Abstract
Logging operations of Liriodendron tulipifera L., as timber trees, and fallen leaves in autumn from ornamental trees produce a large amount of leaf waste. In this study, the allelopathy of L. tulipifera fresh and fallen leaves was investigated for the development of potential applications of leaf waste. The extracts of fresh and fallen leaves of L. tulipifera showed growth inhibitory activity against weed species, Vulpia myuros (L.) C.C.Gmel., Echinochloa crus-galli (L.) P.Beauv., and Lolium multiflorum Lam., under laboratory conditions. The powder of L. tulipifera fresh and fallen leaves also inhibited the germination of E. crus-galli under greenhouse conditions. A potent allelochemical was isolated from fresh and fallen leaf extracts through a bioassay-guided separation process, and was identified as lipiferolide. Lipiferolide inhibited the growth of L. multiflorum and Lepidum sativum in a concentration-dependent manner. This investigation suggests that the leaf waste of L. tulipifera from logging operations and fallen leaves is potentially useful for the purpose of weed control, such as through the use of soil additive materials from leaves or the creation of foliar spray from leaf extracts. The development of weed control materials using L. tulipifera leaf waste may be a means with which to minimize waste, reducing environmental impacts and economic concerns.
1. Introduction
Liriodendron tulipifera L., belonging to the Magnoliaceae family, is a hardwood tree and grows to 25–40 m in height as well as to 1–2 m in trunk diameter. The alternate simple leaves are palmately lobed and grow up to 10–15 cm long and wide [1,2]. The species is native to eastern North America and was introduced to many other countries under temperate climate conditions in Europe and East Asia [3,4,5]. L. tulipifera grows along residential streets as shade trees and in parks as well as gardens as ornamental trees [6,7,8]. L. tulipifera is also valuable as a nectar source for honey production. A 20-year-old tree of L. tulipifera was recorded to produce 3.6 kg of nectar in one season, which is equivalent to 1.8 kg of honey [6]. Since L. tulipifera grows rapidly and has tall and straight trunks, its timber is commercially valuable [2,3,9]. The average dry weight of the timber is 455 kg per m3 (hardness, 2400 N) [9], and the wood of L. tulipifera is used in a wide range of products, such as furniture, interior finishing, and plywood [10,11,12]. However, logging operations of L. tulipifera from tree plantations create a great amount of industrial waste, such as leaves, branches, bark, and stumps. It was recorded that 80% of total tree biomass went to waste during logging operations [13]. Minimizing waste may contribute to the sustainability of timber production systems and reducing the environmental impact [14,15]. The development of possible applications for the waste from timber processing and the minimization of the environmental impact are two tasks to be completed for future timber production systems. Several options of the usage of L. tulipifera waste from logging operations have been investigated, such as charcoal and bio-oil from the wood waste [13,16], solid fuel from the sawdust [17], and bioethanol from the branches and wood [18,19]. In addition, L. tulipifera is a deciduous tree species, and its fallen leaves accumulate on the streets and grounds in parks and gardens in the autumn, where they are grown as shade and ornamental trees. These fallen leaves need to be organized at a high expense [20]. However, the utilization methods of leaf waste from logging operations and fallen leaves from residential areas have not yet been investigated.
The bark of L. tulipifera has been used by Native Americans as an antipyretic for the treatment of malaria [21,22,23,24]. Pharmaceutical investigations have shown that L. tulipifera bark contains several secondary metabolites, such as alkaloids and lignans [25]. Its wood, roots, and leaves have also been reported to contain many secondary metabolites, such as alkaloids, terpens, flavonones, lignans, and steroids, and some of them have shown pharmacological activity [23,24,26,27,28,29,30,31]. Although the allelopathic activity of these identified compounds in L. tulipifera has not yet been investigated, some of these compounds may work as allelopathic agents. Allelopathy is the chemical interaction between donor and receiver plants, and donor plants can release certain secondary metabolites, defined as allelochemicals, to their neighboring environments [32,33,34,35]. Allelochemicals affect the germination, growth, development, and regeneration of plant species nearby, including weed plant species, and most allelochemicals show inhibitory effects [36,37,38,39]. Therefore, allelopathic plants and their tissues, which contain allelochemicals, can potentially be applied for weed control purposes. Some allelopathic plants have shown excellent weed control ability when these plants were incorporated into the soil as soil additives and when their extracts were applied to weeds as a foliar spray [40,41,42,43]. Weed infestation causes significant problems in agricultural production [44,45,46]. However, there is no available information on the allelopathy of L. tulipifera leaves in the literature.
The objective of this research was to develop a possible application of L. tulipifera leaf waste from logging operations from their use as timber trees and from fallen leaves accumulated on the streets and on the grounds of parks and gardens in autumn (Figure 1). Therefore, we investigated the allelopathic activity of L. tulipifera fresh and fallen leaves against the weed species V. myuros, E. crus-galli, and L. multiflorum in laboratory conditions, and characterized the allelochemicals from the leaves. The fresh leaves were used as substitutes for leaf waste from the logging operations of L. tulipifera. V. myuros, E. crus-galli, and L. multiflorum are common weed species in temperate climate conditions in North America, Europe, and Asia [45,46], and E. crus-galli is also a common weed species in tropical and temperate areas worldwide [47]. We also determined the allelopathic activity of the fresh and fallen leaf powder applied in soil under greenhouse conditions.

Figure 1.
L. tulipifera fresh and fallen leaves.
2. Materials and Methods
2.1. Plant Materials
The fallen and fresh leaves of L. tulipifera were collected on the campus of Kagawa University, Faculty of Agriculture, on November 2020 and July 2021, respectively. The fresh leaves are substitutes for the waste from logging operations of L. tulipifera trees. The fallen and fresh leaves were dried under shade conditions. Weed species—Vulpia myuros (L.) C.C.Gmel., Echinochloa crus-galli (L.) P.Beauv., and Lolium multiflorum Lam.—were used as bioassay test plant species for the determination of the allelopathic potential of L. tulipifera fallen and fresh leaves. A dicotyledonous plant species, Lepidum sativum L., was used as a bioassay plant through a bioassay-guided separation process of allelochemicals, because the species is a crop plant and shows a high as well as stable germination rate.
2.2. Determination of the Allelopathic Activity of L. tulipifera Fresh and Fallen Leaves under Laboratory Conditions
The fresh leaves of L. tulipifera were extracted, and the allelopathic activity of the extracts was determined by the procedure described in the published papers [48]. Test plant seeds were germinated in the dark at 25 °C for 48 h, and 10 germinated seeds of V. myuros, E. crus-galli, and L. multiflorum were incubated in Petri dishes with the extracts in darkness at 25 °C. After 48 h incubation, the length of the coleoptiles and roots of these weed species was determined. Petri dishes contained the extracts obtained from 1, 3, 10, 30, or 100 mg L. tulipifera leaves per 1 mL of the assay solution. The experiment was independently repeated four times with 10 test plants for each determination. The fallen leaves of L. tulipifera were extracted, and the allelopathic activity of the extracts was determined with the same procedure as for the fresh leaves.
The allelopathic activity of the fresh and fallen leaf extracts of L. tulipifera was also evaluated using L. sativum. The L. sativum bioassay was carried out with the same procedure described for the weed species except the pre-germination process. L. sativum seeds (not germinated) were incubated with the leaf extracts of L. tulipifera.
2.3. Allelopathic Activity of L. tulipifera Fresh and Fallen Leaves under Greenhouse Conditions
The greenhouse experiments were followed by the procedure described in published papers [48]. The powder of the fresh and fallen leaves of L. tulipifera was separately applied onto the soil surface in the pots at powder dosages of 0 (control), 0.21, 0.7, 2.1, 7, and 21 g per pot. After these pots were kept in a greenhouse for 9 days, 100 seeds of E. crus-galli were sown on the soil in the pots. The seeds were germinated under the conditions of natural daylight (average daylight of 12.5 h), temperature (average of 14.7 °C), and watering (500 mL, every two days). After 14 days of incubation, the germinated seeds of E. crus-galli were counted. Control seeds were germinated with exactly the same process except for the L. tulipifera leaf powder. The experiment was independently repeated four times with 100 seeds of E. crus-galli for each determination.
