Tin–Phosphorus Alloy: The Impact of Temperature on Alloy Formation and the Influence of the Dross Amount on the Solder Bath Surface
Abstract
1. Introduction
2. Materials and Methods
- The preparation of samples of the SnP2 alloy under laboratory conditions;
- An analysis of the dross amount formed on the solder bath surface.
2.1. Preparation of Samples of the SnP2 Alloy under Laboratory Conditions
- A total of 10 g of P4 (Figure 1A) red phosphorus in form of dust, stored in a vacuum desiccator;
- Interlayer—fine silica sand and bentonite in a ratio of 1:1 (Figure 1B);
- Tin, with 99.98% purity, in the form of cut wire with a 5 mm diameter (Figure 1C);
- Top layer—crushed coal (Figure 1D);
- Sibral—temperature-resistant insulation material.
- -
- Electrical conductivity: The sample must be electrically conductive, without impurities on the surface.
- -
- Strength: The sample has to be solid and non-porous, free from bubbles, cracks, and dirt.
- -
- Homogeneity: The sample has to be homogeneous throughout its volume, and there is not any segregation of individual elements. The surface of the sample has to be flat, clean, dry, and unoxidized as well as larger than the hole above the electrode in order to cover and seal it completely.
- -
- Cleanliness: The sanded side of the sample surface is not allowed to be placed on the table to avoid contamination of the surface to be analysed.
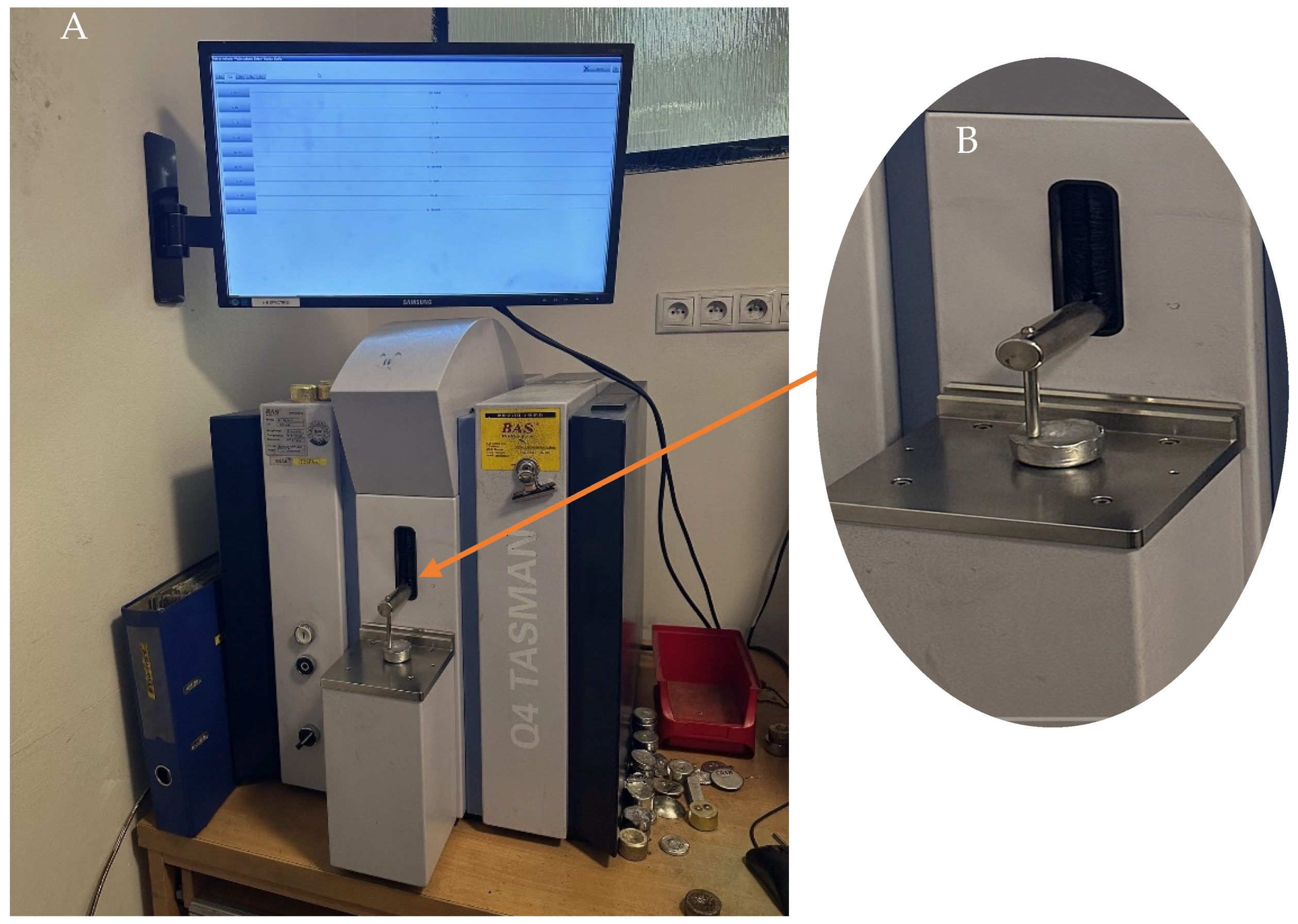
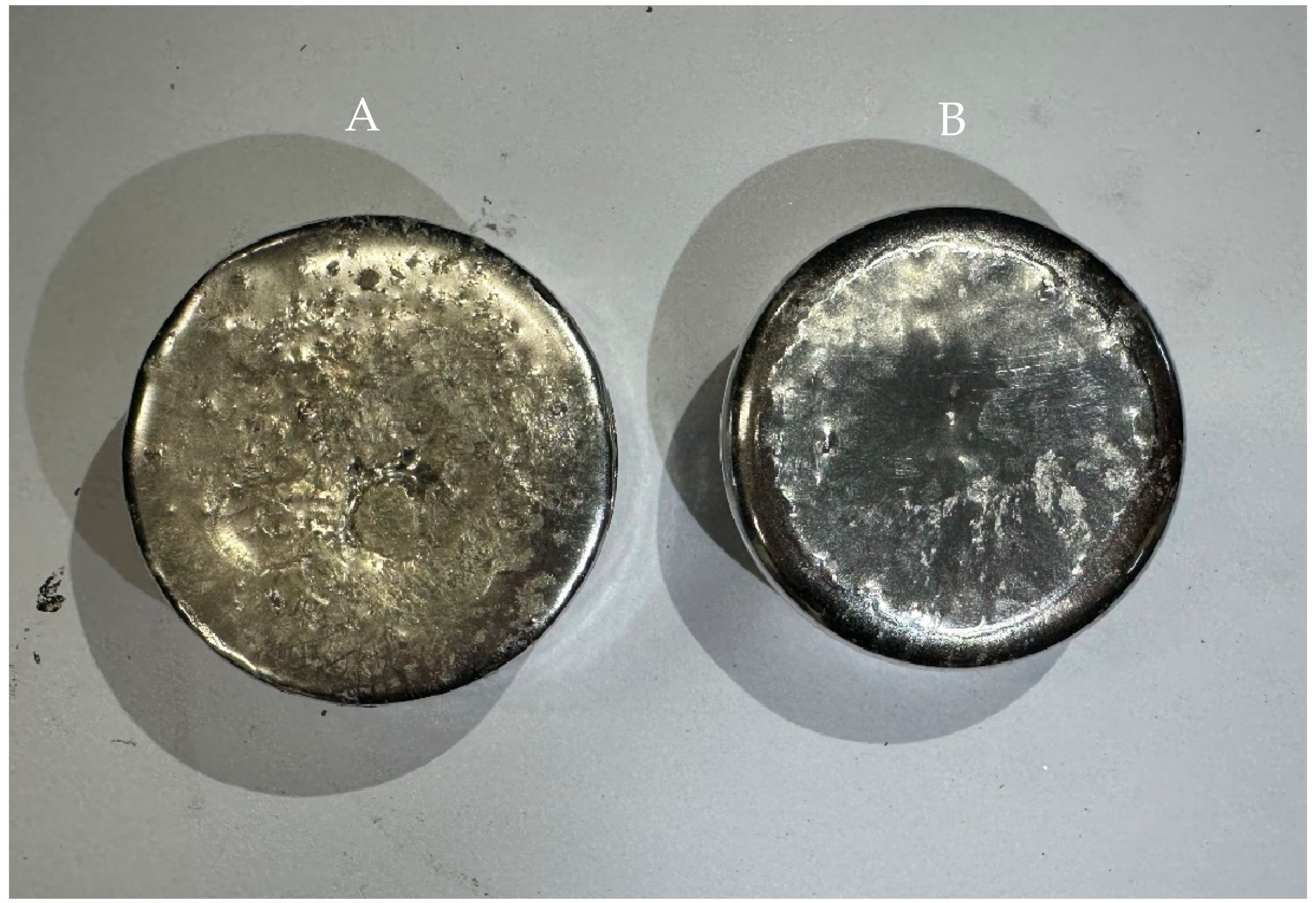
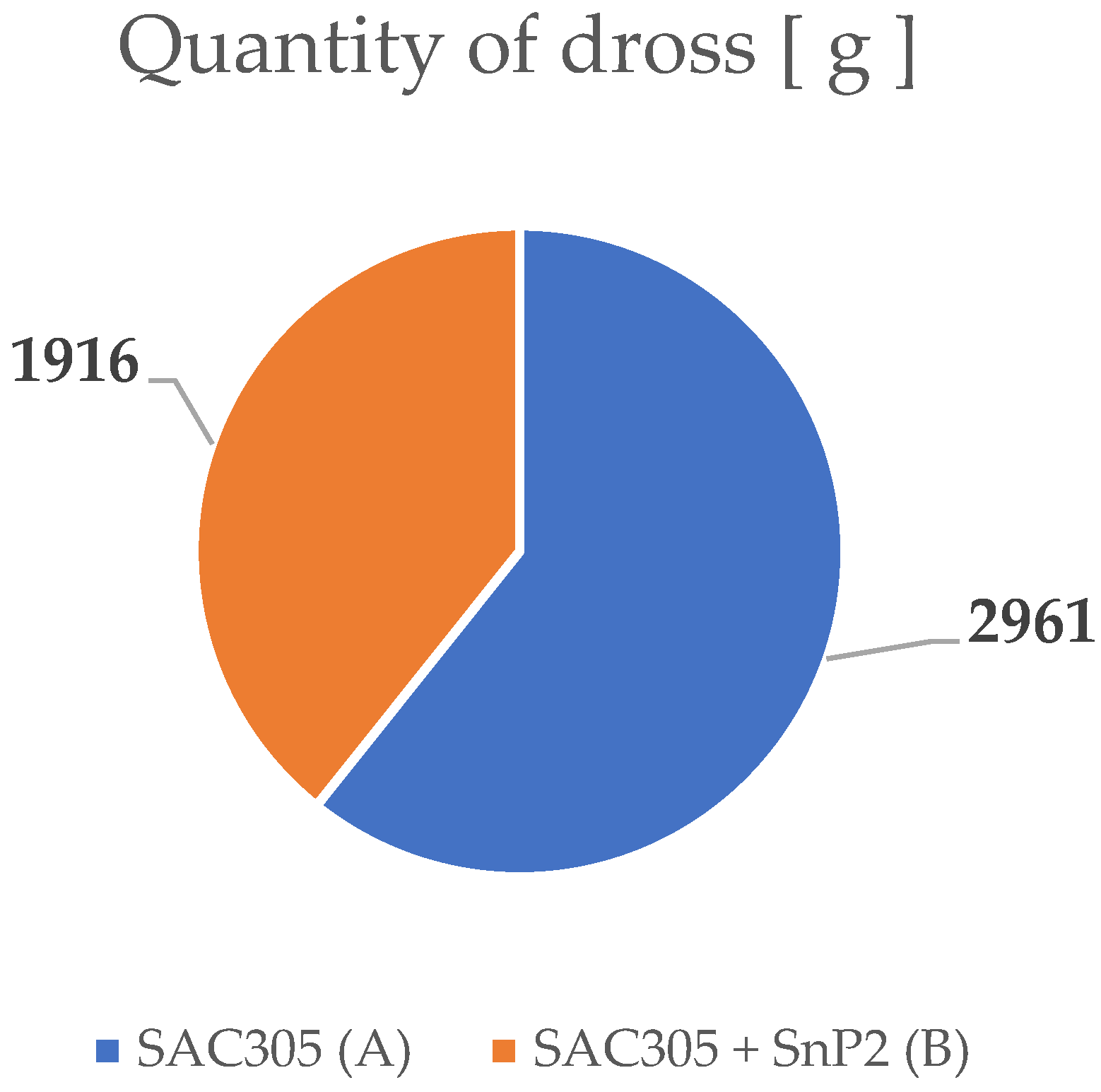
2.2. Analysis of Dross Amount Formed on the Solder Bath Surface
- Tin (Sn), with 99.98% purity, in the form of bars—288.45 kg.
