Evaluation of a Three-Dimensional Printed Interventional Simulator for Cardiac Ablation Therapy Training
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
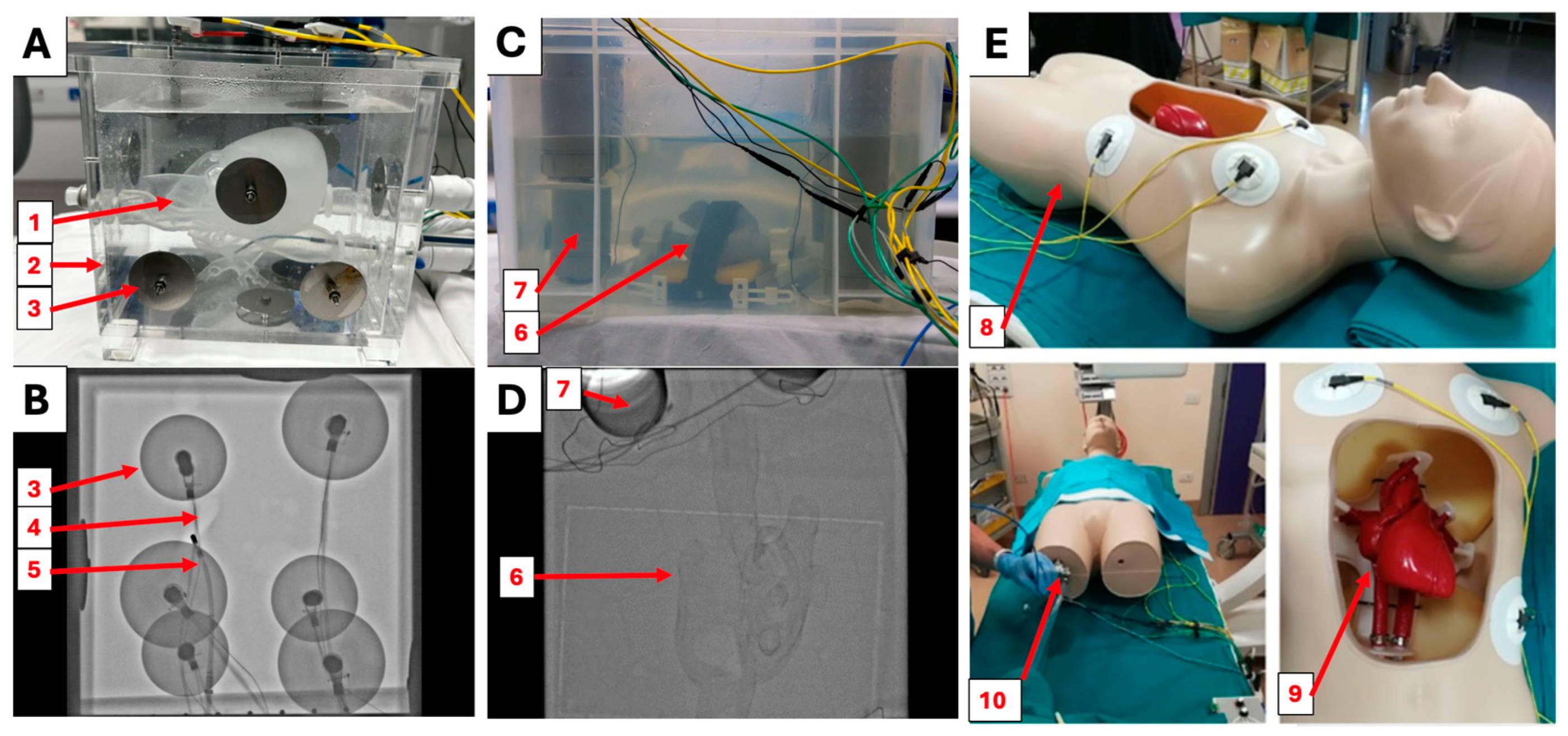
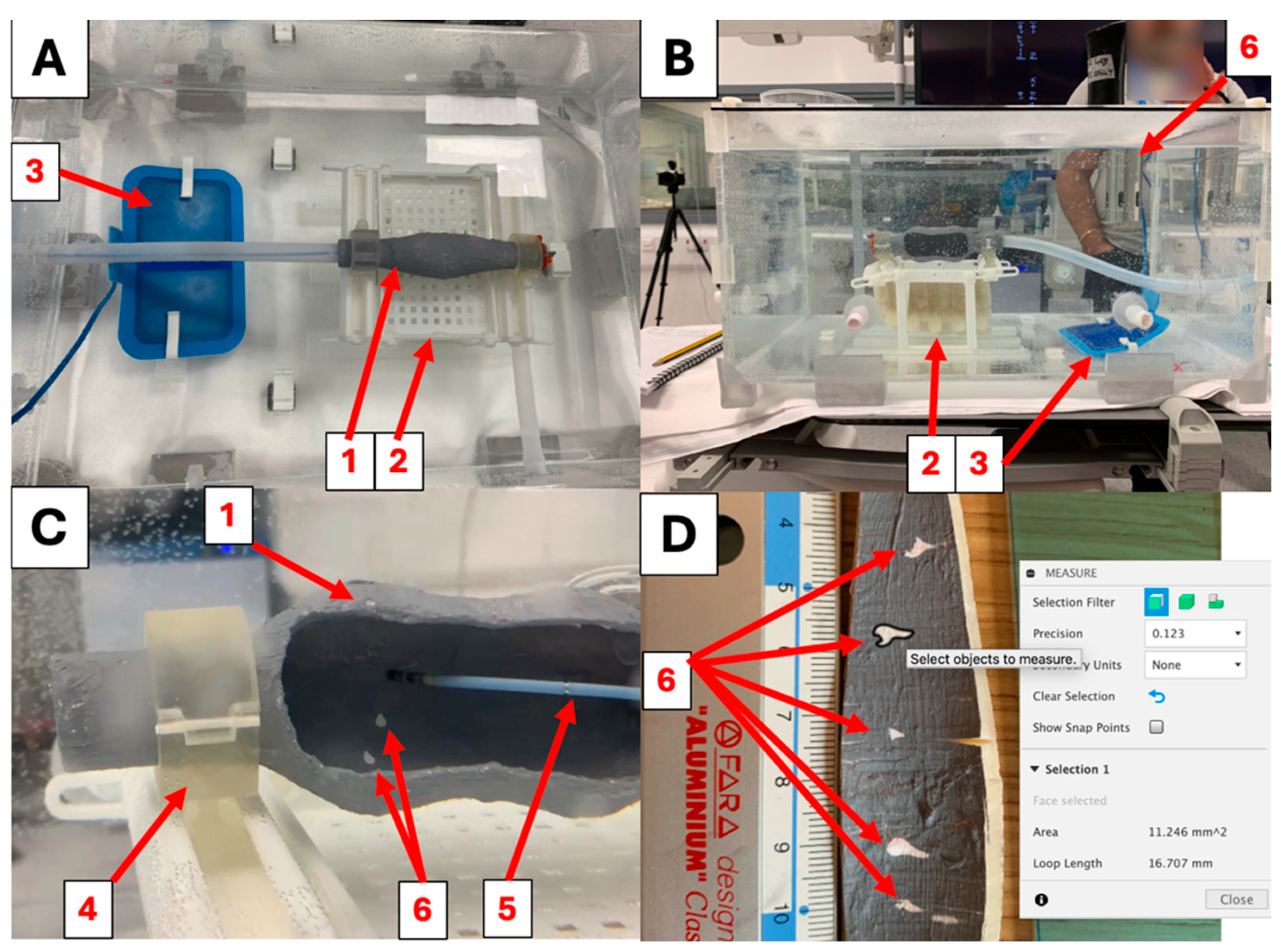
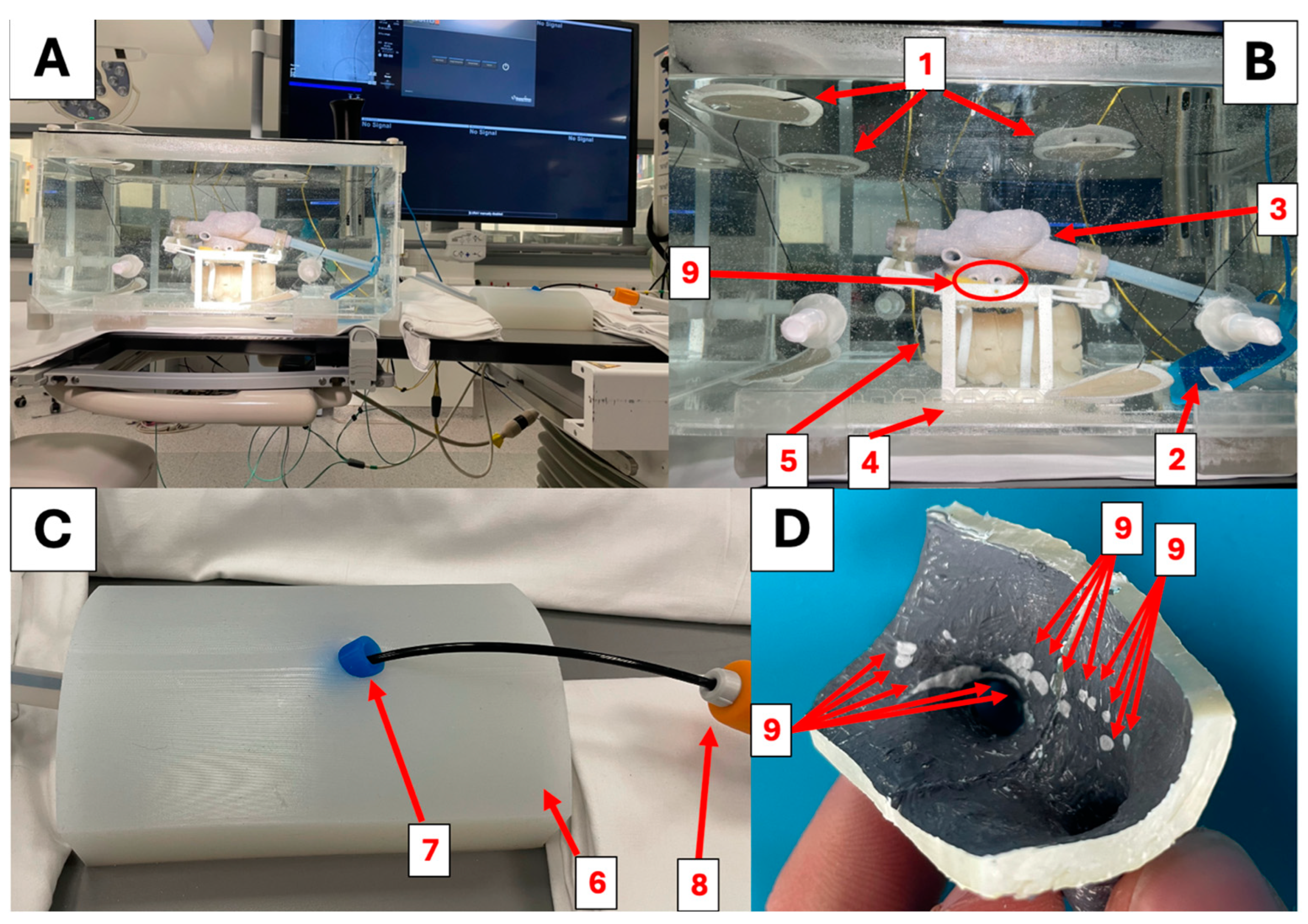
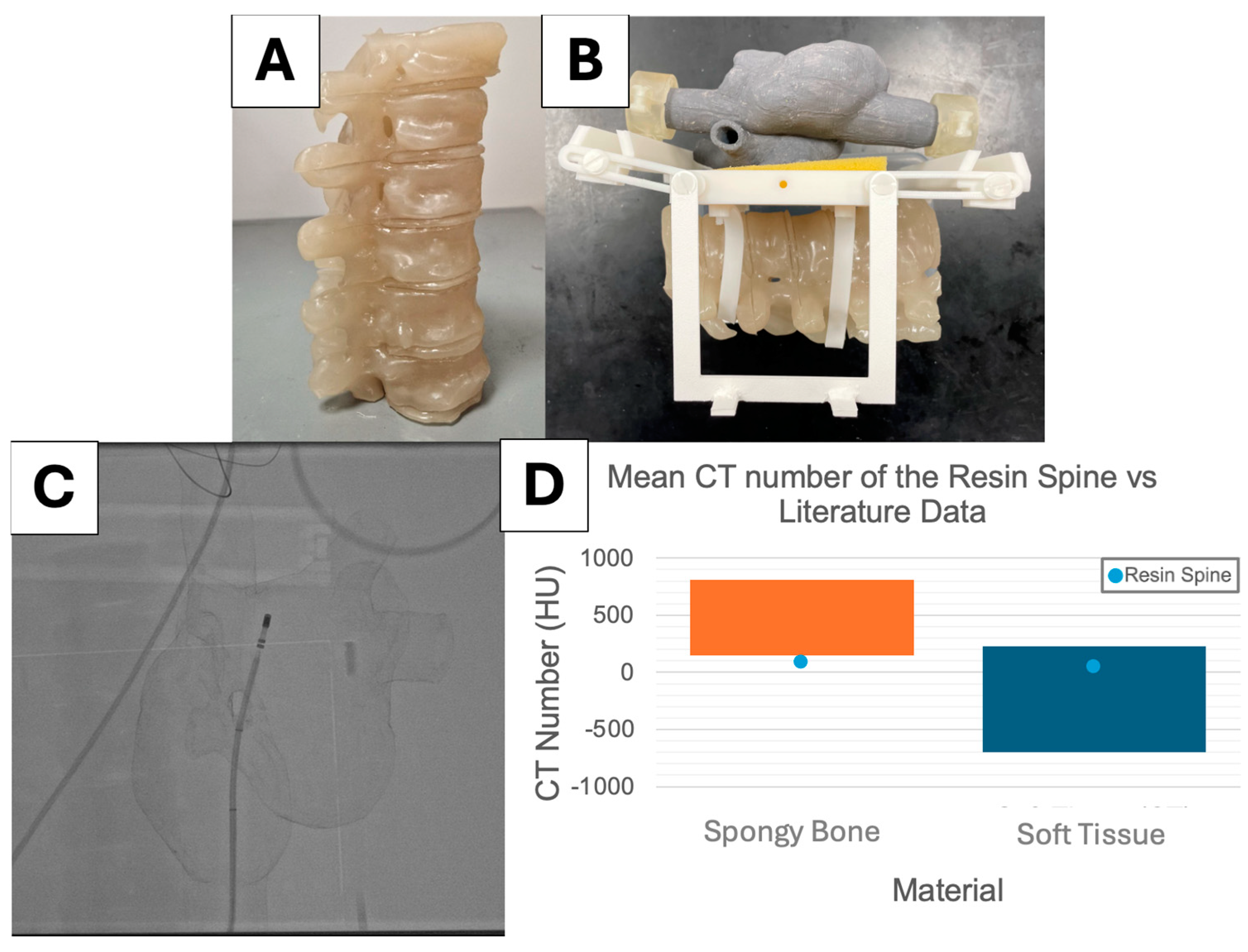
2.1. Simulator Construction
2.2. X-ray Visibility
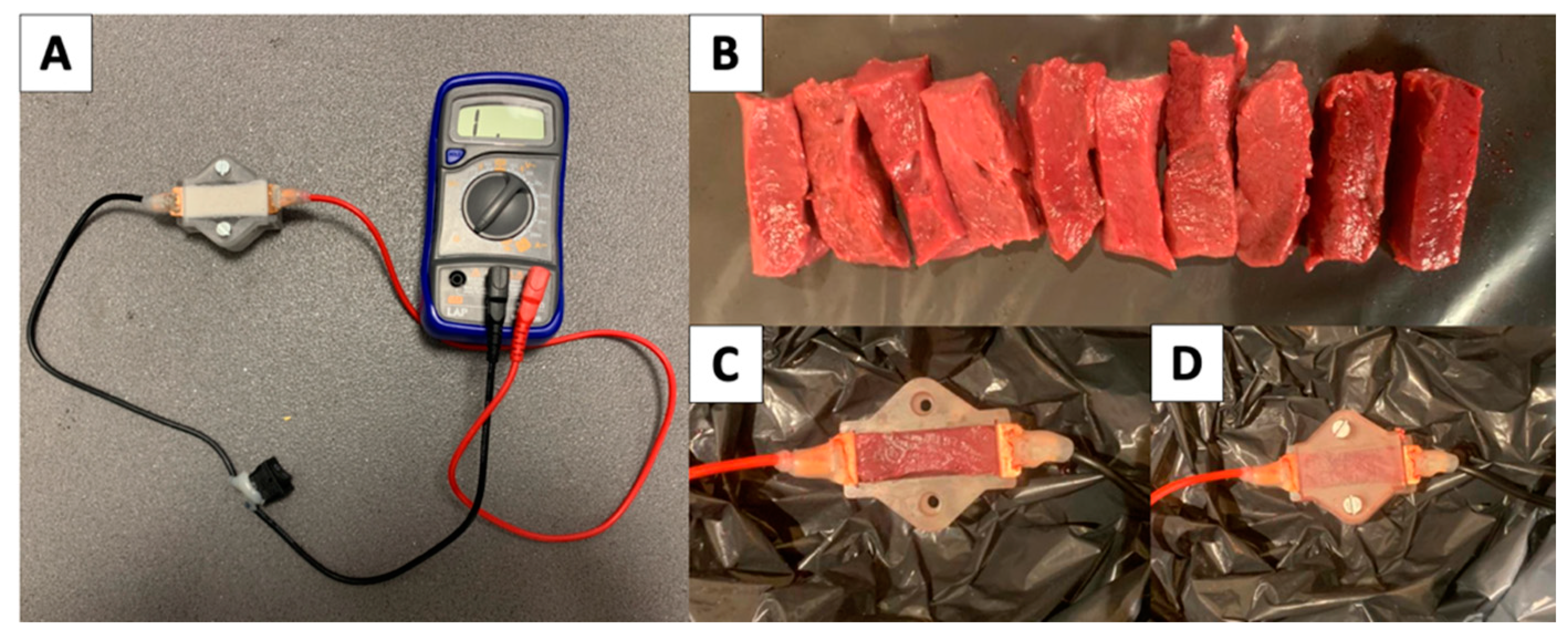
2.3. Electrical Resistivity
2.4. System Impedance
2.5. Ablation Settings
2.6. Clinical Evaluation
3. Results
3.1. X-ray Visibility
3.2. Electrical Properties