2.4. Isolation of an Allelochemical from L. tulipifera Fresh and Fallen Leaves
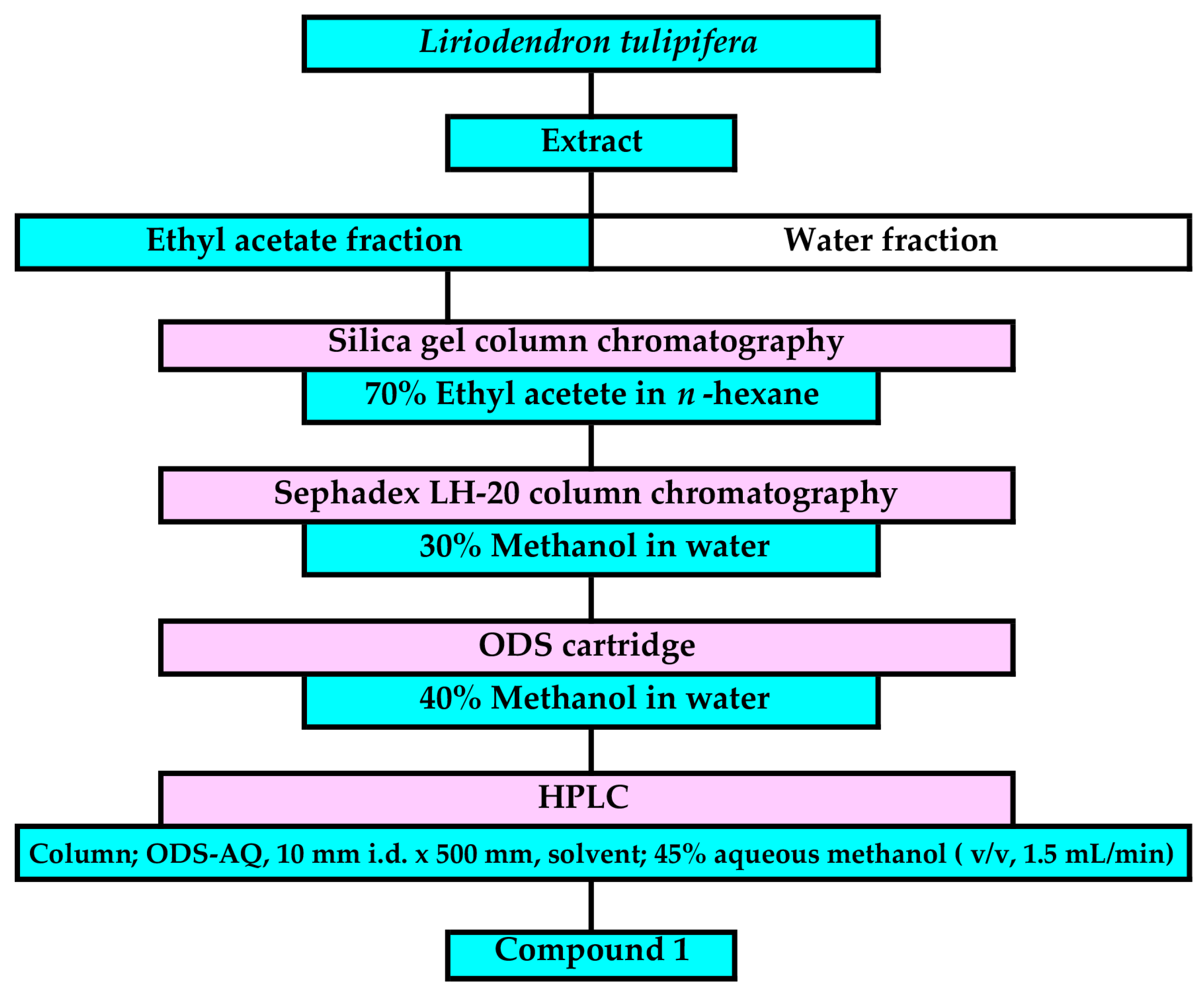
The fresh L. tulipifera leaves (100 g dry weight) were extracted, and the extracts were purified through a bioassay-guided separation process based on the procedure described in published papers [48]. The ethyl acetate fraction was separated using silica gel chromatography, Sephadex LH-20 chromatography, reverse-phase ODS cartridges (Figure 2, Figures S1–S6). The allelopathic activity of all fractions was determined using a L. sativum bioassay. An active compound, 1, was finally isolated using reverse-phase HPLC in a fraction eluted between 168 and 172 min. The chemical structure of compound 1 was determined by the analysis of spectrum data of 1H-NMR (400 MHz, CDCl3), HRESI-MS, and optical rotation.
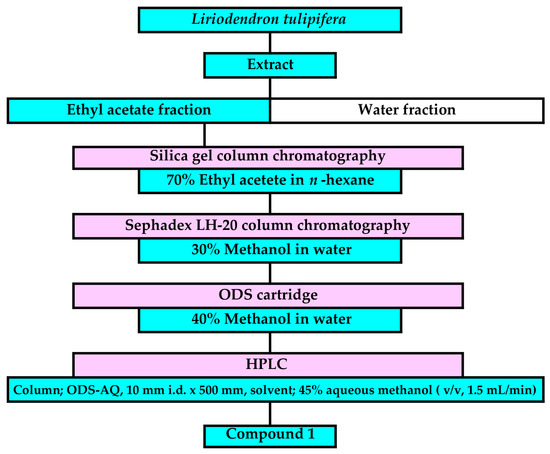
Figure 2.
Separation process of compound 1 from L. tulipifera leaf extracts.
The fallen leaves of L. tulipifera were extracted and purified as described for the fresh leaves, and the same active compound was detected in a fraction eluted between 168 and 172 min of the HPLC. The active compound isolated from the fallen leaves is identical with compound 1 based on its chromatographic property.
2.5. Allelopathic Activity of the Isolated Compound
The allelopathic activity of compound 1 isolated from L. tulipifera leaves was determined using a bioassay of L. multiflorum and L. sativum. The concentrations of lipiferolide in the bioassay were 0.01, 0.03, 0.1, 0.3, and 1 mM. The bioassay was independently repeated four times with 10 germinated seeds (L. multiflorum) and 10 seeds (L. sativum) for each determination.
2.6. Statistical Analysis
All bioassay treatments were set in a randomized block design, and repeated four times. The means ± SE (standard error) of the collected data were calculated. Significant differences were evaluated by an ANOVA one-way analysis (SPSS, version 16.0) and a post hoc analysis with Tukey’s HSD test at a p < 0.05 level. The values of the concentration causing 50% growth inhibition (IC50) of test plant species in the respective bioassay were obtained using GraphPad Prism 6.0.
3. Results
3.1. Allelopathic Activity of L. tulipifera Fresh and Fallen Leaves under Laboratory Conditions
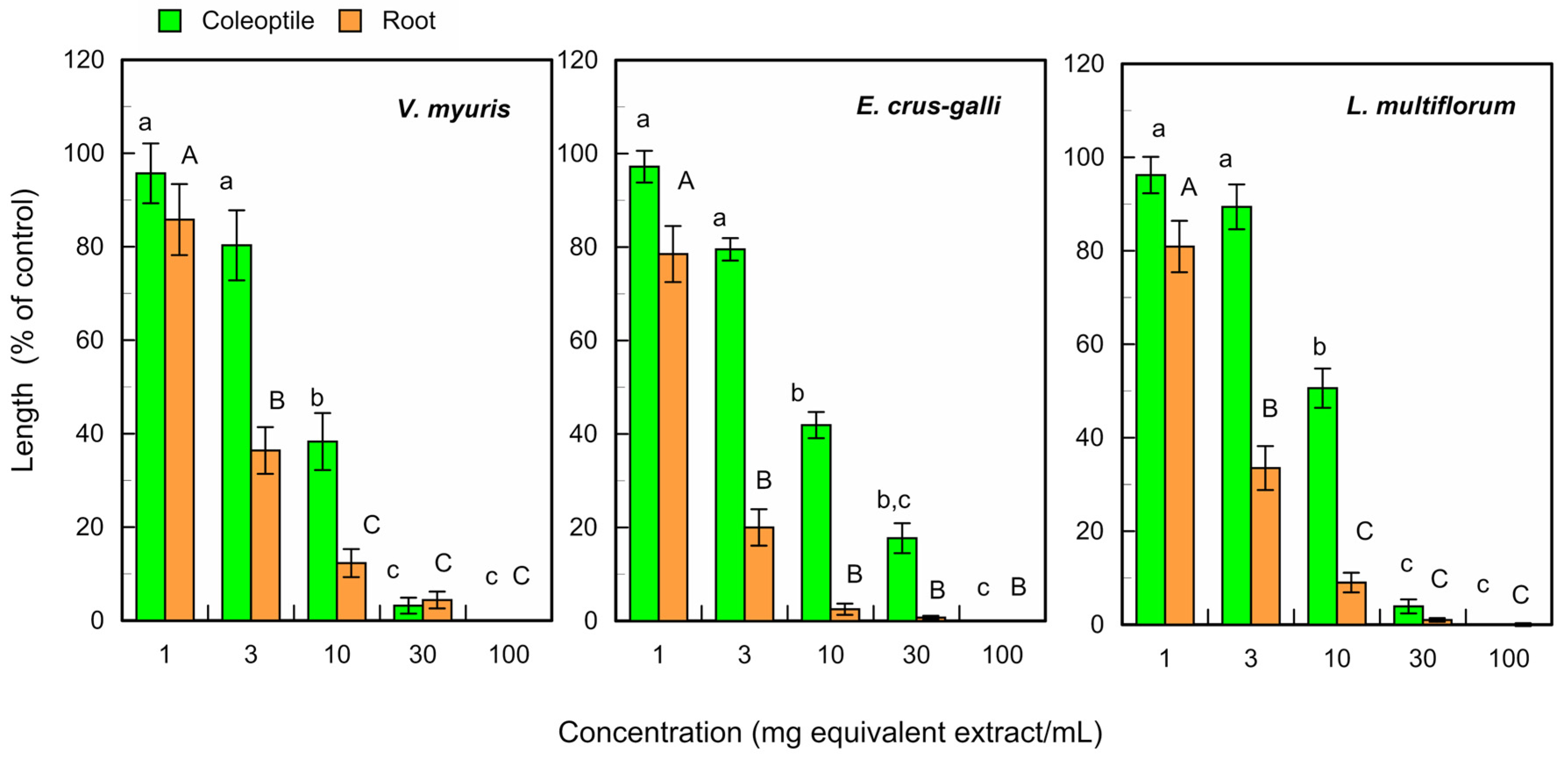
The fresh leaf extracts of L. tulipifera significantly suppressed the growth of coleoptiles of V. myuros, E. crus-galli, and L. multiflorum at concentrations ≥ 10 mg equivalent leaf extracts, and suppressed the root growth of these weed species at concentrations ≥ 3 mg equivalent extracts per mL (Figure 3).
