Diversity of Host Species and Optimized Cultivation Practices for Enhanced Bioactive Compound Production in Cordyceps militaris
Abstract
:1. Introduction
2. Host Diversity for Cordyceps militaris Cultivation
3. Factors Influencing Yield and Quality
4. Selection of Host Species
4.1. Genetic Variability
4.2. Geographic Origin
4.3. Environmental Conditions
5. Bioactive Compounds in C. militaris
6. Industrial Production of C. militaris
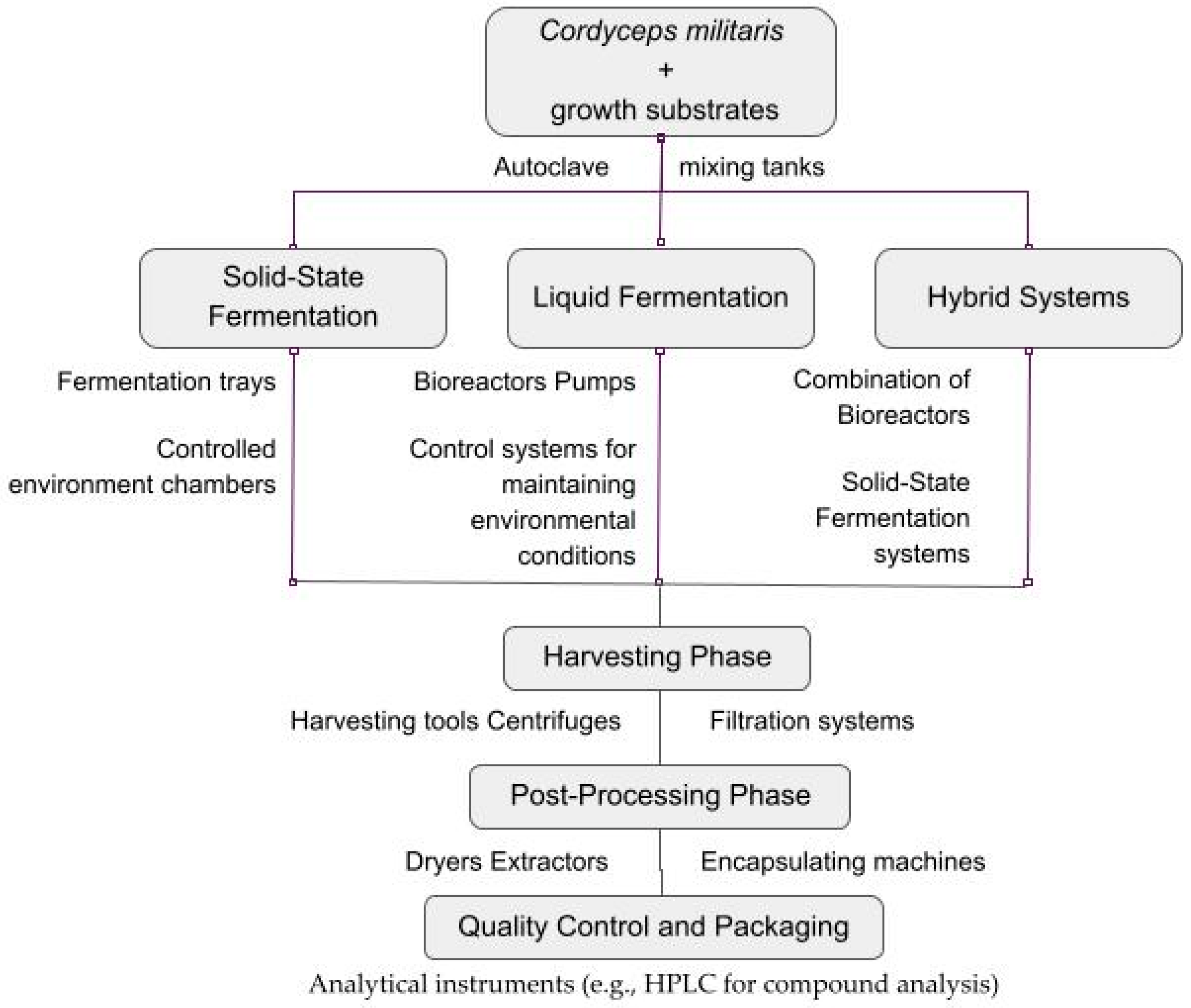
6.1. Overview of Production Methods
6.2. Differences between Natural and Industrial Production of C. militaris
7. Research Prospects
- Substrate Optimization: Utilizing substrates enriched with complex carbohydrates or proteins can enhance the levels of specific metabolites such as polysaccharides and bioactive peptides, crucial for the medicinal properties of C. militaris. Investigating different substrate combinations and their effects on metabolite synthesis can lead to more effective and economical cultivation practices.
