Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement
Abstract
1. Introduction
2. Materials and Methods
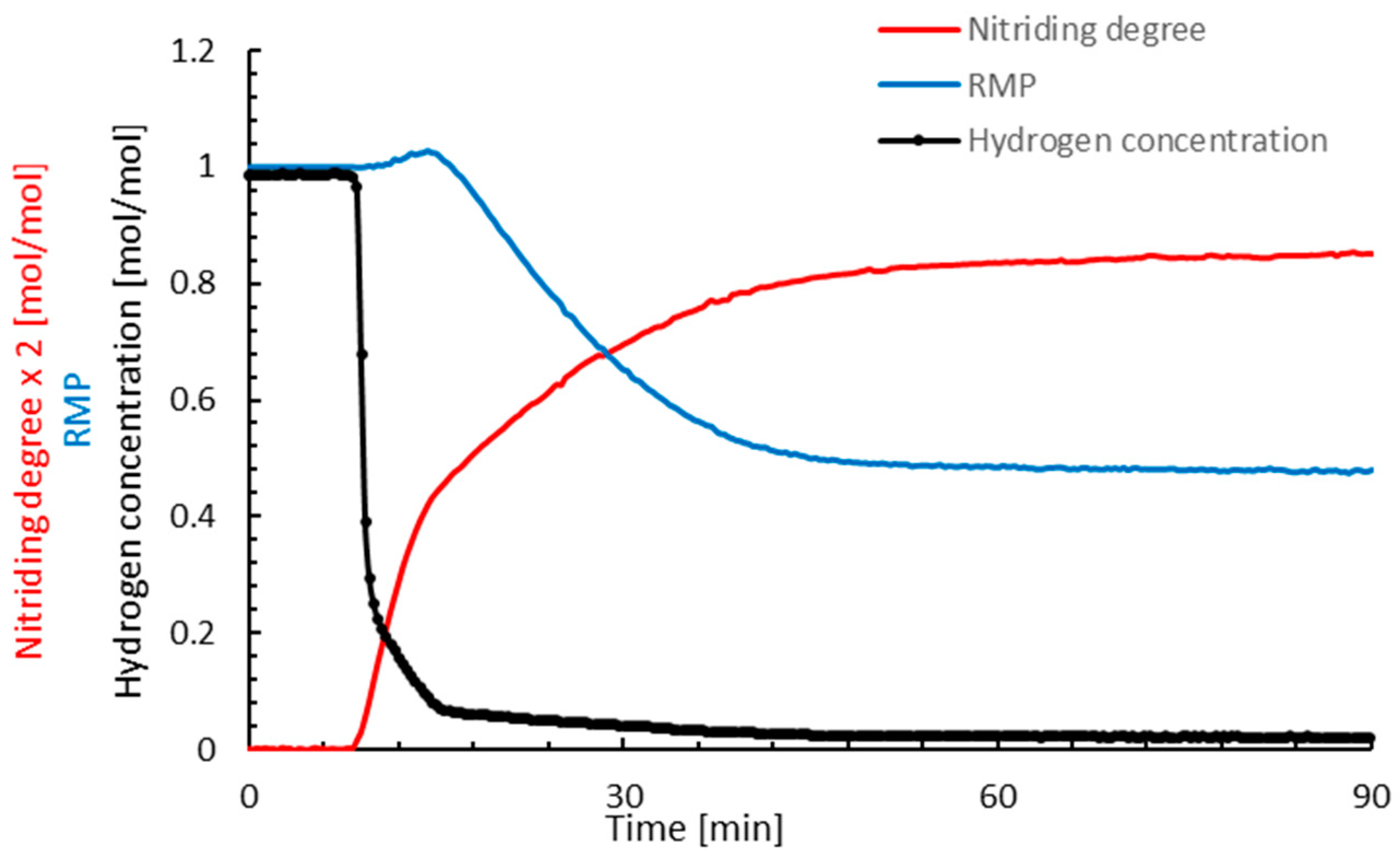
3. Results
4. Discussion
4.1. Thermodynamics of Phase Transformation
- ∆GFe,ib,α-[γ]*–Gibbs free energy change for crystal lattice transformation of phases α-Fe(N) → [γ’]*-Fe4−xN;
- ∆GN,ib–Gibbs free energy change of nitrogen dissolved in a volume of iron nanocrystallite;