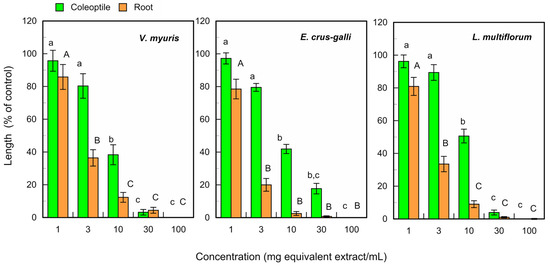
Figure 3.
Effects of L. tulipifera fresh leaf extracts against the coleoptile and root growth of V. myuros, E. crus-galli, and L. multiflorum under laboratory conditions. The concentrations on the X-axis indicate the substances obtained from 1, 3, 10, 30, and 100 mg of L. tulipifera leaves in 1 mL of the assay solution. The means ± SE were calculated from four independent experiments with 10 seedlings for each determination. Different letters on the bars in the same panels indicate significant differences at a p < 0.05 level.
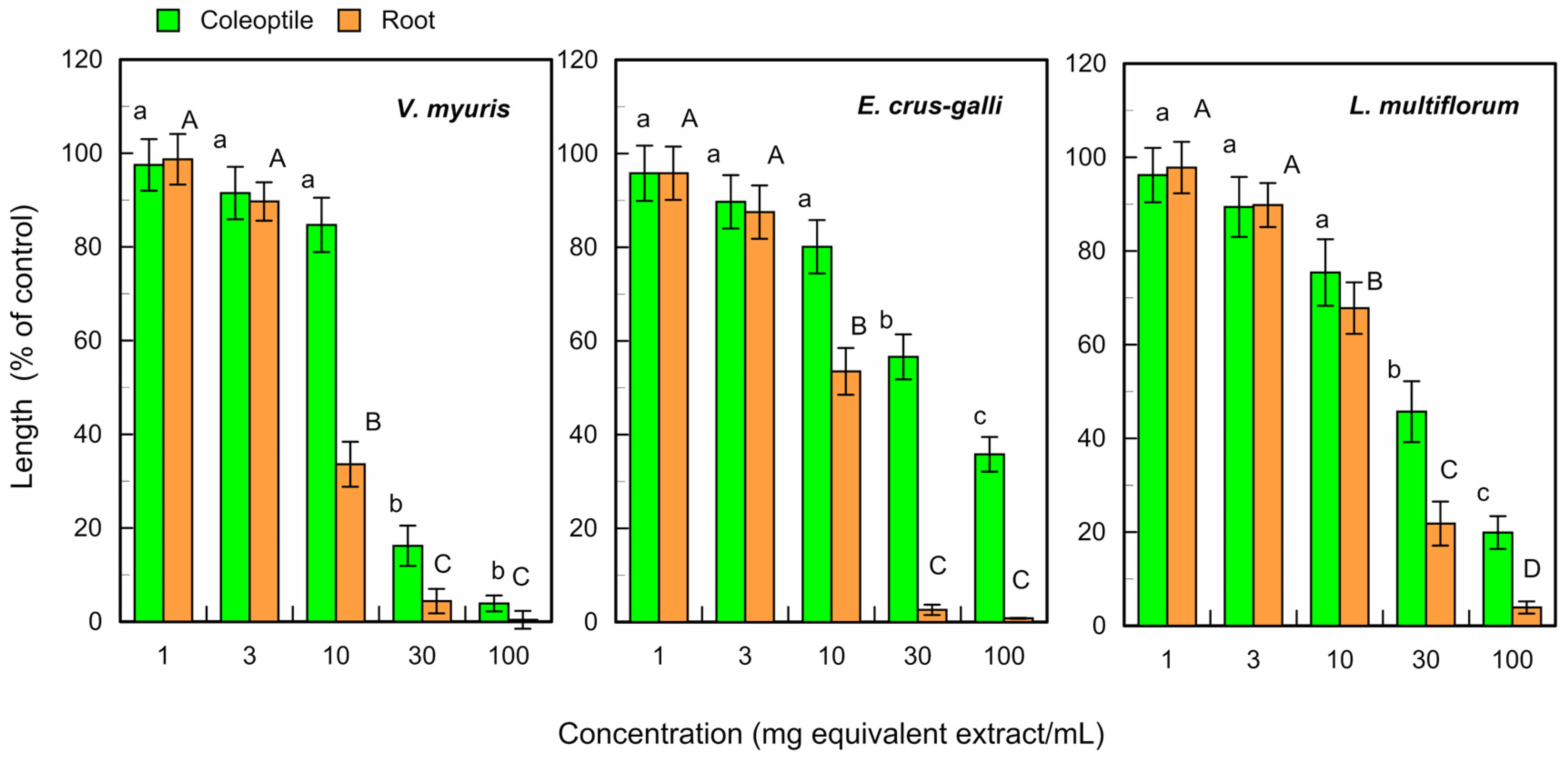
The fallen leaf extracts of L. tulipifera also suppressed the growth of V. myuros, E. crus-galli, and L. multiflorum coleoptiles at concentrations ≥ 30 mg equivalent leaf extracts per mL, and suppressed the growth of V. myuros, E. crus-galli, and L. multiflorum roots at concentrations ≥ 10 mg equivalent leaf extracts per mL (Figure 4). The IC50 value of the fresh and fallen leaf extracts for the coleoptile and root growth was shown in Table 1.
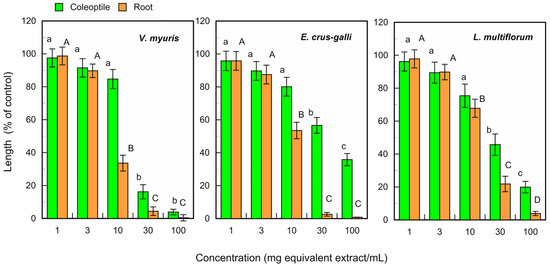
Figure 4.
Effects of the L. tulipifera fallen leaf extracts against the coleoptile and root growth of V. myuros, E. crus-galli, and L. multiflorum under laboratory conditions. Other conditions were the same as those described in Figure 3.

Table 1.
IC50 values of the fresh and fallen leaf extracts of L. tulipifera on the coleoptile and root growth of bioassay test plant species. The values were determined by the regression equation of the concentration response as described in the text.
