Nano-CeO2 for the Photocatalytic Degradation of the Complexing Agent Citric Acid in Cu Chemical Mechanical Polishing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Characterization of CeO2
2.3. Characterization of CeO2 Photocatalytic Degradation
2.4. Electrochemical Measurements
2.5. Copper Ion Concentration Testing
3. Results and Discussion
3.1. CeO2 Morphologies and Crystal Structures
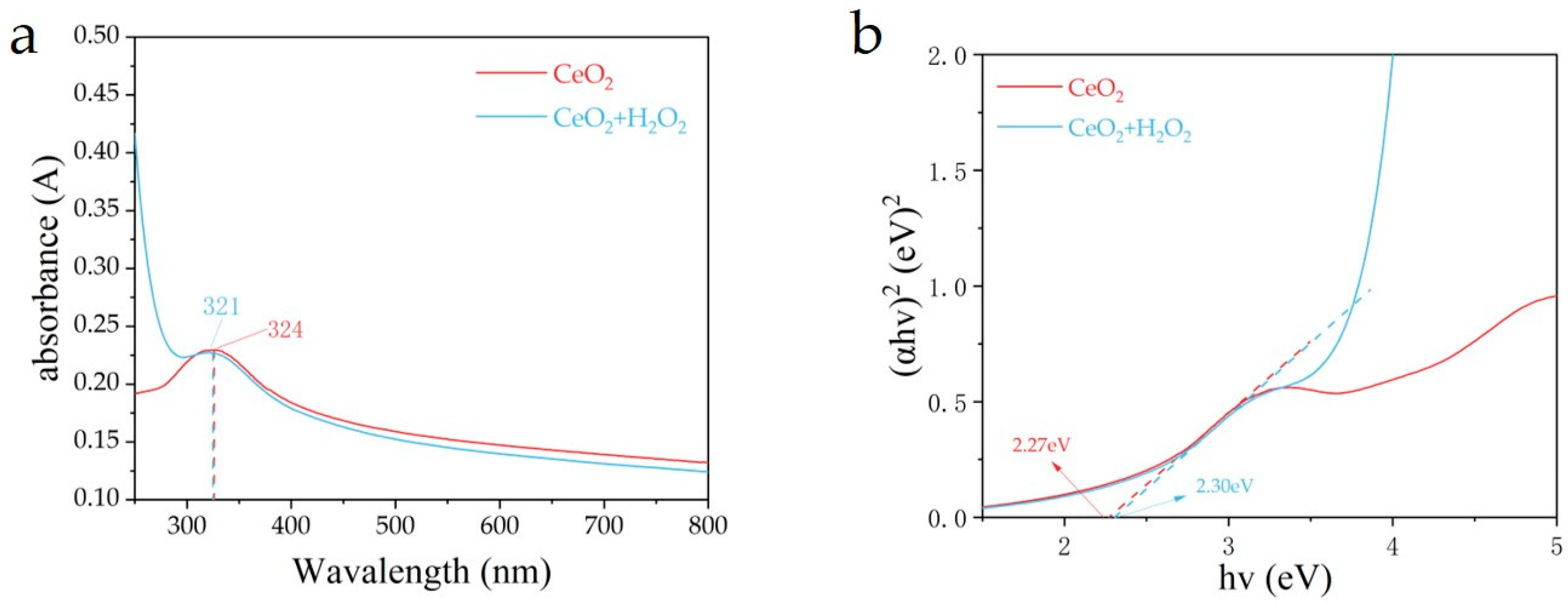
3.2. The Optical Characteristics of the Photocatalysts
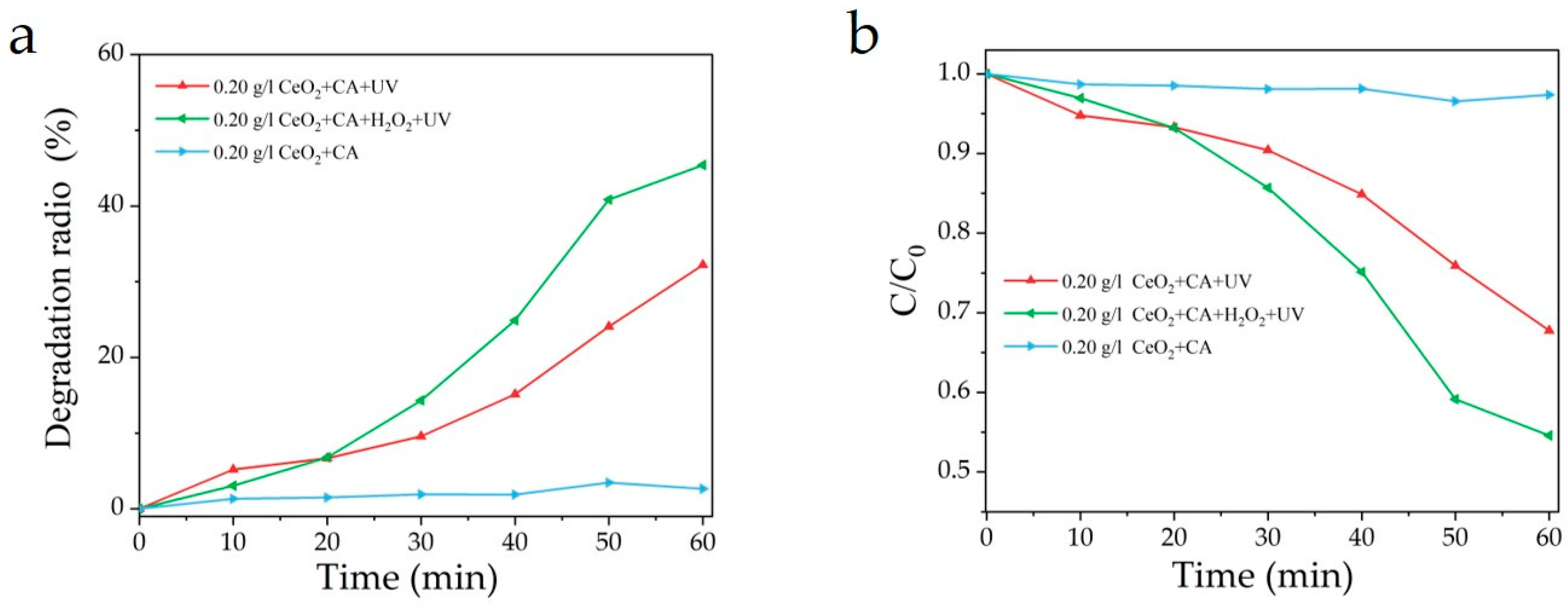
3.3. Performance of CeO2 in the Photocatalytic Degradation of Citric Acid
3.4. Corrosion Behavior of Cu Wafer under Photocatalysis
3.5. Photocatalytic Cleaning Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Y.; Xu, M.; Wen, J.; Wan, Y.; Zhao, Q.; Cao, X.; Ding, Y.; Wang, Z.; Li, H.; Bian, Z. Selective recovery of precious metals through photocatalysis. Nat. Sustain. 2021, 4, 618–626. [Google Scholar] [CrossRef]
- Yan, H.; Niu, X.; Qu, M.; Luo, F.; Zhan, N.; Liu, J.; Zou, Y. A review: Research progress of chemical-mechanical polishing slurry for copper interconnection of integrated circuits. Int. J. Adv. Manuf. Technol. 2023, 125, 47–71. [Google Scholar] [CrossRef]
- Ein-Eli, Y.; Starosvetsky, D. Review on copper chemical-mechanical polishing (CMP) and post-CMP cleaning in ultra large system integrated (ULSI)—An electrochemical perspective. Electrochim. Acta 2007, 52, 1825–1838. [Google Scholar] [CrossRef]
- Hu, L.; Pan, G.; Chen, Q.; Li, L.; Ma, Y.; Zhang, Y. Experimental and computational investigation of complexing agents on copper dissolution for chemical mechanical polishing process. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 664, 131142. [Google Scholar] [CrossRef]
- Ma, B.; Jiang, Y.; Tan, B.; Yuan, J.; Li, W.; Zhang, S.; Ji, J. Complexing agents for potassium oleate removal after cobalt chemical-mechanical polishing: Prediction, verification and mechanism. J. Mol. Liq. 2023, 383, 122077. [Google Scholar] [CrossRef]
