The Structural Modification of Jackfruit Leaf Proteins (Artocarpus heterophyllus Lam.) by High-Intensity Ultrasound Alters Their Techno-Functional Properties and Antioxidant Capacity
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Material
2.2. Chemical Substances
2.3. Protein Ultrasound-Assisted Extraction (UAE) and the Modification of Protein by High-Intensity Ultrasound (HIU)
2.4. Amino Acid Profile by Gas Chromatography–Mass Spectrometry (GC-MS)
2.5. Techno-Functional Properties Characterization
2.5.1. Loss of Hydrophobicity (Solubility)
2.5.2. Foaming Properties
2.5.3. Emulsifying Properties and Droplet Size Distribution
2.5.4. Size Distribution
2.5.5. Antioxidant Properties
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Yield of Protein and the Amino Acid Composition
3.2. The Techno-Functional Properties of Proteins
3.2.1. Loss of Hydrophobicity (Solubility)
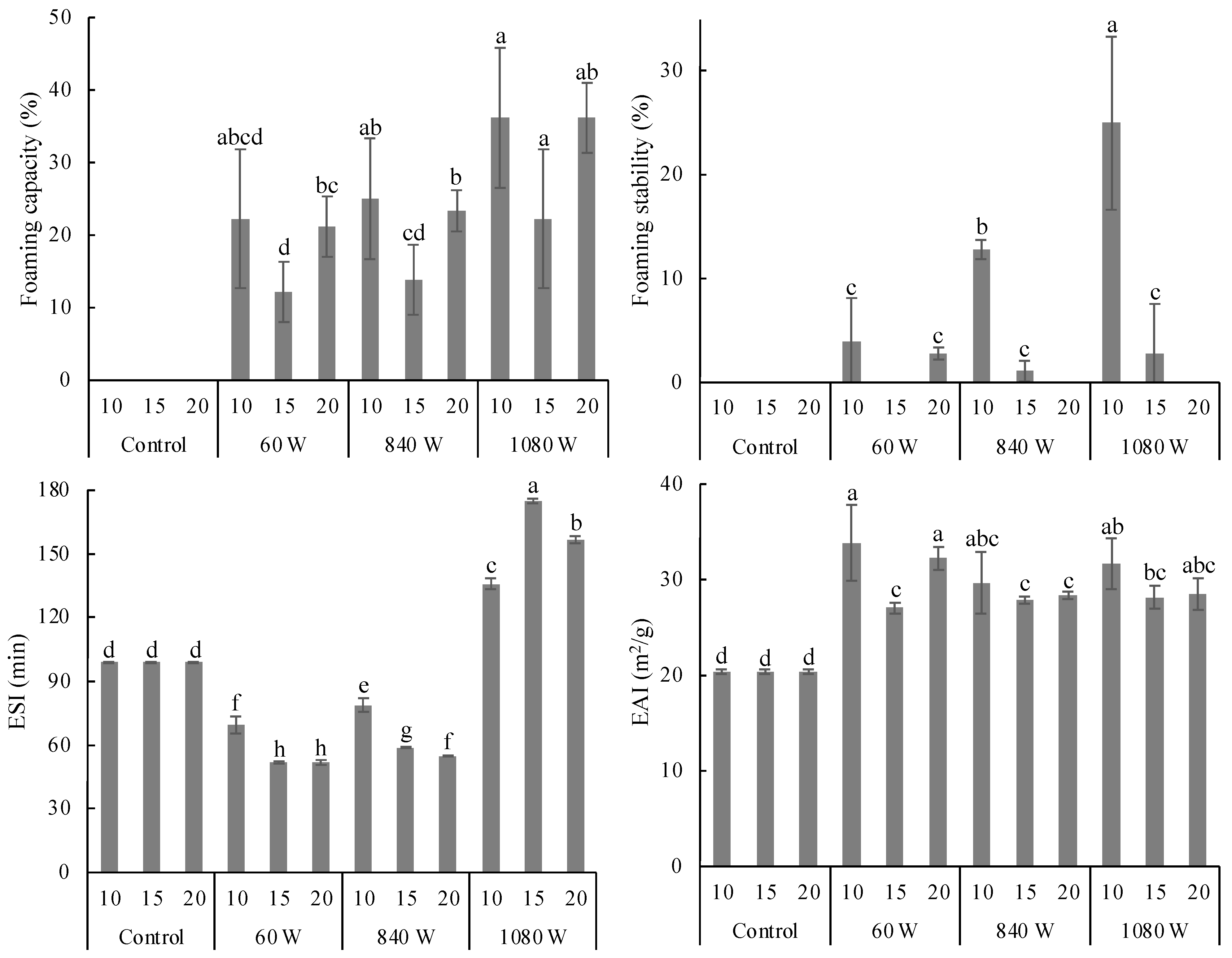
3.2.2. Foaming Properties
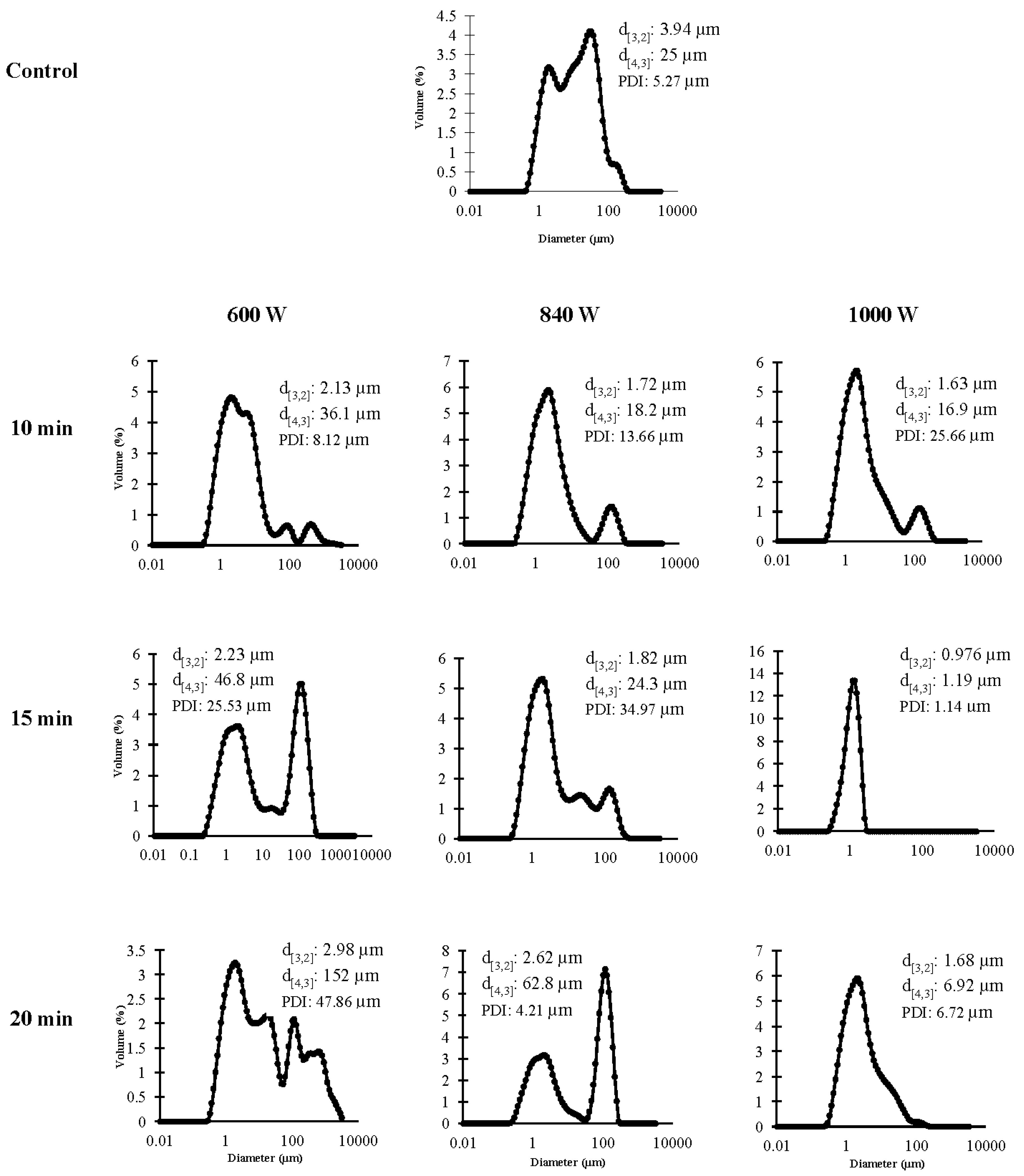
3.2.3. Emulsifying Properties
3.2.4. Particle Size Distribution
3.2.5. Antioxidant Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Damasceno-Gomes, S.; Ragazzo-Sánchez, J.A. Use of Jackfruit Leaf (Artocarpus heterophyllus L.) Protein Hydrolysates as a Stabilizer of the Nanoemulsions Loaded with Extract-Rich in Pentacyclic Triterpenes Obtained from Coccoloba uvifera L. Leaf. Food Chem. X 2021, 12, 100138. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Herman-Lara, E.; Ragazzo-Sánchez, J.A. Jackfruit (Artocarpus heterophyllus Lam) Leaf as a New Source to Obtain Protein Hydrolysates: Physicochemical Characterization, Techno-Functional Properties and Antioxidant Capacity. Food Hydrocoll. 2021, 112, 106319. [Google Scholar] [CrossRef]
- Ranasinghe, R.A.S.N.; Maduwanthi, S.D.T.; Marapana, R.A.U.J. Nutritional and Health Benefits of Jackfruit (Artocarpus heterophyllus Lam.): A Review. Int. J. Food Sci. 2019, 2019, 4327183. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Nájera, L.C.; Ragazzo-Sánchez, J.A.; Gastón-Peña, C.R.; Calderón-Santoyo, M. Green Technologies for the Extraction of Proteins from Jackfruit Leaves (Artocarpus heterophyllus Lam). Food Sci. Biotechnol. 2020, 29, 1675–1684. [Google Scholar] [CrossRef]
- Brion-Espinoza, I.A.; Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Barros-Castillo, J.C.; Calderón-Chiu, C.; Calderón-Santoyo, M. Edible Pectin Film Added with Peptides from Jackfruit Leaves Obtained by High-Hydrostatic Pressure and Pepsin Hydrolysis. Food Chem. X 2021, 12, 100170 Contents. [Google Scholar] [CrossRef]