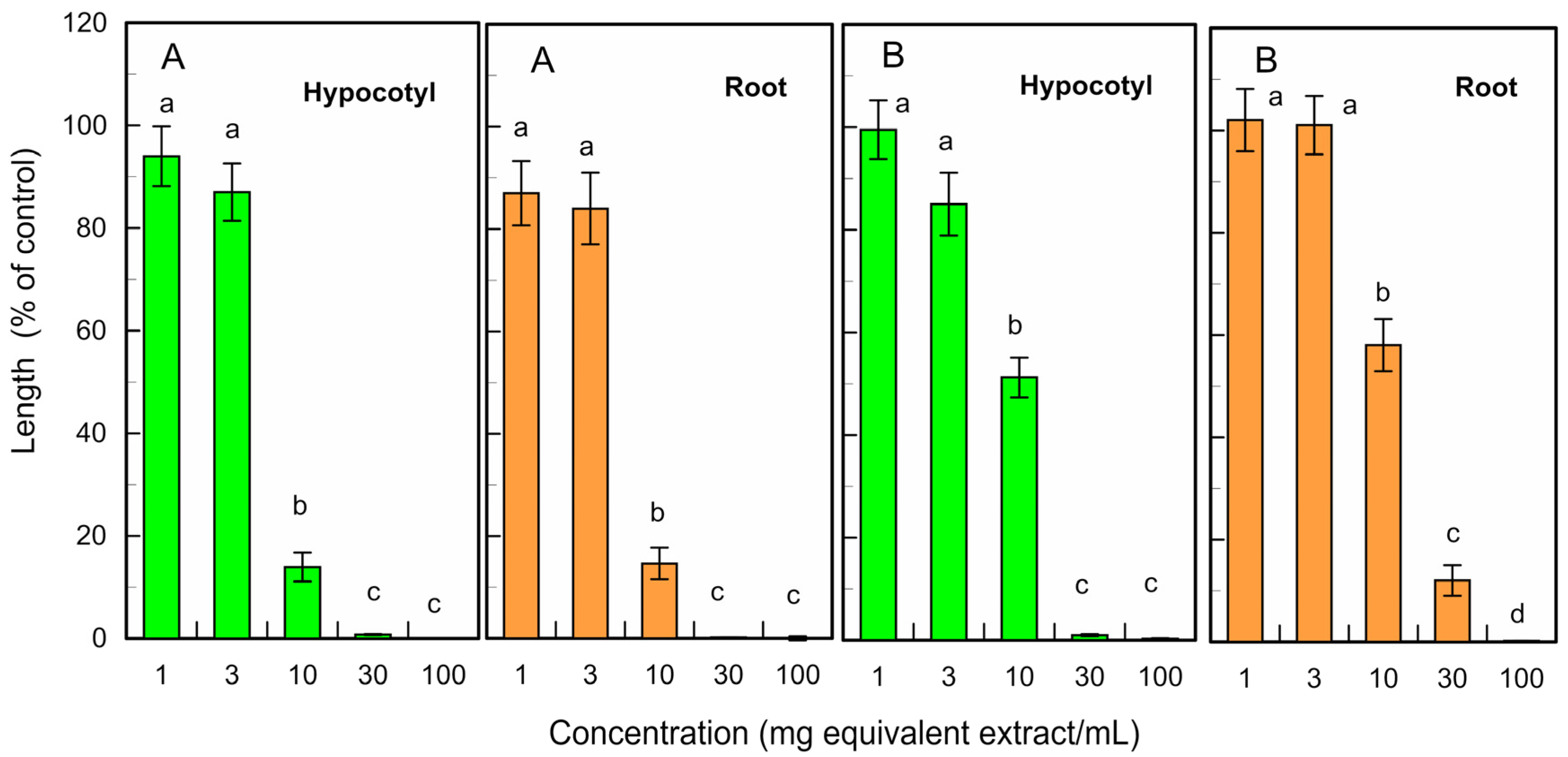
The fresh and fallen leaf extracts of L. tulipifera also suppressed the hypocotyl and root growth of L. sativum at concentrations ≥ 10 mg leaf equivalent extracts per mL at a p < 0.05 level (Figure 5). The IC50 value of the fresh leaf extracts for the L. sativum hypocotyl and root growth was 4.7 mg and 5.1 mg equivalent extracts per mL, respectively, and that of the fallen leaf extracts for the L. sativum hypocotyl and root growth was 10.2 mg and 18.8 mg equivalent extracts per mL, respectively.
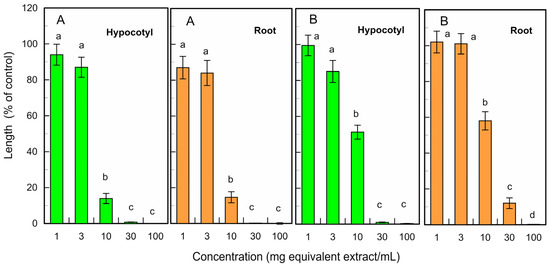
Figure 5.
Effects of the fresh (A) and fallen (B) L. tulipifera leaf extracts against the growth of L. sativum hypocotyls and roots. Other conditions were the same as those described in Figure 3.
3.2. Allelopathic Activity of L. tulipifera Fresh and Fallen Leaves under Greenhouse Conditions
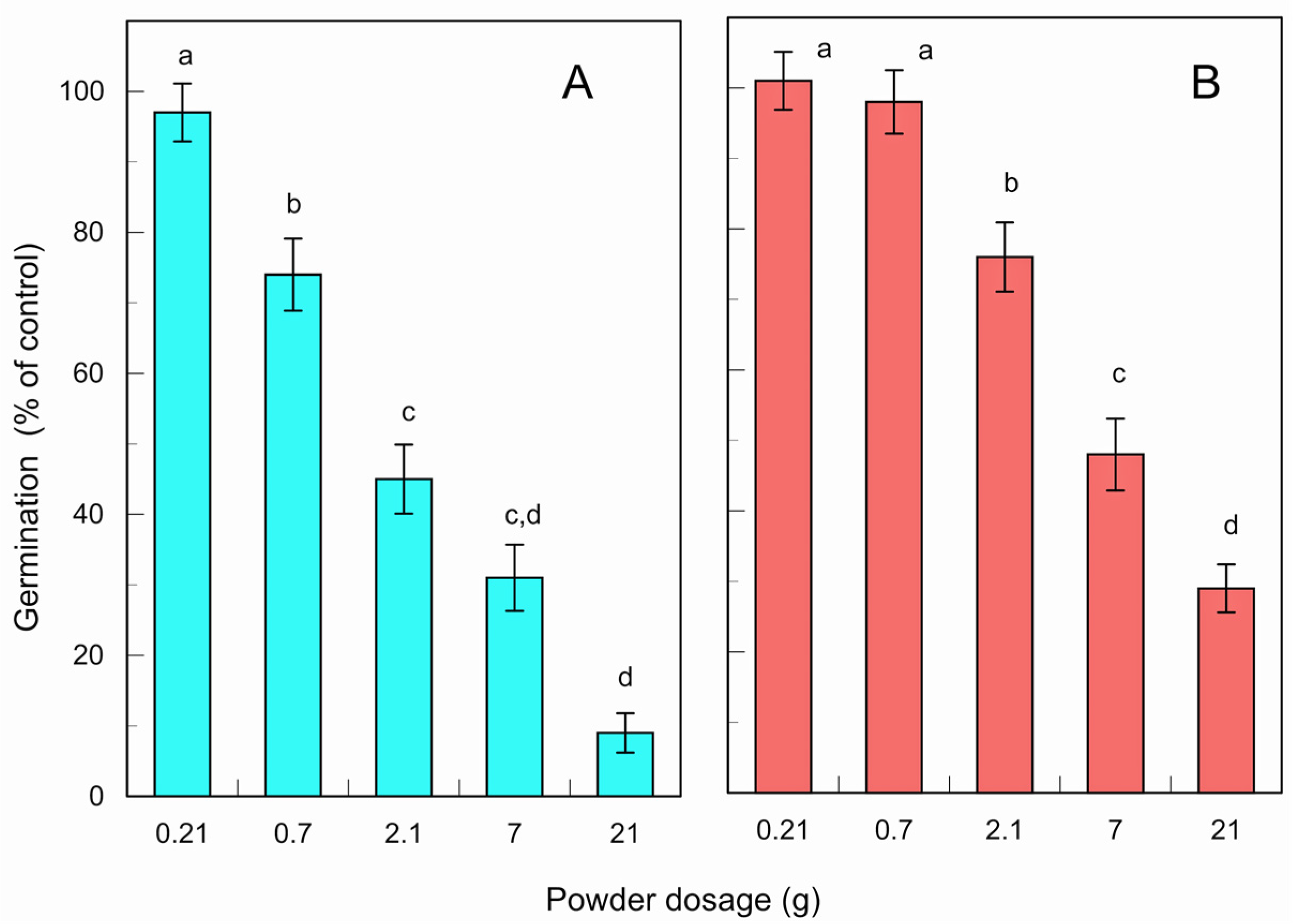
The fresh and fallen leaf powder of L. tulipifera suppressed E. crus-galli germination under greenhouse conditions at dosages ≥ 0.7 g and 2.1 g powder per pot at a p < 0.05 level, respectively (Figure 6). The suppression of germination was increased with an increase in the powder dosage. The powder dosages at 0.21, 0.7, 2.1, 7, and 21 g per pot (area: 0.07 m2) corresponded to nearly 0.03, 0.1, 0.3, 1, and 3 tons of the powder per hectare (10,000 m2), respectively. The IC50 values of the leaf powder for E. crus-galli germination was 1.7 g and 5.2 g per pot for fresh and fallen leaves, respectively. E. crus-galli is one of the problematic weeds in agricultural production [47,49].
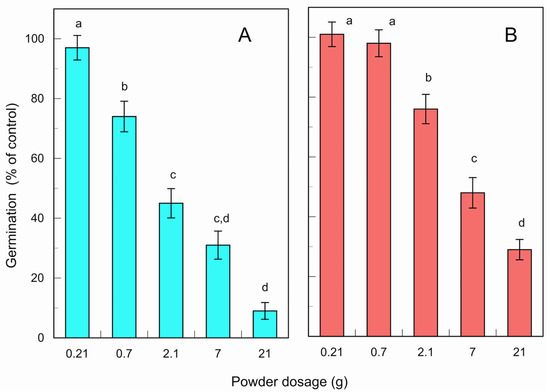
Figure 6.
