Long-Term Monitoring and Management of Genetically Modified Canola in Natural Environments: A 15-Year Study
Abstract
:1. Introduction
2. Materials and Methods
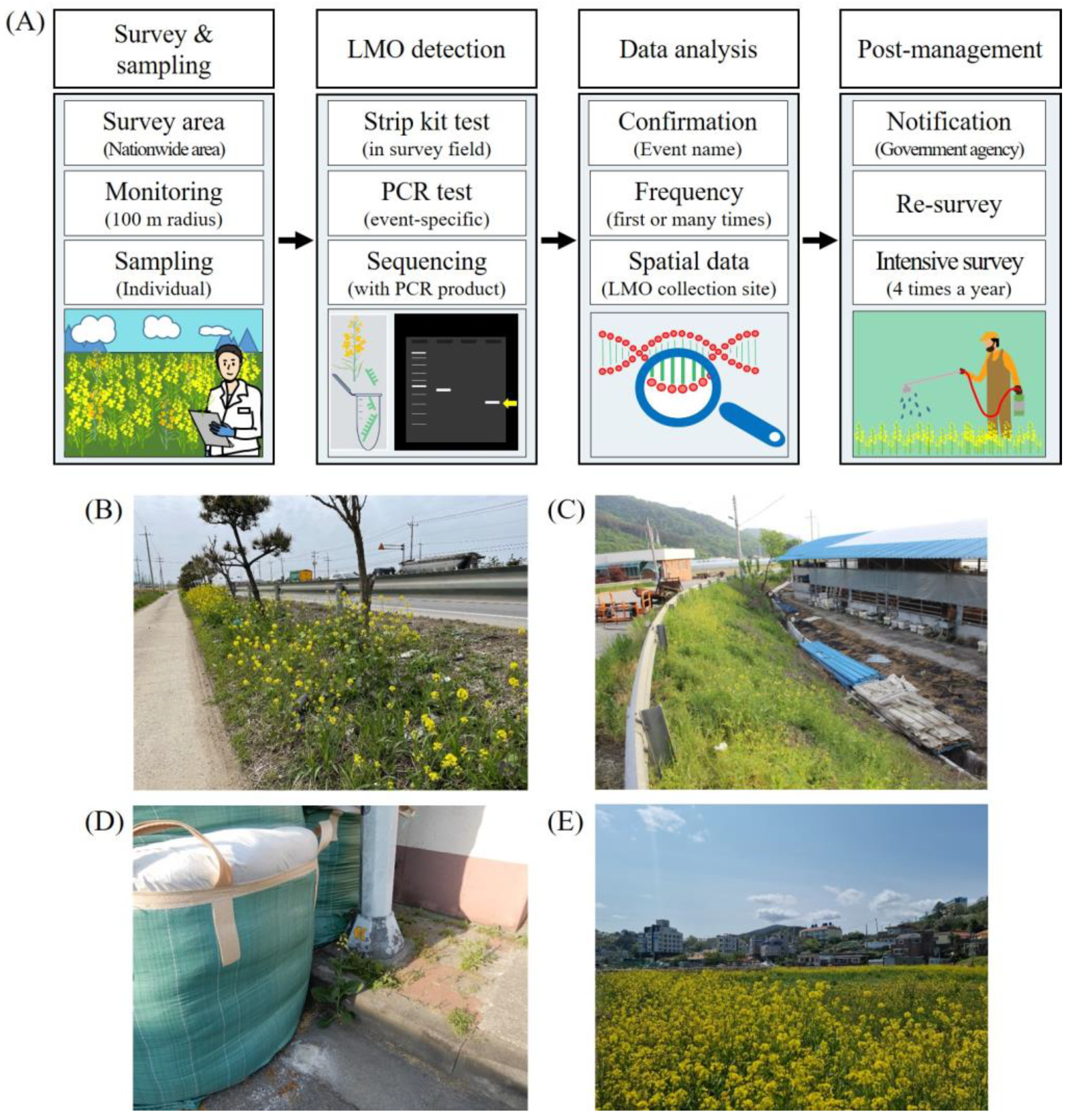
2.1. LMO Monitoring for LM Canola in Natural Environments
2.2. Identification of Collected LM Canola Samples
| Target Event (Or Gene) | Primer Name | Sequence (5′-3′) | Product Size (bp) | Reference |
|---|---|---|---|---|
| Topas 19/2 | Topas 19/2-F | CGACCGGCGCTGATATATGA | 95 | [47] |
| Topas 19/2-R | GTTGCGGTTCTGTCAGTTCC | |||
| Rf3 | Rf3-F | AGCATTTAGCATGTACCATCAGACA | 267 | [48] |
| Rf3-R | ATCAATATCATTGCAACGGAAAAGG | This study | ||
| MON88302 | MON88302-F | TCAGATTGTCGTTTCCCGCCTTCA | 304 | [49] |
| MON88302-R | GTCTTTGCTTTTGGCTCTTACTTTTGCG | [50] | ||
| Ms8 | Ms8-F | CCAAATAGCCTCCCACCCTATA | 249 | [23] |
| Ms8-R | GGAGGGTGTTTTTGGTTATC | [51] | ||
| GT73 | GT73-F | GCTTATACGAAGGCAAGAAAAGGA | 321 | [52] |
| GT73-R | GAAGTTTCTCATCTAAGCCCCCATTTG | This study | ||
| DP-073496-4 | DP-073496-4-F | GCTGGTCCAATTCAGATATGGT | 350 | This study |