- Controlled Environmental Conditions:
- -
- Maintaining temperatures between 20 and 22 °C is essential for optimal fungal growth and metabolite production.
- -
- Controlling humidity and light exposure is critical to optimize fungal metabolism and secondary metabolite synthesis. Specific light regimes can be employed to stimulate the production of targeted bioactive compounds.
- Biotechnological Advances:
- -
- Modifying genetic pathways in C. militaris via genetic engineering can enhance the production of targeted metabolites like cordycepin. This approach can also improve the strain’s resilience to environmental stressors, thereby increasing overall yield.
- -
- Both solid-state and submerged fermentation technologies can be optimized to improve biomass production and consistency of bioactive compounds. Advances in these technologies can also reduce production costs and enhance scalability.
- Bioreactor Utilization: Employing bioreactors in submerged fermentation setups allows for automated and precise monitoring of growth conditions, significantly boosting production efficiency and consistency in bioactive compound profiles. Bioreactors can provide controlled environments conducive to large-scale production, ensuring high-quality outputs.
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paterson, R.R.M. Cordyceps—A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.S.; Leyva-Gómez, G.; Prado-Audelo, M.L.D.; Cortes, H.; Singh, Y.D.; Patra, J.K. Cordyceps spp.: A review on its immune-stimulatory and other biological potentials. Front. Pharmacol. 2021, 11, 602364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Sharma, N.; An, S.S.A. Unique Bioactives from Zombie Fungus (Cordyceps) as Promising Multitargeted Neuroprotective Agents. Nutrients 2023, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Ahmed, M.; Park, H.J. Cordyceps militaris as a Bio Functional Food Source: Pharmacological Potential, Anti-Inflammatory Actions and Related Molecular Mechanisms. Microorganisms 2022, 10, 405. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, Extraction and Biomedical Properties of Polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef]
- Prasain, J.K. Pharmacological effects of Cordyceps and its bioactive compounds. Stud. Nat. Prod. Chem. 2013, 40, 453–468. [Google Scholar]
- Chiriví, J.; Danies, G.; Sierra, R.; Schauer, N.; Trenkamp, S.; Restrepo, S.; Sanjuan, T. Metabolomic profile and nucleoside composition of Cordyceps nidus sp. nov.(Cordycipitaceae): A new source of active compounds. PLoS ONE 2017, 12, e0179428. [Google Scholar] [CrossRef]
- Xiao, J.H.; Xiong, Q. Nucleosides, a valuable chemical marker for quality control in traditional Chinese medicine Cordyceps. Recent Pat. Biotechnol. 2013, 7, 153–166. [Google Scholar] [CrossRef]
- Dutta, D.; Singh, N.S.; Aggarwal, R.; Verma, A.K. Cordyceps militaris: A Comprehensive Study on Laboratory Cultivation and Anti-cancer Potential in Dalton’s Ascites Lymphoma Tumor Model. Anti-Cancer Agents Med. Chem. 2024, 24, 668–690. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Siddiqui, A.J.; Patel, M.; Awadelkareem, A.M.; Snoussi, M.; Hadi, S. Cordycepin for health and wellbeing: A potent bioactive metabolite of an entomopathogenic medicinal fungus Cordyceps with its nutraceutical and therapeutic potential. Molecules 2020, 25, 2735. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, Y.; Liu, C.; Li, H.; Du, X.; Meng, J.; Li, Q. Elucidation of antioxidant activities of intracellular and extracellular polysaccharides from Cordyceps militaris in vitro and their protective effects on ulcerative colitis in vivo. Int. J. Biol. Macromol. 2024, 267, 131385. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Lee, S.; Lee, K.; Shin, Y.S.; Kang, H.; Cho, H. Anti-cancer effect of Cordyceps militaris in human colorectal carcinoma RKO cells via cell cycle arrest and mitochondrial apoptosis. DARU J. Pharm. Sci. 2015, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Olalde, J.; Antoshechkin, A.; del Castillo, O.; Guzmán, R.; Améndola, F. Design and Evaluation of a Complex Phytoceutical Formulation for Circulatory Diseases. In Medical Complications of Type 2 Diabetes; IntechOpen: London, UK, 2011. [Google Scholar]
