An Environmentally Sustainable Approach for Raw Whey Treatment through Sequential Cultivation of Macrophytes and Microalgae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Macrophytes and Microalgae
2.2. Characterization of the Raw Whey Wastewater
2.3. Flask Culture Experiments
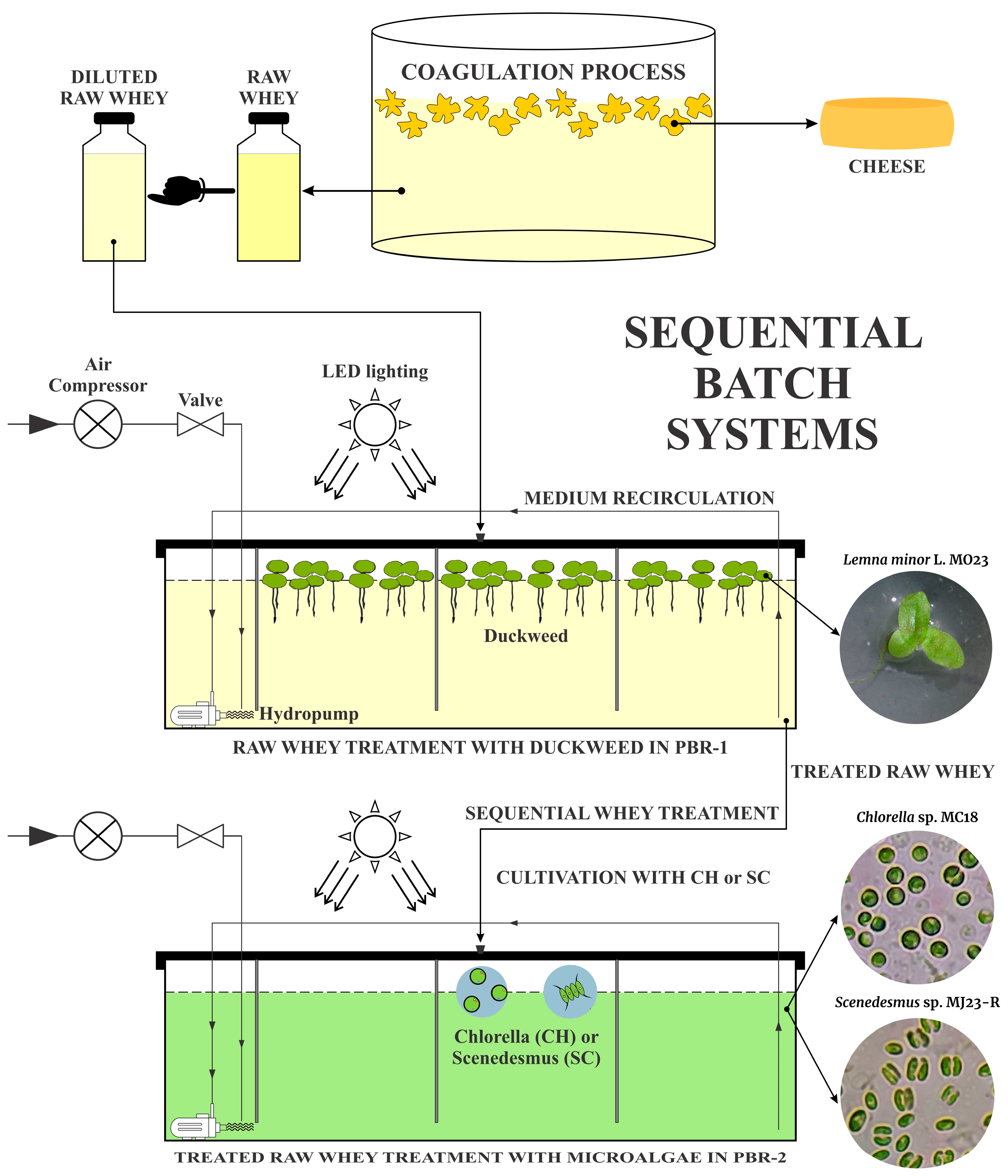
2.4. Sequential Batch Photobioreactor Experimentation
2.5. Growth Kinetic Data and Parameters
2.6. Other Analytical Measurements
2.7. Statistical Analysis
3. Results and Discussion
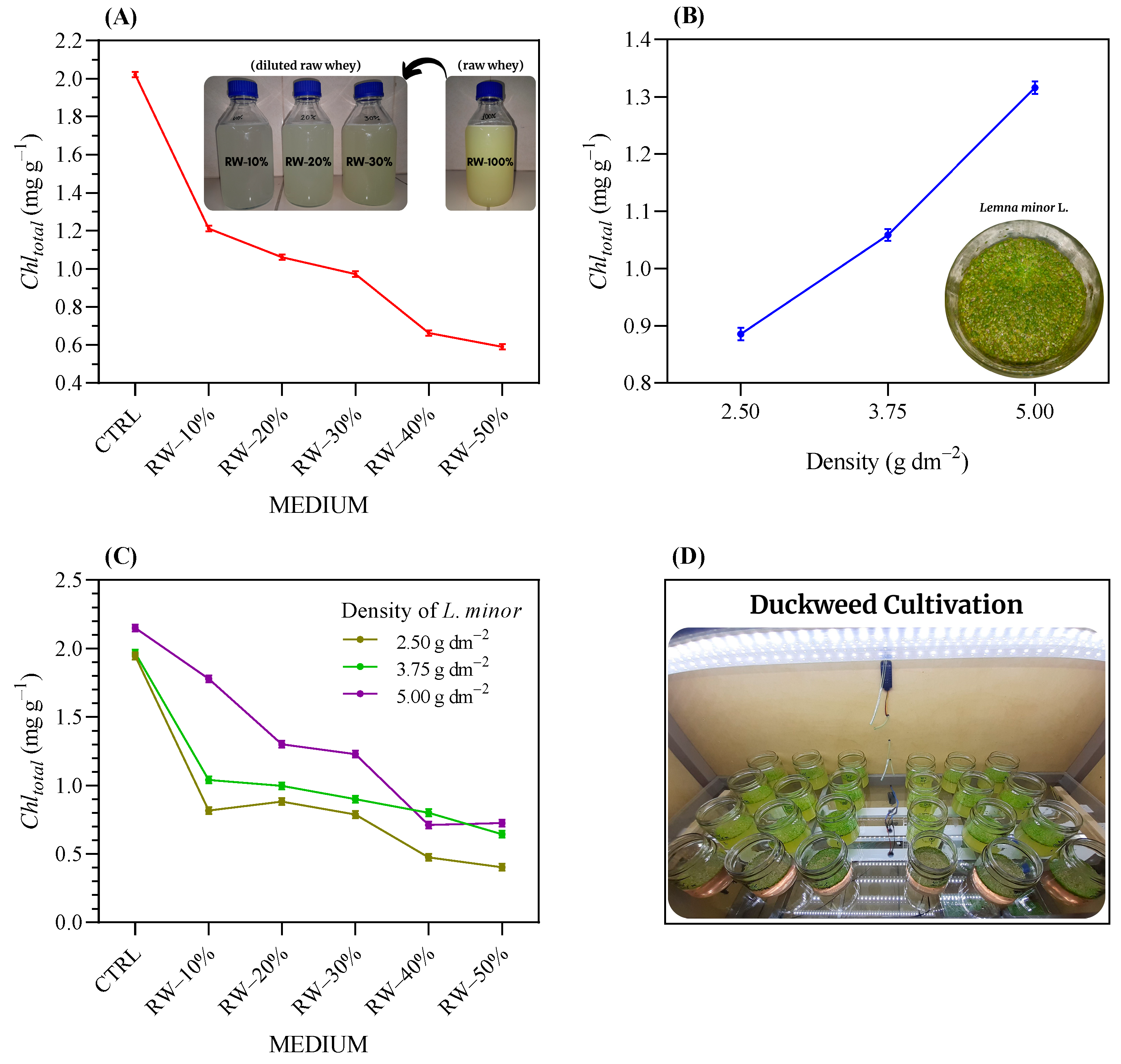
3.1. Tolerance of Duckweed to Raw Whey (RW) Wastewater
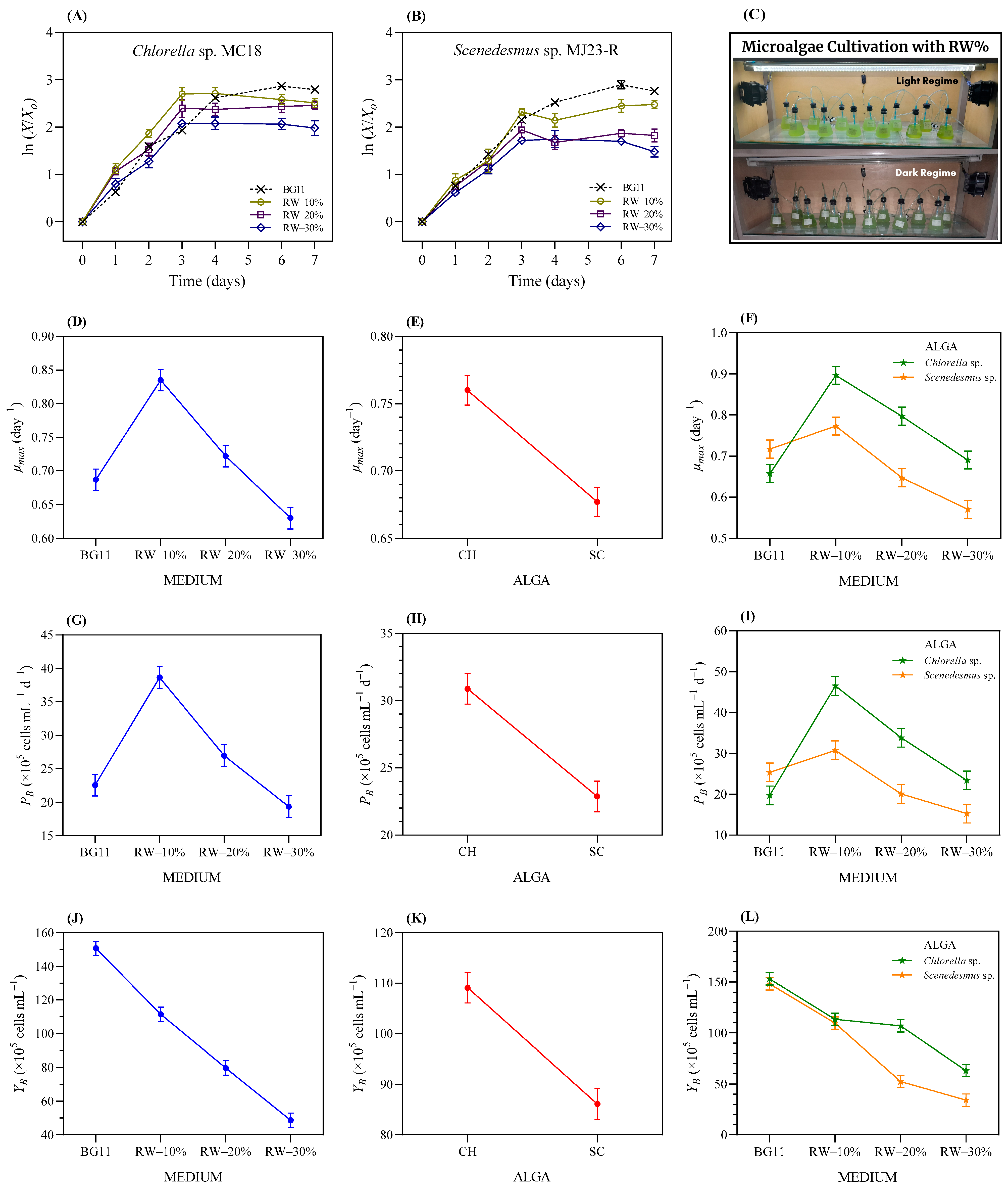
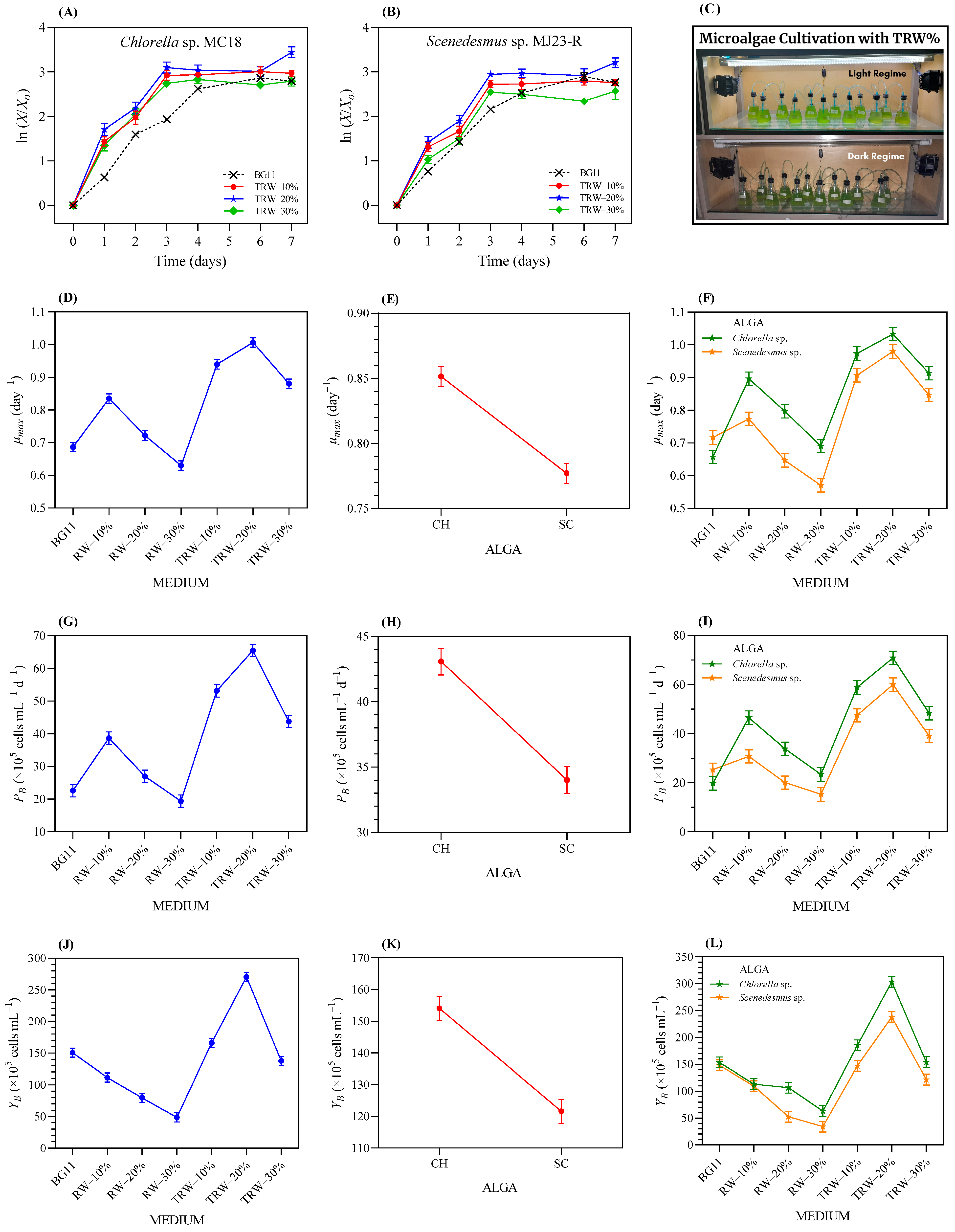
3.2. Tolerance of the Microalgae to Raw Whey (RW) Wastewater
3.3. Sequential Batch Photobioreactor Experimentation with Duckweed and Microalgae
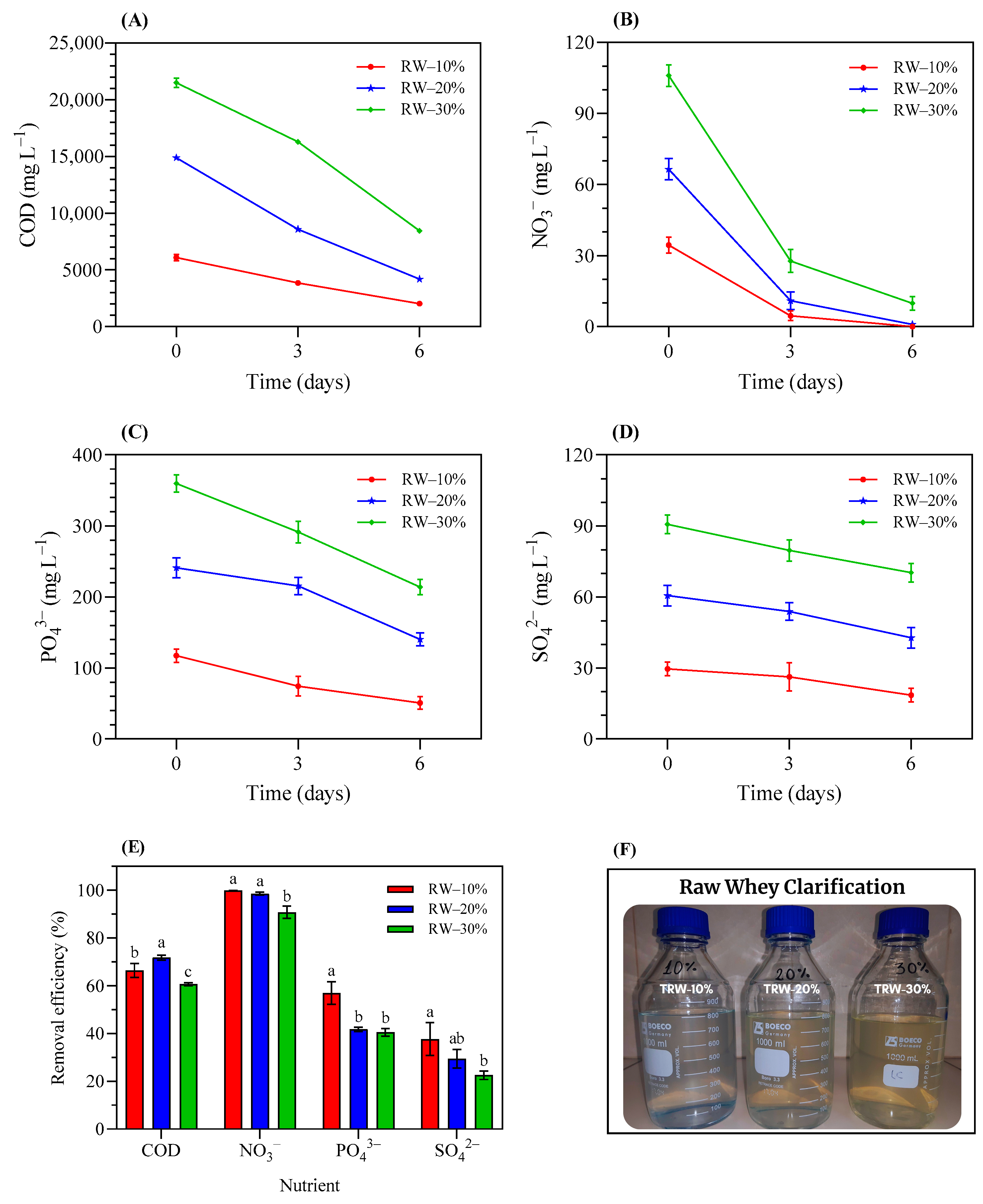
3.3.1. Bioremediation Potential of Duckweed in RW-Based Media
3.3.2. Tolerance Test of the Microalgae to RW-Based Media Pre-Treated with Duckweed
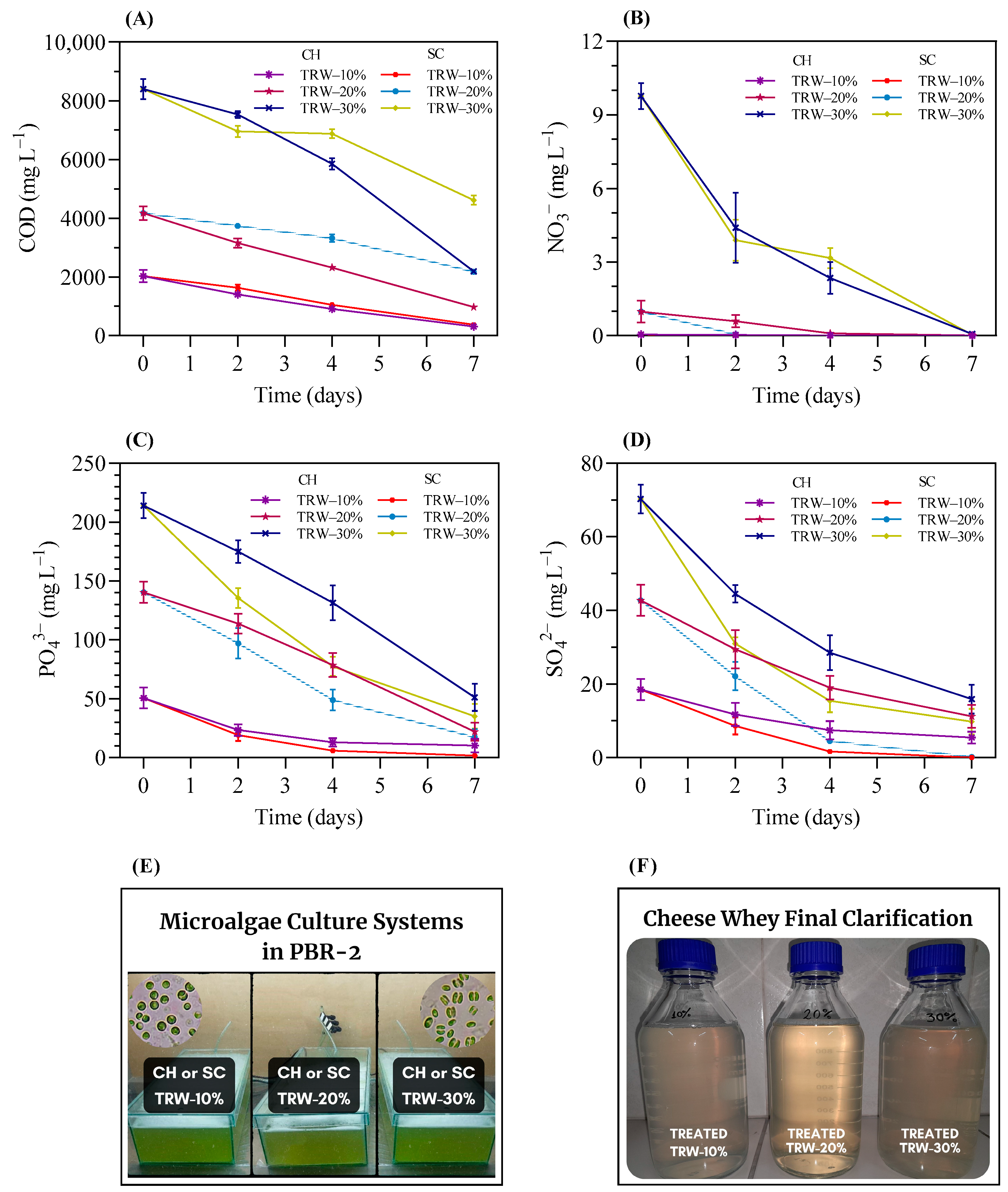
3.3.3. Microalgae Bioremediation in Duckweed-Treated RW Media
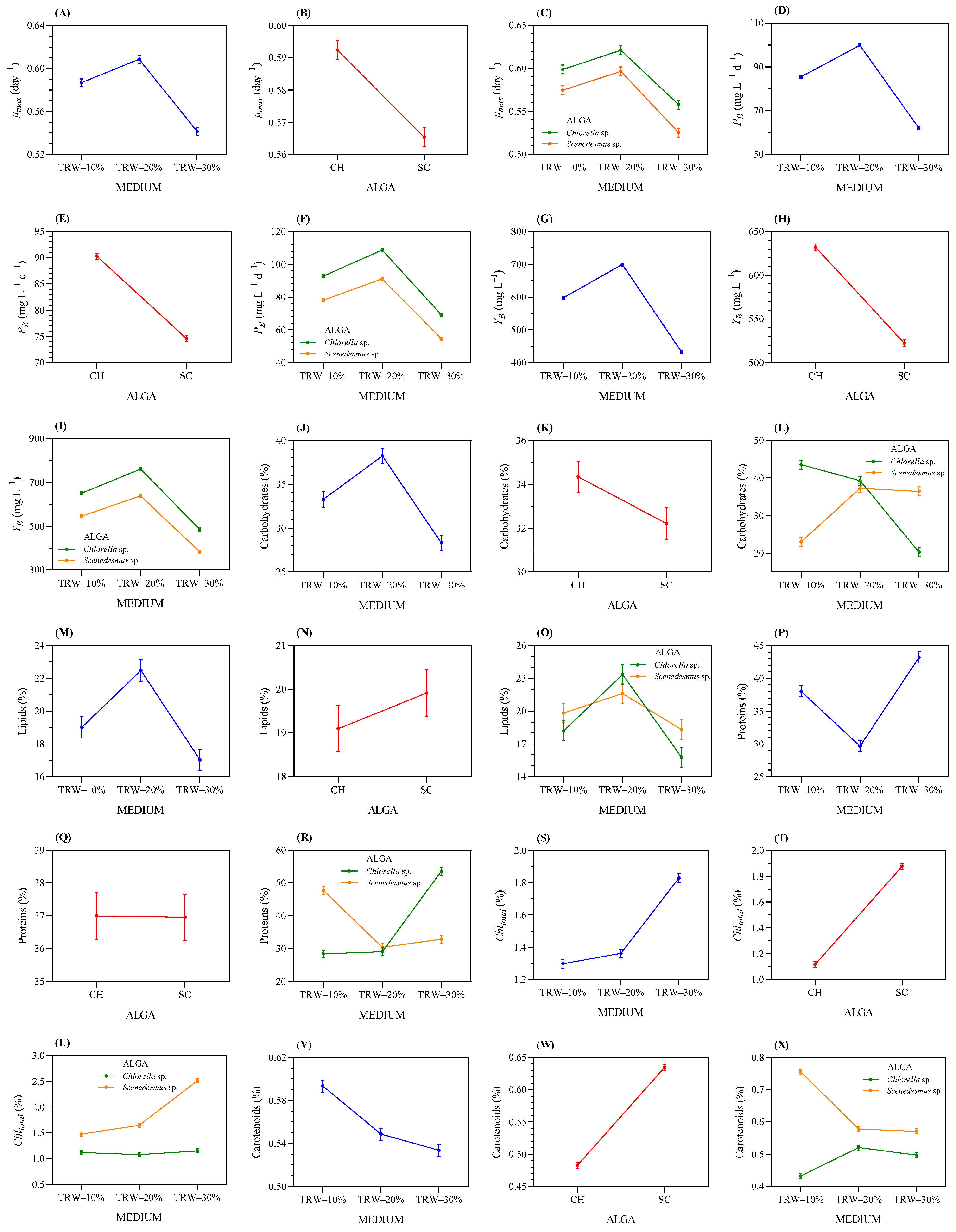
3.3.4. Effect on Biomass Production and the Proximate Chemical Composition of Microalgae
3.3.5. Final RW% Composition after Each Treatment with Duckweed and Microalgae