| DP-073496-4-R | CAAACCTCCATAGAGTTCAACATCTTAA | [53] | ||
| T45 | T45-F | CAAGCGTGTCGTGCTCCACCATGTT | 378 | [23] |
| T45-R | GAACATAGATCGAGTCTCCCA | [54] | ||
| MS11 | MS11-F | CAAGATGGGAATTAACATCTACAAATTG | 535 | [55] |
| MS11-R | GCAGCACTGCTACTGGTCAA | This study | ||
| Cru A | Cru A -F | GTCAAGGCCAAGGACAACAG | 154 | This study |
| Cru A-R | CCGTCGTTGTAGAACCATTGG | [55] |
2.3. Classification of LM Canola Collection Sites
2.4. Post-Management of LM Canola Collection Site
3. Results and Discussion
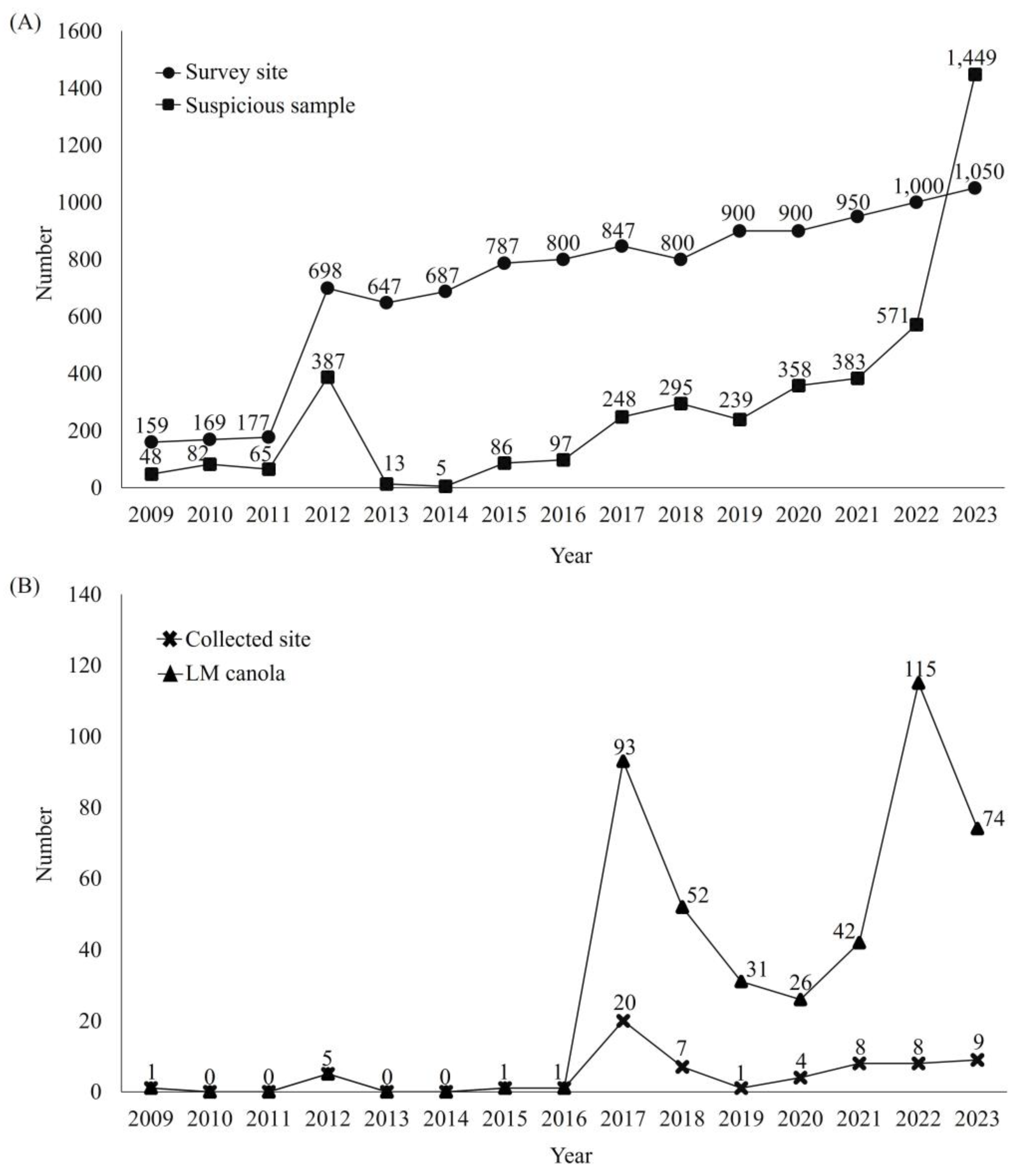
3.1. The LMO Monitoring Project for LM Canola
3.2. Analysis of LM Canola Survey Data from 2009 to 2023
3.3. Analysis of LM Canola Monitoring Results over Fifteen Years
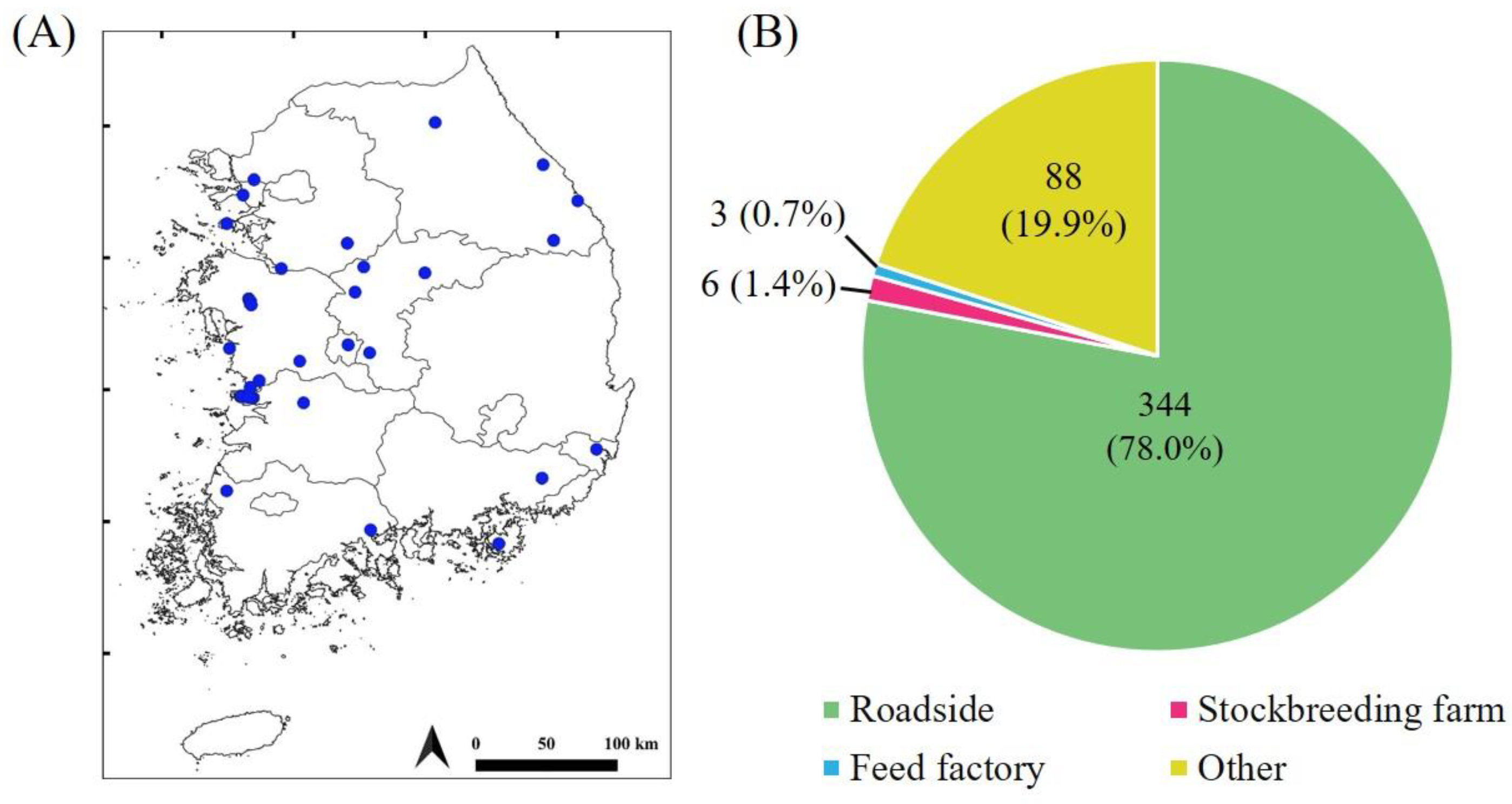
3.4. Classification of LM Canola Collection Sites from 2009 to 2023
3.5. Analysis of LM Canola Re-Collection Sites from 2014 to 2021
3.6. Post-Management System of LM Canola