3.3. Ablation Lesions
3.4. Clinical Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arrhythmia—NHS. Available online: https://www.nhs.uk/conditions/arrhythmia/ (accessed on 11 September 2024).
- Dai, H.; Zhang, Q.; Much, A.A.; Maor, E.; Segev, A.; Beinart, R.; Adawi, S.; Lu, Y.; Bragazzi, N.L.; Wu, J. Global, regional, and national prevalence, incidence, mortality, and risk factors for atrial fibrillation, 1990–2017: Results from the global burden of disease study 2017. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 7, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Kwan, G.F.; Benjamin, E.J. Global Epidemiology of Atrial Fibrillation. Nat. Rev. Cardiol. 2014, 11, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Mujović, N.; Marinković, M.; Lenarczyk, R.; Tilz, R.; Potpara, T.S. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Adv. Ther. 2017, 34, 1897–1917. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Haines, D.E.; Boersma, L.V.; Sood, N.; Natale, A.; Marchlinski, F.E.; Calkins, H.; Sanders, P.; Packer, D.L.; Kuck, K.-H.; et al. Pulsed Field Ablation for the Treatment of Atrial Fibrillation: PULSED AF Pivotal Trial. Circulation 2023, 147, 19. [Google Scholar] [CrossRef] [PubMed]
- Gill, J.S. How to perform pulmonary vein isolation. EP Eur. 2003, 6, 83–91. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, R.; Marazzi, R.; Ghiringhelli, S.; Salerno-Uriarte, J.A.; Calkins, H.; Cheng, A. Superiority of simulator-based training compared with conventional training methodologies in the performance of Transseptal Catheterization. J. Am. Coll. Cardiol. 2011, 58, 359–363. [Google Scholar] [CrossRef] [PubMed]
- De Ponti, R.; Marazzi, R.; Doni, L.A.; Tamborini, C.; Ghiringhelli, S.; Salerno-Uriarte, J.A. Simulator training reduces radiation exposure and improves trainees’ performance in placing electrophysiologic catheters during patient-based procedures. Heart Rhythm 2012, 9, 1280–1285. [Google Scholar] [CrossRef] [PubMed]
- Haiser, A.; Aydin, A.; Kunduzi, B.; Ahmed, K.; Dasgupta, P. A systematic review of simulation-based training in Vascular surgery. J. Surg. Res. 2022, 279, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Angio Mentor Surgical Science. Available online: https://surgicalscience.com/simulators/angio-mentor/ (accessed on 11 September 2024).