3.3.6. Scalability Considerations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feil, A.A.; Schreiber, D.; Haetinger, C.; Haberkamp, Â.M.; Kist, J.I.; Rempel, C.; Maehler, A.E.; Gomes, M.C.; da Silva, G.R. Sustainability in the dairy industry: A systematic literature review. Environ. Sci. Pollut. Res. 2020, 27, 33527–33542. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, S.; Majeed, F.; Jose, E.; Mohan, A. Different treatment methodologies and reactors employed for dairy effluent treatment-A review. J. Water Process Eng. 2020, 46, 102622. [Google Scholar] [CrossRef]
- de Almeida Pires, T.; Cardoso, V.L.; Batista, F.R.X. Feasibility of Chlorella vulgaris to waste products removal from cheese whey. J. Environ. Sci. Technol. 2022, 19, 4713–4722. [Google Scholar] [CrossRef]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Kumar, N.; Heena; Dixit, A.; Mehra, M.; Daniloski, D.; Petkoska, A.T. Utilization of Whey: Sustainable Trends and Future Developments. In Whey Valorization: Innovations, Technological Advancements and Sustainable Exploitation; Springer Nature: Singapore, 2023; pp. 47–62. [Google Scholar] [CrossRef]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.; Prazeres, A.R.; Rivas, J. Cheese whey wastewater: Characterization and treatment. Sci. Total Environ. 2013, 445, 385–396. [Google Scholar] [CrossRef]
- Tabelini, D.B.; Lima, J.P.P.; Borges, A.C.; Aguiar, A. A review on the characteristics and methods of dairy industry wastewater treatment in the state of Minas Gerais, Brazil. J. Water Process Eng. 2023, 53, 103779. [Google Scholar] [CrossRef]