- Isokauppila, T.; Broida, D.R. Healing Adaptogens: The Definitive Guide to Using Super Herbs and Mushrooms for Your Body’s Restoration, Defense, and Performance; Hay House, Inc.: Carlsbad, CA, USA, 2024. [Google Scholar]
- Ray, P.; Kundu, S.; Paul, D. Exploring the Therapeutic Properties of Chinese Mushrooms with a Focus on their Anti-Cancer Effects: A Systemic review. Pharmacol. Res.-Mod. Chin. Med. 2024, 11, 100433. [Google Scholar] [CrossRef]
- Hobbs, C. The health and clinical benefits of medicinal fungi. In Biochemical Engineering and Biotechnology of Medicinal Mushrooms; Springer International Publishing: Cham, Switzerland, 2023; pp. 285–356. [Google Scholar]
- Vu, T.X.; Tran, T.B.; Vu, H.H.; Le, Y.T.H.; Nguyen, P.H.; Do, T.T.; Tran, V.T. Ethanolic extract from fruiting bodies of Cordyceps militaris HL8 exhibits cytotoxic activities against cancer cells, skin pathogenic yeasts, and postharvest pathogen Penicillium digitatum. Arch. Microbiol. 2024, 206, 97. [Google Scholar] [CrossRef]
- Quy, N.N.; Nhut, P.T.; Nhi, T.T.Y.; Tien, N.M.; Chung, D.D.; Phuong, T.T.M. Effect of Time and Temperature on the Extraction of Cordyceps militaris in Pilot Scale. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1092, 012079. [Google Scholar]
- Minh, T.N.; Anh, L.V.; Trung, N.Q.; Minh, B.Q.; Xuan, T.D. Efficacy of green extracting solvents on antioxidant, xanthine oxidase, and plant inhibitory potentials of solid-based residues (SBRs) of Cordyceps militaris. Stresses 2022, 3, 11–21. [Google Scholar] [CrossRef]
- Quy, T.N.; Xuan, T.D. Xanthine oxidase inhibitory potential, antioxidant and antibacterial activities of Cordyceps militaris (L.) Link fruiting body. Medicines 2019, 6, 20. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Nguyen, V.T.; Nguyen, M.T.; Thanh, L.V.; Phuong, T.T.M.; Duong, D.C. Screening of extraction conditions by Plackett–Burman design for extraction of Cordyceps militaris Cordycipitaceae. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012017. [Google Scholar] [CrossRef]
- Shrestha, B.; Zhang, W.; Zhang, Y.; Liu, X. The medicinal fungus Cordyceps militaris: Research and development. Mycol. Prog. 2012, 11, 599–614. [Google Scholar] [CrossRef]
- Shrestha, B.; Han, S.K.; Sung, J.M.; Sung, G.H. Fruiting body formation of Cordyceps militaris from multi-ascospore isolates and their single ascospore progeny strains. Mycobiology 2012, 40, 100–106. [Google Scholar] [CrossRef]
- López-Rodríguez, L.; Burrola-Aguilar, C.; Estrada-Zúñiga, M.E.; Garibay-Orijel, R.; González-Pedroza, M.G. Characterization of mycelial growth, biomass production, and fruiting bioassays in Cordyceps mexicana. Mycol. Prog. 2023, 22, 68. [Google Scholar] [CrossRef]
- Pradhan, P.; De, J.; Acharya, K. Strategies in Artificial Cultivation of Two Entomopathogenic Fungi Cordyceps militaris and Ophiocordyceps sinensis. In Applied Mycology for Agriculture and Foods; Apple Academic Press: Cambridge, MA, USA, 2024; pp. 315–345. [Google Scholar]
- Xing, P.; Diao, H.; Wang, D.; Zhou, W.; Tian, J.; Ma, R. Identification, Pathogenicity, and Culture Conditions of a New Isolate of Cordyceps javanica (Hypocreales: Cordycipitaceae) from Soil. J. Econ. Entomol. 2023, 116, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Miaomiao, L.; Ruoyao, N.; Guiling, Z.; Yanni, Z.; Peipei, W.; Ruihao, S. Vegetative development and host immune interaction of Ophiocordyceps sinensis within the hemocoel of the ghost moth larva, Thitarodes xiaojinensis. J. Invertebr. Pathol. 2020, 170, 107331. [Google Scholar]
- Elkhateeb, W.A. What medicinal mushroom can do. Chem. Res. J. 2020, 5, 106–118. [Google Scholar]
