsychologia 2008, 46, 1888–1897. [Google Scholar] [CrossRef]
- Mann, L.S.; Wise, T.N.; Trinidad, A.; Kohanski, R. Alexithymia, Affect Recognition, and Five Factors of Personality in Substance Abusers. Percept. Mot. Ski. 1995, 81, 35–40. [Google Scholar] [CrossRef]
- Marco-Garcia, S.; Ferrer-Quintero, M.; Usall, J.; Ochoa, S.; Del Cacho, N.; Huerta-Ramos, E. Facial emotion recognition in neurological disorders: A narrative review. Rev. Neurol. 2019, 69, 207–219. [Google Scholar] [CrossRef]
- Yetkin-Ozden, S.; Ekizoglu, E.; Baykan, B. Face Recognition in Patients with Migraine. Pain Pract. 2015, 15, 319–322. [Google Scholar] [CrossRef]
- Szabó, E.; Galambos, A.; Kocsel, N.; Édes, A.E.; Pap, D.; Zsombók, T.; Kozák, L.R.; Bagdy, G.; Kökönyei, G.; Juhász, G. Association between Migraine Frequency and Neural Response to Emotional Faces: An fMRI Study. NeuroImage: Clin. 2019, 22, 101790. [Google Scholar] [CrossRef] [PubMed]



| MG (n = 45) | CG (n = 25) | |
|---|---|---|
| Age (mean, ±SD) | 39.18 ± 13.20 | 35.56 ± 11.97 |
| Sex | f = 39 | f = 21 |
| Nationality | n = 45 AT | n = 25 AT |
| Employed | Yes: n = 33 No: n = 6 Academic education: n = 6 | Yes: n = 15 No: n = 0 Academic education: n = 10 |
| Highest level of education | Primary school: n = 14 Graduate: n = 17 University: n = 14 | Primary school: n = 0 Graduate: n = 12 University: n = 13 |
| Months with HA (mean, ±SD) | 273.6 ± 145.1 | --- |
| Ø HA days/last 3 months (mean, ±SD) | 37.5 ± 19 | --- |
| MG (n = 45) | CG (n = 25) | ||
|---|---|---|---|
| Current medication | NOPA: n = 34 Triptans: n = 22 Tricyclic AD: n = 9 MAB: n = 9 | Anticonvulsive: n = 5 (of which TPM: n = 3) Beta-Blockers: n = 2 SNRI: n = 2 | --- |
| Acute and previous non-drug treatment(s) | Acute: Physiotherapy: n = 6 Acupuncture: n = 4 Massage: n = 3 Relaxation techniques: n = 2 Psychotherapy: n = 2 Osteopathy: n = 1 Orthodontics: n = 1 | Previous: Physiotherapy: n = 7 Acupuncture: n = 7 Relaxation techniques: n = 3 Osteopathy: n = 3 Botulinum toxin: n = 3 Massage: n = 2 Orthodontics: n = 1 Biofeedback: n = 1 Migraine surgery: n = 1 | --- |
| Additional diagnoses | Cervical-spine shoulder syndrome: n = 10 Depression: n = 3 Anxiety disorder: n = 1 Mb. Hashimoto: n = 3 Hypothyroidism: n = 3 Post-COVID Syndrome: n = 2 Chron. Gastritis: n = 2 Mb. Basedow: n = 1 PCOS: n = 1 Psoriasis: n = 1 | Melkerson-Rosenthal-Syndrome: n = 1 TTH: n = 1 Restless-Leg-Syndrom: n = 1 Irritable bowel syndrome: n = 1 Mb. Raynaud: n = 1 Arterial hypertension: n = 1 Chronic Sinusitis: n = 1 Endometriosis: n = 1 Adipositas: n = 1 CMD: n = 1 | Mb. Basedow: n = 1 PCOS: n = 1 Endometriosis: n = 1 |
| Persistent pain (>3 months) in other body regions | Spine: n = 12 Shoulder: n = 5 Hip: n = 1 | Spine: n = 2 Knee: n = 1 Lower thigh: n = 1 | |
| Nicotine | Yes: n = 6 No: n = 39 | Yes: n = 1 No: n = 24 | |