- Vera-Salgado, J.; Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; López-García, U.M.; Ragazzo-Sánchez, J.A. Ultrasound-Assisted Extraction of Artocarpus heterophyllus L. Leaf Protein Concentrate: Solubility, Foaming, Emulsifying, and Antioxidant Properties of Protein Hydrolysates. Colloids Interfaces 2022, 6, 50. [Google Scholar] [CrossRef]
- Calderón-Chiu, C.; Calderón-Santoyo, M.; Barros-Castillo, J.C.; Díaz, J.A.; Ragazzo-Sánchez, J.A. Structural Modification of Jackfruit Leaf Protein Concentrate by Enzymatic Hydrolysis and Their Effect on the Emulsifier Properties. Colloids Interfaces 2022, 6, 52. [Google Scholar] [CrossRef]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative Applications of High-Intensity Ultrasound in the Development of Functional Food Ingredients: Production of Protein Hydrolysates and Bioactive Peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, Y.; Chen, J.; Zou, J.; Wang, Q.; Ma, M. Influence of High-Intensity Ultrasound on Foaming and Structural Properties of Egg White. Food Res. Int. 2018, 108, 604–610. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of Ultrasound-Assisted Extraction. In Water Extraction of Bioactive Compounds: From Plants to Drug Development; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. ISBN 9780128096154. [Google Scholar]
- Xiong, T.; Xiong, W.; Ge, M.; Xia, J.; Li, B.; Chen, Y. Effect of High Intensity Ultrasound on Structure and Foaming Properties of Pea Protein Isolate. Food Res. Int. 2018, 109, 260–267. [Google Scholar] [CrossRef]
- Li, Y.-D.; Zheng, J.-B.; Wang, X.-L.; Yang, K.-K.; Wang, Y.-Z. Structure and Properties of Soy Protein/Poly(Butylene Succinate) Blends with Improved Compatibility. Biomacromolecules 2008, 9, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.; Martínez, K.D.; Pizones Ruiz-Henestrosa, V.M.; Pilosof, A.M.R. Modification of Foaming Properties of Soy Protein Isolate by High Ultrasound Intensity: Particle Size Effect. Ultrason. Sonochem. 2015, 26, 48–55. [Google Scholar] [CrossRef]
- Zhao, F.; Zhai, X.; Liu, X.; Lian, M.; Liang, G.; Cui, J.; Dong, H.; Wang, W. Effects of High-Intensity Ultrasound Pretreatment on Structure, Properties, and Enzymolysis of Walnut Protein Isolate. Molecules 2022, 27, 208. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, L.; Liu, B.; Wang, W.; Shang, Z.; Dang, H.; Liu, C. Improvement of Foaming Properties of Ovalbumin: Insights into the Synergistic Effect of Preheating and High-Intensity Ultrasound on Physicochemical Properties and Structure Analysis. Ultrason. Sonochem. 2023, 101, 106672. [Google Scholar] [CrossRef]