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brookes, G. Genetically Modified (GM) Crop Use 1996–2020: Environmental Impacts Associated with Pesticide Use Change. GM Crops Food 2022, 13, 262–289. [Google Scholar] [CrossRef] [PubMed]
- Stein, A.; Rodríguez-Cerezo, E. Low-Level Presence of New GM Crops: An Issue on the Rise for Countries Where They Lack Approval. AgBioForum 2010, 13, 173–182. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC54768 (accessed on 19 August 2024).
- Pellegrino, E.; Bedini, S.; Nuti, M.; Ercoli, L. Impact of Genetically Engineered Maize on Agronomic, Environmental and Toxicological Traits: A Meta-Analysis of 21 Years of Field Data. Sci. Rep. 2018, 8, 3113. [Google Scholar] [CrossRef]
- Brookes, G. GM Crops: Global Socio-Economic and Environmental Impacts 1996–2020. 2022. Available online: https://pgeconomics.co.uk/pdf/Globalimpactbiotechcropsfinalreportoctober2022.pdf (accessed on 19 August 2024).
- Kim, J.-H.; Kim, E.-H.; Yea, M.-C.; Kim, H.-Y. Validation of A Multiplex PCR Detection Kit for Screening of Herbicide-Tolerant Genes in Genetically Modified Crops. J. Korean Soc. Appl. Biol. Chem. 2013, 56, 251–254. [Google Scholar] [CrossRef]
