Comprehensive Characterization and Metamorphic Control Analysis of Full Apertures in Different Coal Ranks within Deep Coal Seams
Abstract
:1. Introduction
2. Methods and Models
2.1. Sample Source and Basic Parameters
2.2. Methods and Plans
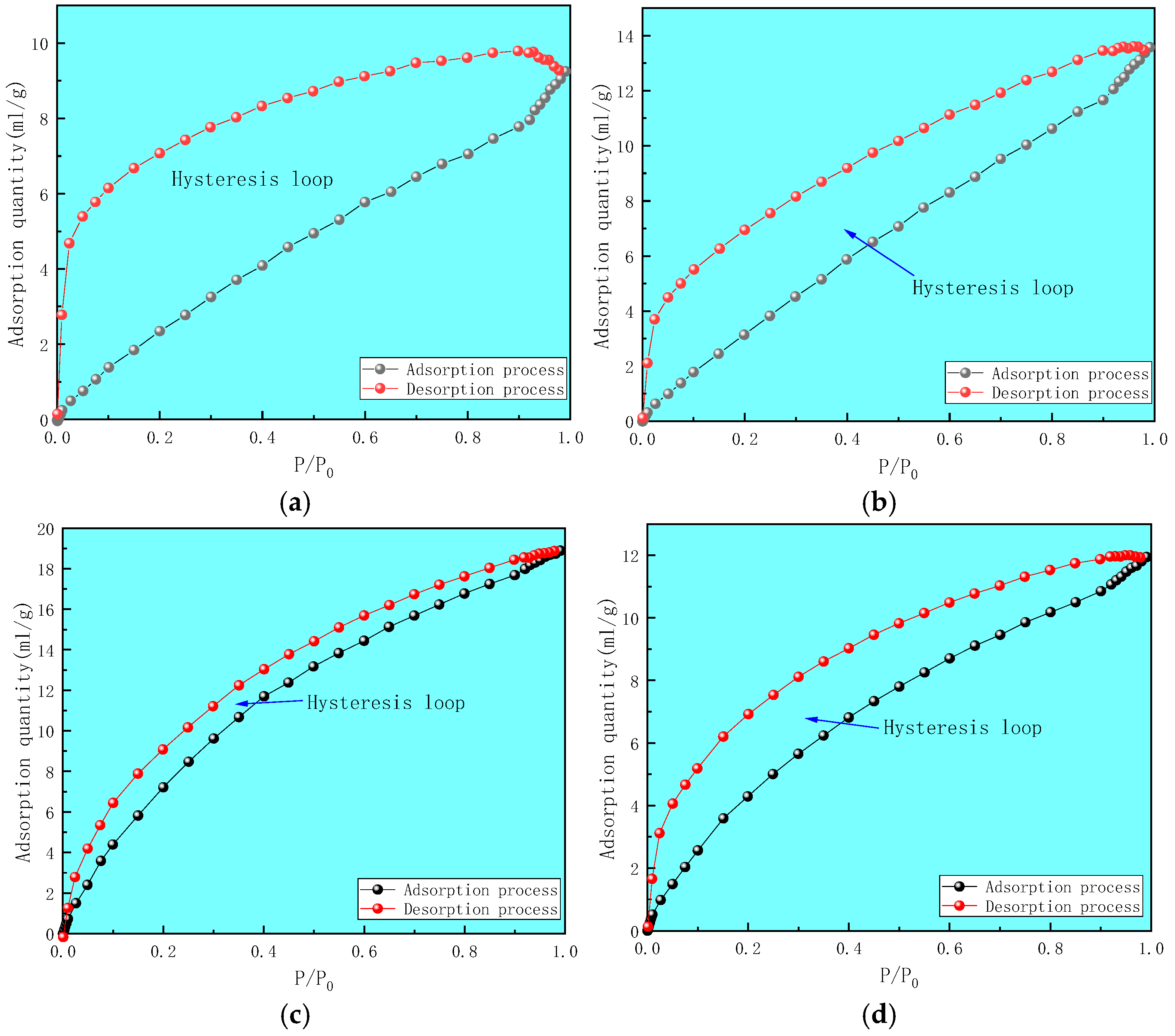
2.2.1. Mercury Intrusion Method
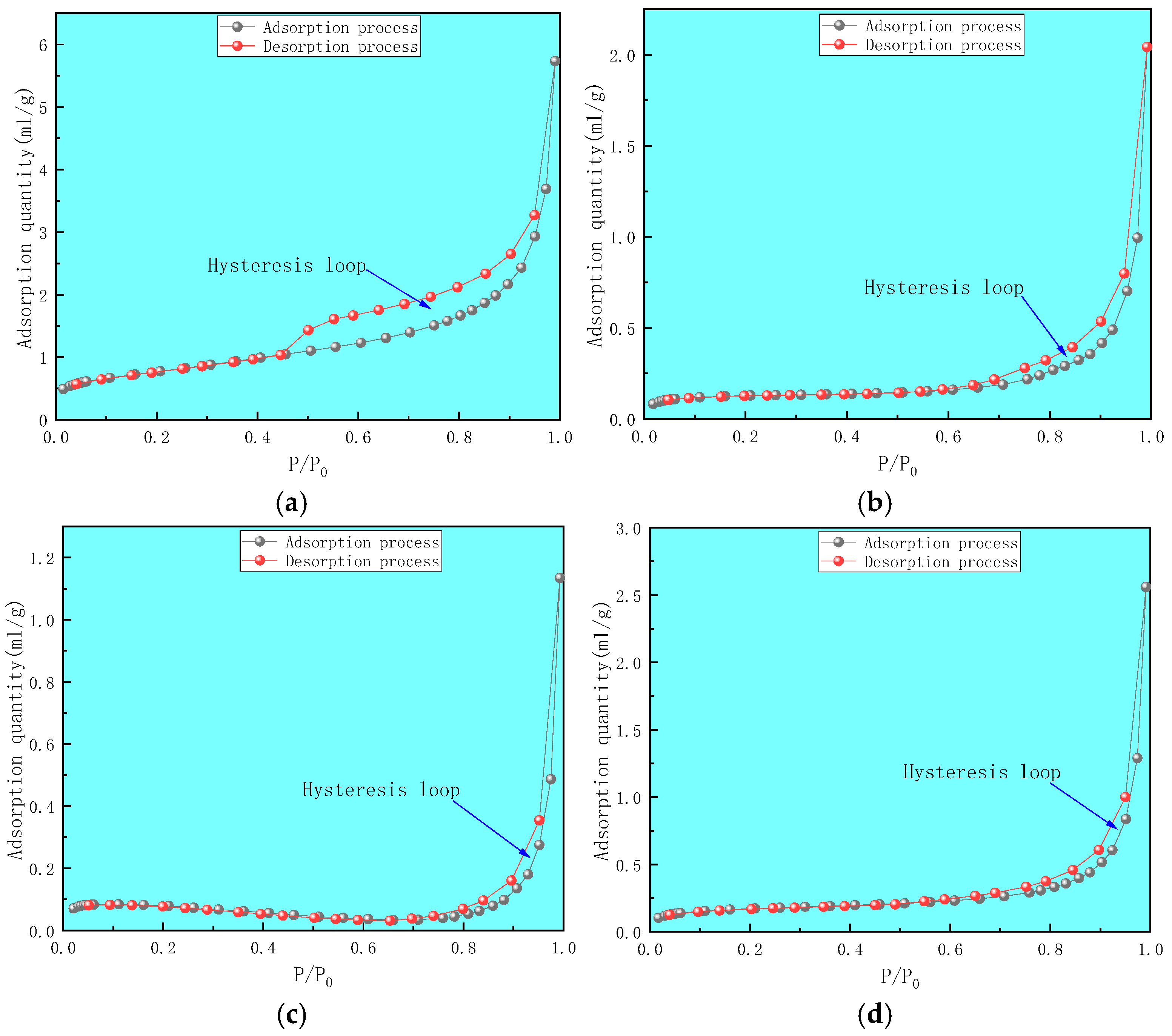
2.2.2. Liquid Nitrogen Adsorption Method