- Yang, T.; Guo, M.; Yang, H.; Guo, S.; Dong, C. The blue-light receptor CmWC-1 mediates fruit body development and secondary metabolism in Cordyceps militaris. Appl. Microbiol. Biotechnol. 2016, 100, 743–755. [Google Scholar] [CrossRef]
- de Castro, A.S.C.B. Study of Antitumor and Immunomodulatory Activities of Wild Mushroom Extracts. Doctoral Dissertation, Universidade do Minho, Braga, Portugal, 2014. [Google Scholar]
- Powell, M. Medicinal Mushrooms—A Clinical Guide; eBook Partnership: London, UK, 2015. [Google Scholar]
- Deshmukh, L.; Sharma, A.K.; Sandhu, S.S. Contrive Himalayan soft gold Cordyceps species: A lineage of Eumycota bestowing tremendous pharmacological and therapeutic potential. Curr. Pharmacol. Rep. 2020, 6, 155–166. [Google Scholar] [CrossRef]
- Kontogiannatos, D.; Koutrotsios, G.; Xekalaki, S.; Zervakis, G.I. Biomass and cordycepin production by the medicinal mushroom Cordyceps militaris—A review of various aspects and recent trends towards the exploitation of a valuable fungus. J. Fungi 2021, 7, 986. [Google Scholar] [CrossRef]
- Li, W.; Zou, G.; Bao, D.; Wu, Y. Current Advances in the Functional Genes of Edible and Medicinal Fungi: Research Techniques, Functional Analysis, and Prospects. J. Fungi 2024, 10, 311. [Google Scholar] [CrossRef]
- Thomas, L.; Mago, P. Unearthing the therapeutic benefits of culinary-medicinal mushrooms for humans: Emerging sustainable bioresources of 21st century. J. Basic Microbiol. 2024, 64, e2400127. [Google Scholar] [CrossRef]
- Krishna, K.V.; Balasubramanian, B.; Park, S.; Bhattacharya, S.; Kadanthottu Sebastian, J.; Liu, W.C.; Malaviya, A. Conservation of Endangered Cordyceps sinensis through Artificial Cultivation Strategies of Cordyceps militaris, an Alternate. Mol. Biotechnol. 2024, 1–16. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef] [PubMed]
- Bode, H.B. Insect-associated microorganisms as a source for novel secondary metabolites with therapeutic potential. In Insect Biotechnology; Vilcinskas, A., Ed.; Biologically-Inspired Systems; Springer: Dordrecht, 2011; Volume 2, pp. 77–93. [Google Scholar]
- Chaubey, R.; Singh, J.; Baig, M.M.; Kumar, A. Recent advancement and the way forward for Cordyceps. In Recent Advancement in White Biotechnology Through Fungi; Yadav, A., Singh, S., Mishra, S., Gupta, A., Eds.; Fungal Biology; Springer: Cham, Switzerland, 2019; pp. 441–474. [Google Scholar]
- Xie, B.; Zhu, Y.; Chu, X.; Pokharel, S.S.; Qian, L.; Chen, F. Research Progress and Production Status of Edible Insects as Food in China. Foods 2024, 13, 1986. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, S.L.; Chen, H.Y.; Zou, Y.; Zheng, Q.W.; Guo, L.Q.; Wu, G.H.; Lu, J.; Lin, J.F.; Ye, Z.W. Enhancement of carotenoid production and its regulation in edible mushroom Cordyceps militaris by abiotic stresses. Enzyme Microb. Technol. 2021, 148, 109808. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Luo, L.; Zhang, L. A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo. Molecules 2023, 28, 3867. [Google Scholar] [CrossRef] [PubMed]
- Baral, B. Entomopathogenicity and Biological Attributes of Himalayan Treasured Fungus Ophiocordyceps sinensis (Yarsagumba). J. Fungi 2017, 3, 4. [Google Scholar] [CrossRef]
- Raethong, N.; Laoteng, K.; Vongsangnak, W. Uncovering global metabolic response to cordycepin production in Cordyceps militaris through transcriptome and genome-scale network-driven analysis. Sci. Rep. 2018, 8, 9250. [Google Scholar] [CrossRef]
- Sripilai, K.; Chaicharoenaudomrung, N.; Phonchai, R.; Chueaphromsri, P.; Kunhorm, P.; Noisa, P. Development of an animal-free nitrogen source for the liquid surface culture of Cordyceps militaris. Lett. Appl. Microbiol. 2023, 76, ovad053. [Google Scholar] [CrossRef]
- Choi, J.; Paje, L.A.; Kwon, B.; Noh, J.; Lee, S. Quantitative analysis of cordycepin in Cordyceps militaris under different extraction methods. J. Appl. Biol. Chem. 2021, 64, 153–158. [Google Scholar] [CrossRef]