- Chavan, P.; Sharma, P.; Sharma, S.R.; Mittal, T.C.; Jaiswal, A.K. Application of High-Intensity Ultrasound to Improve Food Processing Efficiency: A Review. Foods 2022, 11, 122. [Google Scholar] [CrossRef]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying Properties of Proteins: Evaluation of a Turbidimetric Technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Cortés-Muñoz, M.; Chevalier-Lucia, D.; Dumay, E. Characteristics of Submicron Emulsions Prepared by Ultra-High Pressure Homogenisation: Effect of Chilled or Frozen Storage. Food Hydrocoll. 2009, 23, 640–654. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Huang, M.; Hayat, K.; Kurtz, N.C.; Wu, X.; Ahmad, M.; Zheng, F. Ultrasound-Assisted Alkaline Proteinase Extraction Enhances the Yield of Pecan Protein and Modifies Its Functional Properties. Ultrason. Sonochem. 2021, 80, 105789. [Google Scholar] [CrossRef]
- Rahman, M.M.; Lamsal, B.P. Ultrasound-Assisted Extraction and Modification of Plant-Based Proteins: Impact on Physicochemical, Functional, and Nutritional Properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1457–1480. [Google Scholar] [CrossRef] [PubMed]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.T.; Show, P.L. Microalgae: A Potential Alternative to Health Supplementation for Humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- Liestianty, D.; Rodianawati, I.; Arfah, R.A.; Assa, A.; Patimah; Sundari. Muliadi Nutritional Analysis of Spirulina Sp to Promote as Superfood Candidate. IOP Conf. Ser. Mater. Sci. Eng. 2019, 509, 012031. [Google Scholar] [CrossRef]
- Su, J.; Cavaco-Paulo, A. Effect of Ultrasound on Protein Functionality. Ultrason. Sonochem. 2021, 76, 105653. [Google Scholar] [CrossRef]
- Higuera-Barraza, O.A.; Del Toro-Sanchez, C.L.; Ruiz-Cruz, S.; Márquez-Ríos, E. Effects of High-Energy Ultrasound on the Functional Properties of Proteins. Ultrason. Sonochem. 2016, 31, 558–562. [Google Scholar] [CrossRef]
- Meurer, M.C.; de Souza, D.; Ferreira Marczak, L.D. Effects of Ultrasound on Technological Properties of Chickpea Cooking Water (Aquafaba). J. Food Eng. 2020, 265, 109688. [Google Scholar] [CrossRef]
- Wali, A.; Ma, H.; Shahnawaz, M.; Hayat, K.; Xiaong, J.; Jing, L. Impact of Power Ultrasound on Antihypertensive Activity, Functional Properties, and Thermal Stability of Rapeseed Protein Hydrolysates. J. Chem. 2017, 2017, 4373859. [Google Scholar] [CrossRef]
- Uluko, H.; Liu, L.; Li, H.; Cui, W.; Zhang, S.; Zhao, L.; Xue, H.; Lv, J. Effect of Power Ultrasound Pretreatment on Peptidic Profiles and Angiotensin Converting Enzyme Inhibition of Milk Protein Concentrate Hydrolysates. J. Sci. Food Agric. 2014, 94, 2420–2428. [Google Scholar] [CrossRef]