| Distance to last menstrual period | <1 Week: n = 5 <2 Weeks: n = 9 <3 Weeks: n = 1 >4 Weeks resp. menopause: n = 9 No information: n = 15 | <1 Week: n = 3 <2 Weeks: n = 4 <3 Weeks: n = 4 >4 Weeks resp. menopause: n = 5 No information: n = 5 | |
| Hormonal treatment | Thyroid medication: n = 9 Oral contraception: n = 6 Other: n = 5 Hormone coil: n = 3 None: n = 23 | Thyroid medication: n = 1 Oral contraception: n = 4 Other: n = 0 Hormone coil: n = 1 None: n = 20 |
| Emotion | MG Mean/SD | CG Mean/SD | p-Value |
|---|---|---|---|
| Fear | 24.51/19.23 | 37.72/25.66 | 1.00 |
| Disgust | 59.29/17.94 | 69.00/15.26 | 0.54 |
| Surprised | 85.38/16.07 | 77.12/21.44 | 1.00 |
| Happy | 96.22/8.82 | 95.48/7.92 | 1.00 |
| Sad | 72.64/22.21 | 87.48/10.48 | 0.12 |
| Angry | 75.00/15.42 | 80.56/10.24 | 1.00 |
| Total number of wrong answers | 24.40/6.54 | 20.00/6.14 | 1.00 |
| Total number unanswered | 6.76/6.86 | 5.00/4.95 | 1.00 |
| Average response time | 3.12/0.24 | 3.18/0.22 | 1.00 |
| Emotion | Migraine Group Spearman’s ρ (rho) Significance p Values | Control Group Spearman’s ρ (rho) Significance p Values |
|---|---|---|
| Fear | −0.24 * (p = 0.11) | −0.44 * (p = 0.03) |
| Disgust | 0.03 (p = 0.16) | −0.21 * (p = 0.33) |
| Surprised | −0.22 * (p = 0.16) | −0.26 * (p = 0.21) |
| Happy | −0.21 * (p = 0.16) | 0.03 (p = 0.88) |
| Sad | −0.27 * (p = 0.07) | 0.04 (p = 0.87) |
| Angry | −0.24 * (p = 0.11) | −0.06 (p = 0.77) |
| Total number of wrong answers | 0.21 * (p = 0.16) | 0.48 * (p = 0.02) |
| Total number unanswered | 0.19 (p = 0.21) | 0.06 (p = 0.77) |
| Average response time | 0.08 (p = 0.59) | −0.05 (p = 0.81) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taxer, B.; von Piekartz, H.; Lauth, W.; Christova, M.; Leis, S. Exploring Facial Somatosensory Distortion in Chronic Migraine: The Role of Laterality and Emotion Recognition—A Cross-Sectional Study. Appl. Sci. 2024, 14, 8102. https://doi.org/10.3390/app14188102
Taxer B, von Piekartz H, Lauth W, Christova M, Leis S. Exploring Facial Somatosensory Distortion in Chronic Migraine: The Role of Laterality and Emotion Recognition—A Cross-Sectional Study. Applied Sciences. 2024; 14(18):8102. https://doi.org/10.3390/app14188102
Chicago/Turabian StyleTaxer, Bernhard, Harry von Piekartz, Wanda Lauth, Monica Christova, and Stefan Leis. 2024. "Exploring Facial Somatosensory Distortion in Chronic Migraine: The Role of Laterality and Emotion Recognition—A Cross-Sectional Study" Applied Sciences 14, no. 18: 8102. https://doi.org/10.3390/app14188102
APA StyleTaxer, B., von Piekartz, H., Lauth, W., Christova, M., & Leis, S. (2024). Exploring Facial Somatosensory Distortion in Chronic Migraine: The Role of Laterality and Emotion Recognition—A Cross-Sectional Study. Applied Sciences, 14(18), 8102. https://doi.org/10.3390/app14188102