- Dewedar, A.; Bahgat, M. Fate of faecal coliform bacteria in a waste water retention reservoir containing Lemna gibba L. Water Res. 1995, 29, 2598–2600. [Google Scholar] [CrossRef]
- Kamyab, H.; Chelliapan, S.; Din, M.F.M.; Shahbazian-Yassar, R.; Rezania, S.; Khademi, T.; Kumar, A.; Azimi, M. Evaluation of Lemna minor and Chlamydomonas to treat palm oil mill effluent and fertilizer production. J. Water Process Eng. 2017, 17, 229–236. [Google Scholar] [CrossRef]
- Coughlan, N.E.; Walsh, É.; Bolger, P.; Burnell, G.; O’Leary, N.; O’Mahoney, M.; Paolacci, S.; Wall, D.; Jansen, M.A.K. Duckweed bioreactors: Challenges and opportunities for large-scale indoor cultivation of Lemnaceae. J. Clean. Prod. 2022, 336, 130285. [Google Scholar] [CrossRef]
- Shahid, A.; Malik, S.; Zhu, H.; Xu, J.; Nawaz, M.Z.; Nawaz, S.; Asraful Alam, M.; Mehmood, M.A. Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci. Total Environ. 2020, 704, 135303. [Google Scholar] [CrossRef]
- Condori, M.A.M.; Condori, M.M.; Gutierrez, M.E.V.; Choix, F.J.; García-Camacho, F. Bioremediation potential of the Chlorella and Scenedesmus microalgae in explosives production effluents. Sci. Total Environ. 2024, 920, 171004. [Google Scholar] [CrossRef] [PubMed]
- Landolt, E. The Family of Lemnaceae—A Monographic Study; Veroffentlichungen des Geobotanischen Institutes der ETH, Stiftung Rubel: Zürich, Switzerland, 1986. [Google Scholar]