- Li, R.; Chen, X.; Chang, Y.; Zhang, L.; Zhang, Y.; Zhu, Y.; Wang, T. Increase of Bubble Size Playing a Critical Role in Foam-Induced Protein Aggregation: Aggregation of BSA in Foam Fractionation. Chem. Eng. Sci. 2017, 174, 387–395. [Google Scholar] [CrossRef]
- Qiao, X.; Miller, R.; Schneck, E.; Sun, K. Adsorption, Surface Viscoelasticity, and Foaming Properties of Silk Fibroin at the Air/Water Interface. Colloids Interfaces 2022, 6, 40. [Google Scholar] [CrossRef]
- Singh, A.M.; Dalgleish, D.G. The Emulsifying Properties of Hydrolyzates of Whey Proteins. J. Dairy. Sci. 1998, 81, 918–924. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A Comprehensive Review on Emulsions and Emulsion Stability in Chemical and Energy Industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Ruiz-Montañez, G.; Calderón-Santoyo, M.; Chevalier-Lucia, D.; Picart-Palmade, L.; Jimenez-Sánchez, D.E.; Ragazzo-Sánchez, J.A. Ultrasound-Assisted Microencapsulation of Jackfruit Extract in Eco-Friendly Powder Particles: Characterization and Antiproliferative Activity. J. Dispers. Sci. Technol. 2019, 40, 1507–1515. [Google Scholar] [CrossRef]
- Ho, T.M.; Razzaghi, A.; Ramachandran, A.; Mikkonen, K.S. Emulsion Characterization via Microfluidic Devices: A Review on Interfacial Tension and Stability to Coalescence. Adv. Colloid Interface Sci. 2022, 299, 102541. [Google Scholar] [CrossRef]
- Lu, W.C.; Huang, D.W.; Wang, C.C.R.; Yeh, C.H.; Tsai, J.C.; Huang, Y.T.; Li, P.H. Preparation, Characterization, and Antimicrobial Activity of Nanoemulsions Incorporating Citral Essential Oil. J. Food Drug Anal. 2018, 26, 82–89. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Rubiolo, A.C.; Santiago, L.G. Characterisation of Freeze-Dried Flaxseed Oil Microcapsules Obtained by Multilayer Emulsions. Powder Technol. 2017, 319, 238–244. [Google Scholar] [CrossRef]
- Kaganyuk, M.; Mohraz, A. Impact of Particle Size on Droplet Coalescence in Solid-Stabilized High Internal Phase Emulsions. Langmuir 2019, 35, 12807–12816. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Luo, L.J.; Lai, J.Y. Effects of Shell Thickness of Hollow Poly(Lactic Acid) Nanoparticles on Sustained Drug Delivery for Pharmacological Treatment of Glaucoma. Acta Biomater. 2020, 111, 302–315. [Google Scholar] [CrossRef]
- Ling, Z.; Ai, M.; Zhou, Q.; Guo, S.; Zhou, L.; Fan, H.; Cao, Y.; Jiang, A. Fabrication Egg White Gel Hydrolysates-Stabilized Oil-in-Water Emulsion and Characterization of Its Stability and Digestibility. Food Hydrocoll. 2020, 102, 105621. [Google Scholar] [CrossRef]