- ∆GN,is–Gibbs free energy change of nitrogen adsorbed on iron nanocrystallite surface;
- ∆GFe,is–surface energy change of iron nanocrystallite during phase transition process;
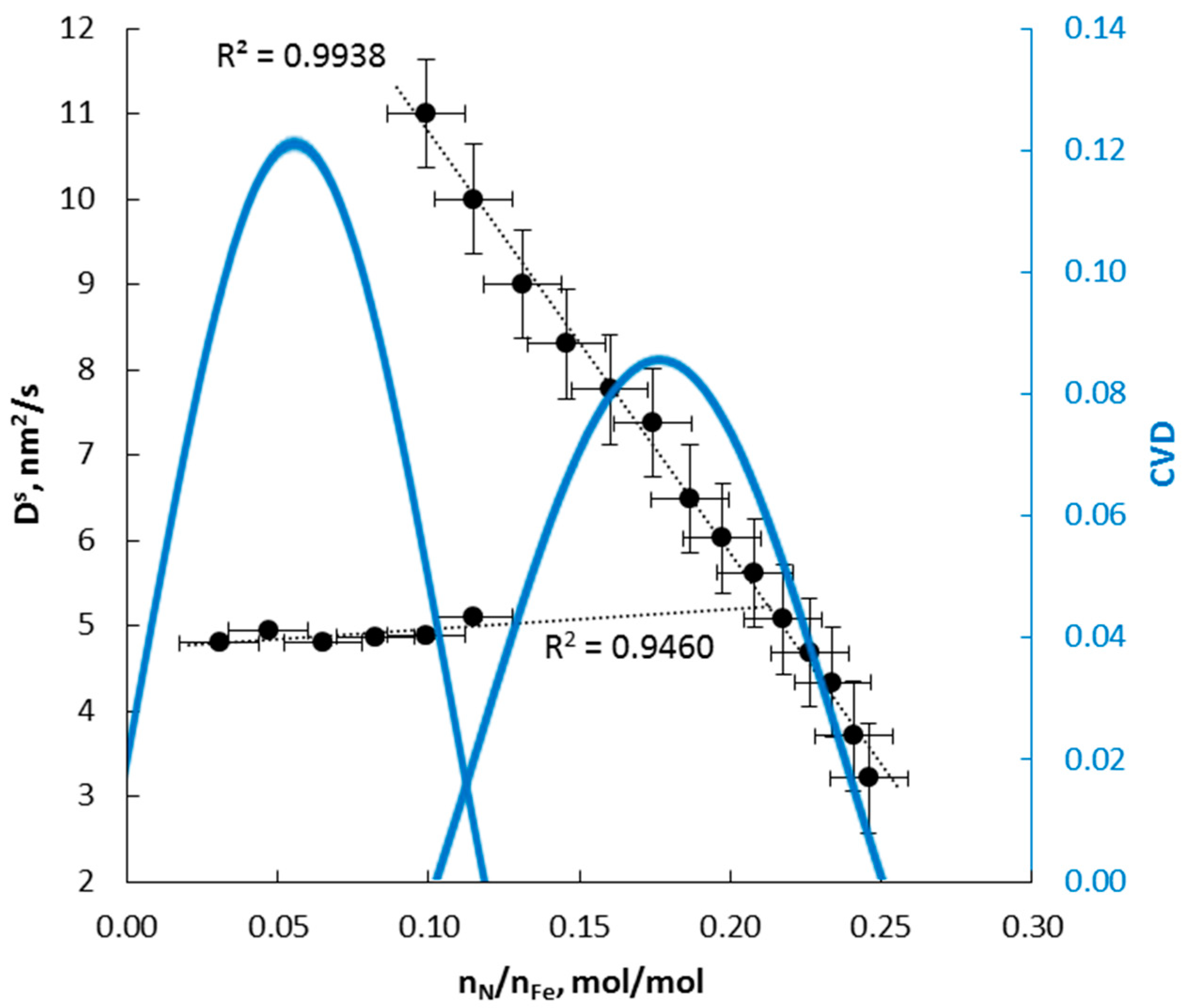
4.2. Kinetics of Phase Transformation
- for small nanocrystallites and low concentrations (nitrogen solution in the γ’ lattice)
- for large nanocrystallites and high concentrations:
