Comparison of Three Biological Control Models of Pycnoporus sanguineus on Phytopathogenic Fungi
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Descriptions
2.2. Model 1: Dual Tests of Antagonistic Activity
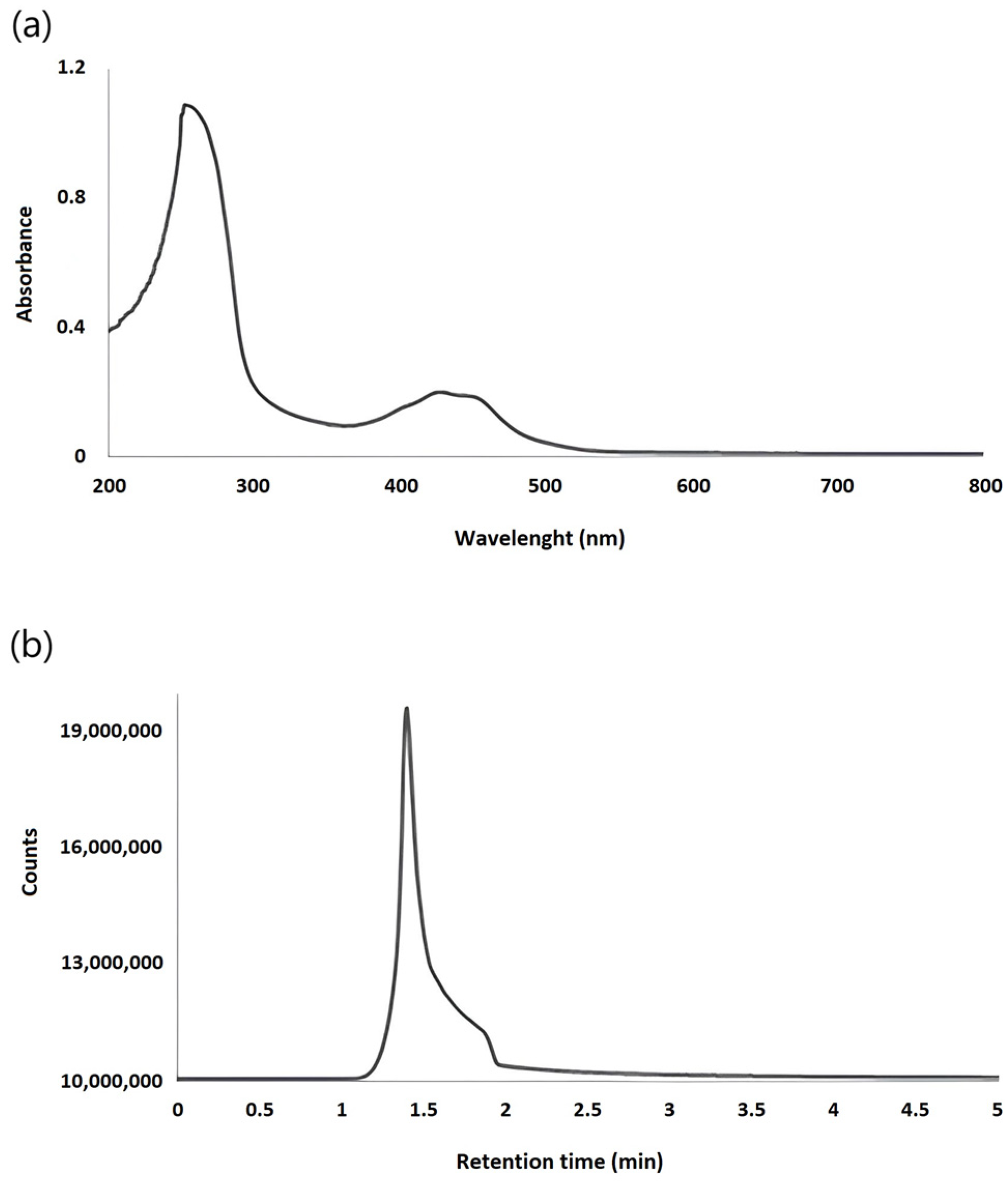
2.3. Model 2: Antifungal Effectiveness Tests of Cinnabarin
2.4. Model 3: Antifungal Effectiveness Tests of Full P. sanguineus Extract
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pal, K.K.; Gardener, B.M. Biological control of plant pathogens. Plant Health Instr. 2006, 6, 1–25. [Google Scholar] [CrossRef]
- Serrano, L.; Galindo, E. Control biológico de organismos fitopatógenos: Un reto multidisciplinario. Ciencia 2007, 77–88. Available online: https://www.amc.edu.mx/revistaciencia/images/revista/58_1/PDF/controlBiologico.pdf (accessed on 15 January 2024).