- Ai, M.; Zhang, Z.; Fan, H.; Cao, Y.; Jiang, A. High-Intensity Ultrasound Together with Heat Treatment Improves the Oil-in-Water Emulsion Stability of Egg White Protein Peptides. Food Hydrocoll. 2021, 111, 106256. [Google Scholar] [CrossRef]
- Malik, M.A.; Sharma, H.K.; Saini, C.S. High Intensity Ultrasound Treatment of Protein Isolate Extracted from Dephenolized Sunflower Meal: Effect on Physicochemical and Functional Properties. Ultrason. Sonochem. 2017, 39, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Saricaoglu, F.T.; Atalar, I.; Gul, L.B.; Tornuk, F.; Simsek, S. Structural Characterization, Technofunctional and Rheological Properties of Sesame Proteins Treated by High-Intensity Ultrasound. Foods 2023, 12, 1791. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Vazquez, J.A.; Ulloa, J.A.; Urías-Silvas, J.E.; Bautista-Rosales, P.U.; Ramírez-Ramírez, J.C.; Rosas-Ulloa, P.; González-Torres, L. Effect of High-Intensity Ultrasound on the Technofunctional Properties and Structure of Jackfruit (Artocarpus heterophyllus) Seed Protein Isolate. Ultrason. Sonochem. 2017, 37, 436–444. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of Divergent Ultrasound Pretreatment on the Structure of Watermelon Seed Protein and the Antioxidant Activity of Its Hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef]
- Aluko, R.E. Amino Acids, Peptides, and Proteins as Antioxidants for Food Preservation; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; ISBN 9781782420972. [Google Scholar]
- Fadimu, G.J.; Gill, H.; Farahnaky, A.; Truong, T. Investigating the Impact of Ultrasound Pretreatment on the Physicochemical, Structural, and Antioxidant Properties of Lupin Protein Hydrolysates. Food Bioproc. Technol. 2021, 14, 2004–2019. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and Identification of Novel Antioxidant Peptides from Watermelon Seed Protein Hydrolysates and Their Cytoprotective Effects on H2O2-Induced Oxidative Stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef]
- Mahdavi-Yekta, M.; Nouri, L.; Azizi, M.H. The Effects of Hydrolysis Condition on Antioxidant Activity of Protein Hydrolyzate from Quinoa. Food Sci. Nutr. 2019, 7, 930–936. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The Importance of Antioxidants and Place in Today’s Scientific and Technological Studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- Gallo, M.; Ferrara, L.; Naviglio, D. Application of Ultrasound in Food Science and Technology: A Perspective. Foods 2018, 7, 164. [Google Scholar] [CrossRef]
| Treatment | Time | Protein Content (%, w/w) | Solubility (%) |