- Ceschin, S.; Leacche, I.; Pascucci, S.; Abati, S. Morphological study of Lemna minuta Kunth, an alien species often mistaken for the native L. minor L. (Araceae). Aquat. Bot. 2016, 131, 51–56. [Google Scholar] [CrossRef]
- Al-Dakhil, M.; Alghamdi, S.; Migdadi, H.; Afzal, M.; Ali, A.A. Morphological characterization and DNA barcoding of Duckweed species in Saudi Arabia. Plants 2021, 10, 2438. [Google Scholar] [CrossRef]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Hutner, S.H. Comparative physiology of heterotrophic growth in higher plants. In Growth and Differentiation in Plants; Iowa State College Press: Ames, IA, USA, 1953; pp. 417–447. [Google Scholar]
- Ceschin, S.; Crescenzi, M.; Iannelli, M.A. Phytoremediation potential of the duckweeds Lemna minuta and Lemna minor to remove nutrients from treated waters. Environ. Sci. Pollut. Res. 2020, 27, 15806–15814. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, G.; Xiao, G.; Duan, Z.; Dai, C.; Hu, J.; Wang, Y.; Yang, Y.u.; Jiang, X. Enhancing CO2 photo-biochemical conversion in a newly-designed attached photobioreactor characterized by stacked horizontal planar waveguide modules. Sci. Total Environ. 2021, 760, 144041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gu, L.; Zheng, P.; Chen, Z.; Dou, X.; Qin, Q.; Cai, X. Improvement of cell counting method for Neubauer counting chamber. J. Clin. Lab. Anal. 2020, 34, e23024. [Google Scholar] [CrossRef]