- Silver (Ag), with 99.98% purity in the form of cut wire with 5 mm diameter—9.0 kg.
- Copper (Cu), with 99.98% purity in the form of thin wire with 0.44 mm*—1.5 kg.
3. Results
3.1. Preparation of SnP2 Alloy Samples under Laboratory Conditions
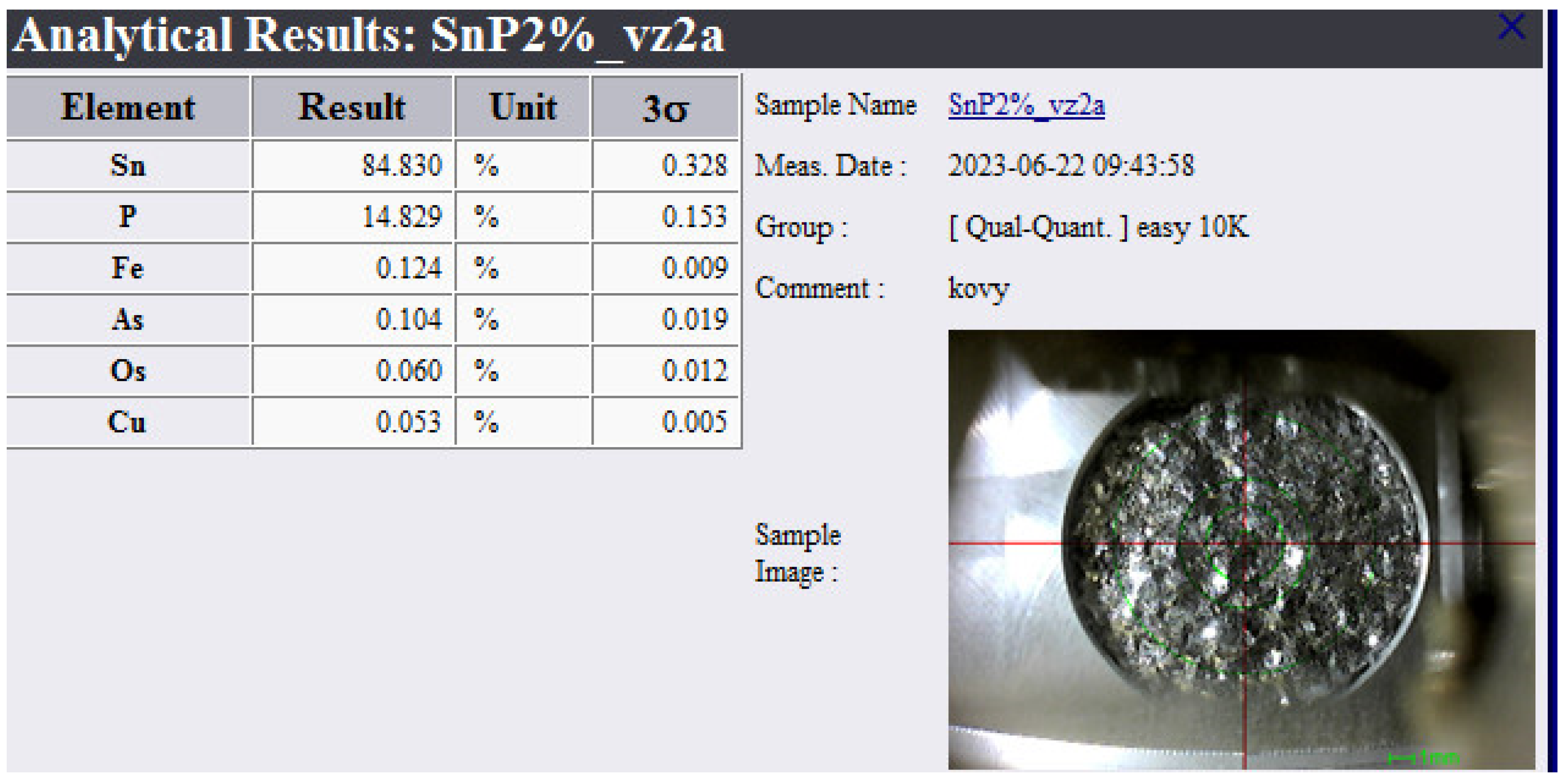
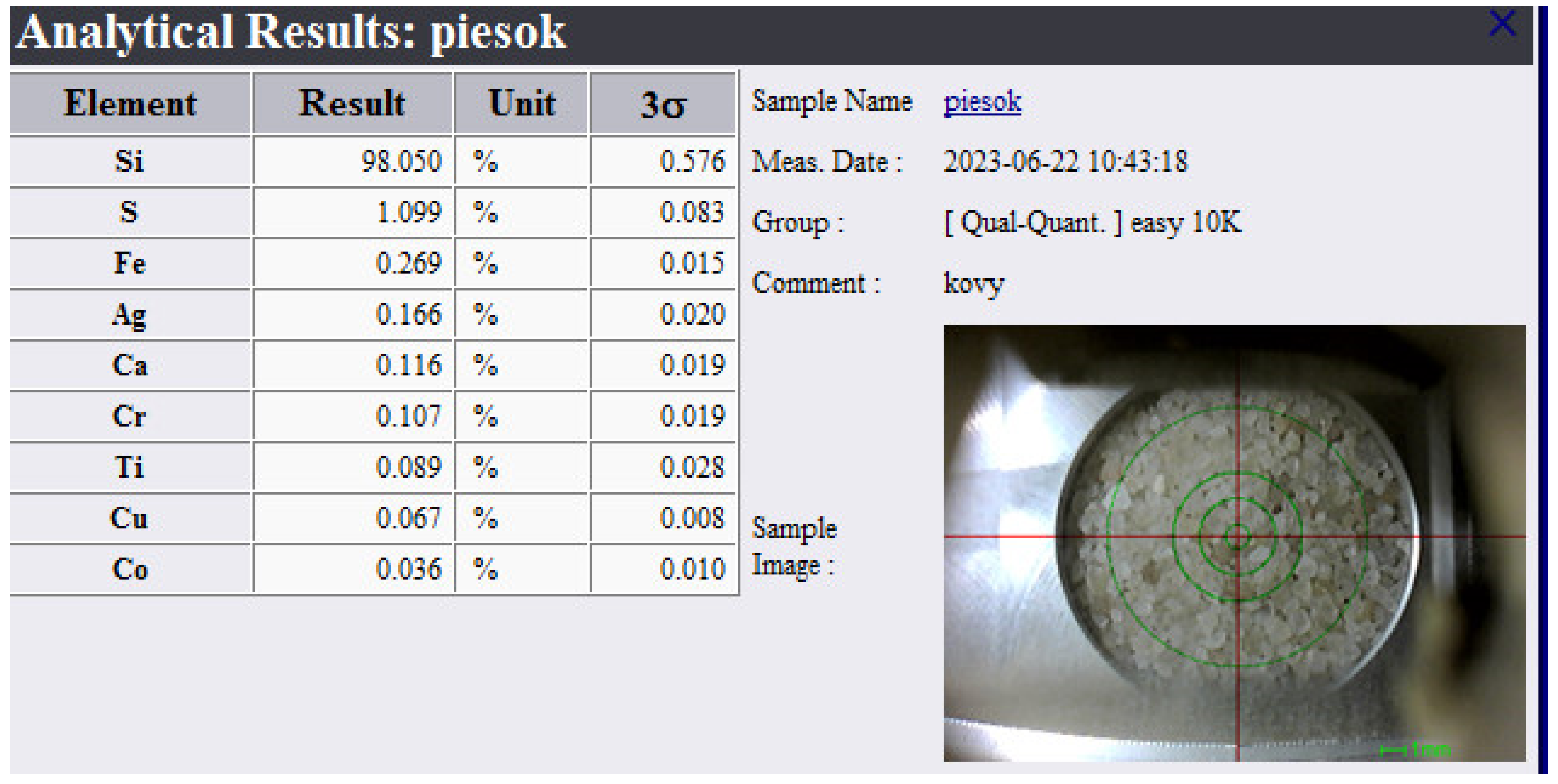
3.2. Chemical Analysis of Dross Amount Formed on the Solder Bath Surface
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, J.; Xiao, W.; Wang, J.; Zhang, F.; Li, X.; He, H.; Wang, L. Effects of alloying elements on the interfacial segregation of bismuth in tin-based solders. Mater. Today Commun. 2023, 35, 105713. [Google Scholar] [CrossRef]
- Huang, W.S. Stannum; Metallurgical Industry Press: Beijing, China, 2000. [Google Scholar]
- Campbell, J. Complete Casting Handbook Metal Casting Processes, Metallurgy, Techniques and Design, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2015; ISBN 978-0-444-63509-9. [Google Scholar]
- Kurdyumov, A.V.; Belov, V.D.; Pikunov, M.V. Casting Production from Nonferrous Metal Alloys: Textbook; MISiS: Moscow, Russia, 2011. [Google Scholar]
- Smiryagin, A.P. Industrial Nonferrous Metals and Alloys; Ripol Klassik: Moscow Russia, 2013. [Google Scholar]
- He, S.; Nishikawa, H. Effect of substrate metallization on the impact strength of Sn-Ag-Cu solder bumps fabricated in a formic acid atmosphere. In Proceedings of the 2017 International Conference on Electronics Packaging (ICEP), Yamagata, Japan, 19–22 April 2017; pp. 381–385. [Google Scholar] [CrossRef]