Effects of the fresh (A) and fallen (B) leaf powder of L. tulipifera against the germination of E. crus-galli under greenhouse conditions. The means ± SE were calculated from four independent experiments with 100 seeds for each determination. Different letters on the bars in the same panels indicate significant differences at a p < 0.05 level.
3.3. Isolation and Identification of Allelochemicals in L. tulipifera Fresh and Fallen Leaves
The fresh leaf extract of L. tulipifera was purified through the bioassay-guided separation process (Figure 2); 12 and 4 mg of compound 1 were isolated from 100 g dry weight of the fresh and fallen leaves, respectively.
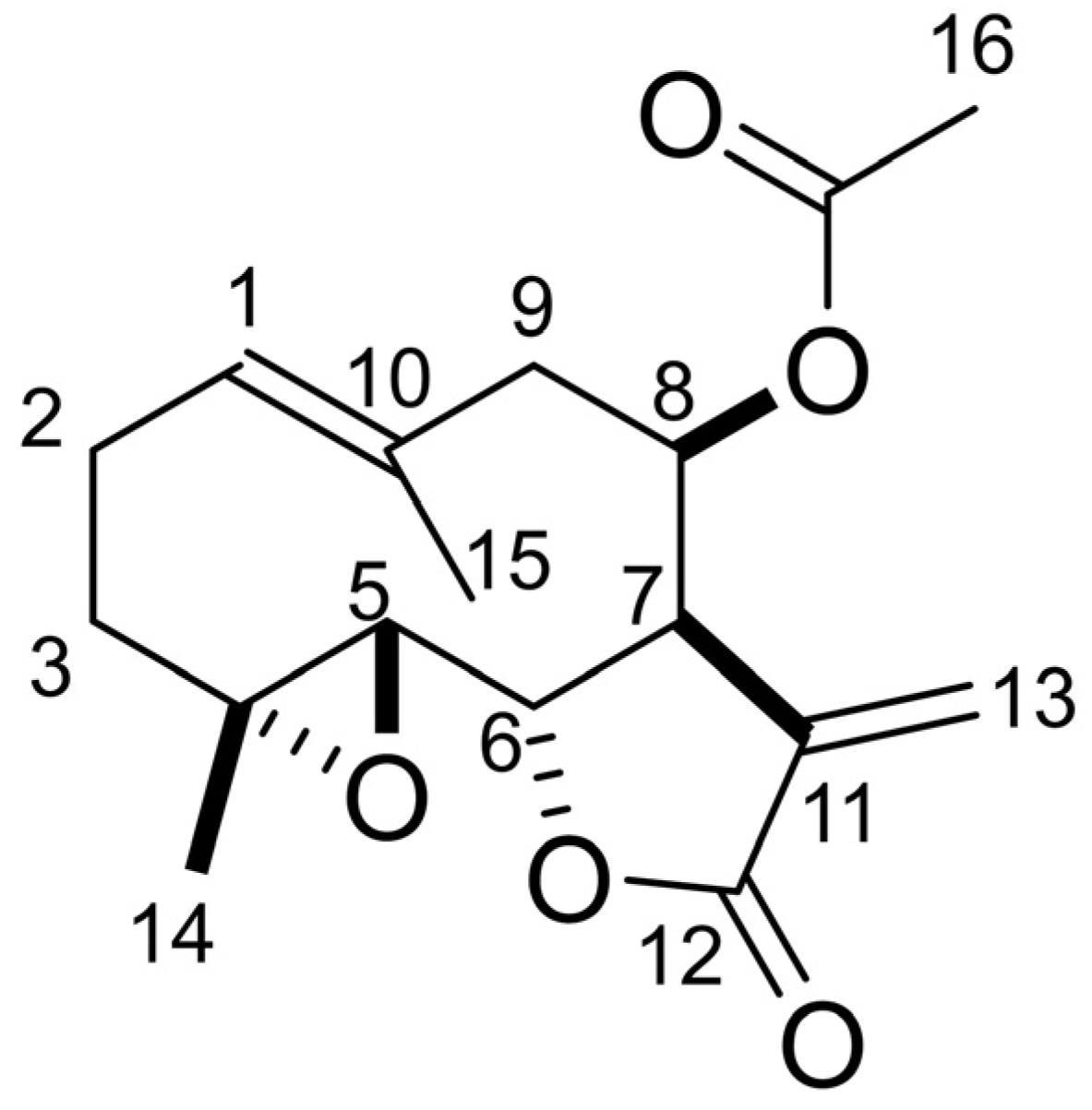
The molecular formula of compound 1 was determined as C17H22O5 via HRESI-MS analysis. The 1H-NMR spectrum showed δH 6.39 (d, J = 3.6 Hz, 1 H, H13), 5.71 (d, J = 3.1 Hz, 1 H, H13), 5.69 (brd, J = 4.9 Hz, 1 H, H8), 5.29 (dd, J = 12.6, 2.7 Hz, 1 H, H1), 4.40 (t, J = 8.3 Hz, 1 H, H6), 3.13 (dt, J = 7.2, 3.4 Hz, 1 H, H7), 2.82 (d, J = 8.8 Hz, 1 H, H5), 2.75 (dd, J = 14.4, 4.9 Hz, 1 H, H9), 2.47 (m, 1 H, H2), 2.36 (brd, J = 14.4 Hz, 1 H, H9), 2.24 (m, 1 H, H2), 2.19 (ddd, J = 13.0, 6.5, 1.8 Hz, 1 H, H3), 2.06 (s, 3 H, H16), 1.76 (s, 3 H, H15), 1.37 (s, 3 H, H14), 1.29 (m, 1 H, H3). The optical rotation was [α]D27 −104 (c 0.1, CH3OH). Compound 1 was determined to be lipiferolide (sesquiterpene lactone) (Figure 7) based on the comparison of its spectrum data with the spectrum data in publication [50].
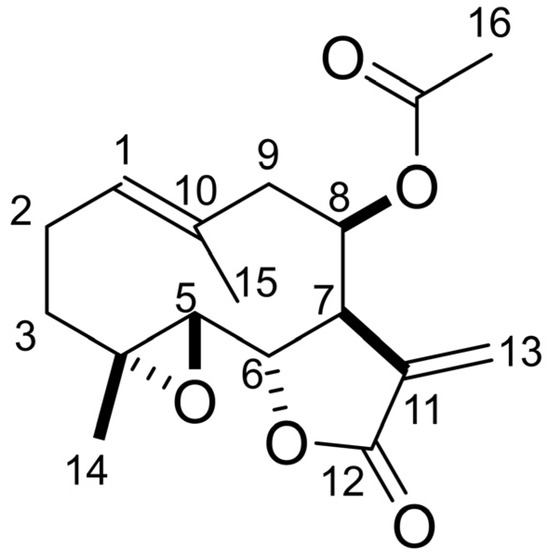
Figure 7.
Chemical structure of lipiferolide.