- Waszczuk, U.; Zapora, E. Arboreal Fungi in Biological Control against Soil Fungi. Environ. Sci. Proc. 2021, 9, 31. [Google Scholar] [CrossRef]
- Rossbacher, S.; Vorburger, C. Prior adaptation of parasitoids improves biological control of symbiont-protected pests. Evol. Appl. 2020, 13, 1868–1876. [Google Scholar] [CrossRef] [PubMed]
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant diseases: The European situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Cruz-Muñoz, R. Producción de Extractos de Pycnoporus sanguineus con Actividad Antimicrobiana en Hongos y Bacterias Fitopatógenas. Master’s Thesis, Instituto Politécnico Nacional, Mexico City, México, 2012. Available online: http://www.repositoriodigital.ipn.mx/handle/123456789/15754 (accessed on 20 February 2024).
- Adrio, J.L.; Demain, A.L. Fungal biotechnology. Int. Microbiol. 2003, 6, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.; Ferreira, I.F.R.; Dias, J.; Teixeira, V.; Martins, A.; Pintado, M. A review on antimicrobial activity of mushroom (basidiomycetes) extracts and isolated compounds. Planta Med. 2012, 78, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Sivanandhan, S.; Khusro, A.; Paulraj, M.G.; Ignacimuthu, S.; Al-Dhabi, N.A. Biocontrol properties of basidiomycetes: An overview. J. Fungi 2017, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Muñoz, R.; Piña-Guzmán, A.B.; Yáñez-Fernández, J.; Valencia-Del Toro, G.; Bautista-Baños, S.; Villanueva-Arce, R. Producción de pigmentos de Pycnoporus sanguineus en medio de cultivo sólido. Agrociencia 2015, 49, 347–359. [Google Scholar]
- Smânia, A.; Delle Monache, F.; Smânia, E.F.A.; Gil, M.L.; Benchetrit, L.C.; Cruz, F.S. Antibacterial activity of a substance produced by the fungus Pycnoporus sanguineus (Fr.) Murr. J. Ethnopharmacol. 1995, 45, 177–181. [Google Scholar] [CrossRef]
- Smânia, E.F.A.; Smânia, A., Jr.; Loguercio-Leite, C.; Gil, M.L. Optimal parameters for Cinnabarin synthesis by Pycnoporus sanguineus. J. Chem. Technol. Biotechnol. Int. Res. Process. Environ. Clean Technol. 1997, 70, 57–59. [Google Scholar]
- Hwang, H.J.; Kim, S.W.; Xu, C.P.; Choi, J.W.; Yun, J.W. Morphological and rheological properties of the three different species of basidiomycetes Phellinus in submerged cultures. J. Appl. Microbiol. 2004, 96, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Tuong, T.D.; Chu, D.X.; Dieu, B.T.M. Antioxidant Activity of Fruiting Body Extracts from Pycnoporus sanguineus Mushroom. Vietnam. J. Sci. Technol. 2020, 58, 143–151. [Google Scholar]
- Acosta-Urdapilleta, L.; Alonso Paz, G.A.; Rodríguez, A.; Adame, M.; Salgado, D.; Salgado, J.; Montiel-Peña, M.; Medrano-Vega, F.; Villegas Villarreal, E.C. Pycnoporus sanguineus, a fungus having biotechnological potential. In Hacia un Desarrollo Sostenible del Sistema de Producción-Consumo de los Hongos Comestibles y Medicinales en Latinoamérica: Avances y Perspectivas en el Siglo XXI; Martínez-Carrera, D., Curvetto, N., Sobal, M., Morales, P., Mora, V.M., Eds.; Red Latinoamericana de Hongos Comestibles y Medicinales; Porfirio MORALES: Moca, Espaillat, 2010; pp. 189–220. [Google Scholar]