- ISO 9001:2015; Quality Management Systems—Requirements. ISO: Geneva, Switzerland, 2015.
- ISO 14001:2015; Environmental Management Systems—Requirements with Guidance for Use. ISO: Geneva, Switzerland, 2015.
- ISO 45001:2018; Occupational Health and Safety Management Systems—Requirements with Guidance for Use. ISO: Geneva, Switzerland, 2018.
- ISO 37001:2016; Anti-Bribery Management Systems—Requirements with Guidance for Use. ISO: Geneva, Switzerland, 2016.
- Rice, E.W.; Baird, R.B.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association (APHA), American Water Works Association (AWWA) and Water Environment Federation (WEF): Washington, DC, USA, 2017. [Google Scholar]
- Van Wychen, S.; Laurens, L. Determination of total carbohydrates in algal biomass. Contract 2013, 303, 275–3000. [Google Scholar]
- Lourenço, S.O.; Barbarino, E.; Lavín, P.L.; Lanfer Marquez, U.M.; Aidar, E. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 2004, 39, 17–32. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Method 1664, review B: n-Hexane Extractable Material (HEM; Oil and Grease) and Silica Gel Treated n-Hexane Extractable Material (SGT-HEM; Non-polar Material) by Extraction and Gravimetry. 2010, 4303, 1–30. Available online: https://www.epa.gov/sites/default/files/2015-08/documents/method_1664b_2010.pdf (accessed on 18 January 2024).