- Platforms (2023) Surgical Science. Available online: https://surgicalscience.com/simulators/angio-mentor/platforms/ (accessed on 11 September 2024).
- EP Model (2024) Heartroid. Available online: https://www.heartroid.com/ep/ (accessed on 6 September 2024).
- Wang, S.; Saija, C.; Choo, J.; Ou, Z.; Birsoan, M.; Germanos, S.; Rothwell, J.; Vakili, B.; Kotadia, I.; Xu, Z.; et al. Cardiac radiofrequency ablation simulation using a 3D-printed bi-atrial thermochromic model. Appl. Sci. 2022, 12, 6553. [Google Scholar] [CrossRef]
- Mass, P.N.; Contento, J.M.; Opfermann, J.D.; Sumihara, K.; Kumthekar, R.N.; Berul, C.I. An infant Phantom for pediatric pericardial access and electrophysiology training. Heart Rhythm O2 2022, 3, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Rossi, L.; Penela, D.; Doni, L.; Marazzi, R.; Napoli, V.; Napoli, L.; Vilotta, M.; Villani, G.Q.; De Ponti, R. Development of simulation combining a physical heart model and three-dimensional system for electrophysiology training. Pacing Clin. Electrophysiol. 2018, 41, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Bourier, F.; Reents, T.; Ammar-Busch, S.; Buiatti, A.; Grebmer, C.; Telishevska, M.; Brkic, A.; Semmler, V.; Lennerz, C.; Kaess, B.; et al. Sensor-based Electromagnetic Navigation (Mediguide): How accurate is it? A phantom model study. J. Cardiovasc. Electrophysiol. 2015, 26, 1140–1145. [Google Scholar] [CrossRef] [PubMed]
- Safety Data Sheet: Anycubic 3D Printing UV Sensitive Resin (2021). Available online: https://cdn.shopify.com/s/files/1/0245/5519/2380/files/EN_SDS_3D_Printing_UV_Sensitive_Resin___SDS_SGS_GHS_ANNEXII_CLP_2021616_SGS-SHENZHEN-03_Shenzhen_Anycubic_Technology_Co._Ltd.pdf?v=1660036934 (accessed on 11 September 2024).
- X-ray Mass Attenuation Coefficients—Table 3 NIST. Available online: https://physics.nist.gov/PhysRefData/XrayMassCoef/tab3.html (accessed on 11 September 2024).
- ICRP. Adult Reference Computational Phantoms; ICRP Publication 110; ICRP: Stockholm, Sweden, 2009; Volume 39. [Google Scholar]
- Britannica, The Editors of Encyclopaedia. “Resistivity”. Available online: https://www.britannica.com/science/resistivity (accessed on 6 September 2024).
- Chougule, V.; Mulay, A.; Ahuja, B.B. Clinical Case Study: Spine Modeling for Minimum Invasive Spine Surgeries (MISS) using Rapid Prototyping. In Proceedings of the COPENA, Chennai, India, 7–9 December 2017. [Google Scholar]
- Barkagan, M.; Rottmann, M.; Leshem, E.; Shen, C.; Buxton, A.E.; Anter, E. Effect of baseline impedance on ablation lesion dimensions. Circ. Arrhythm. Electrophysiol. 2018, 11, e006690. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, K.; Porterfield, J.E.; Kottam, A.T.G.; Feldman, M.D.; Escobedo, D.; Valvano, J.W.; Pearce, J.A. Electrical conductivity and permittivity of murine myocardium. IEEE Trans. Biomed. Eng. 2009, 56, 2044–2053. [Google Scholar] [CrossRef] [PubMed]
- Miklavčič, D.; Pavšelj, N.; Hart, F.X. Electric properties of tissues. Wiley Encycl. Biomed. Eng. 2006. [Google Scholar] [CrossRef]