- Lomascolo, A.; Uzan-Boukhris, E.; Herpoël-Gimbert, I.; Sigoillot, J.C.; Lesage-Meessen, L. Peculiarities of Pycnoporus species for applications in biotechnology. Appl. Microbiol. Biotechnol. 2011, 92, 1129–1149. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Insuasti, J.A.; Gómez-Andrade, W.E.; Duarte-Trujillo, A.S.; Soto-Arroyave, C.P.; Pineda-Soto, C.A.; Fierro-Ramos, F.J.; Álvarez-Ramos, S.E. Producción de Pycnoporus spp. y sus metabolitos secundarios: Una revisión. ICIDCA Sobre Deriv. Caña Azúcar 2017, 51, 60–69. [Google Scholar]
- Imtiaj, A.; Lee, T.S. Screening of antibacterial and antifungal activities from Korean wild mushrooms. World J. Agric. Sci. 2007, 3, 316–321. [Google Scholar]
- Imtiaj, A.; Jayasinghe, C.; Lee, G.W.; Lee, T.S. Antibacterial and Antifungal Activities of Stereum ostrea, an Inedible Wild Mushroom. Mycobiology 2007, 35, 210. [Google Scholar] [CrossRef]
- Arfi, Y.; Levasseur, A.; Record, E. Differential gene expression in Pycnoporus coccineus during interspecific mycelial interactions with different competitors. Appl. Environ. Microbiol. 2013, 79, 6626–6636. [Google Scholar] [CrossRef]
- Figueiredo, Á.; Castro, E.; Silva, A. Atividade “in vitro” de extratos de Pycnoporus sanguineus e Lentinus crinitus sobre o fitopatógeno Fusarium sp. Acta Amaz. 2014, 44, 1–8. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. HPLC and NMR Studies of Phenoxazone Alkaloids from Pycnoporus cinnabarinus. Nat. Prod. Commun. 2009, 4, 489–498. [Google Scholar] [CrossRef]
- Romero-Arenas, O.; Jara y Rivera, A.P.; Valencia de Ita, M.A.; Lezama, C.P.; Villa-Ruano, N.; Rivera, A. In Vitro Antimicrobial Activity of Cinnabarin on Xanthomonas campestris Isolated from Bean Crops of Puebla, Mexico. Appl. Sci. 2021, 11, 5391. [Google Scholar] [CrossRef]
- Al-Fatimi, M.; Schröder, G.; Kreisel, H.; Lindequist, U. Biological activities of selected basidiomycetes from Yemen. Pharmazie 2013, 68, 221–226. [Google Scholar]
- Teoh, Y.P.; Don, M.M.; Ujang, S. Media selection for mycelia growth, antifungal activity against wood-degrading fungi, and GC-MS study by Pycnoporus sanguineus. BioResources 2011, 6, 2719–2731. [Google Scholar] [CrossRef]
- Viecelli, C.A.; Stangarlin, J.R.; Kuhn, O.J.; Schwan-Estrada, K.R.F. Resistance induction in bean plants against angular leaf spot by extracts from Pycnoporus sanguineus mycelium. Summa. Phytopathol. 2010, 36, 73–80. [Google Scholar]
- Ojeda, K.; Suescum, J. Evaluación de Cinco Cepas de Hongos Nativos como Controladores Biológicos de la Enfermedad “Ojo de Pollo”(Mycena citricolor) en café (Coffea arabica) en Condiciones in vitro. Bachelor’s Thesis, Universidad Técnica Particular de Loja, Loja, Ecuador, 2012. [Google Scholar]
- Nascimento, V.C.; Rodrigues-Santos, K.C.; Carvalho-Alencar, K.L.; Castro, M.B.; Kruger, R.H.; Lopes, F.A.C. Trichoderma: Biological control efficiency and perspectives for the Brazilian Midwest states and Tocantins. Braz. J. Biol. 2022, 82, e260161. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Smânia, A.; Marques, C.J.S.; Smânia, E.F.A.; Zanetti, C.R.; Carobrez, S.G.; Tramonte, R.; Loguercio-Leite, C. Toxicity and Antiviral Activity of Cinnabarin Obtained from Pycnoporus sanguineus (Fr.) Murr. Phyther. Res. 2003, 17, 1069–1072. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Muñoz, R. Caracterización de los Pigmentos de Pycnoporus sanguineus y su Citotoxicidad Sobre Hongos y Bacterias Fitopatógenas. Ph.D. Thesis, Instituto Politécnico Nacional, Mexico City, México, 2015. Available online: http://tesis.ipn.mx/bitstream/handle/123456789/19567/TesisDoctoralROCIOCRUZM.pdf?sequence=1&isAllowed=y (accessed on 12 March 2024).