2.2.3. Low-Temperature Carbon Dioxide Adsorption Method
2.3. Pore Fractal Model
2.3.1. Sponge Model Based on the Mercury Intrusion Method
2.3.2. FHH Model Based on Liquid Nitrogen Method
2.3.3. Density Function Model Based on the Carbon Dioxide Adsorption Method
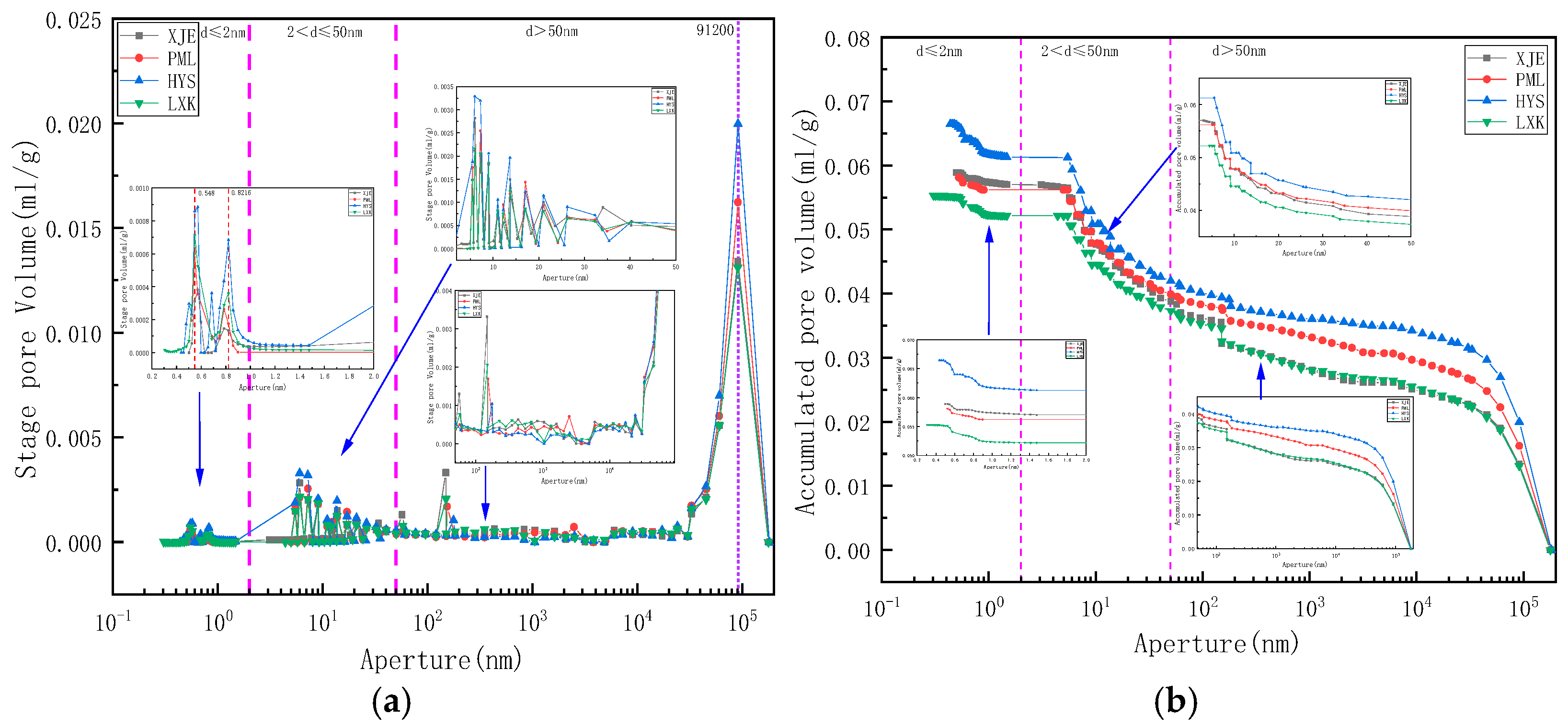
3. Results
4. Discussion
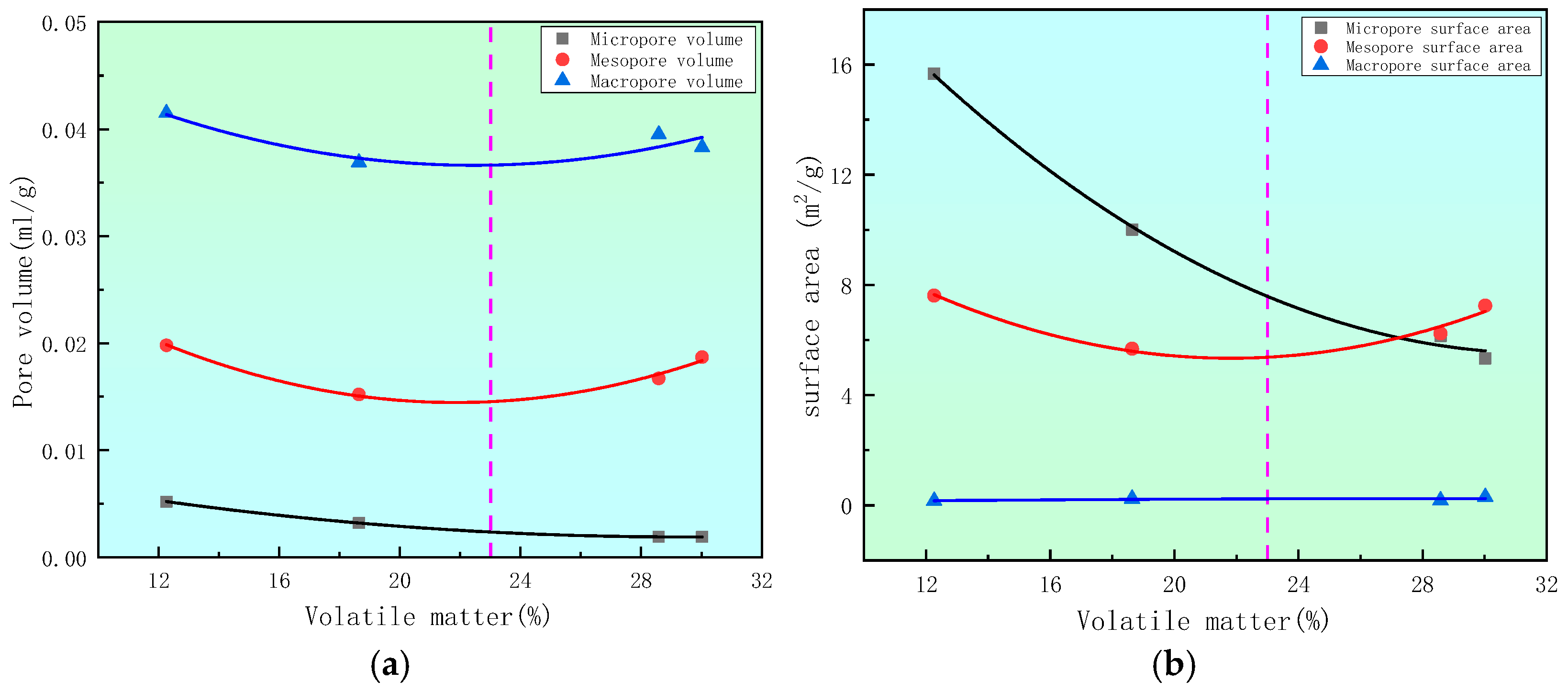
4.1. The Influence of the Degree of Deterioration on Pore Volume and Specific Surface Area
4.2. Influence of Metamorphic Degree on Fractal Characteristics
5. Conclusions
- Using mercury intrusion porosimetry, liquid nitrogen adsorption, and low-temperature carbon dioxide adsorption methods to comprehensively characterize the full pore size of deep coal samples of different coal grades, the pore volume distribution relationship of each coal sample was found to be macropore→mesopore→micropore, indicating that the connectivity of macropores is relatively good. The specific surface area of micropores and mesopores is the main component, whereas the specific surface area of macropores is relatively small. The development characteristics of micropores and microcracks control the adsorption performance and development potential of deep gas.