- Klamann, L.; Dutta, R.; Ghazaryan, L.; Sela-Adler, M.; Khozin-Goldberg, I.; Gillor, O. Cobalamin production in phototrophic and mixotrophic cultures of nine duckweed species (Lemnaceae). bioRxiv 2023, 10, 564895. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Z.; Zhou, Z.; Yang, J.; Xia, M.; Chen, Y.; Li, X.; Ba, S.; Lim, B.L.; Zhao, X.; et al. Boosting starch productivity of mixotrophic duckweed via light and organic carbon treatment. Biomass Bioenergy 2023, 173, 106795. [Google Scholar] [CrossRef]
- Frick, H.; Morley, K. Metabolism of lactose by Lemna minor L. (duckweed) callus. Process Biochem. 1995, 30, 57–62. [Google Scholar] [CrossRef]
- Sun, Z.; Guo, W.; Yang, J.; Zhao, X.; Chen, Y.; Yao, L.; Hou, H. Enhanced biomass production and pollutant removal by duckweed in mixotrophic conditions. Bioresour. Technol. 2020, 317, 124029. [Google Scholar] [CrossRef] [PubMed]
- Kinnersley, A.M.; Scott, T.C.; Yopp, J.H.; Whitten, G.H. Promotion of plant growth by polymers of lactic acid. Plant Growth Regul. 1990, 9, 137–146. [Google Scholar] [CrossRef]
- Mazorra-Manzano, M.A.; Robles-Porchas, G.R.; González-Velázquez, D.A.; Torres-Llanez, M.J.; Martínez-Porchas, M.; García-Sifuentes, C.O.; González-Córdova, A.F.; Vallejo-Córdoba, B. Cheese whey fermentation by its native microbiota: Proteolysis and bioactive peptides release with ACE-inhibitory activity. Fermentation 2020, 6, 19. [Google Scholar] [CrossRef]
- Valdiviezo-Marcelo, J.; Arana-Torres, N.M.; Vega-Portalatino, E.J.; Ruiz-Flores, L.A.; Tamariz-Angeles, C.; Olivera-Gonzales, P.; Rosales-Cuentas, M.M.; Espinoza-Espinoza, L.A. Technological potential of native lactic acid bacteria isolated from Swiss-type artisanal cheese (Ancash, Peru) for their application in food. Front. Sustain. Food Syst. 2023, 7, 1212229. [Google Scholar] [CrossRef]
- Dash, K.K.; Fayaz, U.; Dar, A.H.; Shams, R.; Manzoor, S.; Sundarsingh, A.; Deka, P.; Khan, S.A. A comprehensive review on heat treatments and related impact on the quality and microbial safety of milk and milk-based products. Food Chem. Adv. 2022, 1, 100041. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Ortega-Anaya, J.; Wang, K.; Kosmerl, E.; Jiménez-Flores, R. Lactic acid bacteria isolated from dairy products as potential producers of lipolytic, proteolytic and antibacterial proteins. Appl. Microbiol. Biotechnol. 2019, 103, 5243–5257. [Google Scholar] [CrossRef]
- Walsh, É.; Coughlan, N.E.; O’Brien, S.; Jansen, M.A.; Kuehnhold, H. Density dependence influences the efficacy of wastewater remediation by Lemna minor. Plants 2021, 10, 1366. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Zanette, C.M.; Mariano, A.B.; Yukawa, Y.S.; Mendes, I.; Spier, M.R. Microalgae mixotrophic cultivation for β-galactosidase production. J. Appl. Phycol. 2019, 31, 1597–1606. [Google Scholar] [CrossRef]
- Li, Y.; Miros, S.; Kiani, H.; Eckhardt, H.G.; Blanco, A.; Mulcahy, S.; McDonnell, H.; Tiwari, B.K.; Halim, R. Mechanism of lactose assimilation in microalgae for the bioremediation of dairy processing side-streams and co-production of valuable food products. J. Appl. Phycol. 2023, 35, 1649–1661. [Google Scholar] [CrossRef]
- Girard, J.M.; Roy, M.L.; Hafsa, M.B.; Gagnon, J.; Faucheux, N.; Heitz, M.; Tremblay, R.; Deschênes, J.S. Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Res. 2014, 5, 241–248. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, C.Y.; Liao, Q.; Zhu, X.; Chang, J.S. Photoheterotrophic growth of Chlorella vulgaris ESP6 on organic acids from dark hydrogen fermentation effluents. Bioresour. Technol. 2013, 145, 331–336. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.J.; Zhong, C.Q. Cultivating Scenedesmus dimorphus in lactic acid wastewater for cost-effective biodiesel production. Sci. Total Environ. 2021, 792, 148428. [Google Scholar] [CrossRef] [PubMed]
- Hochman, G.; Palatnik, R.R. The economics of aquatic plants: The case of algae and duckweed. Annu. Rev. Resour. Econ. 2022, 14, 555–577. [Google Scholar] [CrossRef]
- Ward, R.E.; German, J.B.; Corredig, M. Composition, applications, fractionation, technological and nutritional significance of milk fat globule membrane material. In Advanced Dairy Chemistry; Springer: Boston, MA, USA, 2006; Volume 2, pp. 213–244. [Google Scholar] [CrossRef]
- Brighenti, M.; Govindasamy-Lucey, S.; Jaeggi, J.J.; Johnson, M.E.; Lucey, J.A. Effect of substituting whey cream for sweet cream on the textural and rheological properties of cream cheese. J. Dairy Sci. 2021, 104, 10500–10512. [Google Scholar] [CrossRef]
- Sahi, W.; Megateli, S. Evaluation of Lemna minor phytoremediation performance for the treatment of dairy wastewater. Water Pract. Technol. 2023, 18, 1138–1147. [Google Scholar] [CrossRef]
- Genualdi, S.; Jeong, N.; DeJager, L. Determination of endogenous concentrations of nitrites and nitrates in different types of cheese in the United States: Method development and validation using ion chromatography. Food Addit. Contam Part A 2018, 35, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Hemalatha, M.; Mohan, S.V. Duckweed biorefinery–Potential to remediate dairy wastewater in integration with microbial protein production. Bioresour. Technol. 2022, 346, 126499. [Google Scholar] [CrossRef] [PubMed]
- Li, J.M.; Du, A.P.; Liu, P.H.; Tian, X.P.; Jin, Y.L.; Yi, Z.L.; He, K.Z.; Fang, Y.; Zhao, H. High starch accumulation mechanism and phosphorus utilization efficiency of duckweed (Landoltia punctata) under phosphate starvation. Ind. Crop Prod. 2021, 167, 113529. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.; Chen, P.; Min, M.; Chen, Y.; Zhu, J.; Ruan, R.R. Anaerobic digested dairy manure as a nutrient supplement for cultivation of oil-rich green microalgae Chlorella sp. Bioresour. Technol. 2010, 101, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.B.; Costanza-Robinson, M.S.; Spatafora, G.A. Neochloris oleoabundans grown on anaerobically digested dairy manure for concomitant nutrient removal and biodiesel feedstock production. Biomass Bioenergy 2011, 35, 40–49. [Google Scholar] [CrossRef]