5. Conclusions
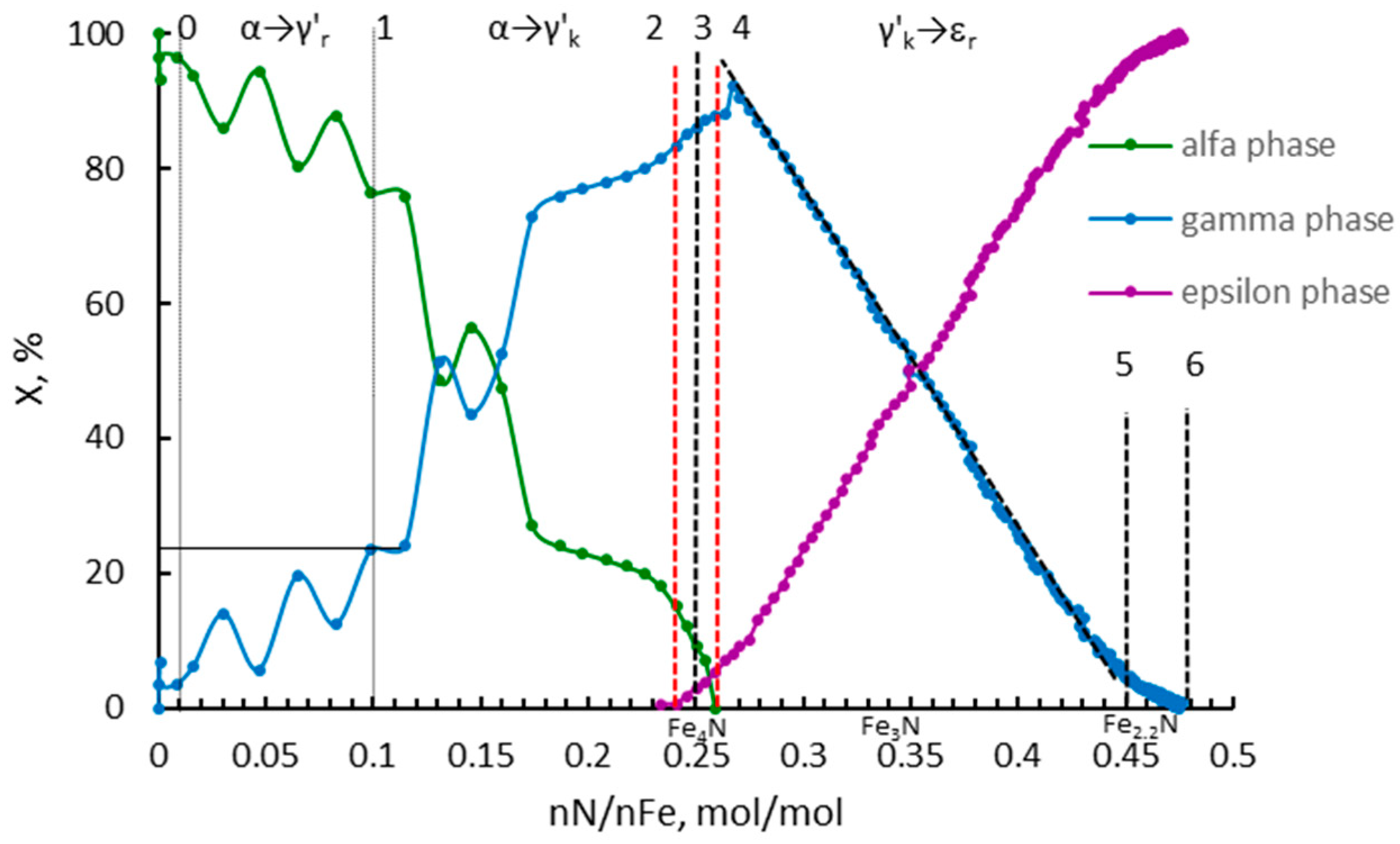
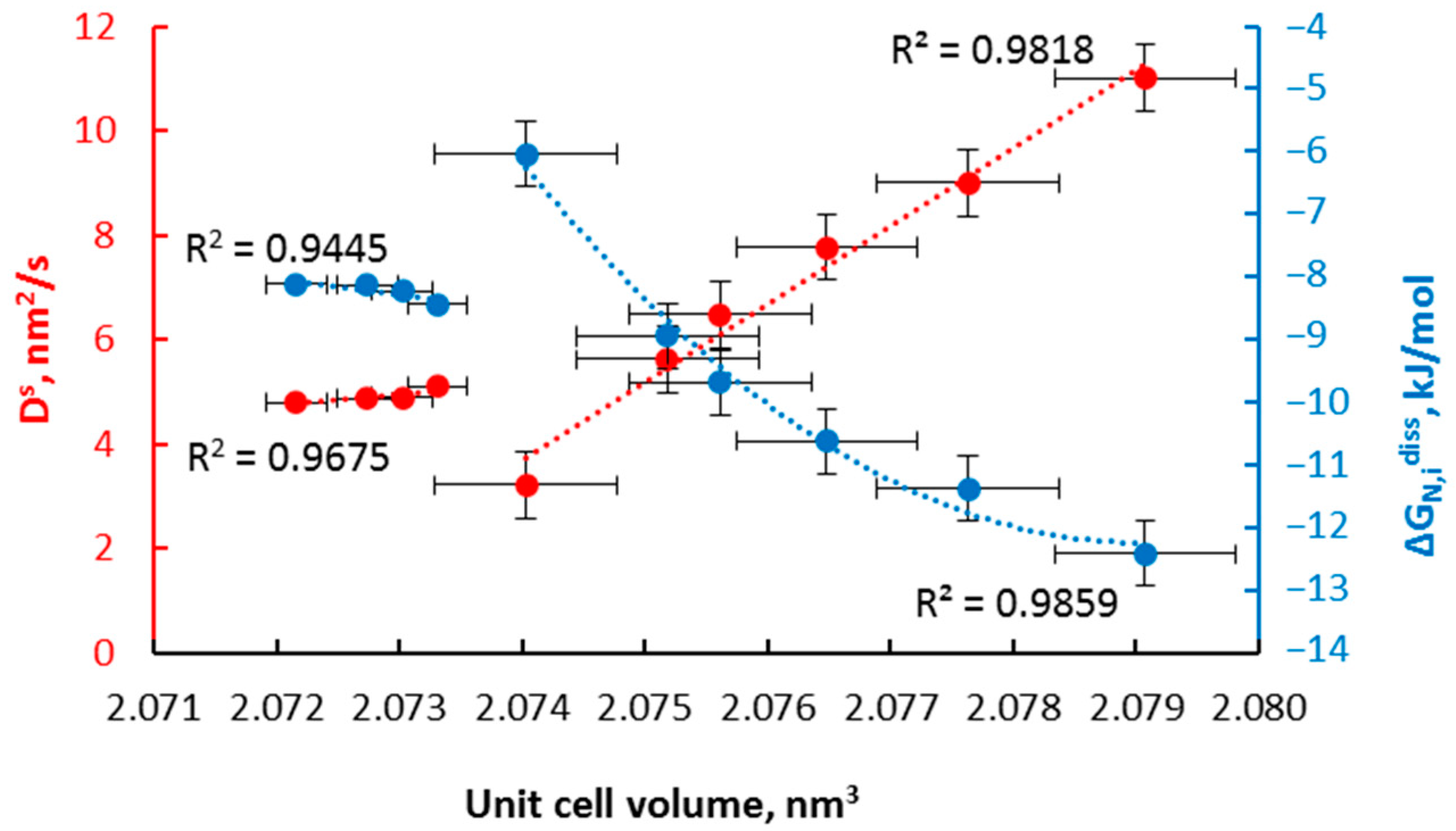
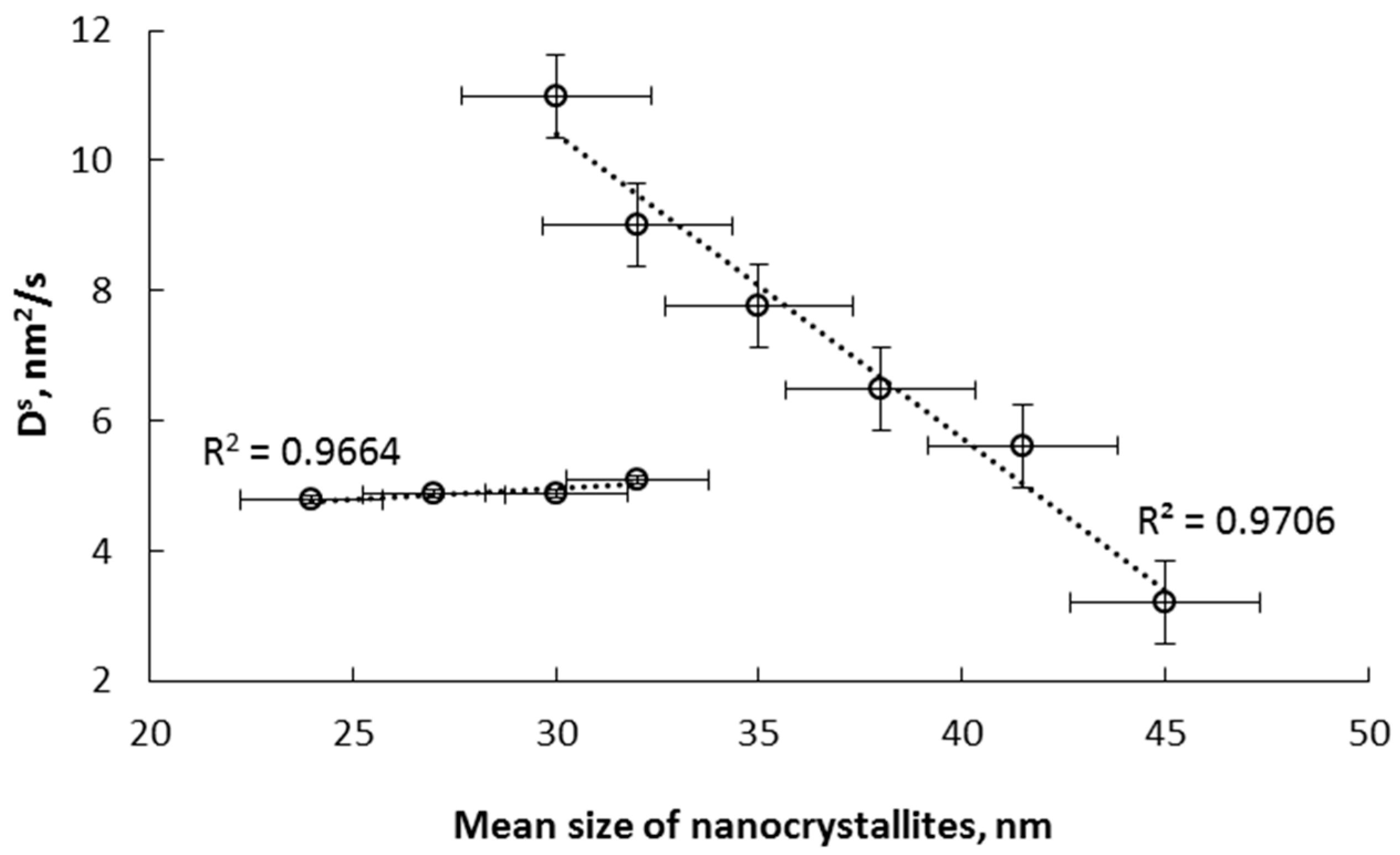
- α→γ’r (smaller nanocrystallites, relative magnetic permeability of 1.02); the dependencies typical for solutions are observed; the relationships of diffusion coefficient and unit cell volume are linear, with negligible variation in the diffusion coefficient, the volume of unit cells, and relative magnetic permeability,
- α→γ’k (bigger nanocrystallites, relative magnetic permeability of 1.00); the relationships are also described by a linear function with a significant variation in the diffusion coefficient, the volume of unit cells, mean size of nanocrystallites, and relative magnetic permeability. In the γ‘k area, changes are observed that indicate step changes in the crystal lattice.