- Lee, H.H.; Kang, N.; Park, I.; Park, J.; Kim, I.; Kim, J.; Seo, Y.S. Characterization of newly bred Cordyceps militaris strains for higher production of cordycepin through HPLC and URP-PCR analysis. J. Microbiol. Biotechnol. 2017, 27, 1223–1232. [Google Scholar] [CrossRef]
- Xie, C.Y.; Gu, Z.X.; Fan, G.J.; Gu, F.R.; Han, Y.B.; Chen, Z.G. Production of cordycepin and mycelia by submerged fermentation of Cordyceps militaris in mixture natural culture. Appl. Biochem. Biotechnol. 2009, 158, 483–492. [Google Scholar] [CrossRef]
- Liu, W.; Dun, M.; Liu, X.; Zhang, G.; Ling, J. Effects on total phenolic and flavonoid content, antioxidant properties, and angiotensin I-converting enzyme inhibitory activity of beans by solid-state fermentation with Cordyceps militaris. Int. J. Food Prop. 2022, 25, 477–491. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, Y.; Song, J.; Ma, M.; Xiao, Y.; Zeng, B. Advancing Cordyceps militaris Industry: Gene Manipulation and Sustainable Biotechnological Strategies. Bioengineering 2024, 11, 783. [Google Scholar] [CrossRef] [PubMed]
- Bawadekji, A.; Al Ali, K.; Al Ali, M. A review of the bioactive compound and medicinal value of Cordyceps militaris. AlShamal Basic Appl. Sci. 2016, 1, 69–76. [Google Scholar] [CrossRef]
- Pan, Z. Research advancement of insect origin fungus Cordyceps. Trends Insect Mol. Biol. Biotechnol; Kumar, D., Gong, C., Eds.; Springer: Cham, Switzerland, 2018; pp. 253–282. [Google Scholar] [CrossRef]
- Kryukov, V.Y.; Yaroslavtseva, O.N.; Dubovskiy, I.M.; Tyurin, M.V.; Kryukova, N.A.; Glupov, V.V. Insecticidal and immunosuppressive effect of ascomycete Cordyceps militaris on the larvae of the Colorado potato beetle Leptinotarsa decemlineata. Biol. Bull. 2014, 41, 276–283. [Google Scholar] [CrossRef]
- Da Silva, J.M.X. Using Different Cereals to Cultivate Cordyceps militaris; Student’s Report (B.S.)-Assumption University: Bangkok, Thailand, 2019. [Google Scholar]
- Raethong, N.; Wang, H.; Nielsen, J.; Vongsangnak, W. Optimizing cultivation of Cordyceps militaris for fast growth and cordycepin overproduction using rational design of synthetic media. Comput. Struct. Biotechnol. J. 2020, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cai, G.; He, Y.I.; Tong, G. Separation of cordycepin from Cordyceps militaris fermentation supernatant using preparative HPLC and evaluation of its antibacterial activity as an NAD+-dependent DNA ligase inhibitor. Exp. Ther. Med. 2016, 12, 1812–1816. [Google Scholar] [CrossRef]
- Masuda, M.; Urabe, E.; Sakurai, A.; Sakakibara, M. Production of cordycepin by surface culture using the medicinal mushroom Cordyceps militaris. Enzyme Microb. Technol. 2006, 39, 641–646. [Google Scholar] [CrossRef]
- Jiaojiao, Z.; Fen, W.; Kuanbo, L.; Qing, L.; Ying, Y.; Caihong, D. Heat and light stresses affect metabolite production in the fruit body of the medicinal mushroom Cordyceps militaris. Appl. Microbiol. Biotechnol. 2018, 102, 4523–4533. [Google Scholar] [CrossRef]
- Zeng, Z.; Mou, D.; Luo, L.; Zhong, W.; Duan, L.; Zou, X. Different cultivation environments affect the yield, bacterial community and metabolites of Cordyceps cicadae. Front. Microbiol. 2021, 12, 669785. [Google Scholar] [CrossRef]
- Fuller, K.K.; Loros, J.J.; Dunlap, J.C. Fungal photobiology: Visible light as a signal for stress, space and time. Curr. Genet. 2015, 61, 275–288. [Google Scholar] [CrossRef]
- Soommat, P.; Raethong, N.; Ruengsang, R.; Thananusak, R.; Laomettachit, T.; Laoteng, K.; Saithong, T.; Vongsangnak, W. Light-exposed metabolic responses of Cordyceps militaris through transcriptome-integrated genome-scale modeling. Biology 2024, 13, 139. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.; Yamamoto, K.I.; An, Y.; Saeki, J.; Itagaki, T.; Kofujita, H.; Suzuki, K. CO2 anesthesia enhances infection rate of Cordyceps militaris (Hypocreales: Clavicipitaceae) on pupae of the silkworm, Bombyx mori. J. Insect Biotechnol. Seric. 2014, 83, 77–81. [Google Scholar]