- Hazarika, J.; Gupta, A.; Rajaraman, P.V. Review—Post-Chemical Mechanical Planarization Cleaning Technology. ECS J. Solid State Sci. Technol. 2023, 12, 114002. [Google Scholar] [CrossRef]
- Du, H.; Wang, F.; Wang, X.; Tan, B.; Shi, Y.; Liu, R.; Han, X. Synergistic Effect of Composite Complex Agent on BTA Removal in Post-Cu-CMP: Experimental and Theoretical Analysis. ECS J. Solid State Sci. Technol. 2023, 12, 124003. [Google Scholar] [CrossRef]
- Li, W.; Tan, B.; Zhang, S.; Gao, B.; Ma, B.; Guo, L.; Du, H.; Wang, F.; Wang, X. Application of an optimized alkaline cleaning solution for inhibitor removal during the post-CMP process: Performance evaluation and mechanism analysis. J. Mol. Liq. 2023, 369, 120892. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Tao, Y.; Shen, L.; Xu, Z.; Bian, Z.; Li, H. Challenges of photocatalysis and their coping strategies. Chem Catal. 2022, 2, 1315–1345. [Google Scholar] [CrossRef]
- Xueling, B.; Qian, Y.; Yang, G.; Baoqiang, H.; Renyuan, Z.; Ran, D.; Jing, L. Alkyl halide formation from degradation of carboxylic acids in the presence of Fe(III) and halides under light irradiation. Water Res. 2023, 235, 119842. [Google Scholar] [CrossRef]
- Xiuling, Z.; Yuanfeng, L.; Linshan, Z.; Qichun, Z.; Congju, L. Simultaneous degradation of high concentration of citric acid coupled with electricity generation in dual-chamber microbial fuel cell. Biochem. Eng. J. 2021, 173, 108095. [Google Scholar] [CrossRef]
- Milica, P.; Tijana, J.; Saša, R.; Janez, K.; Nena, V.; Slobodan, N.; Miloš, K.; Aleksandar, B. Plasma modified electrosynthesized cerium oxide catalyst for plasma and photocatalytic degradation of RB 19 dye. J. Environ. Chem. Eng. 2022, 10, 107931. [Google Scholar] [CrossRef]