- γ’k→εr: in the range of nitriding degree of 0.25–0.45 mol/mol there is a phase transformation of iron nitride γ’-Fe4N to the εr-FexN solution with concentration x = 0.45 mol/mol in the order of the crystal size from the smallest to the largest, with an approximately constant value of the relative magnetic permeability of 0.42. With the further nitriding process, only the nitrogen concentration in the solution phase εr increases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siddiquee, S.; Hong, M.G.J.; Rahman, M.M. Nanotechnology: Applications in Energy, Drug and Food, 1st ed.; Springer International Publishing: New York, NY, USA, 2019. [Google Scholar]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Damodharan, J. Nano-materials in medicine—An overview. Mater. Today Proc. 2021, 37, 382–385. [Google Scholar] [CrossRef]
- Wang, Z.; Colombi Ciacchi, L.; Wei, G. Recent Advances in the Synthesis of Graphene-Based Nanomaterials for Controlled Drug Delivery. Appl. Sci. 2017, 7, 1175. [Google Scholar] [CrossRef]
- Thwala, L.N.; Ndlovu, S.C.; Mpofu, K.T.; Lugongolo, M.Y.; Mthunzi-Kufa, P. Nanotechnology-Based Diagnostics for Diseases Prevalent in Developing Countries: Current Advances in Point-of-Care Tests. Nanomaterials 2023, 13, 1247. [Google Scholar] [CrossRef]
- Mansuriya, B.D.; Altintas, Z. Graphene Quantum Dot-Based Electrochemical Immunosensors for Biomedical Applications. Materials 2020, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, X. Unique Role of Self-Assembled Monolayers in Carbon Nanomaterial-Based Field-Effect Transistors. Small 2013, 9, 1144–1159. [Google Scholar] [CrossRef]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for printed electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef]
- Gohar, O.; Khan, M.Z.; Bibi, I.; Bashir, N.; Tariq, U.; Bakhtiar, M.; Motola, M. Nanomaterials for advanced energy applications: Recent advancements and future trends. Mater. Des. 2024, 241, 112930. [Google Scholar] [CrossRef]
- Singh, S.; Kadyan, S.; Sheoran, S.; Mari, B.; Singh, D. Synthesis and optical investigations of Eu3+ activated MYAlO4 (M = Ca and Sr) as promising display nanomaterials. Optik 2020, 208, 164552. [Google Scholar] [CrossRef]
- Thiruppathi, A.R.; Sidhureddy, B.; Boateng, E.; Soldatov, D.V.; Chen, A. Synthesis and Electrochemical Study of Three-Dimensional Graphene-Based Nanomaterials for Energy Applications. Nanomaterials 2020, 10, 1295. [Google Scholar] [CrossRef]
- Santangelo, S. Electrospun Nanomaterials for Energy Applications: Recent Advances. Appl. Sci. 2019, 9, 1049. [Google Scholar] [CrossRef]
- Liao, L.; Wang, M.; Li, Z.; Wang, X.; Zhou, W. Recent Advances in Black TiO2 Nanomaterials for Solar Energy Conversion. Nanomaterials 2023, 13, 468. [Google Scholar] [CrossRef] [PubMed]
- Lou, D.; Chen, S.; Langrud, S.; Razzaq, A.A.; Mao, M.; Younes, H.; Xing, W.; Lin, T.; Hong, H. Scalable Fabrication of Si-Graphene Composite as Anode for Li-ion Batteries. Appl. Sci. 2022, 12, 10926. [Google Scholar] [CrossRef]
- Khalil, M.; Kadja, G.T.; Ilmi, M.M. Advanced nanomaterials for catalysis: Current progress in fine chemical synthesis, hydrocarbon processing, and renewable energy. J. Ind. Eng. Chem. 2021, 93, 78–100. [Google Scholar] [CrossRef]
- Utsev, T.; Tiza, T.M.; Mogbo, O.; Singh, S.K.; Chakravarti, A.; Shaik, N.; Pal Singh, S. Application of nanomaterials in civil engineering. Mater. Today Proc. 2022, 62, 5140–5146. [Google Scholar] [CrossRef]
- Papadaki, D.; Kiriakidis, G.; Tsoutsos, T. Applications of Nanotechnology in Construction Industry in Micro and Nano Technologies. In Fundamentals of Nanoparticles, 1st ed.; Barhoum, A., Makhlouf, A.S.H., Eds.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2018; pp. 343–370. [Google Scholar] [CrossRef]
- Lazaro, A.; Yu, Q.L.; Brouwers, H.J.H. Nanotechnologies for Sustainable Construction in Sustainability of Construction Materials, 2nd ed.; Khatib, J.M., Ed.; Woodhead Publishing: Cambridge, United Kingdom, 2016; pp. 55–78. [Google Scholar] [CrossRef]
- Kirthika, S.K.; Goel, G.; Matthews, A.; Goel, S. Review of the untapped potentials of antimicrobial materials in the construction sector. Prog. Mater. Sci. 2023, 133, 101065. [Google Scholar] [CrossRef]
- Mohajerani, A.; Burnett, L.; Smith, J.V.; Kurmus, H.; Milas, J.; Arulrajah, A.; Horpibulsuk, S.; Abdul Kadir, A. Nanoparticles in Construction Materials and Other Applications, and Implications of Nanoparticle Use. Materials 2019, 12, 3052. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Soldado, E.; Borsoi, G.; Mendes, M.P.; Flores-Colen, I. Nanomaterials Applied in the Construction Sector: Environmental, Human Health, and Economic Indicators. Appl. Sci. 2023, 13, 12896. [Google Scholar] [CrossRef]