- The influence of metamorphic degree on the pore structure of deep coal was analyzed. The micropore volume and specific surface area both decreased with the increase in volatile matter, highlighting a nonlinear decreasing trend. The metamorphic process of coal promoted the development of micropores. The pore volume and specific surface area of mesopores and macropores first decreased and then increased with the increase in volatile matter, and the curve exhibited a U-shaped change, with the lowest values appearing at approximately 23% of volatile matter. Marked differences in the micropore, mesopore, and macropore structures of coal were observed with different degrees of metamorphism, which are controlled by the degree of metamorphism.
- The fractal dimensions of the pores in each sample were calculated based on the sponge, FHH, and density function models. The HYS sample (lean coal) with the highest degree of metamorphism exhibited the smallest fractal dimension D1, the largest D2, and the smallest D3, among which the pore complexity was the highest. The XJE sample (gas coal) with the lowest degree of metamorphism had the highest fractal dimension D1, the lowest D2, and the highest D3, and the distribution of mesopores and throats was relatively regular. The fractal dimension D1 value based on the sponge model exhibited an inverted U-shaped change with the increase in volatile matter, first increasing and then decreasing. The fractal dimension D2 value based on the FHH model decreased nonlinearly with the increase in volatile matter, and the higher the degree of metamorphism, the more complex the mesoporous structure and the larger the fractal dimension. The fractal dimension D3 value based on the density function model increased nonlinearly with the increase in volatile matter, and the degree of metamorphism intensified, resulting in the development of a more regular microporous structure. Although the degree of metamorphism had different effects on the fractal dimension, they all exhibited certain regular changes, indicating that the degree of metamorphism has a controlling effect on the evolution of the pore fractal dimension. Therefore, further research on the pore structure and fractal characteristics of all coal types is needed to comprehensively and accurately determine the controlling effect of metamorphic degree on pore size distribution.
- China’s fv deep coal seams. The findings can contribute to the future of deep coal and gas coal mining technology, as well as coal and gas outburst prevention and control engineering.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, J.; Yue, Y.; Liu, Y. Analysis and Characterization of Micro–Nano Pores in Coal Reservoirs of Different Coal Ranks. Appl. Sci. 2024, 14, 5198. [Google Scholar] [CrossRef]
- Yao, Y.D.; Liu, D.M.; Che, Y.; Tang, D.; Tang, S.; Huang, W. Non-destructive characterization of coal samples from China using microfocus X-ray computed tomography. Int. J. Coal Geol. 2009, 80, 113–123. [Google Scholar] [CrossRef]
- Gilman, A.; Beckie, R. Flow of coal-bed methane to a gallery. Transp. Porous Media 2000, 1, 1–16. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Lenos, M.J. The measurement of coal porosity with different gases. Int. J. Coal Geol. 2002, 3, 245–281. [Google Scholar] [CrossRef]