- Natalia, Q.; María, E.M.; Raquel, T.G.; Michèle, B.; Marta, I.L. Photocatalytic degradation of citric acid under different conditions: TiO2 heterogeneous photocatalysis against homogeneous photolytic processes promoted by Fe(III) and H2O2. Appl. Catal. B Environ. 2007, 71, 117–124. [Google Scholar] [CrossRef]
- Costa-Silva, M.; Francisca, P.A.; Guerra, Y.; Bartolomeu, C.V.; Edson, C.S.-F.; Josy, A.O.; Luciano, C.A.; Skovroinski, E.; Peña-Garcia, R. Photocatalytic, structural and optical properties of Ce–Ni co-doped ZnO nanodisks-like self-assembled structures. Mater. Chem. Phys. 2022, 292, 126814. [Google Scholar] [CrossRef]
- Zheng, Z.; Tian, S.; Feng, Y.; Zhao, S.; Li, X.; Wang, S.; He, Z. Recent advances of photocatalytic coupling technologies for wastewater treatment. Chin. J. Catal. 2023, 54, 88–136. [Google Scholar] [CrossRef]
- Hu, T.; Chiu, S.; Dai, B.; Tsai, M.; Tung, I.; Feng, M. Nitric acid-based slurry with citric acid as an inhibitor for copper chemical mechanical polishing. Mater. Chem. Phys. 1999, 61, 169–171. [Google Scholar] [CrossRef]
- Monika, S.; Akhilesh Kumar, S. Studies on structural, morphological, and electrical properties of Ga3+ and Cu2+ co-doped ceria ceramics as solid electrolyte for IT-SOFCs. Int. J. Hydrogen Energy 2020, 45, 24014–24025. [Google Scholar] [CrossRef]
- You, H.; Wu, Z.; Zhang, L.; Ying, Y.; Liu, Y.; Fei, L.; Chen, X.; Jia, Y.; Wang, Y.; Wang, F.; et al. Harvesting the Vibration Energy of BiFeO3 Nanosheets for Hydrogen Evolution. Angew. Chem. Int. Ed. 2019, 58, 11779–11784. [Google Scholar] [CrossRef]
- Liu, X.; Xiao, L.; Zhang, Y.; Sun, H. Significantly enhanced piezo-photocatalytic capability in BaTiO3 nanowires for degrading organic dye. J. Mater. 2020, 6, 256–262. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Samiee, S.; Nancarrow, P. Fabrication of cerium oxide nanoparticles: Characterization and optical properties. J. Colloid Interface Sci. 2011, 356, 473–480. [Google Scholar] [CrossRef]