- Macías-Silva, M.A.; Cedeño-Muñoz, J.S.; Morales-Paredes, C.A.; Tinizaray-Castillo, R.; Perero-Espinoza, G.A.; Rodríguez-Díaz, J.M.; Jarre-Castro, C.M. Nanomaterials in construction industry: An overview of their properties and contributions in building house. Case Stud. Chem. Environ. Eng. 2024, 10, 100863. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Konstantopoulos, C.; Poelman, H.; Marin, G.B. Fe-Based Nano-Materials in Catalysis. Materials 2018, 11, 831. [Google Scholar] [CrossRef]
- Franco, A.; Balu, A.M.; Luque, R. Nanostructured Porous Materials: Synthesis and Catalytic Applications in Nanotechnology in Catalysis: Applications in the Chemical Industry, Energy Development, and Environment Protection, 1st ed.; Van de Voorde, M., Sels, B., Eds.; Wiley-VCH: Weinheim, Germany, 2017; Volume 1, pp. 119–144. [Google Scholar]
- Yentekakis, I.V.; Gournis, D.P.; Karakassides, M.A. Nanomaterials in Catalysis Applications. Catalysts 2023, 13, 627. [Google Scholar] [CrossRef]
- Gómez-López, P.; Puente-Santiago, A.; Castro-Beltrán, A.; do Nascimento, L.A.S.; Balu, A.M.; Luque, R.; Alvarado-Beltrán, C.G. Nanomaterials and catalysis for green chemistry. Curr. Opin. Green Sustain. Chem. 2020, 24, 48–55. [Google Scholar] [CrossRef]
- Fiuza, T.E.; Gonçalves, D.S.; Kokumai, T.M.; Resende, K.A.; Destro, P.; Zanchet, D. Nanomaterials and catalysis in Materials Nanoarchitectonics, 1st ed.; Ariga, K., Azzaroni, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 519–559. [Google Scholar] [CrossRef]
- Zhong, C.J.; Maye, M.M.; Luo, J.; Han, L.; Kariuki, N. Nanoparticles in Catalysis. In Nanoparticles. Nanostructure Science and Technology, 1st ed.; Rotello, V., Ed.; Springer: Boston, MA, USA, 2004; pp. 113–143. [Google Scholar] [CrossRef]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Segawa, T.F.; Igarashi, R. Nanoscale quantum sensing with Nitrogen-Vacancy centers in nanodiamonds—A magnetic resonance perspective. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 134, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Nusantara, A.C.; Hamoh, T.; Mzyk, A.; Tian, X.; Martinez, F.P.; Li, R.; Permentier, H.P.; Schirhagl, R. Functionalized Fluorescent Nanodiamonds for Simultaneous Drug Delivery and Quantum Sensing in HeLa Cells. ACS Appl. Mater. Interfaces 2022, 14, 38421–39680. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, M.; Nguyen, N.-V.; Cleuziou, J.-P.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W. Molecular Quantum Spintronics: Supramolecular Spin Valves Based on Single-Molecule Magnets and Carbon Nanotubes. Int. J. Mol. Sci. 2011, 12, 6656–6667. [Google Scholar] [CrossRef]
- Lee, J.; Jeon, D.J.; Yeo, J.S. Quantum plasmonics: Energy transport through plasmonic gap. Adv. Mater. 2021, 33, 2006606. [Google Scholar] [CrossRef]
- Iyengar, S.A.; Puthirath, A.B.; Swaminathan, V. Realizing Quantum Technologies in Nanomaterials and Nanoscience. Adv. Mater. 2023, 35, 2107839. [Google Scholar] [CrossRef]
- Kloeffel, C.; Loss, D. Prospects for Spin-Based Quantum Computing in Quantum Dots. Annu. Rev. Condens. Matter Phys. 2013, 4, 51–81. [Google Scholar] [CrossRef]
- Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. [Google Scholar] [CrossRef]
- Jandt, K.D.; Watts, D.C. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent. Mater. 2020, 36, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Bhardwaj, A. Summary and Future Perspectives of Nanomaterials and Technologies: Special Emphasis on Energy and Environment in Nanomaterials for Sustainable Energy and Environmental Remediation, 1st ed.; Naushad, M., Saravanan, R., Kumar, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 333–353. [Google Scholar] [CrossRef]
- Siddique, J.A.; Numan, A. Perspective Future Development of Nanomaterials. In Contemporary Nanomaterials in Material Engineering Applications, 1st ed.; Mubarak, N.M., Khalid, M., Walvekar, R., Numan, A., Eds.; Springer: Cham, Switzerland, 2021; pp. 319–343. [Google Scholar] [CrossRef]
- Liang, W.; Zhou, C.; Bai, J.; Zhang, H.; Long, H.; Jiang, B.; Dai, H.; Wang, J.; Zhang, H.; Zhao, J. Current developments and future perspectives of nanotechnology in orthopedic implants: An updated review. Front. Bioeng. Biotechnol. 2024, 12, 1342340. [Google Scholar] [CrossRef] [PubMed]
- Rahimi-Iman, A. Advances in functional nanomaterials science. Ann. Der Phys. 2020, 532, 2000015. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Rajakumar, G.; Chung, I.M. Nanotechnology: Current uses and future applications in the food industry. 3 Biotech 2018, 8, 74. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Cheema, S.A.; ur Rehman, H.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E.; et al. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef]
- Fadel, T.R.; Steevens, J.A.; Thomas, T.A.; Linkov, I. The challenges of nanotechnology risk management. Nano Today 2015, 10, 6–10. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, P. Assessing the nanotechnology on the grounds of costs, benefits, and risks. Beni Suef Univ. J. Basic Appl. Sci. 2020, 9, 63. [Google Scholar] [CrossRef]