- Lin, J.Y.; Tsai, H.L.; Sang, W.C. Implementation and performance evaluation of integrated wireless multisensor module for aseptic incubator of Cordyceps militaris. Sensors 2020, 20, 4272. [Google Scholar] [CrossRef]
- Massonnet, M.; Morales-Cruz, A.; Minio, A.; Figueroa-Balderas, R.; Lawrence, D.P.; Travadon, R.; Cantu, D. Whole-genome resequencing and pan-transcriptome reconstruction highlight the impact of genomic structural variation on secondary metabolite gene clusters in the grapevine esca pathogen Phaeoacremonium minimum. Front. Microbiol. 2018, 9, 354712. [Google Scholar] [CrossRef]
- Zeb, U.; Aziz, T.; Azizullah, A.; Zan, X.Y.; Khan, A.A.; Bacha, S.A.S.; Cui, F.J. Complete mitochondrial genomes of edible mushrooms: Features, evolution, and phylogeny. Physiol. Plant. 2024, 176, e14363. [Google Scholar] [CrossRef]
- Liu, Q.; Meng, G.; Wang, M.; Li, X.; Liu, M.; Wang, F.; Dong, C. Safe-harbor-targeted CRISPR/Cas9 system and Cmhyd1 overexpression enhances disease resistance in Cordyceps militaris. J. Agric. Food Chem. 2023, 71, 15249–15260. [Google Scholar] [CrossRef]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese Cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef]
- Chai, L.; Li, J.; Guo, L.; Zhang, S.; Chen, F.; Zhu, W.; Li, Y. Genomic and Transcriptome Analysis Reveals the Biosynthesis Network of Cordycepin in Cordyceps militaris. Genes 2024, 15, 626. [Google Scholar] [CrossRef]
- Gani, M.; Hassan, T.; Saini, P.; Gupta, R.K.; Bali, K. Molecular phylogeny of entomopathogens. Microbes Sustain. Insect Pest Manag. Eco-Friendly Approach 2019, 1, 43–113. [Google Scholar]
- Lusakunwiwat, P.; Thananusak, R.; Nopgason, R.; Laoteng, K.; Vongsangnak, W. Holistic transcriptional responses of Cordyceps militaris to different culture temperatures. Gene 2024, 923, 148574. [Google Scholar] [CrossRef]
- Wichadakul, D.; Kobmoo, N.; Ingsriswang, S.; Tangphatsornruang, S.; Chantasingh, D.; Luangsa-Ard, J.J.; Eurwilaichitr, L. Insights from the genome of Ophio Cordyceps polyrhachis-furcata to pathogenicity and host specificity in insect fungi. BMC Genom. 2015, 16, 881. [Google Scholar] [CrossRef] [PubMed]
- Woodall, H.; Bullock, J.M.; White, S.M. Modelling the harvest of an insect pathogen. Ecol. Modell. 2014, 287, 16–26. [Google Scholar] [CrossRef]
- Nahas, H.H.A.; Abdel-Rahman, M.A.; Gupta, V.K.; Abdel-Azeem, A.M. Myco-antioxidants: Insights into the natural metabolic treasure and their biological effects. Sydowia 2023, 75, 151. [Google Scholar]
- Pintathong, P.; Chomnunti, P.; Sangthong, S.; Jirarat, A.; Chaiwut, P. The feasibility of utilizing cultured Cordyceps militaris residues in cosmetics: Biological activity assessment of their crude extracts. J. Fungi 2021, 7, 973. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Q.; Huang, S.; Chen, J. Economic analysis of using diverse hosts in Cordyceps militaris production. Fungal Econ. Rev. 2017, 9, 130–142. [Google Scholar]
- Jiang, Y.; Li, S.; Li, X. Quality control issues in the commercial cultivation of Cordyceps militaris. Mycologia 2016, 108, 415–424. [Google Scholar]
- Zhang, Y.; Li, E.; Wang, C.; Li, Y.; Liu, X. Comparative study of Cordyceps militaris cultivated on different hosts and its effect on yield. Mycobiology 2014, 42, 215–220. [Google Scholar]
- Kim, S.W.; Lee, K.R.; Park, Y.J. Cultivation of Cordyceps militaris on grain substrates and artificial media: A comparative study. J. Ind. Microbiol. Biotechnol. 2018, 45, 573–582. [Google Scholar]
- Chen, S.; Zhang, Y.; Zhang, W. Utilizing plant-based substrates for the cultivation of Cordyceps militaris: Implications for fungal yield and commercial production. J. Agric. Food Chem. 2019, 67, 2541–2549. [Google Scholar]
- Li, H.; Zhou, X.; Li, L.; Chen, J. The influence of host species on cordycepin and polysaccharide content in Cordyceps militaris fruiting bodies. J. Fungal Res. 2016, 14, 195–202. [Google Scholar]