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.; Li, Y.; Cheng, J.; Wu, C.; Cao, J.; Jin, S. Herbicide Resistance: Another Hot Agronomic Trait for Plant Genome Editing. Plants 2021, 10, 621. [Google Scholar] [CrossRef]
- Christ, B.; Hochstrasser, R.; Guyer, L.; Francisco, R.; Aubry, S.; Hörtensteiner, S.; Weng, J.-K. Nonspecific Activities of the Major Herbicide-Resistance Gene BAR. Nat. Plants 2017, 3, 937–945. [Google Scholar] [CrossRef]
- Braxton, L.B.; Richburg, J.S.; York, A.C.; Culpepper, A.S.; Haygood, R.A.; Lovelace, M.L.; Perry, D.H.; Walton, L.C. Resistance of EnlistTM (AAD-12) Cotton to Glufosinate. Weed Technol. 2017, 31, 380–386. [Google Scholar] [CrossRef]
- Prakash, N.R.; Chaudhary, J.R.; Tripathi, A.; Joshi, N.; Padhan, B.K.; Yadav, S.; Kumar, S.; Kumar, R. Breeding for Herbicide Tolerance in Crops: A Review. Res. J. Biotechnol. 2020, 15, 154–162. Available online: https://worldresearchersassociations.com/Archives/RJBT/Vol(15)2020/April2020.aspx (accessed on 19 August 2024).