- Truffault, L.; Ta, M.-T.; Devers, T.; Konstantinov, K.; Harel, V.; Simmonard, C.; Andreazza, C.; Nevirkovets, I.P.; Pineau, A.; Verona, O.; et al. Application of nanostructured Ca doped CeO2 for ultraviolet filtration. Mater. Res. Bull. 2010, 45, 527–535. [Google Scholar] [CrossRef]
- Li, F.; Yang, L.; Zou, L.; Wu, Y.; Hu, C.; He, J.; Yang, X. Decreasing Crystallinity is Beneficial to the Superoxide Dismutase-like Activity of Ceria Nanoparticles. Chemnanomat 2022, 8, e202100466. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, J.; Yan, B.; Liu, L.; Chen, D.; Liu, X. Regulation of Ce (III)/Ce (IV) ratio of cerium oxide for antibacterial application. Iscience 2021, 24, 102226. [Google Scholar] [CrossRef] [PubMed]
- Parida, V.K.; Srivastava, S.K.; Chowdhury, S.; Gupta, A.K. Facile synthesis of 2D/0D Bi2O3/MnO2 Z-scheme heterojunction for enhanced visible light-assisted photocatalytic degradation of acetaminophen. Chem. Eng. J. 2023, 472, 144969. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic Nanoscaled Fe3O4/CeO2 Composite as an Efficient Fenton-Like Heterogeneous Catalyst for Degradation of 4-Chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef]
- Murugan, R.; Kashinath, L.; Subash, R.; Sakthivel, P.; Byrappa, K.; Rajendran, S.; Ravi, G. Pure and alkaline metal ion (Mg, Ca, Sr, Ba) doped cerium oxide nanostructures for photo degradation of methylene blue. Mater. Res. Bull. 2018, 97, 319–325. [Google Scholar] [CrossRef]
- Deng, H.; Jia, Y.; Wang, W.; Zhong, S.; Hao, R.; Fan, L.; Liu, X. Defect and Crystallinity-Mediated Charge Separation in Carbon Nitride for Synergistically Boosted Solar-Driven Hydrogen Evolution. Acs Sustain. Chem. Eng. 2023, 11, 13736–13746. [Google Scholar] [CrossRef]
- Li, L.; Hu, S.-z.; Huang, T.; Zhang, N.; Wang, Y. Fabricating the ternary CeO2@CNTs/CdSe composite with synchronously enhanced adsorption and photocatalytic activity toward water-soluble pollutants removal. Chem. Eng. J. 2023, 476, 146574. [Google Scholar] [CrossRef]