- Van Doan, H.; Hoseinifar, S.H.; Tapingkae, W.; Chitmanat, C.; Mekchay, S. Effects of Cordyceps militaris spent mushroom substrate on mucosal and serum immune parameters, disease resistance and growth performance of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2017, 67, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Q.; He, X.; Wang, Y.; Zhu, G.; Yu, L. Antimicrobial, antioxidant, anti-inflammatory, and cytotoxic activities of Cordyceps militaris spent substrate. PLoS ONE 2023, 18, e0291363. [Google Scholar] [CrossRef]
- Wu, T.F.; Chan, Y.Y.; Shi, W.Y.; Jhong, M.T. Uncovering the molecular mechanism of anti-allergic activity of silkworm pupa-grown Cordyceps militaris fruit body. Am. J. Chin. Med. 2017, 45, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ćirić, A.; Van Griensven, L.J.; Soković, M.; Ferreira, I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef]
- Kała, K.; Jędrejko, K.; Sułkowska-Ziaja, K.; Muszyńska, B. Cordyceps militaris and its Applications. In Bioprospects of Macrofungi; CRC Press: Boca Raton, FL, USA, 2023; pp. 345–368. [Google Scholar]
- Chen, B.X.; Xue, L.N.; Wei, T.; Ye, Z.W.; Li, X.H.; Guo, L.Q.; Lin, J.F. Enhancement of ergothioneine production by discovering and regulating its metabolic pathway in Cordyceps militaris. Microb. Cell Fact. 2022, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Kim, G.Y.; Choi, Y.H. Induction of apoptosis by aqueous extract of Cordyceps militaris through activation of caspases and inactivation of Akt in human breast cancer MDA-MB-231 Cells. J. Microbiol. Biotechnol. 2008, 18, 1997–2003. [Google Scholar]
- Xu, L.; Wang, F.; Zhang, Z.; Terry, N. Optimization of polysaccharide production from Cordyceps militaris by solid-state fermentation on rice and its antioxidant activities. Foods 2019, 8, 590. [Google Scholar] [CrossRef]
- Jiapeng, T.; Yiting, L.; Li, Z. Optimization of fermentation conditions and purification of cordycepin from Cordyceps militaris. Prep. Biochem. Biotechnol. 2014, 44, 90–106. [Google Scholar] [CrossRef]
- Wen, T.C.; Li, G.R.; Kang, J.C.; Kang, C.; Hyde, K.D. Optimization of solid-state fermentation for fruiting body growth and cordycepin production by Cordyceps militaris. Chiang Mai J. Sci. 2014, 41, 858–872. [Google Scholar]
- Cui, J.D. Biotechnological production and applications of Cordyceps militaris, a valued traditional Chinese medicine. Crit. Rev. Biotechnol. 2015, 35, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Holliday, J. Cordyceps: A highly coveted medicinal mushroom. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Berlin/Heidelberg, Germany, 2017; pp. 59–91. [Google Scholar]
- Bach, M.X.; Minh, T.N.; Anh, D.T.N.; Anh, H.N.; Anh, L.V.; Trung, N.Q.; Xuan, T.D. Protection and Rehabilitation Effects of In Cordyceps militaris Fruit Body Extract and Possible Roles of Cordycepin and Adenosine. Compounds 2022, 2, 388–403. [Google Scholar] [CrossRef]
- Adnan, M.; Ashraf, S.A.; Khan, S.; Alshammari, E.; Awadelkareem, A.M. Effect of pH, temperature and incubation time on cordycepin production from Cordyceps militaris using solid-state fermentation on various substrates. CyTA J. Food 2017, 15, 617–621. [Google Scholar] [CrossRef]
- Trung, N.Q.; Dat, N.T.; Anh, H.N.; Tung, Q.N.; Nguyen, V.T.H.; Van, H.N.B.; Van, N.M.N.; Minh, T.N. Substrate Influence on Enzymatic Activity in Cordyceps militaris for Health Applications. Chemistry 2024, 6, 517–530. [Google Scholar] [CrossRef]
- Sitara, U.; Baloch, P.A.; Pathan, A.U.K.; Bhatti, M.I. Optimization and effect of different grain sources on production of liquid spawn and fruiting body of Cordyceps militaris. Int. J. Agric. Biol. 2022, 28, 67–74. [Google Scholar]



| Host Type | Impact on Fungal Growth and Bioactive Compounds | Ref. |
|---|---|---|
| Lepidoptera (e.g., ghost moth) | Rich in lipids and proteins, conducive to substantial fungal growth and high levels of cordycepin | [33] |
| Coleoptera (e.g., beetles) | Chitinous exoskeleton aids in producing chitinase, affecting anti-inflammatory and anti-tumor activities | [34] |
| Hymenoptera (e.g., wasps, bees) | Supplies unique fatty acids and sterols, enhancing pharmacological value | [35] |