- Zhang, C.; Guan, P.; Zhang, J.H.; Liang, X.; Ding, X.; You, Y. Progress in fractal theory characterization of unconventional oil and gas reservoir pore structure characteristics. J. Peking Univ. 2023, 5, 897–908. [Google Scholar] [CrossRef]
- Mandelbrot, B.B. How long is the coast of Britain statistical selfsimilarity and fractional dimension. Science 1967, 3775, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Mandelbrot, B.B. On the geometry of homogeneous turbulence, with stress on the fractal dimension of the iso-surfaces of scalars. J. Fluid. Mech. 2006, 3, 401–416. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, S.; Si, C.X.; Qi, L.; Kang, N. The fractal characteristics of coal pore structure with different degrees of metamorphism and their impact on gas adsorption. Coal Sci. Technol. 2020, 48, 118–124. [Google Scholar] [CrossRef]
- Wang, X.J.; Yao, H.F.; Li, W.; Zhang, X.; Yan, J. Fractal characterization of coal pore structure based on thermodynamic models. Coal. Geol. Explor. 2014, 6, 20–23. [Google Scholar] [CrossRef]
- Yang, S.Y.; Yao, Y.B.; Wei, R.; Zhang, J. Study on fractal characteristics of seepage pores and pores in coal reservoirs in the Hedong mining area of Urumqi. Coal Sci. Technol. 2020, 8, 175–183. [Google Scholar] [CrossRef]
- Liu, H.Q.; Wang, L.; Xie, G.X.; Yuan, Q.; Zhu, C.; Jiao, Z. Comprehensive characterization of coal pore structure and full aperture fractal characteristics. J. Min. Saf. Eng. 2022, 3, 458–469+479. [Google Scholar] [CrossRef]
- Xue, H.T.; Li, X.J.; Chen, L.Y.; IU, Y. Microscopic pore fractal characteristics of Qianxi outburst coal and their impact on permeability. Coal Sci. Technol. 2021, 49, 118–122. [Google Scholar] [CrossRef]
- Zhou, S.D.; Liu, D.M.; Cai, Y.D.; Yao, Y.; Jiao, Y.; Ren, S. Characteristics and fractal characterization of adsorption pores in low rank coal. Geol. Oil Gas 2018, 2, 373–383. [Google Scholar] [CrossRef]
- Xiong, Y.H.; Zhou, S.W.; Jiao, P.F.; Yang, M.W.; Zhou, J.P.; Wei, W.E.I.; Cai, J.C. Fractal analysis of coal and shale microporous structures based on low-temperature CO2 adsorption. Nat. Gas Geosci. 2020, 7, 1028–1040. [Google Scholar] [CrossRef]
- GB/T 212-2008; Industrial Analysis Methods for Coal. National Coal Standardisation Technical Committee: Beijing, China, 2008.
- GB/T 5751-2009; China Coal Classification. National Coal Standardisation Technical Committee: Beijing, China, 2009.
- Chu, Y.P.; Zhang, D.M.; Wang, M.; Liu, H.; Wu, X.; Zhai, P.; Shen, T. Experimental study on the damage evolution law of pore structure in liquid nitrogen freeze-thaw coal based on nuclear magnetic resonance technology and mercury intrusion method. J. Rock Mech. Eng. 2022, 9, 1820–1831. [Google Scholar] [CrossRef]
- GB T 21650.1-2008; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption. Part 1: Mercury Porosimetry. National Technical Committee for the Standardisation of Sieve Mesh Sieving and Particle Sorting Methods (SAC/TC 168): Beijing, China, 2008.
- Yang, M.; Liu, L.; Liu, J.J.; Mao, J.; Chai, P. Joint characterization of nitrogen adsorption mercury intrusion nuclear magnetic resonance in the pore structure of intermediate rank coal. Coal Sci. Technol. 2021, 5, 67–74. [Google Scholar] [CrossRef]
- GB T 21650.2-2008; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption. Part 2: Analysis of Mesopores and Macropores by Gas Adsorption. National Technical Committee for the Standardisation of Sieve Mesh Sieving and Particle Sorting Methods (SAC/TC 168): Beijing, China, 2008.