- Green, J.M.; Owen, M.D.K. Herbicide-Resistant Crops: Utilities and Limitations for Herbicide-Resistant Weed Management. J. Agric. Food Chem. 2011, 59, 5819–5829. [Google Scholar] [CrossRef]
- George, D.G. Engineering Insect-Resistant Crops: A Review. Afr. J. Biotechnol. 2013, 12. Available online: https://www.ajol.info/index.php/ajb/article/view/132064 (accessed on 19 August 2024).
- Pardo-López, L.; Muñoz-Garay, C.; Porta, H.; Rodríguez-Almazán, C.; Soberón, M.; Bravo, A. Strategies to Improve the Insecticidal Activity of Cry Toxins from Bacillus Thuringiensis. Peptides 2009, 30, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Tabashnik, B.E.; Van Rensburg, J.B.J.; Carrière, Y. Field-Evolved Insect Resistance to Bt Crops: Definition, Theory, and Data. J. Econ. Entomol. 2009, 102, 2011–2025. [Google Scholar] [CrossRef] [PubMed]
- Estruch, J.J.; Warren, G.W.; Mullins, M.A.; Nye, G.J.; Craig, J.A.; Koziel, M.G. Vip3A, a Novel Bacillus Thuringiensis Vegetative Insecticidal Protein with a Wide Spectrum of Activities against Lepidopteran Insects. Proc. Natl. Acad. Sci. USA 1996, 93, 5389–5394. [Google Scholar] [CrossRef] [PubMed]
- Talakayala, A.; Katta, S.; Garladinne, M. Genetic Engineering of Crops for Insect Resistance: An Overview. J. Biosci. 2020, 45, 114. Available online: https://www.ias.ac.in/article/fulltext/jbsc/045//0114 (accessed on 19 August 2024). [CrossRef]
- Shen, C.-H.; Tang, M.; Li, X.-F.; Zhu, L.; Li, W.; Deng, P.; Zhai, Q.; Wu, G.; Yan, X.-H. Evaluation of Reference Genes for Quantitative Expression Analysis in Mylabris Sibirica (Coleoptera, Meloidae). Front. Physiol. 2024, 15, 1345836. [Google Scholar] [CrossRef]
- Knispel, A.L.; McLachlan, S.M.; Acker, R.C.V.; Friesen, L.F. Gene Flow and Multiple Herbicide Resistance in Escaped Canola Populations. Weed Sci. 2008, 56, 72–80. [Google Scholar] [CrossRef]
- Kawata, M.; Murakami, K.; Ishikawa, T. Dispersal and Persistence of Genetically Modified Oilseed Rape around Japanese Harbors. Environ. Sci. Pollut. Res. Int. 2009, 16, 120–126. [Google Scholar] [CrossRef]
- Andow, D.A.; Zwahlen, C. Assessing Environmental Risks of Transgenic Plants. Ecol. Lett. 2006, 9, 196–214. [Google Scholar] [CrossRef]
- Schütte, G.; Eckerstorfer, M.; Rastelli, V.; Reichenbecher, W.; Restrepo-Vassalli, S.; Ruohonen-Lehto, M.; Saucy, A.-G.W.; Mertens, M. Herbicide Resistance and Biodiversity: Agronomic and Environmental Aspects of Genetically Modified Herbicide-Resistant Plants. Environ. Sci. Eur. 2017, 29, 5. [Google Scholar] [CrossRef]
- Convention on Biological Diversity|JNCC–Adviser to Government on Nature Conservation. Available online: https://jncc.gov.uk/our-work/convention-on-biological-diversity/ (accessed on 19 August 2024).
- Unit, B. Handling, Transport, Packaging and Identification. Available online: https://bch.cbd.int/protocol/cpb_art18.shtml (accessed on 19 August 2024).
- Kim, I.R.; Lim, H.S.; Choi, W.; Kang, D.I.; Lee, S.Y.; Lee, J.R. Monitoring Living Modified Canola Using an Efficient Multiplex PCR Assay in Natural Environments in South Korea. Appl. Sci. 2020, 10, 7721. [Google Scholar] [CrossRef]
- Kim, E.-S. Technocratic Precautionary Principle: Korean Risk Governance of Genetically Modified Organisms. New Genet. Soc. 2014, 33, 204–224. [Google Scholar] [CrossRef]
- Korea Biosafety Cleaning House. Available online: https://www.biosafety.or.kr/ (accessed on 19 August 2024).