- Singh, S.; Nalwa, H.S. Nanotechnology and health safety–toxicity and risk assessments of nanostructured materials on human health. J. Nanosci. Nanotechnol. 2007, 7, 3048–3070. [Google Scholar] [CrossRef]
- Pérez-Hernández, H.; Pérez-Moreno, A.; Sarabia-Castillo, C.R.; García-Mayagoitia, S.; Medina-Pérez, G.; López-Valdez, F.; Campos-Montiel, R.G.; Jayanta-Kumar, P.; Fernández-Luqueño, F. Ecological Drawbacks of Nanomaterials Produced on an Industrial Scale: Collateral Effect on Human and Environmental Health. Water Air Soil Pollut. 2021, 232, 435. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, K.; Dixit, S.; Srivastava, S. A review on positive and negative impacts of nanotechnology in agriculture. Int. J. Environ. Sci. Technol. 2019, 16, 2175–2184. [Google Scholar] [CrossRef]
- Fojtů, M.; Teo, W.Z.; Pumera, M. Environmental impact and potential health risks of 2D nanomaterials. Environ. Sci. Nano 2017, 4, 1617–1633. [Google Scholar] [CrossRef]
- Bhattacharyya, S. Iron Nitride Family at Reduced Dimensions: A Review of Their Synthesis Protocols and Structural and Magnetic Properties. J. Phys. Chem. C 2015, 119, 1601–1622. [Google Scholar] [CrossRef]
- Pelka, R.; Kiełbasa, K.; Arabczyk, W. Catalytic Ammonia Decomposition During Nanocrystalline Iron Nitriding at 475 °C with NH3/H2 Mixtures of Different Nitriding Potential. J. Phys. Chem. C 2014, 118, 6178–6185. [Google Scholar] [CrossRef]
- Karch, J.; Birringer, R.; Gleiter, H. Ceramics Ductile at Low Temperature. Nature 1987, 330, 556–558. [Google Scholar] [CrossRef]
- Ruhle, M.; Dosch, H.; Mittemejer, E.J.; van de Voorde, M.H. European White Book on Fundamental Research in Materials Science; Max-Planck Institute for Metals Research: Stuttgart, Germany, 2001. [Google Scholar]
- Lojkowski, W.; Fecht, H.J. Structure of Intercrystalline Interfaces. Prog. Mater. Sci. 2000, 45, 339–568. [Google Scholar] [CrossRef]
- Poliakoff, M.; King, P. Phenomenal Fluids. Nature 2001, 412, 125. [Google Scholar] [CrossRef]
- Beck, I.E.; Bukhtiyarov, V.I.; Pakharukov, I.Y.; Zaikovsky, V.I.; Kriventsov, V.V.; Parmon, V.N. Platinum Nanoparticles on Al2O3: Correlation Between the Particle Size and Activity in Total Methane Oxidation. J. Catal. 2009, 268, 60–67. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Size-dependent transformation of α-Fe into γ’-Fe4N in nanocrystalline the Fe-NH3-H2 system. J. Phys. Chem. C 2016, 120, 17989. [Google Scholar] [CrossRef]
- Jennings, J.R. Catalytic Ammonia Synthesis Fundamentals and Practice; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Liu, H. Ammonia Synthesis Catalysts: Innovation and Practice; World Scientific: Singapore, 2013. [Google Scholar]
- Nielsen, A. Ammonia: Catalysis and Manufacture; Springer-Verlag: Berlin, Germany, 1995. [Google Scholar]
- Schlögl, R. Handbook of Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Kuznetsov, L.D.; Lachinov, S.S. The Effect of Al2O3 and K2O Promoters on the Activity of Iron Catalyst in the Synthesis of Ammonia. Zhurnal Fiz. Khimii 1959, 33, 2542. [Google Scholar]
- Boudart, M. Ammonia synthesis: The bellwether reaction in heterogeneous catalysis. Top. Catal. 1994, 1, 405. [Google Scholar] [CrossRef]
- Nørskov, J.K. From quantum physics to heterogeneous catalysis. Top. Catal. 1994, 1, 385. [Google Scholar] [CrossRef]
- Somorjai, G.A. Introduction to Surface Chemistry and Catalysis; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Ozaki, A.; Aika, K. Catalysis—Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1982; Volume 1. [Google Scholar]
- Ertl, G. Surface Science and Catalysis—Studies on the Mechanism of Ammonia Synthesis: The P. H. Emmett Award Address. Catal. Rev. Sci. Eng. 1980, 21, 201–223. [Google Scholar] [CrossRef]
- Ertl, G. Physical characterization of industrial catalysts: The mechanism of ammonia synthesis. Stud. Surf. Sci. Catal. 1989, 44, 315. [Google Scholar] [CrossRef]
- Ertl, G. Reactions at Solid Surfaces; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Somorjai, G.A.; Materer, N. Surface structure in ammonia synthesis. Top. Catal. 1994, 1, 215. [Google Scholar] [CrossRef]
- Mbaraka, I.; Shanks, B. Conversion of Oils and Fats Using Advanced Mesoporous Heterogeneous Catalysts. J. Am. Oil Chem. Soc. 2006, 83, 79–91. [Google Scholar] [CrossRef]
- Borges, M.E.; Díaz, L. Recent Developments on Heterogeneous Catalysts for Biodiesel Production by Oil Esterification and Transesterification Reactions: A Review, Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Savchenko, V.I.; Makaryan, I.A. Palladium Catalyst for the Production of Pure Margarine. Platin. Met. Rev. 1999, 43, 74–82. [Google Scholar] [CrossRef]
- Heck, R.M.; Farrauto, R.J.; Gulati, S.T. Catalytic Air Pollution Control; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Aika, K.; Christiansen, L.J.; Dybkjaer, I.; Hansen, J.B.; Nielsen, P.E.H.; Nielsen, A.; Stolze, P.; Tamaru, K. Ammonia Catalysis and Manufacture; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Farrauto, R.J.; Bartholomew, C.H. Fundamentals of Industrial Catalytic Processes; Chapman & Hall: London, UK, 1997. [Google Scholar]