|---|---|---|---|
| Control | 0 | 22.0 ± 3.1 e | 6.88 ± 0.23 e |
| 600 W | 10 | 25.4 ± 2.9 de | 7.94 ± 1.09 de |
| 15 | 29.3 ± 3.1 d | 9.16 ± 0.89 d | |
| 20 | 30.1 ± 4.3 d | 9.41 ± 0.56 d | |
| 840 W | 10 | 27.9 ± 1.1 d | 8.72 ± 0.43 d |
| 15 | 38.6 ± 2.6 c | 12.06 ± 1.25 c | |
| 20 | 49.4 ± 5.1 b | 15.44 ± 0.98 b | |
| 1080 W | 10 | 53.0 ± 5.2 ab | 16.56 ± 1.56 ab |
| 15 | 58.0 ± 2.4 a | 18.13 ± 1.33 a | |
| 20 | 50.2 ± 4.1 b | 15.69 ± 0.76 b |
| Amino Acid (g/100 g Protein) | TBDMS-Derivatized Amino Acid | LRI | Extraction Time (min) | Suggested Intake (mg/kg of Weight) | ||
|---|---|---|---|---|---|---|
| 10 | 15 | 20 | ||||
| Alanine | L-Alanine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1521 | 3.54 ± 0.009 a | 11.42 ± 0.091 d | 19.83 ± 0.134 f | NA |
| Glycine | Glycine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1546 | 0.79 ± 0.004 j | 6.32 ± 0.023 i | 13.22 ± 0.032 k | NA |
| Valine | L-Valine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1635 | 1.73 ± 0.008 d | 8.51 ± 0.011 e | 20.47 ± 0.122 e | 24 |
| Leucine | L-Leucine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1674 | 1.71 ± 0.005 e | 10.26 ± 0.026 d | 26.75 ± 0.321 c | 42 |
| Isoleucine | L-Isoleucine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1706 | 1.06 ± 0.002 i | 7.18 ± 0.009 h | 16.94 ± 0.105 g | 19 |
| Proline | L-Proline, 1-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1742 | 3.22 ± 0.001 b | 28.91 ± 0.102 a | 25.94 ± 0.438 d | NA |
| Methionine | L-Methionine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1938 | ND | 1.26 ± 0.002 l | 3.79 ± 0.058 l | 19 |
| Serine | L-Serine, N,O-bis(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1956 | 1.17 ± 0.003 h | 7.52 ± 0.023 g | 13.94 ± 0.098 j | NA |
| Threonine | L-Threonine, N,O-bis(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 1982 | 1.46 ± 0.002 g | 8.56 ± 0.045 e | 15.37 ± 0.110 i | 20 |
| Phenylalanine | L-Phenylalanine, N-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 2053 | 0.69 ± 0.001 k | 6.15 ± 0.018 j | 16.50 ± 0.201 h | 33 |
| Aspartic acid | L-Aspartic acid, N-(tert-butyldimethylsilyl)-, bis(tert-butyldimethylsilyl) ester | 2120 | 1.88 ± 0.005 c | 14.89 ± 0.029 c | 29.00 ± 0.348 b | NA |
| Hydroxyproline | L-Proline, 4-[(tert-butyldimethylsilyl)oxy]-1-(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 2147 | ND | 1.08 ± 0.008 m | 2.08 ± 0.065 m | NA |
| Glutamic acid | L-Glutamic acid, N-(tert-butyldimethylsilyl)-, bis(tert-butyldimethylsilyl) ester | 2237 | 1.53 ± 0.007 f | 21.09 ± 0.128 b | 40.16 ± 0.984 a | NA |