- Nie, B.S.; Ma, Y.K.; He, X.Q.; Zhao, D.; Liu, X.; Deng, B.; Li, X.; Meng, Y.; Song, D. Exploring the Micro Mechanism of Coal and Gas Outburst. J. China Univ. Min. Technol. 2022, 2, 207–2020. [Google Scholar] [CrossRef]
- GB T 21650.3-2011; Pore Size Distribution and Porosity of Solid Materials by Mercury Porosimetry and Gas Adsorption. Part 3: Analysis of Micropores by Gas Adsorption. National Technical Committee for the Standardisation of Sieve Mesh Sieving and Particle Sorting Methods (SAC/TC 168): Beijing, China, 2011.
- Song, Y.; Jiang, B.; Li, F.L.; Yan, G.Y.; Yao, Y.P. Applicability and fractal characteristics of low-middling coal level structural coal nano pore fractal model. Earth Sci. 2018, 5, 1611–1622. [Google Scholar] [CrossRef]
- Pfeifer, P.; Avnir, D. Chemistry in noninteger dimensions between two and three. I Fractal theory of heterogeneous surfaces. J. Chem. Phys. 1983, 7, 3558–3565. [Google Scholar] [CrossRef]
- Avnir, D.; Jaroniec, M. An isotherm equation for adsorption on fractal surfaces of heterogeneous porous materials. Langmuir 1989, 6, 1431–1433. [Google Scholar] [CrossRef]
- Jaroniec, M. Evaluation of the fractal dimension from a single adsorption isotherm. Langmuir 1995, 6, 2316–2317. [Google Scholar] [CrossRef]
- Avnir, D.; Pfeifer, P. Fractal Dimension in Chemistry, An intensive characterization of surface irregularity. Nouv. J. Chim. New J. Chem. 1983, 2, 71–72. [Google Scholar]
- Jaroniec, M.; Gilpin, R.K.; Choma, J. Correlation between microporosity and fractal dimension of active carbons. Carbon 1993, 2, 325–331. [Google Scholar] [CrossRef]