- J-STD-006C; Requirements for Electronic Grade Solder Alloys and Fluxed and Non-Fluxed Solid Solders for Electronic Soldering Applications. Institute of Electrical and Electronics Engineers (IEEE): Piscataway, NJ, USA; Association Connecting Electronics Industries (IPC): Bannockburn, IL, USA, 2017.
- Chen, Y.; Huang, J.; Huang, Y.; Li, Q.; Zeng, H.; Tian, L.; Li, J.; Wang, S.; He, W.; Hong, Y. Microstructure evolution and growth kinetics of intermetallic compound in SAC305/Ag and SAC305/Cu solder joints during solid-state aging. J. Mater. Sci. Mater. Electron. 2024, 35, 297. [Google Scholar] [CrossRef]
- Diepstraten, G. Wave/Selective Soldering. In Lead-Free Soldering Process Development and Reliability; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Yang, M.; Ji, H.; Wang, S.; Ko, Y.-H.; Lee, C.-W.; Wu, J.; Li, M. Effects of Ag content on the interfacial reactions between liquid Sn–Ag–Cu solders and Cu substrates during soldering. J. Alloys Compd. 2016, 679, 18–25. [Google Scholar] [CrossRef]
- Sweatman, K.W.; Nishimura, T. The effects of phosphorus in lead-free solders. In Proceedings of the SMTA International, Rosemont, IL, USA, 27 September–1 October 2015. [Google Scholar]
- Vianco, P.T.; Hosking, F.M.; Rejent, J.A. Wettability Analysis of Tin-Based, Lead Free Solders; Sandia National Labs.: Albuquerque, NM, USA, 1992. [Google Scholar]
- Xian, A.P.; Gong, G.L. Oxidation Behavior of Molten Tin Doped with Phosphorus. J. Electron. Mater. 2007, 36, 1669–1678. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, H.; Sekulic, D.P.; Qian, Y. A Comparative Study of Reactive Wetting of Lead and Lead-Free Solders on Cu and (Cu6Sn5/Cu3Sn)/Cu Substrates. J. Electron. Mater. 2008, 37, 1640–1647. [Google Scholar] [CrossRef]