| Grains (e.g., rice, wheat) | More sustainable and consistent but yields lower levels of key metabolites like cordycepin compared to natural hosts | [36] |
| Synthetic media | Tailored with specific nutrients to control growth conditions, enhancing understanding of metabolic pathways | [37] |
| Host Species | Yield of Cordycepin (mg/g Dry Weight) | Bioactive Compounds | Ref. |
|---|---|---|---|
| Bombyx mori Pupae (silkworm pupae) | 4.37 ± 2.32 | Cordycepin, Polysaccharides, Adenosine | [44] |
| Brown rice | 2.89 ± 1.99 | Primarily Polysaccharides | [44] |
| Brown rice paste, beerwort, and soybean meal juice | 2.17 ± 0.09 | - | [45] |
| Soybean | 8.33 ± 0.44 | Cordycepin | [46] |
| Chickpea | 11.12 ± 0.76 | - | [46] |
| Black bean | 10.43 ± 0.37 | - | [46] |
| Mung bean | 6.64 ± 0.14 | - | [46] |
| Potato Dextrose | 1.16 ± 1.23 | Low Levels of Cordycepin | [44] |
| Wheat standard substrate and pupal (Bombyx mori Pupae) injection | ~1.2 | - | [47] |
| Generation of ΔMAT1-1-2; injection of 107 ΔMAT1-1-2xΜAΤ1-2 spores/mL into the Chinese Tussah Silkworm pupae | Up to 16.77 | - | [48] |
| Cicada Larvae | - | Cordycepin, peptides, mannitol | [49] |
| Pupa Substrate | - | Cordycepin, polysaccharides, vitreoscilla hemoglobin | [47] |
| Beetles | - | Lower levels of cordycepin, ergosterol | [50] |
| Soybean Powder | - | Cordycepin | [46] |
| Wheat Bran | - | Moderate levels of cordycepin, various vitamins | [51] |
| Potato Dextrose | - | Low levels of cordycepin | [44] |
| Synthetic Media | - | Designed to mimic natural substrates, varies based on formulation | [52] |
| Bioactive Compound | Influential Factor | Effects | Ref. |
|---|---|---|---|
| Polysaccharides | Cultivation substrate, extraction method | Immunomodulating, antitumor, antioxidant | [8] |
| Beta-glucan | Host species, extraction method | Enhances immune response, lowers cholesterol | [79] |
| Cordycepin | Host species, cultivation conditions | Anti-cancer, anti-inflammatory, potential antiviral | [7] |
| Adenosine | Cultivation methods, enzyme activity | Energy metabolism, neurotransmission, cardiovascular protection | [16] |
| Sterols | Host species, growth stage | Anti-inflammatory, cholesterol management | [65] |
| Saponins | Cultivation substrate | Antifungal, antitumor, immunomodulatory | [79] |
| Triterpenoids | Host species, extraction process | Hepatoprotective, anti-inflammatory, antiviral | [80] |
| Ergosterol | Cultivation environment | Antioxidant, precursor for Vitamin D2 synthesis | [81] |
| Mannitol | Host nutrient availability | Diuretic, free radical scavenging | [81] |
| γ-Aminobutyric acid (GABA) | Fermentation conditions | Reduces anxiety, enhances mood, improves sleep | [82] |
| Ergothioneine | Specific enzymatic pathways | Antioxidant, cellular protector against oxidative stress | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung, N.Q.; Quyen, P.D.T.; Ngoc, N.T.T.; Minh, T.N. Diversity of Host Species and Optimized Cultivation Practices for Enhanced Bioactive Compound Production in Cordyceps militaris. Appl. Sci. 2024, 14, 8418. https://doi.org/10.3390/app14188418
Trung NQ, Quyen PDT, Ngoc NTT, Minh TN. Diversity of Host Species and Optimized Cultivation Practices for Enhanced Bioactive Compound Production in Cordyceps militaris. Applied Sciences. 2024; 14(18):8418. https://doi.org/10.3390/app14188418
Chicago/Turabian StyleTrung, Nguyen Quang, Phan Duong Thuc Quyen, Nguyen Thi Thanh Ngoc, and Truong Ngoc Minh. 2024. "Diversity of Host Species and Optimized Cultivation Practices for Enhanced Bioactive Compound Production in Cordyceps militaris" Applied Sciences 14, no. 18: 8418. https://doi.org/10.3390/app14188418
APA StyleTrung, N. Q., Quyen, P. D. T., Ngoc, N. T. T., & Minh, T. N. (2024). Diversity of Host Species and Optimized Cultivation Practices for Enhanced Bioactive Compound Production in Cordyceps militaris. Applied Sciences, 14(18), 8418. https://doi.org/10.3390/app14188418