- Lim, Y.; Yook, M.J.; Zhang, C.J.; Nah, G.; Park, S.; Kim, D.S. Dormancy Associated Weedy Risk of the F1 Hybrid Resulted from Gene Flow from Oilseed Rape to Mustard. Weed Turfgrass Sci. 2015, 4, 35–43. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Zhang, X.; Gill, R.A.; Hu, M.; Bai, Z.; Zhao, C.; Zhang, Y.; Liu, Y.; Hu, Q.; et al. Functional and Evolutionary Study of MLO Gene Family in the Regulation of Sclerotinia Stem Rot Resistance in Brassica Napus, L. Biotechnol. Biofuels Bioprod. 2023, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Friedt, W.; Tu, J.; Fu, T. Academic and Economic Importance of Brassica Napus Rapeseed. In The Brassica napus Genome; Liu, S., Snowdon, R., Chalhoub, B., Eds.; Springer International Publishing: Cham, Germany, 2018; pp. 1–20. ISBN 978-3-319-43694-4. [Google Scholar]
- Woodfield, H.K.; Harwood, J.L. Oilseed Crops: Linseed, Rapeseed, Soybean, and Sunflower. In Encyclopedia of Applied Plant Sciences, 2nd ed.; Thomas, B., Murray, B.G., Murphy, D.J., Eds.; Academic Press: Oxford, UK, 2017; pp. 34–38. ISBN 978-0-12-394808-3. [Google Scholar]
- Howell, E.C.; Kearsey, M.J.; Jones, G.H.; King, G.J.; Armstrong, S.J. A and C Genome Distinction and Chromosome Identification in Brassica Napus by Sequential Fluorescence in Situ Hybridization and Genomic in Situ Hybridization. Genetics 2008, 180, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.-K.; Chen, H.-W.; Tseng, K.-Y.; Lin, Y.-C.; Kuo, B.-J. Morphological and Genetic Characteristics of F1 Hybrids Introgressed from Brassica Napus to B. Rapa in Taiwan. Bot. Stud. 2020, 61, 1. [Google Scholar] [CrossRef]
- Kwon, H.-Y.; Choi, S.-I.; Park, H.-I.; Choi, S.-H.; Sim, W.-S.; Yeo, J.-H.; Cho, J.-H.; Lee, O.-H. Comparative Analysis of the Nutritional Components and Antioxidant Activities of Different Brassica Juncea Cultivars. Foods 2020, 9, 840. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.; Hur, O.; Kang, M.-J.; Ro, N.; Hyun, D.-Y.; Cho, G.-T.; Choi, Y.-M.; Gwag, J.; Baek, H.-J.; Kim, C.-Y. Collect. Charact. Leaf Mustard (Brassica Juncea) Korea Poster Board #429. 2012. Available online: https://cdn.ymaws.com/ashs.org/resource/resmgr/files/2012ashsfullconfprogram.pdf (accessed on 19 August 2024).
- Kwon, D.-E.; Kim, K.-S.; Hwang, E.-J.; Park, J.-C.; Lee, J.-E.; Lee, Y.-H. Changes in Growth and Productivity Characteristics by Sowing Date on Spring Sowing Rapeseed (Brassica napus L.) in Paddy Field of Southern Region of South Korea. Korean J. Crop Sci. 2021, 66, 80–86. [Google Scholar] [CrossRef]
- Amro, A.M. Pollinators and Pollination Effects on Three Canola (Brassica Napus, L.) Cultivars: A Case Study in Upper Egypt. J. King Saud Univ. Sci. 2021, 33, 101240. [Google Scholar] [CrossRef]
- Stewart, A. A Review of Brassica Species, Cross-Pollination and Implications for Pure Seed Production in New Zealand. 2002. Available online: https://www.agronomysociety.org.nz/uploads/94803/files/2002_9._Review_-_Brassica_cross-pollination.pdf (accessed on 19 August 2024).
- Salisbury, P.A. The Myths of Gene Transfer—A Canola Case Study. PlantProtection Q. 2000, 15, 2. Available online: https://caws.org.nz/PPQ131415/PPQ%2015-2%20pp071-76%20Salisbury.pdf (accessed on 19 August 2024).