| Lysine | L-Lysine, N2,N6-bis(tert-butyldimethylsilyl)-, tert-butyldimethylsilyl ester | 2339 | ND | 2.74 ± 0.002 k | 25.34 ± 0.872 d | 38 |
| HAA | - | - | 12.05 | 73.85 | 126.94 | - |
| AAA | - | - | 0.69 | 6.15 | 16.50 | - |
| EAA | - | - | 6.64 | 44.64 | 125.16 | - |
| NCAA | - | - | 3.41 | 35.98 | 69.15 | - |
| TAAC | - | - | 18.77 | 135.87 | 269.32 | - |
| Time (min) | Power (W) | %RSA | mg TE/g Sample | IC50 Values of ABTS (mg/mL) |
|---|---|---|---|---|
| 10 | Control | 36.99 ± 1.01 h | 4.52 ± 0.21 b | ND |
| 10 | 600 | 79.25 ± 0.56 c | 4.70 ± 0.53 ab | 0.032 |
| 10 | 840 | 87.60 ± 1.42 b | 5.42 ± 0.02 a | 0.029 |
| 10 | 1080 | 74.98 ± 1.05 e | 1.99 ± 0.01 f | 0.033 |
| 15 | Control | 28.71 ± 1.09 i | 0.6 ± 0.08 h | ND |
| 15 | 600 | 77.28 ± 1.03 d | 2.59 ± 0.33 e | 0.032 |
| 15 | 840 | 96.65 ± 0.56 a | 1.04 ± 0.07 g | 0.026 |
| 15 | 1080 | 64.72 ± 1.13 g | 3.59 ± 0.28 d | 0.039 |
| 20 | Control | 34.04 ± 1.28 h | 2.34 ± 0.43 e | ND |
| 20 | 600 | 74.66 ± 0.68 e | 2.35 ± 0.12 e | 0.033 |
| 20 | 840 | 71.09 ± 0.28 f | 4.02 ± 0.05 c | 0.035 |
| 20 | 1080 | 64.78 ± 0.71 g | 4.02 ± 0.03 c | 0.039 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragazzo-Calderón, F.Z.; Iñiguez-Moreno, M.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A. The Structural Modification of Jackfruit Leaf Proteins (Artocarpus heterophyllus Lam.) by High-Intensity Ultrasound Alters Their Techno-Functional Properties and Antioxidant Capacity. Appl. Sci. 2024, 14, 8301. https://doi.org/10.3390/app14188301
Ragazzo-Calderón FZ, Iñiguez-Moreno M, Calderón-Santoyo M, Ragazzo-Sánchez JA. The Structural Modification of Jackfruit Leaf Proteins (Artocarpus heterophyllus Lam.) by High-Intensity Ultrasound Alters Their Techno-Functional Properties and Antioxidant Capacity. Applied Sciences. 2024; 14(18):8301. https://doi.org/10.3390/app14188301
Chicago/Turabian StyleRagazzo-Calderón, Frida Zoé, Maricarmen Iñiguez-Moreno, Montserrat Calderón-Santoyo, and Juan Arturo Ragazzo-Sánchez. 2024. "The Structural Modification of Jackfruit Leaf Proteins (Artocarpus heterophyllus Lam.) by High-Intensity Ultrasound Alters Their Techno-Functional Properties and Antioxidant Capacity" Applied Sciences 14, no. 18: 8301. https://doi.org/10.3390/app14188301
APA StyleRagazzo-Calderón, F. Z., Iñiguez-Moreno, M., Calderón-Santoyo, M., & Ragazzo-Sánchez, J. A. (2024). The Structural Modification of Jackfruit Leaf Proteins (Artocarpus heterophyllus Lam.) by High-Intensity Ultrasound Alters Their Techno-Functional Properties and Antioxidant Capacity. Applied Sciences, 14(18), 8301. https://doi.org/10.3390/app14188301