- Mansingh, S.; Padhi, D.K.; Parida, K.M. Enhanced visible light harnessing and oxygen vacancy promoted N, S co-doped CeO2 nanoparticle: A challenging photocatalyst for Cr(VI) reduction. Catal. Sci. Technol. 2017, 7, 2772–2781. [Google Scholar] [CrossRef]
- Ghassempour, A.; Najafi, N.M.; Amiri, A.A. Determination of citric acid in fermentation media by pyrolysis mass spectrometry. J. Anal. Appl. Pyrolysis 2003, 70, 251–261. [Google Scholar] [CrossRef]
- Tekin, N.; Cebe, M. Investigation of dissociation properties of oxalic acid–solvent systems by UV-spectrophotometry. J. Mol. Liq. 2005, 122, 65–68. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Shi, Y.; Ma, T.; Zhou, J.; Wang, R.; Wang, H.; Zeng, N. Improvement in chemical mechanical polishing of 4H-SiC wafer by activating persulfate through the synergistic effect of UV and TiO2. J. Mater. Process. Technol. 2021, 295, 117150. [Google Scholar] [CrossRef]
- Steigerwald, J.M.; Murarka, S.P.; Gutmann, R.J.; Duquette, D.J. Chemical processes in the chemical mechanical polishing of copper. Mater. Chem. Phys. 1995, 41, 217–228. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Gao, F.; Liang, H. Material removal mechanisms in electrochemical-mechanical polishing of tantalum. Electrochim. Acta 2009, 54, 6808–6815. [Google Scholar] [CrossRef]
- Gorantla, V.R.K.; Assiongbon, K.A.; Babu, S.V.; Roy, D. Semiconductor Devices, Materials, and Processing—Citric Acid as a Complexing Agent in CMP of Copper—Investigation of Surface Reactions Using Impedance Spectroscopy. J. Electrochem. Soc. 2005, 152, G404. [Google Scholar] [CrossRef]
- Guo, X.; Yuan, S.; Gou, Y.; Wang, X.; Guo, J.; Jin, Z.; Kang, R. Study on chemical effects of H2O2 and glycine in the Copper CMP process using ReaxFF MD. Appl. Surf. Sci. 2020, 508, 145262. [Google Scholar] [CrossRef]