3.4. Allelopathic Activity of the Isolated Compound
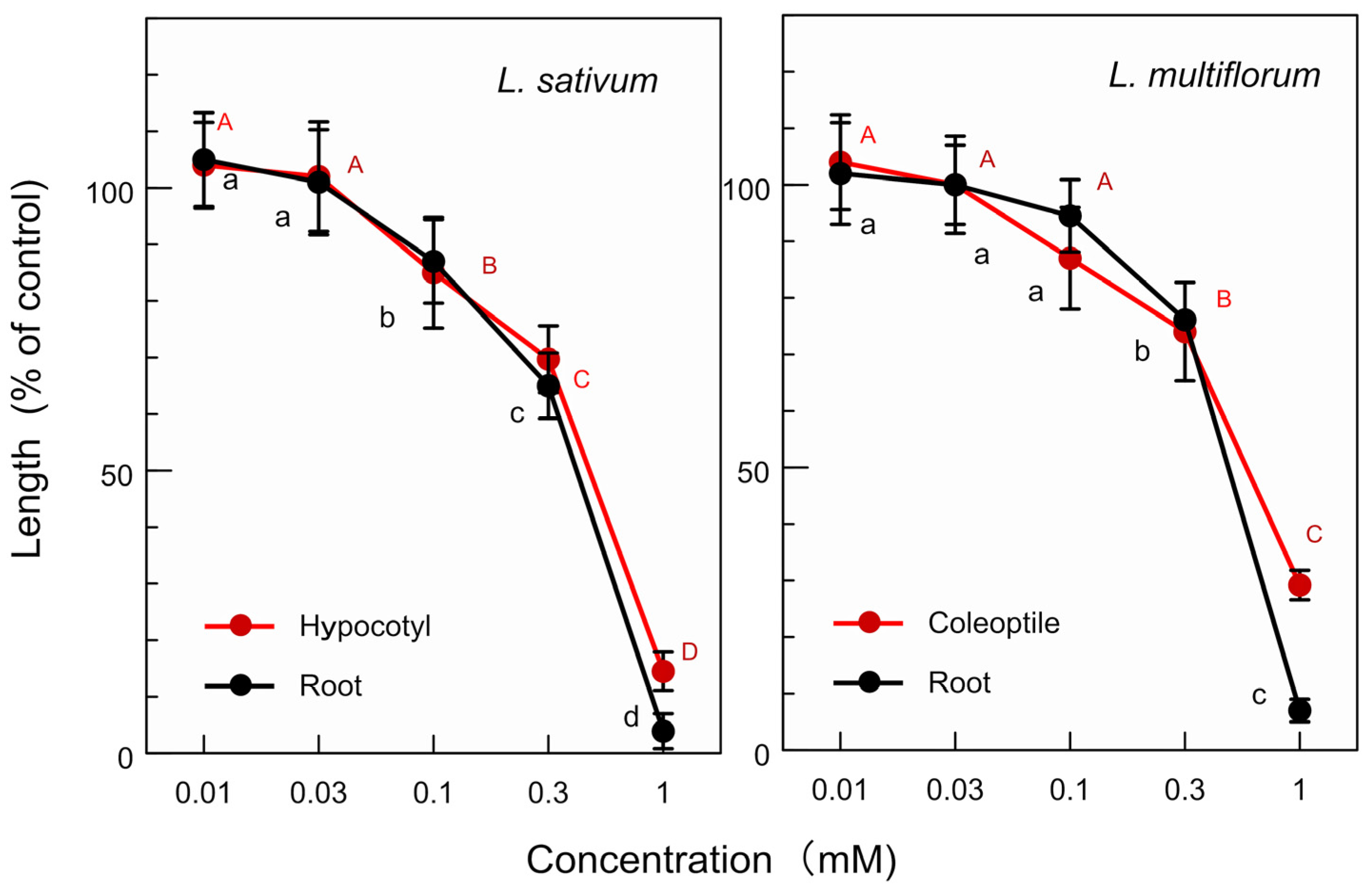
Lipiferolide showed significant growth inhibition at a p < 0.05 level on the L. sativum hypocotyls and roots at concentrations ≥ 0.1 mM, as well as on the L. multiflorum coleoptiles and roots at concentrations ≥ 0.3 mM (Figure 8). The IC50 value of lipiferolide for the L. sativum hypocotyl and root growth was 0.51 mM and 0.4 mM, respectively, and its IC50 value for the L. multiflorum coleoptile and root growth was 0.61 mM and 0.47 mm, respectively.
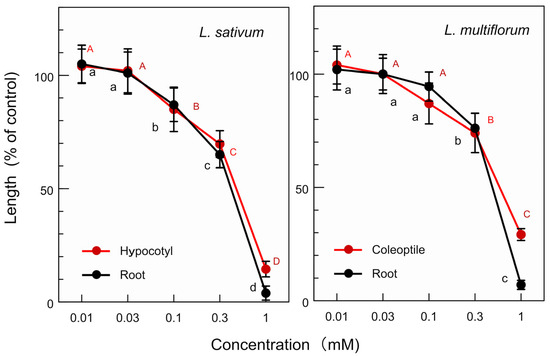
Figure 8.
Effects of lipiferolide on the L. sativum hypocotyl and root growth, as well as on the L. multiflorum coleoptile and root growth. The means ± SE were calculated from four independent experiments with 10 seeds (L. sativum) and 10 germinated seeds (L. multiflorum) for each determination. Different letters on the bars in the same panels indicate significant differences at a p < 0.05 level.
4. Discussion
4.1. Allelopathic Activity of the Fresh and Fallen Leaves of L. tulipifera
L. tulipifera is a popular timber species, and logging operations of the trees produce a large amount of leaf waste [1,2,3,6,7,8,9]. L. tulipifera is also grown in residential areas as shade and ornamental trees, and the fallen leaves accumulate on the streets and ground in the autumn. These fallen leaves need to be organized at a large expense [20]. However, there is no information available on the allelopathic activity and allelochemicals in the leaves of L. tulipifera on the literature. Therefore, we have examined the allelopathic activity of L. tulipifera fresh and fallen leaves under laboratory and greenhouse conditions. The fresh leaves were used as substitutes for leaf waste from the logging operations of L. tulipifera. The fresh leaf extracts showed significant growth inhibition on the roots and coleoptiles of weed plant species—V. myuros, E. crus-galli, and L. multiflorum—under laboratory conditions (Figure 3). The fallen leaf extracts also showed significant growth inhibition on these weed species (Figure 4). However, the sensitivity of these weed species differed to the fresh and fallen leaf extracts, and differed in the organs of test plant species (coleoptiles and roots). Comparing their IC50 values, the sensitivity of the E. crus-galli roots to the fresh leaf extracts was the highest among the roots and coleoptiles of these weed species, and that of the L. multiflorum coleoptiles to the fallen leaf extracts was the lowest (Table 1). The IC50 values of the fallen leaf extracts were 2- and 2.8-fold, 4.3- and 7.2-fold, and 6.5- and 6.6-fold greater than those of the fresh leaf extracts for the coleoptiles and roots of V. myuros, E. crus-galli, and L. multiflorum, respectively, which indicates that the allelopathic activity of the fresh leaves is greater than that of the fallen leaves. These results suggest that the L. tulipifera fresh and fallen leaves may contain extractable allelochemicals, and the fresh leaves may contain more allelochemicals than the fallen leaves.
The fresh and fallen leaf powder of L. tulipifera significantly suppressed the germination of E. crus-galli under greenhouse conditions (Figure 6). The IC50 value of the fallen leaf powder was 3.1-fold greater than that of the fresh leaf powder, which consists of the IC50 values of the fresh and fallen leaf extracts (Table 1). As described in Materials and Methods (Section 2.3.), E. crus-galli seeds were sown 9 days after the leaf powder was applied onto the soil. Therefore, allelochemicals in the L. tulipifera leaf powder may be released into soils within 9 days, and may cause the suppression of the germination (Figure 6). In addition, the amount of released allelochemicals from the fresh leaves may be greater than that from the fallen leaves.
As described in the Introduction, when certain plant parts of some allelopathic plant species were incorporated into soil, these plant parts suppressed the germination and growth of several weed species in greenhouse and field conditions due to their allelochemicals [40,41,42,43,44]. When the soaking water and extracts of some allelopathic plant leaves were applied to several weed species as a foliar spray and/or irrigation water, the germination and growth of several weed species were restricted [40,41,42,43,44,51,52,53,54,55,56,57]. The fresh and fallen leaves of L. tulipifera may contain certain allelochemicals (Figure 3, Figure 4 and Figure 5), and some of these allelochemicals in the leaves may be released into soils and cause the suppression of the germination of weed species (Figure 6). Therefore, the fresh and fallen leaves of L. tulipifera are potentially useful as soil additive materials to control weeds in a variety of agricultural practices. The leaf extracts may also be applied as a foliar spray to control weeds. However, the weed control activity of the leaves in a soil additive material and that of the leaf extracts as a foliar spray under large-field condition should be evaluated.
4.2. An Allelochemical in L. tulipifera Leaves
The fresh and fallen leaf extracts of L. tulipifera were purified through the bioassay-guided separation process using silica gel chromatography, Sephadex LH-20 chromatography, an ODS cartridge, and reverse-phase HPLC (Figure 2). During the separation process, the allelopathic activity of all separated fractions was determined, and the most active fraction was applied to the next separation process. L. sativum was selected as a bioassay plant species for the isolation process because of its stable germination rate and easy handling [58]. The germination of L. sativum was high (about 90% germination rate at 48 h after sowing) compared with the weed species—V. myuros, E. crus-galli, and L. multiflorum (20–40% germination rate). The sensitivity of the L. sativum hypocotyls and roots to the L. tulipifera fresh and fallen leaf extracts was not too high and not too low in comparison with the IC50 values of these weed species (Figure 3, Figure 4 and Figure 5 and Table 1).
An active compound, 1, was isolated from both fresh and fallen leaf extracts and characterized by the spectrum data as lipiferolide (Figure 7). Lipiferolide suppressed the growth of L. sativum and L. multiflorum in a concentration-dependent manner (Figure 8). Lipiferolide was first isolated from L. tulipifera leaves [59,60], and has been isolated from other Magnoliaceae species, Liriodendron chinensis (Hemsl.) Sarg., Magnolia sirindhorniae, Michelia yunnanensis Noot & Chalermglin, and Michelia fuscata (Lour.) DC. [61,62,63,64], and the Asteraceae species Vicoa pentanema Aitch. & Hemsl. [65]. Lipiferolide showed cytotoxic activity against human cancer cell lines, such as KB cells and Hepa lclc7 cells [59,66], and antiplasmodial activity [23]. However, it is the first report on the biological activity and allelopathic activity of lipiferolide against plant species, including weed species. The mode of action of lipiferolide on allelopathic activity and its concentration in soil should be evaluated in the furfure. Lipiferolide is only commercially available for laboratories but not for practical use.