- Ummalyma, S.B.; Sukumaran, R.K. Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour. Technol. 2014, 165, 295–301. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, F.; Cao, Y.; Xing, L.; Liu, W.; Mei, S.; Li, S. Cultivation of microalgae in dairy farm wastewater without sterilization. Int. J. Phytoremediation 2015, 17, 222–227. [Google Scholar] [CrossRef]
- da Silva, B.W.; Araújo, B.S.A.; Moura, L.G.; Coutinho Filho, U.; de Resende, M.M.; Cardoso, V.L. Bio-oil production and removal of organic load by microalga Scenedesmus sp. using culture medium contaminated with different sugars, cheese whey and whey permeate. J. Environ. Manag. 2016, 173, 134–140. [Google Scholar] [CrossRef]
- Riaño, B.; Blanco, S.; Becares, E.; García-González, M.C. Bioremediation and biomass harvesting of anaerobic digested cheese whey in microalgal-based systems for lipid production. Ecol. Eng. 2016, 97, 40–45. [Google Scholar] [CrossRef]
- Choi, Y.K.; Jang, H.M.; Kan, E. Microalgal biomass and lipid production on dairy effluent using a novel microalga, Chlorella sp. isolated from dairy wastewater. Biotechnol. Bioproc. Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Brar, A.; Kumar, M.; Pareek, N. Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Front. Microbiol. 2019, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, E.; Zarrinmehr, M.J.; Koutra, E.; Kornaros, M.; Farhadian, O.; Bhatnagar, A. Sequential cultivation of microalgae in raw and recycled dairy wastewater: Microalgal growth, wastewater treatment and biochemical composition. Bioresour. Technol. 2019, 273, 556–564. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Shah, E.; Parikh, B.S.; Patel, A.; Dixit, G.; Gupta, S.; Divecha, J.M. Cultivation of Ascochloris sp. ADW007-enriched microalga in raw dairy wastewater for enhanced biomass and lipid productivity. Int. J. Environ. Sci. Technol. 2019, 16, 943–954. [Google Scholar] [CrossRef]
- Suwal, S.; Bentahar, J.; Marciniak, A.; Beaulieu, L.; Deschênes, J.S.; Doyen, A. Evidence of the production of galactooligosaccharide from whey permeate by the microalgae Tetradesmus obliquus. Algal Res. 2019, 39, 101470. [Google Scholar] [CrossRef]
- Marazzi, F.; Bellucci, M.; Fantasia, T.; Ficara, E.; Mezzanotte, V. Interactions between microalgae and bacteria in the treatment of wastewater from milk whey processing. Water 2020, 12, 297. [Google Scholar] [CrossRef]
- Kallarakkal, K.P.; Muthukumar, K.; Alagarsamy, A.; Pugazhendhi, A.; Mohamed, S.N. Enhancement of biobutanol production using mixotrophic culture of Oscillatoria sp. in cheese whey water. Fuel 2021, 284, 119008. [Google Scholar] [CrossRef]
- Khalaji, M.; Hosseini, S.A.; Ghorbani, R.; Agh, N.; Rezaei, H.; Kornaros, M.; Koutra, E. Treatment of dairy wastewater by microalgae Chlorella vulgaris for biofuels production. Biomass Convers. Biorefin. 2021, 1–7. [Google Scholar] [CrossRef]
- El Ouaer, M.; Turki, N.; Kallel, A.; Halaoui, M.; Trabelsi, I.; Hassen, A. Recovery of landfill leachate as culture medium for two microalgae: Chlorella sp. and Scenedesmus sp. Environ. Dev. Sustain. 2020, 22, 2651–2671. [Google Scholar] [CrossRef]
- Nordin, N.; Yusof, N.; Samsudin, S. Biomass production of Chlorella sp., Scenedesmus sp., and Oscillatoria sp. in nitrified landfill leachate. Waste Biomass Valor. 2017, 8, 2301–2311. [Google Scholar] [CrossRef]
- MVCS. Decreto Supremo 010-2019-VIVIENDA, Maximum Admissible Values of Non-Domestic Wastewater Discharges in the Sanitary Sewage System. Peruvian Ministry of Housing, Construction and Sanitation. 2019, 14862, 17–31. Available online: https://busquedas.elperuano.pe/normaslegales/decreto-supremo-que-aprueba-el-reglamento-de-valores-maximos-decreto-supremo-n-010-2019-vivienda-1748339-3/ (accessed on 15 May 2022).
- Schierano, M.C.; Panigatti, M.C.; Maine, M.A.; Griffa, C.A.; Boglione, R. Horizontal subsurface flow constructed wetland for tertiary treatment of dairy wastewater: Removal efficiencies and plant uptake. J. Environ. Manag. 2020, 272, 111094. [Google Scholar] [CrossRef]
- Schwantes, D.; Gonçalves Jr, A.C.; Schiller, A.D.P.; Manfrin, J.; Campagnolo, M.A.; Veiga, T.G. Salvinia auriculata in post-treatment of dairy industry wastewater. Int. J. Phytoremediation 2019, 21, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, B.; Haddis, A.; Zeine, W. Assessment of the effect of solid waste dump site on surrounding soil and river water quality in Tepi town, Southwest Ethiopia. J. Environ. Public Health 2020, 9, 5157046. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R.; Pandey, A.K.; Ranganathan, P.; Singh, S.; Udayan, A.; Awasthi, M.K.; Hoang, A.T.; Chilakamarry, C.R.; Kim, S.H.; Sim, S.J. Design and applications of photobioreactors-A review. Bioresour. Technol. 2022, 349, 126858. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Unit | RW |
|---|---|---|
| pH | −log [H+] | 5.82 |
| Temperature | °C | 30.7 |
| Electrolytic conductivity (EC) | μS cm−1 | 6823 |
| True color | CU | 4122 |
| Optical density (OD) at 570 nm | AU | 2.40 |
| Turbidity | NTU | 529.33 |
| Total suspended solids (TSS) | mg L−1 | 4091 |
| Biochemical oxygen demand (BOD5) | mg L−1 | 42,188 |
| Chemical oxygen demand (COD) | mg L−1 | 69,531 |
| Oil and grease (OG) | mg L−1 | 1472 |
| Total Kjeldahl Nitrogen (TKN) | mg L−1 | 98.0 |
| Nitrate (NO3−) | mg L−1 | 313 |
| Nitrite (NO2−) | mg L−1 | 3.761 |
| Ammoniacal nitrogen (N–NH3) | mg L−1 | 14.4 |
| Phosphate (PO43−) | mg L−1 | 1186 |