- Shijie, L.; Shiwei, H.; Wei, J.; Yu, L.; Yingtang, Z.; Jianshe, L.; Zhaohui, W. Facile synthesis of cerium oxide nanoparticles decorated flower-like bismuth molybdate for enhanced photocatalytic activity toward organic pollutant degradation. J. Colloid Interface Sci. 2018, 530, 171–178. [Google Scholar] [CrossRef]
- Herzog, A.E.; Michael, T.J.; Dunkelberger, A.D.; Johannes, M.D.; Rolison, D.R.; DeSario, P.A.; Novak, T.G. Nanostructured CeO2 photocatalysts: Optimizing surface chemistry, morphology, and visible-light absorption. Nanoscale 2024, 16, 9659–9679. [Google Scholar] [CrossRef]



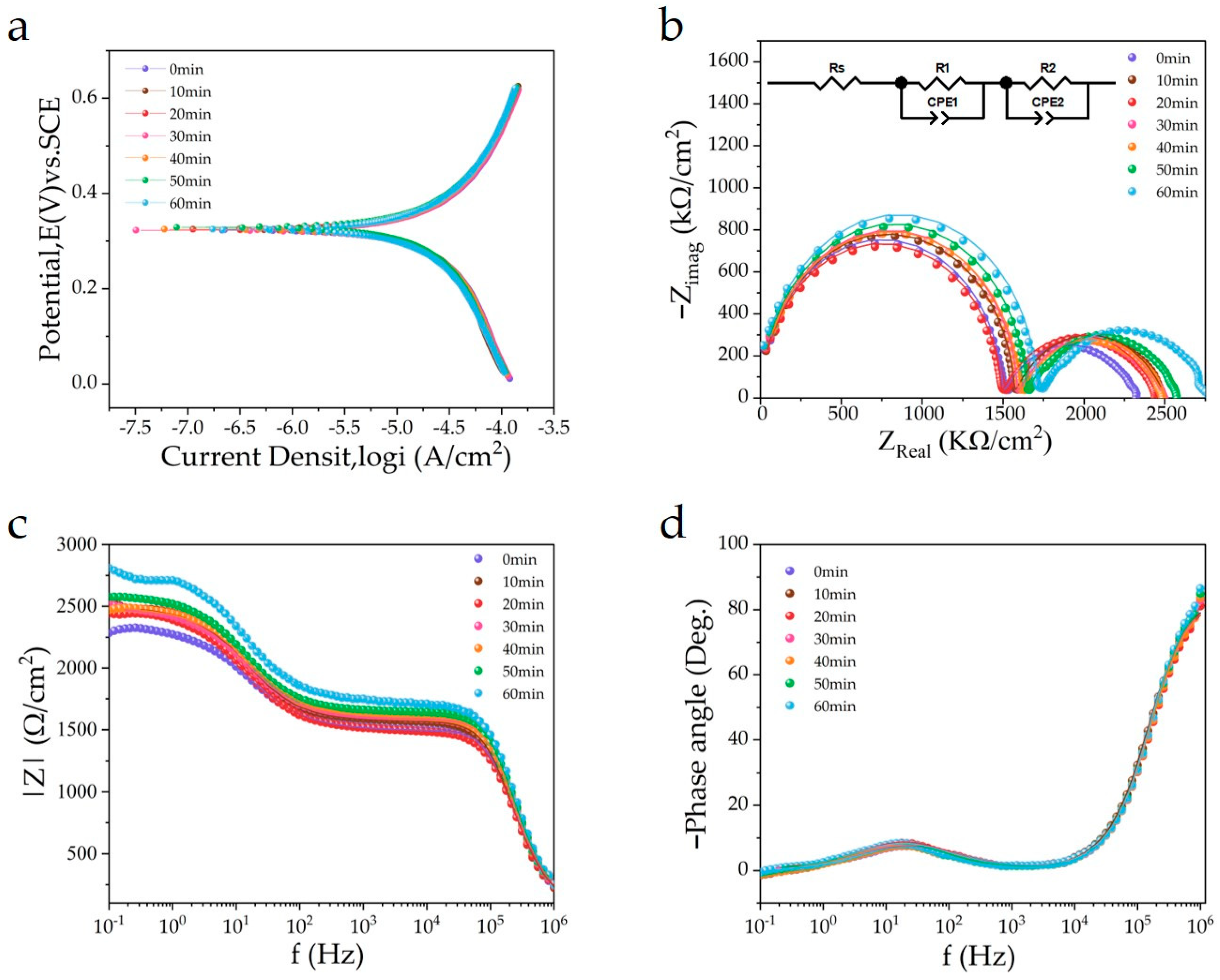

| Electrolyte Illumination Time | Ecorr vs. SCE (V) | Icorr (A/cm2) |
|---|---|---|
| 0 min | −0.323 | |
| 10 min | −0.325 | |
| 20 min | −0.325 | |
| 30 min | −0.323 | |
| 40 min | −0.325 | |
| 50 min | −0.329 | |
| 60 min | −0.325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Lu, Z.; Wang, J.; Lai, J.; Li, Z.; Zhang, C.; Qi, Y. Nano-CeO2 for the Photocatalytic Degradation of the Complexing Agent Citric Acid in Cu Chemical Mechanical Polishing. Appl. Sci. 2024, 14, 8285. https://doi.org/10.3390/app14188285
Liu Y, Lu Z, Wang J, Lai J, Li Z, Zhang C, Qi Y. Nano-CeO2 for the Photocatalytic Degradation of the Complexing Agent Citric Acid in Cu Chemical Mechanical Polishing. Applied Sciences. 2024; 14(18):8285. https://doi.org/10.3390/app14188285
Chicago/Turabian StyleLiu, Yihang, Zongmao Lu, Jiajie Wang, Jinghui Lai, Ziyang Li, Chu Zhang, and Yuhang Qi. 2024. "Nano-CeO2 for the Photocatalytic Degradation of the Complexing Agent Citric Acid in Cu Chemical Mechanical Polishing" Applied Sciences 14, no. 18: 8285. https://doi.org/10.3390/app14188285
APA StyleLiu, Y., Lu, Z., Wang, J., Lai, J., Li, Z., Zhang, C., & Qi, Y. (2024). Nano-CeO2 for the Photocatalytic Degradation of the Complexing Agent Citric Acid in Cu Chemical Mechanical Polishing. Applied Sciences, 14(18), 8285. https://doi.org/10.3390/app14188285