- Légère, A. Risks and Consequences of Gene Flow from Herbicide-Resistant Crops: Canola (Brassica Napus L) as a Case Study. Pest Manag. Sci. 2005, 61, 292–300. [Google Scholar] [CrossRef]
- Damgaard, C.; Kjellsson, G. Gene Flow of Oilseed Rape (Brassica Napus) According to Isolation Distance and Buffer Zone. Agric. Ecosyst. Environ. 2005, 108, 291–301. [Google Scholar] [CrossRef]
- Aono, M.; Wakiyama, S.; Nagatsu, M.; Nakajima, N.; Tamaoki, M.; Kubo, A.; Saji, H. Detection of Feral Transgenic Oilseed Rape with Multiple-Herbicide Resistance in Japan. Env. Biosaf. Res 2006, 5, 77–87. [Google Scholar] [CrossRef] [PubMed]
- The Biology of Brassica Napus, L. (Canola). Department of Health and Ageing Office of the Gene Technology Regulator. Available online: https://bangladeshbiosafety.org/wp-content/uploads/2017/06/Biology_of_Canola_Au.pdf (accessed on 19 August 2024).
- Spontaneous Hybridization between Oilseed Rape (Brassica napus) and Weedy Relatives | International Society for Horticul-tural Science. Available online: http://www.actahort.org/books/407/407_23.htm (accessed on 19 August 2024).
- Lim, H.S.; Kim, I.R.; Lee, S.; Choi, W.; Yoon, A.-M.; Lee, J.R. Establishment and Application of a Monitoring Strategy for Living Modified Cotton in Natural Environments in South Korea. Appl. Sci. 2021, 11, 10259. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, I.R.; Lim, H.S.; Choi, W.; Lee, J.R. Development of a Multiplex PCR Assay to Monitor Living Modified Cottons in South Korea. Appl. Sci. 2019, 9, 2688. [Google Scholar] [CrossRef]
- Lim, H.S.; Yoon, A.-M.; Kim, I.R.; Choi, W.; Jung, Y.J.; Lee, S.; Lee, J.R. Effectiveness of a Priority Management Scheme of Living Modified Organism Re-Collection Areas in Natural Environments of South Korea. Appl. Sci. 2023, 13, 7185. [Google Scholar] [CrossRef]
- Hall, T. Bioedit: A User-Friendly Biological Sequence Alignment Editor and Analysis Program for Windows 95/98/ NT. Available online: https://www.semanticscholar.org/paper/BIOEDIT%3A-A-USER-FRIENDLY-BIOLOGICAL-SEQUENCE-EDITOR-Hall/0ae262d9cf78536754bc064e07113ab5e978f208 (accessed on 19 August 2024).
- Topas 19-2 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/1021/Topas%2019-2 (accessed on 19 August 2024).
- Rf3 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/22/Rf3 (accessed on 19 August 2024).
- MON 88302 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/1132/MON%2088302 (accessed on 19 August 2024).
- Seol, M.-A.; Jo, B.-H.; Choi, W.; Shin, S.Y.; Eum, S.-J.; Kim, I.R.; Song, H.-R.; Lee, J.R. Development of Detection Methods for Six Approved LM Crops in Korea. J. Plant Biotechnol. 2017, 44, 97–106. [Google Scholar] [CrossRef]
- Ms8 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/21/Ms8 (accessed on 19 August 2024).
- GT73 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/24/GT73 (accessed on 19 August 2024).
- Gmomethods | Eurl Gmff. Available online: https://gmo-crl.jrc.ec.europa.eu/gmomethods/entry?db=gmometh&nr=96&rq=ac%3aDP-073496-4 (accessed on 19 August 2024).
- Jo, B.-H.; Lee, J.; Choi, W.; Moon, J.; Shin, S.; Eum, S.J.; Seol, M.-A.; Kim, I.; Song, H.-R. Development of Multiplex PCR-Based Detection Method for Five Approved LM Canola Events in Korea. J. Plant Biotechnol. 2015, 42, 117–122. [Google Scholar] [CrossRef]
- Ms11 Documents | European Union Reference Laboratory for Genetically Modified Food and Feed (EURL GMFF). Available online: https://gmo-crl.jrc.ec.europa.eu/method-validation/details/all/2027/Ms11 (accessed on 19 August 2024).