Lipiferolide in the leaves of L. tulipifera may be involved in the allelopathic activity of the fresh and fallen leaf extracts and the leaf powder (Figure 3, Figure 4 and Figure 6). The fresh leaves may contain greater amounts of lipiferolide than fallen leaves because 12 mg and 4 mg of lipiferolide were isolated from the fresh and fallen leaves, respectively. One of the reasons for the lower amount of lipiferolide in the fallen leaves may be the metabolism of the compound during the defoliation and/or degradation process of the leaves [67,68,69,70]. The concentrations of lipiferolide in the fresh and fallen leaves are consistent with the allelopathic activity of the extracts and powder obtained from the fresh and fallen leaves (Figure 3, Figure 4, Figure 5 and Figure 6). Therefore, lipiferolide in the fresh and fallen leaf powder of L. tulipifera may be liberated into soil at the same proportion and suppress germination.
5. Conclusions
The fresh and fallen leaf extracts and leaf powder of L. tulipifera showed allelopathic activity against weed species—V. myuros, E. crus-galli, and/or L. multiflorum—under laboratory and greenhouse conditions. Lipiferolide was isolated from the fresh and fallen leaves of L. tulipifera as an allelochemical. Lipiferolide inhibited the growth of L. sativum and L. multiflorum in a concentration-dependent manner. Therefore, both fresh and fallen leaf extracts, as well as the leaves themselves, can be applied as a foliar spray and soil mixture, respectively, to suppress the germination and growth of certain weed species. Therefore, the leaves of L. tulipifera are potentially useful for the purpose of weed control. Logging operations of timber trees and the fallen leaves in autumn of ornamental plants produce a large amount of leaf waste. The development of weed control materials using L. tulipifera leaf waste may be one solution to reduce environmental impacts and economic concerns.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14188437/s1, Figure S1: Effects of the fractions separated by a silica gel column chromatography on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 30 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level; Figure S2: Effects of the fractions separated by a silica gel column chromatography on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 100 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level; Figure S3: Effects of the fractions separated by a Sephadex LH-20 column chromatography on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 30 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level; Figure S4: Effects of the fractions separated by a Sephadex LH-20 column chromatography on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 100 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level; Figure S5: Effects of the fractions separated by a ODS cartridge on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 50 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level; Figure S5: Effects of the fractions separated by a ODS cartridge on root and coleoptile growth of L. sativum. Concentration of tested samples corresponded to the extract obtained from 150 mg of L. tulipifera leaves per mL. Means ± SE were calculated from 4 independent experiments with 10 seedlings for each determination. Different letters on the bars indicate significant differences at p < 0.05 level.
Author Contributions
Conceptualization, H.K.-N.; methodology, K.H., A.I., K.S. and H.K.-N.; software K.H.; validation, K.S. and H.K.-N.; formal analysis, K.H.; investigation, K.H. and A.I.; data curation, K.S. and H.K.-N.; writing, H.K.-N.; visualization, H.K.-N.; supervision, H.K.-N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Plant Database. Liriodendron tulipifera. Available online: https://www.wildflower.org/plants/result.php?id_plant=LITU (accessed on 8 July 2024).
- US Forest Service. Liriodendron tulipifera. Available online: https://www.fs.usda.gov/database/feis/plants/tree/lirtul/all.html (accessed on 8 July 2024).
- Ryu, K.O.; Jang, S.S.; Choi, W.Y.; Kim, H.E. Growth performance and adaptation of Liriodendron tulipifera in Korea. J. Korean For. Soc. 2003, 92, 515–525. [Google Scholar]
- Bozsik, A. Spread and occurrence of tulip tree aphid in Europe: New record of Illinoia liriodendri (Monell, 1879) (Hemiptera: Aphididae) from Hungary. EPPO Bull. 2012, 42, 154–157. [Google Scholar] [CrossRef]
- Cao, Y.; Feng, J.; Hwarari, D.; Ahmad, B.; Wu, H.; Chen, J.; Yang, L. Alterations in population distribution of Liriodendron chinense (Hemsl.) Sarg. and Liriodendron tulipifera Linn. caused by climate change. Forests 2022, 13, 488. [Google Scholar] [CrossRef]
- Gilman, E.F.; Watson, D.G. Liriodendron tulipifera: Tuliptree. UF-IFAS 1993, 363, 1–4. [Google Scholar]
- Spongberg, S.A.; Bell, A.C. Liriodendron tulipifera: The tulip tree. New Plantsman 1996, 3, 204–215. [Google Scholar]
- USDA, Forest Service. Yellow-Poplar (Liriodendron tulipifera). Available online: https://www.fs.usda.gov/nrs/atlas/tree/v3/621 (accessed on 8 July 2024).
- The Wood Database (Hardwood). Yellow Poplar. Available online: https://www.wood-database.com/yellow-poplar/ (accessed on 8 July 2024).
- Hernandez, R.; Davalos, J.F.; Sonti, S.S.; Kim, Y.; Moody, R.C. Strength and Stiffness of Reinforced Yellow-Poplar Glued Laminated Beams; US Department of Agriculture, Forest Service, Forest Products Laboratory: Washington, DC, USA, 1997; Volume 554, pp. 1–23. [Google Scholar]
- Williams, R.S.; Feist, W.C. Durability of yellow-poplar and sweetgum and service life of finishes after long-term exposure. For. Prod. J. 2004, 54, 96–101. [Google Scholar]
- Kim, M.J.; Lee, S.J.; Kim, S.; Yang, M.S.; Son, D.W.; Kim, C.K. Study on the combustion characteristics of tulip tree (Liriodendron tulipifera) for use as interior building materials. J. Korean Wood Sci. Technol. 2023, 51, 410–418. [Google Scholar] [CrossRef]
- Dionco-Adetayo, E.A. Utilization of wood wastes in Nigeria: A feasibility overview. Technovation 2001, 21, 55–60. [Google Scholar] [CrossRef]
- Eshun, J.F.; Potting, J.; Leemans, R. Wood waste minimization in the timber sector of Ghana: A systems approach to reduce environmental impact. J. Clean. Prod. 2012, 26, 67–78. [Google Scholar] [CrossRef]
- Adhikari, S.; Ozarska, B. Minimizing environmental impacts of timber products through the production process “From Sawmill to Final Products”. Environ. Syst. Res. 2018, 7, 6. [Google Scholar] [CrossRef]
- Kim, K.H.; Eom, I.Y.; Lee, S.M.; Choi, D.; Yeo, H.; Choi, I.G.; Choi, J.W. Investigation of physicochemical properties of biooils produced from yellow poplar wood (Liriodendron tulipifera) at various temperatures and residence times. J. Anal. Appl. Pyrolysis 2011, 92, 2–9. [Google Scholar] [CrossRef]
- Ahn, B.J.; Chang, H.S.; Lee, S.M.; Choi, D.H.; Cho, S.T.; Han, G.S.; Yang, I. Effect of binders on the durability of wood pellets fabricated from Larix kaemferi C. and Liriodendron tulipifera L. sawdust. Renew. Energy 2014, 62, 18–23. [Google Scholar] [CrossRef]
- Jung, J.Y.; Choi, M.S.; Kim, J.S.; Jeong, M.J.; Kim, Y.W.; Woon, B.T.; Yeo, J.K.; Shin, H.N.; Goo, Y.B.; Ryu, K.O.; et al. Enzymatic hydrolysate from non-pretreated biomass of yellow poplar (Liriodendron tulipifera) is an alternative resource for bioethanol production. J. Korean Soc. For. Sci. 2010, 99, 744–749. [Google Scholar]
- Kim, H.Y.; Gwak, K.S.; Lee, S.Y.; Jeong, H.S.; Ryu, K.O.; Choi, I.G. Biomass characteristics and ethanol production of yellow poplar (Liriodendron tulipifera) treated with slurry composting and biofiltration liquid as fertilizer. Biomass Bioenergy 2012, 42, 10–17. [Google Scholar] [CrossRef]
- Boldrin, A.; Christensen, T.H. Seasonal generation and composition of garden waste in Aarhus (Denmark). Waste Manag. 2010, 30, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Rafinesque, C.S. Medical Flora; Or, Manual of the Medical Botany of the United States of North America; Atkinson & Alexander: Philadelphia, PA, USA, 1828; pp. 1–376. [Google Scholar]