| Settings | 45 W Lower Power Non-Irrigated (LPNI) | 45 W Lower Power Irrigated (LPI) | 75 W Higher Power Irrigated (HPI) |
|---|---|---|---|
| Catheter Used | Thermocool SmartTouch SF | Thermocool SmartTouch SF | Thermocool SmartTouch SF |
| RF Generator | SMARTABLATE Stockert | SMARTABLATE Stockert | SMARTABLATE Stockert |
| Catheter Setting | 4 mm Catheter | SF | 4 mm Catheter |
| Max. Power | 45 W | 45 W | 75 W |
| Time | 30 s | 30 s | 5 s |
| Target Temp. | 65 °C | 65 °C | 65 °C |
| Cut-off Temp. | 85 °C | 85 °C | 85 °C |
| Min. Flow | 0 mL/min | 2 mL/min | 2 mL/min |
| Max. Flow | 0 mL/min | 8 mL/min | 8 mL/min |
| Simulator Fluoroscopy | Patient Fluoroscopy | |
|---|---|---|
| Mean Vertebral Intensity | 107.2 | 106.0 |
| Mean Background Intensity | 108.0 | 111.8 |
| Contrast | 6.2% | 5.3% |
| Questionnaire Section | Experimental Simulator | Commercial Simulator | Unpaired Multi-Variance t-Test p Value |
|---|---|---|---|
| Cather Entry | 3.90 ± 0.88 | 4.13 ± 1.06 | 0.5551 |
| Phantom Geometry | 4.11 ± 0.93 | 3.33 ± 1.56 | 0.1709 |
| X-ray Visibility | 3.66 ± 1.30 | 1.55 ± 1.22 | 0.0001 |
| Mapping and Ablation | 3.41 ± 1.01 | 3.25 ± 1.54 | 0.5864 |
| OVERALL SCORE | 3.65 ± 1.05 | 3.00 ± 1.64 | 0.0033 |
| Number of Ablations | 27 ± 16 | 57 ± 42 | 0.2621 |
| Settings | Experimental Simulator | Commercial Simulator |
|---|---|---|
| Max. Power | 76 W | 5 W |
| Mean Power | 41 W | 5 W |
| Max. Temperature | 45 °C | 30 °C |
| Mean Temperature | 37 °C | 25 °C |
| Max. Impedance | 141 Ω | 171 Ω |
| Mean Impedance | 125 Ω | 149 Ω |
| Min. Impedance | 109 Ω | 134 Ω |
| Max. Impedance Dorp | 23 Ω/s | 22 Ω/s |
| Mean Impedance Drop | 16 Ω/s | 6 Ω/s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saija, C.; Sabu, S.; Leung, L.; Lowe, E.; Al-Bahrani, N.; Coutinho Pinto, M.A.; Herridge, M.; Chowdhury, N.M.; Gibson, G.; Byrne, C.; et al. Evaluation of a Three-Dimensional Printed Interventional Simulator for Cardiac Ablation Therapy Training. Appl. Sci. 2024, 14, 8423. https://doi.org/10.3390/app14188423
Saija C, Sabu S, Leung L, Lowe E, Al-Bahrani N, Coutinho Pinto MA, Herridge M, Chowdhury NM, Gibson G, Byrne C, et al. Evaluation of a Three-Dimensional Printed Interventional Simulator for Cardiac Ablation Therapy Training. Applied Sciences. 2024; 14(18):8423. https://doi.org/10.3390/app14188423
Chicago/Turabian StyleSaija, Carlo, Sachin Sabu, Lisa Leung, Ellie Lowe, Noor Al-Bahrani, Marco Antonio Coutinho Pinto, Mark Herridge, Nadia M. Chowdhury, Gregory Gibson, Calum Byrne, and et al. 2024. "Evaluation of a Three-Dimensional Printed Interventional Simulator for Cardiac Ablation Therapy Training" Applied Sciences 14, no. 18: 8423. https://doi.org/10.3390/app14188423
APA StyleSaija, C., Sabu, S., Leung, L., Lowe, E., Al-Bahrani, N., Coutinho Pinto, M. A., Herridge, M., Chowdhury, N. M., Gibson, G., Byrne, C., Gabbeta, A., Marion, E., Chavan, R., Behar, J., Pontiki, A. A., Berthet-Rayne, P., Housden, R. J., & Rhode, K. (2024). Evaluation of a Three-Dimensional Printed Interventional Simulator for Cardiac Ablation Therapy Training. Applied Sciences, 14(18), 8423. https://doi.org/10.3390/app14188423