- Kim, Y.-C.; Kim, A.; Lim, J.; Kim, T.-S.; Park, S.-G.; Kim, M.; Lee, J.-H.; Lee, J.R.; Lee, D.-H. Distribution and Management of Nutria (Myocastor Coypus) Populations in South Korea. Sustainability 2019, 11, 4169. [Google Scholar] [CrossRef]
- Umurzokov, M.; Jia, W.; Cho, K.M.; Khaitov, B.; Sohn, S.I.; Cho, J.W.; Park, K.W. Persistence, Viability and Emergence Rate of Canola (Brassica Napus L) in Korean Soil. WeedTurfgrass Sci. 2019, 8, 309–318. [Google Scholar] [CrossRef]
- Sparrow, S.D.; Knight, C.W.; Conn, J.S. Canola Seed Survival over Winter in the Field in Alaska. Can. J. Plant Sci. 1990, 70, 799–807. [Google Scholar] [CrossRef]
- Simard, M.-J.; Légère, A.; Pageau, D.; Lajeunesse, J.; Warwick, S. The Frequency and Persistence of Volunteer Canola (Brassica Napus) in Québec Cropping Systems. Weed Technol. 2002, 16, 433–439. [Google Scholar] [CrossRef]
- Belter, A. Long-Term Monitoring of Field Trial Sites with Genetically Modified Oilseed Rape (Brassica Napus, L.) in Saxony-Anhalt, Germany. Fifteen Years Persistence to Date but No Spatial Dispersion. Genes 2016, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Messéan, A.; Sausse, C.; Gasquez, J.; Darmency, H. Occurrence of Genetically Modified Oilseed Rape Seeds in the Harvest of Subsequent Conventional Oilseed Rape over Time. Eur. J. Agron. 2007, 27, 115–122. [Google Scholar] [CrossRef]
- D’Hertefeldt, T.; Jørgensen, R.B.; Pettersson, L.B. Long-Term Persistence of GM Oilseed Rape in the Seedbank. Biol. Lett. 2008, 4, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Assefa, Y.; Prasad, P.V.V.; Foster, C.; Wright, Y.; Young, S.; Bradley, P.; Stamm, M.; Ciampitti, I.A. Major Management Factors Determining Spring and Winter Canola Yield in North America. Crop Sci. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Spring vs. Winter Canola Phenology across Australia: New Insights for WA Growers–GRDC. Available online: https://grdc.com.au/resources-and-publications/grdc-update-papers/tab-content/grdc-update-papers/2022/03/spring-vs-winter-canola-phenology-across-australia-new-insights-for-wa-growers (accessed on 19 August 2024).
- Fiebelkorn, D.; Rahman, M. Development of a Protocol for Frost-Tolerance Evaluation in Rapeseed/Canola (Brassica napus, L.). Crop J. 2016, 4, 147–152. [Google Scholar] [CrossRef]


| 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | 2021 | 2022 | 2023 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Re-collection sites a | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 4 | 1 | 3 | 3 | 4 | 7 | 24 |
| LM canola b | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 28 | 31 | 23 | 7 | 63 | 67 | 243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.S.; Choi, W.; Jung, Y.J.; Yoon, A.-M.; Noh, D.; Lee, J.H.; Kim, C.M.; Lee, J.R. Long-Term Monitoring and Management of Genetically Modified Canola in Natural Environments: A 15-Year Study. Appl. Sci. 2024, 14, 8333. https://doi.org/10.3390/app14188333
Lim HS, Choi W, Jung YJ, Yoon A-M, Noh D, Lee JH, Kim CM, Lee JR. Long-Term Monitoring and Management of Genetically Modified Canola in Natural Environments: A 15-Year Study. Applied Sciences. 2024; 14(18):8333. https://doi.org/10.3390/app14188333
Chicago/Turabian StyleLim, Hye Song, Wonkyun Choi, Young Jun Jung, A-Mi Yoon, Donghyeon Noh, Jeong Hwan Lee, Chul Min Kim, and Jung Ro Lee. 2024. "Long-Term Monitoring and Management of Genetically Modified Canola in Natural Environments: A 15-Year Study" Applied Sciences 14, no. 18: 8333. https://doi.org/10.3390/app14188333