- El-Daly, A.A.; Hammad, A.E. Enhancement of creep resistance and thermal behavior of eutectic Sn–Cu lead-free solder alloy by Ag and In-additions. Mater. Des. 2012, 40, 292–298. [Google Scholar] [CrossRef]
- Goh, Y.; Haseeb, A.S.M.A.; Faizul Mohd Sabri, M. Electrodeposition of lead-free solder alloys. Solder. Surf. Mt. Technol. 2013, 25, 76–90. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alhaji, A.I.; Ryder, K.S.; Horne, M.; Rodopoulos, T. Electrodeposition of Copper-Tin Alloys Using Deep Eutectic Solvents. Trans. Inst. Met. Finish. 2016, 94, 104–113. [Google Scholar] [CrossRef]








| Chemical Element | Content in Alloy [%] | Chemical Element | Content in Alloy [%] |
|---|---|---|---|
| Sn | balance | As | 0.03 max |
| Ag | 3.0 ± 0.2 | Ni | 0.01 max |
| Cu | 0.5 ± 0.1 | Bi | 0.10 max |
| Pb | 0.07 max | Cd | 0.001 max |
| Sb | 0.10 max | Al | 0.001 max |
| Zn | 0.001 max | In | 0.05 max |
| Fe | 0.02 max | - | - |
| Sample No. | Weight of Tin [g] | Weight of P4 [g] | TA [°C] | tA [min] | TB [°C] | tB [min] |
|---|---|---|---|---|---|---|
| 1 | 490.03 | 10.09 | 599 | 21 | 602 | 20 |
| 2 | 490.12 | 9.98 | 709 | 22 | 712 | 21 |
| 3 | 490.08 | 10.03 | 746 | 21 | 750 | 21 |
| 4 | 490.02 | 10.01 | 849 | 21 | 851 | 21 |
| Sample No. | Weight of Tin [g] | Weight of P4 [g] | Starting Temperature [°C] | Ø Casting Temperature [°C] | Weight Sn [%] | Weight P [%] |
|---|---|---|---|---|---|---|
| 1 | 490.03 | 10.09 | 123 | 600.5 | 99.93 | 0.07 |
| 2 | 490.12 | 9.98 | 121 | 710.5 | 99.42 | 0.58 |
| 3 | 490.08 | 10.03 | 120 | 748 | 99.10 | 0.90 |
| 4 | 490.02 | 10.01 | 120 | 850 | 97.96 | 2.04 |