- Henrique Rosa, L.; Gomes Machado, K.M.; Jacob, C.C.; Capelari, M.; Augusto Rosa, C.; Leomar Zani, C. Screening of Brazilian Basidiomycetes for Antimicrobial Activity. Mem. Inst. Oswaldo Cruz 2003, 98, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Téllez-Téllez, M.; Villegas, E.; Rodriguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Diaz-Godinez, G. Mycosphere Essay 11: Fungi of Pycnoporus: Morphological and molecular identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 7, 1500. [Google Scholar]
- Borderes, J.; Costa, A.; Guedes, A.; Tavares, L.B.B. Antioxidant activity of the extracts from Pycnoporus sanguineus mycelium. Brazilian Arch. Biol. Technol. 2011, 54, 1167–1174. [Google Scholar] [CrossRef]
- Potočnik, I.; Vukojević, J.; Stajić, M.; Rekanović, E.; Milijašević, S.; Stepanović, M.; Todorović, B. Toxicity of fungicides with different modes of action to Cladobotryum dendroides and Agaricus bisporus. J. Environ. Sci. Health Part B Pestic. Food Contam. Agric. Wastes 2009, 44, 823–827. [Google Scholar] [CrossRef] [PubMed]
- Avenot, H.F.; Morgan, D.P.; Quattrini, J.; Michailides, T.J. Resistance to Thiophanate-Methyl in Botrytis cinerea Isolates from Californian Vineyards and Pistachio and Pomegranate Orchards. Plant Dis. 2020, 104, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
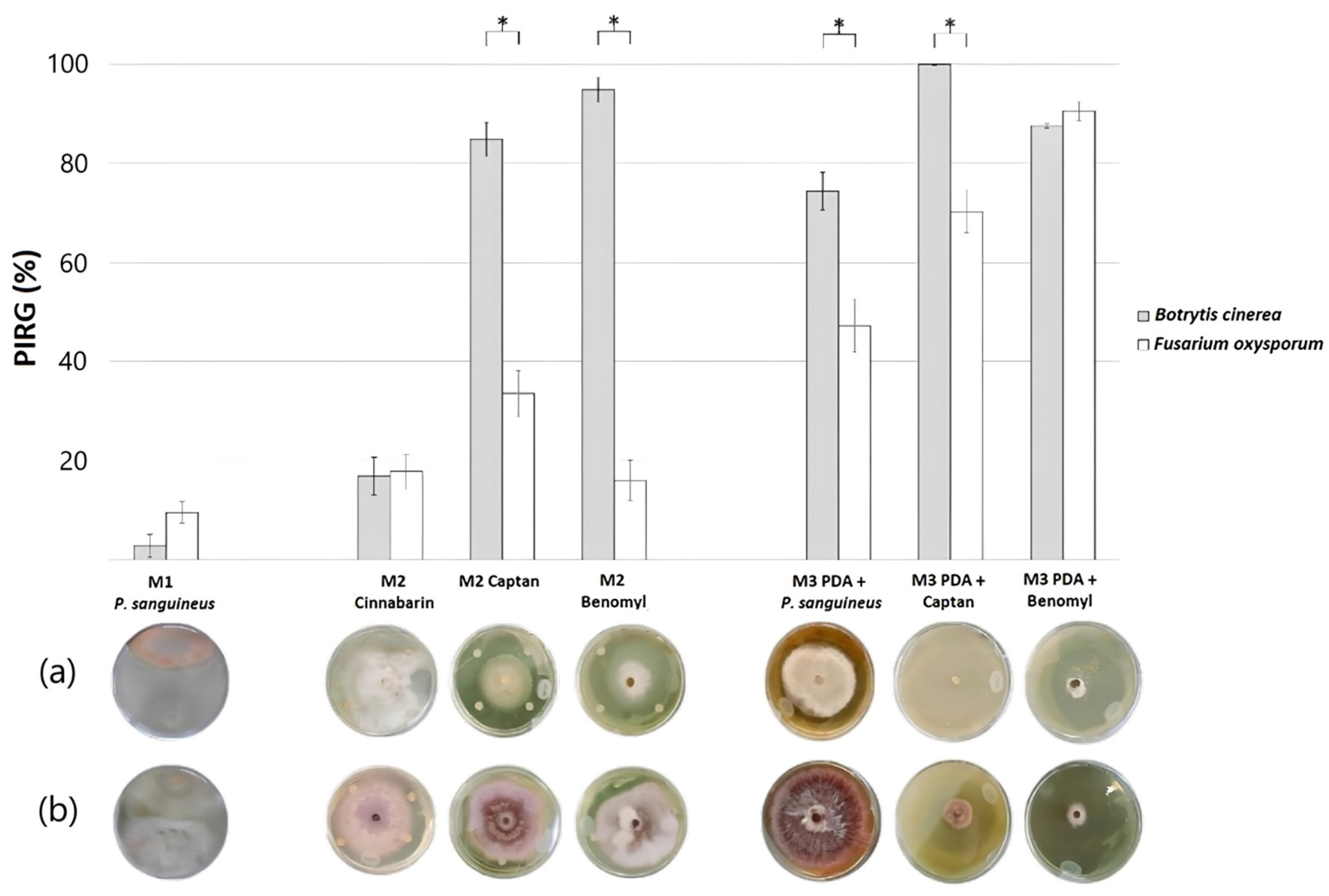
| Model’s | Treatment | Botrytis cinerea | Fusarium oxysporum | ||
|---|---|---|---|---|---|
| PIRG (%) | p ≤ 0.05 | PIRG (%) | p ≤ 0.05 | ||
| Model 1 | Pycnoporus sanguineus | (±2.3) 2.78 | d | (±2.2) 9.54 | d |
| Model 2 | Cinnabarin (CH2OH-COOH-NH2) | (±3.8) 16.81 | c | (±3.5) 17.79 | d |
| Captan (C9H8Cl3NO2S) | (±3.4) 84.85 | b | (±4.6) 33.50 | c | |
| Benomyl (C14H18N4O3) | (±2.4) 94.83 | b | (±4.1) 15.98 | d | |
| Model 3 | PDA + Pycnoporus sanguineus extract | (±3.8) 74.34 | b | (±5.3) 47.14 | c |