- Kunze, J. Nitrogen and Carbon in Iron and Steels Thermodynamics. In Physical research; Akademie Verlag: Berlin, Germany, 1990; Volume 16. [Google Scholar]
- Pelka, R.; Arabczyk, W. Studies of the Kinetics of Reaction Between Iron Catalysts and Ammonia—Nitriding of Nanocrystalline Iron with Parallel Catalytic Ammonia Decomposition. Top. Catal. 2009, 52, 1506–1516. [Google Scholar] [CrossRef]
- Wróbel, R.; Arabczyk, W. Solid-Gas Reaction with Adsorption as the Rate Limiting Step. J. Phys. Chem. A 2006, 110, 9219–9224. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals 2024, 14, 188. [Google Scholar] [CrossRef]
- Moszyńska, I.; Moszyński, D.; Arabczyk, W. Hysteresis in nitriding and reduction in the nanocrystalline iron-ammonia-hydrogen system. Przemysł Chem. 2009, 88, 526–529. [Google Scholar]
- Moszyński, D.; Moszyńska, I. Phase transformations during nitriding of nanocrystalline iron. Przemysł Chem. 2013, 92, 1332–1335. [Google Scholar]
- Moszyński, D. Nitriding of Nanocrystalline Iron in the Atmospheres with Variable Nitriding Potential. J. Phys. Chem. C 2014, 118, 15440–15447. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Wilk, B.; Lendzion-Bieluń, Z. Kinetics and Thermodynamics of the Phase Transformation in the Nanocrystalline Substance—Gas Phase System. Crystals 2024, 14, 129. [Google Scholar] [CrossRef]
- Lehrer, E. Über das Eisen-Waserstoff-Amoniak Gleichgewicht. Z. Für Elektrochem. 1930, 36, 383–392. [Google Scholar]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1975. [Google Scholar]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Reaction Model Taking into Account the Catalyst Morphology and Its Active Specific Surface in the Process of Catalytic Ammonia Decomposition. Materials 2021, 14, 7229. [Google Scholar] [CrossRef]
- Pelka, R.; Nowosielecka, U.; Klimza, K.; Moszyńska, I.; Aidinis, K.; Żołnierkiewicz, G.; Guskos, A.; Guskos, N. Nanocrystalline Iron Oxides with Various Average Crystallite Size Investigated Using Magnetic Resonance Method. Crystals 2024, 14, 363. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Kocemba, I.; Brzoza-Kos, A.; Wyszkowski, A.; Lendzion-Bieluń, Z. Study of Phase Transformation Processes Occurring in the Nanocrystalline Iron/Ammonia/Hydrogen System by the Magnetic Permeability Measurement Method. J. Phys. Chem. C 2022, 126, 7704–7710. [Google Scholar] [CrossRef]
- Arabczyk, W.; Rogowski, J.; Pelka, R.; Lendzion-Bieluń, Z. Application of TOF-SIMS Method in the Study of Wetting the Iron (111) Surface with Promoter Oxides. Molecules 2022, 27, 648. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Jędrzejewski, R.; Ekiert, E.; Arabczyk, W. Heterogeneity of ingot of the fused iron catalyst for ammonia synthesis. Appl. Catal. A: Gen.L 2011, 400, 48–53. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. Modelling of nanocrystalline iron nitriding process—Influence of specific surface area. Chem. Pap. 2011, 65, 198–202. [Google Scholar] [CrossRef]
- Website. Available online: https://www.tf.uni-kiel.de/matwis/amat/iss/kap_5/illustr/s5_2_3d.html (accessed on 21 June 2022).
- Pelka, R. A method of determining nanoparticle size distribution in iron ammonia synthesis catalyst by measuring mass changes during the nitriding process. Catal. Today 2017, 286, 118–123. [Google Scholar] [CrossRef]
- Ekiert, E.; Wilk, B.; Lendzion-Bieluń, Z.; Pelka, R.; Arabczyk, W. Study of Phase Transitions Occurring in a Catalytic System of ncFe-NH3/H2 with Chemical Potential Programmed Reaction (CPPR) Method Coupled with In Situ XRD. Catalysts 2021, 11, 183. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabczyk, W.; Pelka, R.; Brzoza-Kos, A.; Kocemba, I.; Rokicka-Konieczna, P.; Skulmowska-Polok, K.; Klimza, K.; Lendzion-Bieluń, Z. Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement. Appl. Sci. 2024, 14, 8452. https://doi.org/10.3390/app14188452
Arabczyk W, Pelka R, Brzoza-Kos A, Kocemba I, Rokicka-Konieczna P, Skulmowska-Polok K, Klimza K, Lendzion-Bieluń Z. Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement. Applied Sciences. 2024; 14(18):8452. https://doi.org/10.3390/app14188452
Chicago/Turabian StyleArabczyk, Walerian, Rafał Pelka, Agnieszka Brzoza-Kos, Ireneusz Kocemba, Paulina Rokicka-Konieczna, Katarzyna Skulmowska-Polok, Kamila Klimza, and Zofia Lendzion-Bieluń. 2024. "Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement" Applied Sciences 14, no. 18: 8452. https://doi.org/10.3390/app14188452
APA StyleArabczyk, W., Pelka, R., Brzoza-Kos, A., Kocemba, I., Rokicka-Konieczna, P., Skulmowska-Polok, K., Klimza, K., & Lendzion-Bieluń, Z. (2024). Studies of Phase Transformation Kinetics in the System of Nanocrystalline Iron/Ammonia/Hydrogen at the Temperature of 350 °C by Means of Magnetic Permeability In Situ Measurement. Applied Sciences, 14(18), 8452. https://doi.org/10.3390/app14188452