| Chemical Element | Content in Alloy [%] | Chemical Element | Content in Alloy [%] |
|---|---|---|---|
| Sn | 96.39 | As | 0.01 |
| Ag | 2.9 | Ni | 0.001 |
| Cu | 0.6 | Bi | 0.02 |
| Pb | 0.02 | Cd | 0.000 |
| Sb | 0.05 | Al | 0.001 |
| Zn | 0.001 | In | 0.02 |
| Fe | 0.01 | P | 0.000 |
| Chemical Element | Content in Alloy [%] | Chemical Element | Content in Alloy [%] |
|---|---|---|---|
| Sn | 96.42 | As | 0.002 |
| Ag | 3.0 | Ni | 0.005 |
| Cu | 0.4 | Bi | 0.004 |
| Pb | 0.02 | Cd | 0.000 |
| Sb | 0.1 | Al | 0.001 |
| Zn | 0.001 | In | 0.02 |
| Fe | 0.02 | P | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körmendy, J.; Vavro, J., Jr.; Vavro, J. Tin–Phosphorus Alloy: The Impact of Temperature on Alloy Formation and the Influence of the Dross Amount on the Solder Bath Surface. Appl. Sci. 2024, 14, 8257. https://doi.org/10.3390/app14188257
Körmendy J, Vavro J Jr., Vavro J. Tin–Phosphorus Alloy: The Impact of Temperature on Alloy Formation and the Influence of the Dross Amount on the Solder Bath Surface. Applied Sciences. 2024; 14(18):8257. https://doi.org/10.3390/app14188257
Chicago/Turabian StyleKörmendy, Jana, Ján Vavro, Jr., and Ján Vavro. 2024. "Tin–Phosphorus Alloy: The Impact of Temperature on Alloy Formation and the Influence of the Dross Amount on the Solder Bath Surface" Applied Sciences 14, no. 18: 8257. https://doi.org/10.3390/app14188257
APA StyleKörmendy, J., Vavro, J., Jr., & Vavro, J. (2024). Tin–Phosphorus Alloy: The Impact of Temperature on Alloy Formation and the Influence of the Dross Amount on the Solder Bath Surface. Applied Sciences, 14(18), 8257. https://doi.org/10.3390/app14188257