| Sulfate (SO42−) | mg L−1 | 344.8 |
| Potassium (K+) | mg L−1 | 1599 |
| Strain | Wastewater Classification | Treatment | Concentration | Removal Efficiency (RE, %) | Biomass Productivity | Reference | ||
|---|---|---|---|---|---|---|---|---|
| COD | NO3− | PO43− | (mg L−1 d−1) | |||||
| Chlorella sp. | Dairy manure | AED and filtration | 20% (v/v) | 34.3 | 82.5 II | 78.3 | ~81.4 | [54] |
| Neochloris oleoabundans | Dairy manure | AED and sterilization | 2% (v/v) | na | 90−95 | na | 88.3 ± 7.9 | [55] |
| Clorococo sp. | Dairy effluent | Sterilization | 100% (v/v) | 93 | na | na | 53.3 | [56] |
| Chlorella sp. | Dairy farm WW | Filtration and centrifugation | 10% (v/v) | 87.85 | 93.01 III | 90.94 V | 80 | [57] |
| Chlorella sp. | Dairy farm WW | Filtration and centrifugation | 20% (v/v) | 89.7 | 83.20 III | 91.97 V | 95 | [57] |
| Scenedesmus sp. | Cheese whey | No treatment specified | 6.25% (w/w) a | 64.9 I | na | na | ~10.58 | [58] |
| Chlorella sorokiniana | Cheese whey | AED and centrifugation | 10% (v/v) | 41% | 84 III | 71 VI | 60 ± 10 | [59] |
| Chlorella vulgaris | Dairy effluent | Sterilization 1 | 20% (v/v) | Minimum RE | na | na | ~16.25 | [60] |
| Chlorella sp. | Dairy effluent | Sterilization 1 | 50% (v/v) | Partial RE | na | na | ~85.63 | [60] |
| Chlorella pyrenoidosa | Dairy WW | No treatment specified | 75% (v/v) b | 87.5 | 88.91 | 79.02 | 28.44 ± 8.02 | [61] |
| Scenedesmus abundans | Dairy WW | No treatment specified | 75% (v/v) b | 62.5 | 84.72 | 86.51 | 18.72 ± 2.06 | [61] |
| Scenedesmus quadricauda | Dairy WW | Treatment systems 2 | 100% (v/v) | 69.1 ± 4.6 I | 86.7 ± 2.3 IV | 71.2 ± 8.4 | 32.5 | [62] |
| Tetraselmis suecica | Dairy WW | Treatment systems 2 | 100% (v/v) | 40.2 ± 1.9 I | 66.8 ± 5.1 IV | 42.2 ± 0.8 | 45 | [62] |
| Ascochloris sp. | Raw dairy WW | Filtration 3 | 100% (v/v) | 95.1 | 79.7 | 98.1 | 102 ± 3 | [63] |
| Tetradesmus obliquus | Cheese whey permeate | Filtration and sterilization | 100% (w/w) | na | 16.7 | 0.32 | 94 | [64] |
| Scenedesmus acuminatus | Milk whey processing WW | Aerobic digestion 4 | 100% (v/v) | 93 | 88 IV | 90 V | 56 | [65] |
| Oscillatoria sp. | Cheese whey water | Sterilization | 25% (v/v) c | na | na | na | 32 | [66] |
| Chlorella vulgaris | Dairy WW | Sedimentation and filtration | 25% (v/v) | na | 29.27 ± 0.01 | 30.64 ± 0.01 | ~1.72 × 105 i | [67] |
| Chlorella vulgaris | Dairy effluent | Sedimentation and filtration | 25% (v/v) | na | 56.62 ± 0.01 | 51.84 ± 0.01 | ~3.15 × 105 i | [67] |
| Chlorella sp. MC18 | Raw cheese whey | Pretreatment with duckweed | 20% (v/v) | 76.58 ± 1.35 | 98.36 ± 0.64 | 84.48 ± 4.60 | 91.10 ± 1.50 | This study |
| Scenedesmus sp. MJ23-R | Raw cheese whey | Pretreatment with duckweed | 20% (v/v) | 47.83 ± 3.10 | 99.39 ± 0.35 | 87.89 ± 4.19 | 108.67 ± 1.57 | This study |
| Parameters | Environmental Regulations | Raw Whey | Treated Whey (TRW) | Raw Whey | Treated Whey (TRW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peru | Italy | Argentina | Brazil | Swiss | Macrophyte | Microalgae | Macrophyte | Microalgae | |||||
| MAV 1 | ILD 2 | LRL 3 | CSR 4 | SLV 5 | RW–10% | L. minor | Chlorella | Scenedesmus | RW–20% | L. minor | Chlorella | Scenedesmus | |
| pH | 6–9 | 5.5–9.5 | 5–9 | 6.5–8.5 | 7.18 | 7.27 | 8.47 | 8.75 | 7.13 | 6.47 | 8.62 | 8.33 | |
| Temperature | <35 | ≤40 | 24.7 | 27.3 | 22.5 | 21.6 | 24.1 | 27.0 | 21.1 | 21.4 | |||
| OD at 570 nm | 0.284 | 0.015 | 0.059 | 0.028 | 0.549 | 0.032 | 0.079 | 0.051 | |||||
| EC | 1400 | 1107 | 1203 | 1223 | 1327 | 2030 | 2183 | 2203 | 2290 | ||||
| Turbidity | ≤100 | 25 | 64.86 | 6.85 | 14.27 | 9.62 | 128.55 | 10.67 | 22.16 | 13.58 | |||
| True color | 35.6 | 75.1 | 95.8 | 99.2 | 62.1 | 43.0 | 122 | 123 | |||||
| TSS | 500 | 50 | 249 | 10.3 | 12.1 | 9.8 | 358 | 13.8 | 14.9 | 12.7 | |||
| BOD5 | 500 | 125 | <5 | 3505 | 1146 | 194.6 | 217.9 | 9750 | 3620 | 485 | 1188 | ||
| COD | 1000 | 160 | 190 | <5 | 6096.67 | 2039 | 321 | 364 | 14,885 | 4190 | 977 | 2176 | |
| OG | 100 | 180 | <0.5 | <0.5 | <0.5 | 300 | 4 | <0.5 | <0.5 | ||||
| NO3− | 20 | 30 | 25.4 | 0.051 | <0.005 | <0.005 | 60.4 | 0.50 | 0.02 | <0.005 | |||
| NO2− | 0.6 | 3.13 | 0.011 | ND | 0.007 | 3.33 | 0.067 | nd | nd | ||||
| N–NH3 | 80 | 25 | ≤20 | 1.95 | 0.973 | <0.02 | 0.035 | 3.79 | 0.299 | 0.042 | 0.044 | ||
| TKN | 15 | 22.7 | 1.29 | 0.24 | 0.27 | 48.9 | 3.66 | 0.96 | 2.13 | ||||
| PO43− | 10 a | 2 a | ≤0.15 a | 104 | 54.1 | 10.10 | 1.64 | 238 | 141 | 22.04 | 17.22 | ||
| SO42− | 200 | 29.4 | 18.8 | 5.47 | 0.03 | 61.4 | 44.9 | 11.24 | 0.16 | ||||
| K+ | 12 | 167.4 | 135.8 | 115.26 | 119.674 | 326.8 | 265.7 | 218 | 209 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamani Condori, M.A.; Montesinos Pachapuma, K.A.; Gomez Chana, M.P.; Quispe Huillca, O.; Veliz Llayqui, N.E.; López-Rosales, L.; García-Camacho, F. An Environmentally Sustainable Approach for Raw Whey Treatment through Sequential Cultivation of Macrophytes and Microalgae. Appl. Sci. 2024, 14, 8139. https://doi.org/10.3390/app14188139
Mamani Condori MA, Montesinos Pachapuma KA, Gomez Chana MP, Quispe Huillca O, Veliz Llayqui NE, López-Rosales L, García-Camacho F. An Environmentally Sustainable Approach for Raw Whey Treatment through Sequential Cultivation of Macrophytes and Microalgae. Applied Sciences. 2024; 14(18):8139. https://doi.org/10.3390/app14188139
Chicago/Turabian StyleMamani Condori, Marco Alberto, Karen Adriana Montesinos Pachapuma, Maria Pia Gomez Chana, Olenka Quispe Huillca, Nemesio Edgar Veliz Llayqui, Lorenzo López-Rosales, and Francisco García-Camacho. 2024. "An Environmentally Sustainable Approach for Raw Whey Treatment through Sequential Cultivation of Macrophytes and Microalgae" Applied Sciences 14, no. 18: 8139. https://doi.org/10.3390/app14188139