- Thacher, J. American Medical Biography; Or, Memoirs of Eminent Physicians Who Have Flourished in America, to Which is Prefixed a Succinct History of Medical Science in the United States from the First Settlement of the Country; Milford House: New York, NY, USA, 1967; pp. 1–799. [Google Scholar]
- Graziose, R.; Rathinasabapathy, T.; Lategan, C.; Poulev, A.; Smith, P.J.; Grace, M.; Lia, M.A.; Raskin, I. Antiplasmodial activity of aporphine alkaloids and sesquiterpene lactones from Liriodendron tulipifera L. J. Ethnopharmacol. 2011, 133, 26–30. [Google Scholar] [CrossRef]
- Kang, Y.F.; Liu, C.M.; Kao, C.L.; Chen, C.Y. Antioxidant and anticancer constituents from the leaves of Liriodendron tulipifera. Molecules 2014, 19, 4234–4245. [Google Scholar] [CrossRef]
- Chen, L.C.; Chang, H.M. Lignans and aporphine alkaloids in bark of Liriodendron tulipifera. Phytochemistry 1978, 17, 779–782. [Google Scholar] [CrossRef]
- Hufford, C.D.; Funderburk, M.J.; Morgan, J.M.; Robertson, L.W. Two antimicrobial alkaloids from heartwood of Liriodendron tulipifera L. J. Pharm. Sci. 1975, 64, 789–792. [Google Scholar] [CrossRef]
- Muhammad, I.; Hufford, C.D. Phenylpropanoids, sesquiterpenes, and alkaloids from the seeds of Liriodendron tulipifera. J. Nat. Prod. 1989, 52, 1177–1179. [Google Scholar] [CrossRef]
- Lee, C.H.; Chen, H.L.; Hong, Z.L.; Hsieh, C.W.; Juan, S.W.; Huang, J.C.; Wang, H.M.; Chen, C.Y. Chemical constituents of Liriodendron tulipifera. Chem. Nat. Compd. 2013, 49, 398–400. [Google Scholar] [CrossRef]
- Mikašauskaitė, J.; Ragažinskienė, O.; Maruška, A. Variation of total amount of phenolic compounds, radical scavenging activity and volatile compounds of Liriodendron tulipifera L. and Ginkgo biloba L. leaves extracts during different vegetation periods. Biologija 2013, 59, 175–186. [Google Scholar] [CrossRef]
- Yadav, A.K.; Kim, S.H.; Kang, S.C. Chemical composition, antioxidant potential and cyto-protecting activity of essential oil of Liriodendron tulipifera L. leaves. Korea J. Herbol. 2015, 30, 1–9. [Google Scholar] [CrossRef]
- Quassinti, L.; Maggi, F.; Ortolani, F.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Miano, A.; Bramucci, M. Exploring new applications of tulip tree (Liriodendron tulipifera L.): Leaf essential oil as apoptotic agent for human glioblastoma. Environ. Sci. Pollut. Res. 2019, 26, 30485–30497. [Google Scholar] [CrossRef]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.G.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and Its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef]
- Narwal, S.S. Allelopathy in weed management. In Allelopathy Update; Basic and Applied Aspects; Narwal, S.S., Ed.; Science Publishers Inc.: Enfield, NH, USA, 1999; Volume 2, pp. 203–254. [Google Scholar]
- Global Biodiversity Information Facility. Vulpia myuros. Available online: https://www.gbif.org/species/5289590 (accessed on 8 July 2024).
- Global Biodiversity Information Facility. Lolium multiflorum. Available online: https://www.gbif.org/species/4129708 (accessed on 8 July 2024).
- Global Biodiversity Information Facility. Echinochloa crus-galli. Available online: https://www.gbif.org/species/2702808 (accessed on 8 July 2024).
- Kato-Noguchi, H.; Mori, K.; Iwasaki, A.; Suenaga, K. Allelopathy and allelochemicals in Chamaecyparis obtusa leaves for the development of sustainable agriculture. Agronomy 2024, 14, 1557. [Google Scholar] [CrossRef]
- Rao, A.N.; Johnson, D.E.; Sivaprasad, B.; Ladha, J.K.; Mortimer, A.M. Weed management in direct-seeded rice. Adv. Agron. 2007, 93, 153–255. [Google Scholar]
- Hirai, N.; Yoshida, R.; Todoroki, Y.; Ohigashi, H. Biosynthesis of abscisic acid by the non-mevalonate pathway in plants, and by the mevalonate pathway in fungi. Biosci. Biotechnol. Biochem. 2000, 64, 1448–1458. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef]
- Heděnec, P.; Novotný, D.; Ust’ak, S.; Honzík, R.; Kovářová, M.; Šimáčková, H.; Frouz, J. Allelopathic effect of new introduced biofuel crops on the soil biota: A comparative study. Eur. J. Soil Biol. 2014, 63, 14–20. [Google Scholar] [CrossRef]
- Inderjit, S.; Dakshini, K.M.M. Investigations on some aspects of chemical ecology of cogongrass, Imperata cylindrica (L.) Beauv. J. Chem. Ecol. 1991, 17, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Hui-Qiong, Z.; Ning, W.; Liu-Fa, W.; Ping, H.E. Effects of Lantana camara leaf extract on the activity of superoxide dismutase and accumulation of H2O2 in water hyacinth leaf. J. Plant Physiol. Mol. Biol. 2006, 32, 189–194. [Google Scholar]
- Poonpaiboonpipat, T.; Poolkum, S. Utilization of Bidens pilosa var. radiata (Sch. Bip.) Sherff integrated with water irrigation for paddy weed control and rice yield production. Weed Biol. Manag. 2019, 19, 31–38. [Google Scholar] [CrossRef]
- Duke, S.O.; Dayan, F.E.; Romagni, J.G.; Rimando, A.M. Natural products as sources of herbicide, current status and future trends. Weed Res. 2000, 40, 99–111. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hamada, Y.; Kojima, M.; Kumagai, S.; Iwasaki, A.; Suenaga, K. Allelopathic substances of Osmanthus spp. for developing sustainable agriculture. Plants 2023, 12, 376. [Google Scholar] [CrossRef]
- Doskotch, R.W.; Keely, S.L., Jr.; Hufford, C.D.; El-Feraly, F.S. New sesquiterpene lactones from Liriodendron tulipifera. Phytochemistry 1975, 14, 769–773. [Google Scholar] [CrossRef]
- Barbosa, P.; Gross, P.; Provan, G.J.; Pacheco, D.Y.; Stermitz, F.R. Allelochemicals in foliage of unfavored tree hosts of the gypsy moth, Lymantria dispar L. 1. Alkaloids and other components of Liriodendron tulipifera L. (Magnoliaceae), Acer rubrum L. (Aceraceae), and Cornus florida L. (Cornaceae). J. Chem. Ecol. 1990, 16, 1719–1730. [Google Scholar] [CrossRef]
- Iida, T.; Ito, K. Sesquiterpene lactones from Michelia fuscata. Phytochemistry 1982, 21, 701–703. [Google Scholar] [CrossRef]
- Ding, L.F. Sesquiterpenes constituents from fruits of Michelia yunnanensis. Chin. Tradit. Herb. Drugs 2017, 48, 2608–2613. [Google Scholar]
- Katekunlaphan, T.; Chalermglin, R.; Rukachaisirikul, T.; Chalermglin, P. Sesquiterpene lactones from the leaves of Magnolia sirindhorniae. Biochem. Sstem. Ecol. 2014, 57, 152–154. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, L.J.; Qian, J.; Wang, P.P.; Wang, X.J.; Ma, G.L.; Zeng, H.; Hu, J.F. Structurally diverse sesquiterpenoids from the endangered ornamental plant Michelia shiluensis. J. Nat. Prod. 2018, 81, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Mossa, J.S.; El-Feraly, F.S.; Muhammad, I.; Zaw, K.; Mbwambo, Z.H.; Pezzuto, J.M.; Fong, H.H. Sesquiterpene lactones and thymol esters from Vicoa pentanema. J. Nat. Prod. 1997, 60, 550–555. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, D.; Huang, J.; Zhang, P. Sesquiterpenes with quinone reductase-inducing activity from Liriodendron chinense. Nat. Prod. Commun. 2009, 4, 467–468. [Google Scholar] [CrossRef]
- Moon, M.K.; Oh, H.M.; Kwon, B.-M.; Baek, N.-I.; Kim, S.-H.; Kim, J.S.; Kim, D.K. Farnesyl protein transferase and tumor cell growth inhibitory activities of lipiferolide isolated from Liriodendron tulipifera. Arch. Pharm. Res. 2007, 30, 299–302. [Google Scholar] [CrossRef]
- Macías, F.A.; Marín, D.; Oliveros-Bastidas, A.; Simonet, A.M.; Molinillo, J.M. Ecological relevance of the degradation processes of allelochemicals. In Allelopathy; Fujii, Y., Hiradate, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 91–107. [Google Scholar]
- Jilani, G.; Mahmood, S.; Chaudhry, A.N.; Hassan, I.; Akram, M. Allelochemicals: Sources, toxicity and microbial transformation in soil—A review. Ann. Microbiol. 2008, 58, 351–357. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kimura, F.; Ohno, O.; Suenaga, K. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).