| PDA + Captan (C9H8Cl3NO2S) | (±0.2) 100 | a | (±4.2) 70.25 | b | |
| PDA + Benomyl (C14H18N4O3) | (±0.5) 87.55 | b | (±1.9) 90.53 | a | |
| Model’s | Treatment | Botrytis cinerea | Fusarium oxysporum | ||
|---|---|---|---|---|---|
| GR (mm/day) | p ≤ 0.05 | GR (mm/day) | p ≤ 0.05 | ||
| Model 1 | Control (PDA) | (±1.5) 7.84 | a | (±0.4) 3.47 | a |
| Pycnoporus sanguineus | (±1.4) 7.02 | a | (±0.5) 3.18 | a | |
| Model 2 | Control (PDA) | (±1.3) 7.77 | a | (±0.8) 4.25 | a |
| Cinnabarin (CH2OH-COOH-NH2) | (±1.6) 6.60 | ab | (±0.6) 3.88 | a | |
| Captan (C9H8Cl3NO2S) | (±0.8) 1.94 | ce | (±0.4) 3.16 | a | |
| Benomyl (C14H18N4O3) | (±0.6) 1.46 | ce | (±0.4) 3.48 | a | |
| Model 3 | Control (PDA) | (±1.5) 7.61 | a | (±1.2) 4.28 | a |
| PDA + Pycnoporus sanguineus extract | (±0.8) 3.85 | bc | (±0.3) 2.99 | a | |
| PDA + Captan (C9H8Cl3NO2S) | (±0.02) 0.02 | d | (±0.2) 1.22 | b | |
| PDA + Benomyl (C14H18N4O3) | (±0.2) 0.93 | e | (±0.2) 0.50 | c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-López, R.I.; Romero-Arenas, O.; Parraguirre Lezama, C.; Romero López, A.; Rivera, A.; Cedillo Ramírez, L. Comparison of Three Biological Control Models of Pycnoporus sanguineus on Phytopathogenic Fungi. Appl. Sci. 2024, 14, 8263. https://doi.org/10.3390/app14188263
Pérez-López RI, Romero-Arenas O, Parraguirre Lezama C, Romero López A, Rivera A, Cedillo Ramírez L. Comparison of Three Biological Control Models of Pycnoporus sanguineus on Phytopathogenic Fungi. Applied Sciences. 2024; 14(18):8263. https://doi.org/10.3390/app14188263
Chicago/Turabian StylePérez-López, Ricardo Irving, Omar Romero-Arenas, Conrado Parraguirre Lezama, Anabel Romero López, Antonio Rivera, and Lilia Cedillo Ramírez. 2024. "Comparison of Three Biological Control Models of Pycnoporus sanguineus on Phytopathogenic Fungi" Applied Sciences 14, no. 18: 8263. https://doi.org/10.3390/app14188263