- Jaroniec, M.; Lu, X.; Madey, R.; Choma, J. Evaluation of structural heterogeneities and surface irregularities of microporous solids. Mater. Chem. Phys. 1990, 1, 87–97. [Google Scholar] [CrossRef]
- Dai, F.Y.; Hu, H.Y.; Zhang, A.H. Applicability of fractal models for organic shale pores. Coal Sci. Technol. 2019, 2, 168–175. [Google Scholar] [CrossRef]
- Stoeckli, F.; Ballerini, L.; Sbernardini, D. On the evolution of micropore widths and areas in the course of activation. Carbon 1989, 3, 501–502. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 2, 235–241. [Google Scholar] [CrossRef]
- Choma, J.; Jaroniec, M.; Piotrowska, J. On the mesopore correction of adsorption data used for characterizing microporous structure of activated carbons. Mater. Chem. Phys. 1987, 4, 409–421. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and po rosity (Recommendations 1984). Pure Appl. Chem. 1985, 4, 603–619. [Google Scholar] [CrossRef]
- International Union of Pure and Applied Chemistry. Physical chemistry division commission on colloid and surface chemistry, subcommittee on characterization of porous solids: Recommendations for the characterization of porous solids (Technical report). Pure Appl. Chem. 1994, 8, 1739–1758. [Google Scholar]
- Li, Y.H.; Zhao, J.G.; Li, W.S. Prediction of the Location of Concealed Pluton on the Spatial Variation Characteristics of Volatile Matter in Coal Seams. Geofluids 2024, 2024, 9455853. [Google Scholar] [CrossRef]
- Zheng, C.S.; Han, F.L.; Jiang, B.Y.; Xue, S.; Li, G.; Liu, S. Coal multi-scale pore fracture variations and permeability sharp increase characteristics induced by in-situ hydraulic injection. J. China Univ. Min. Technol. 2024, 4, 710–725. [Google Scholar]


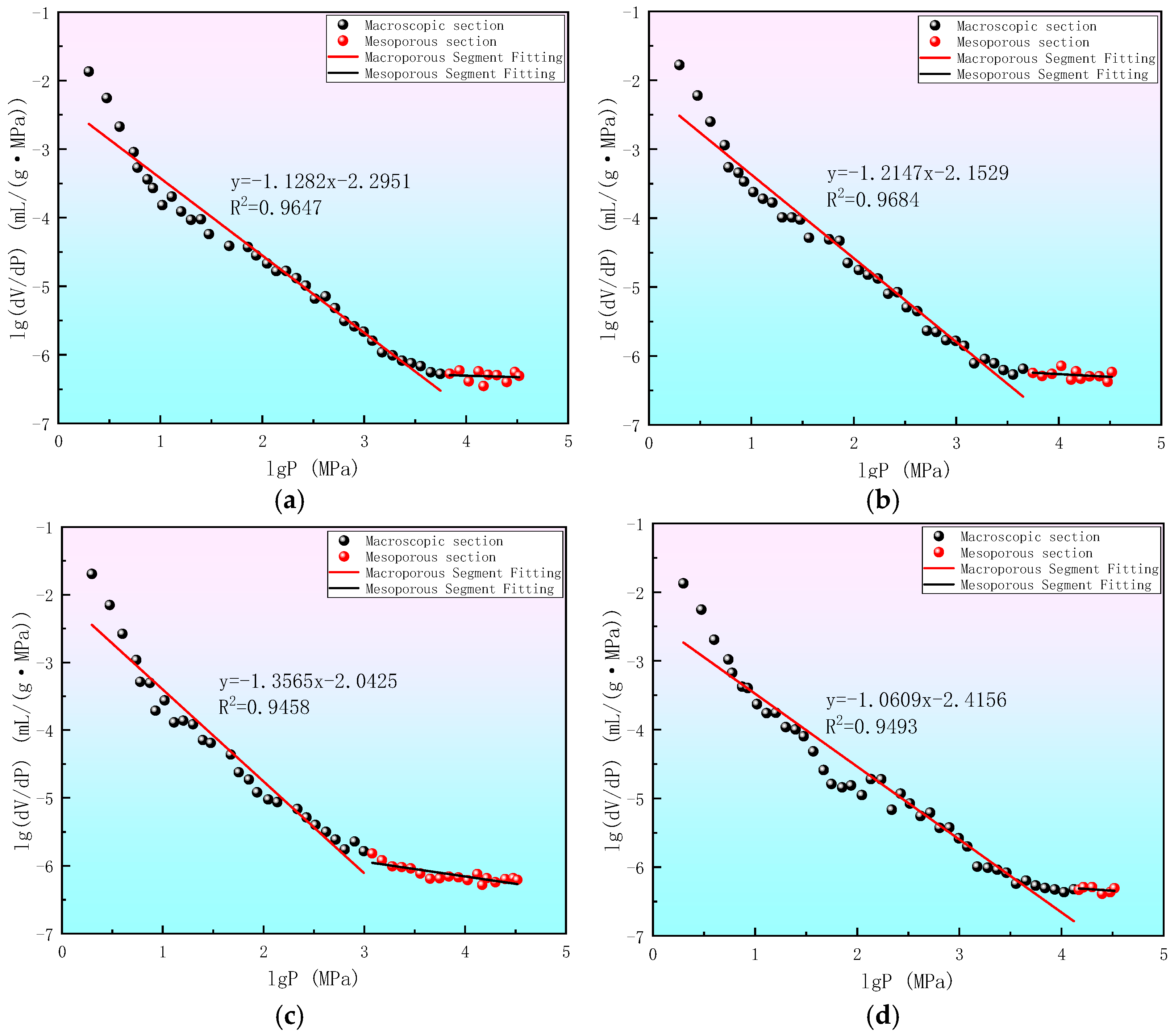
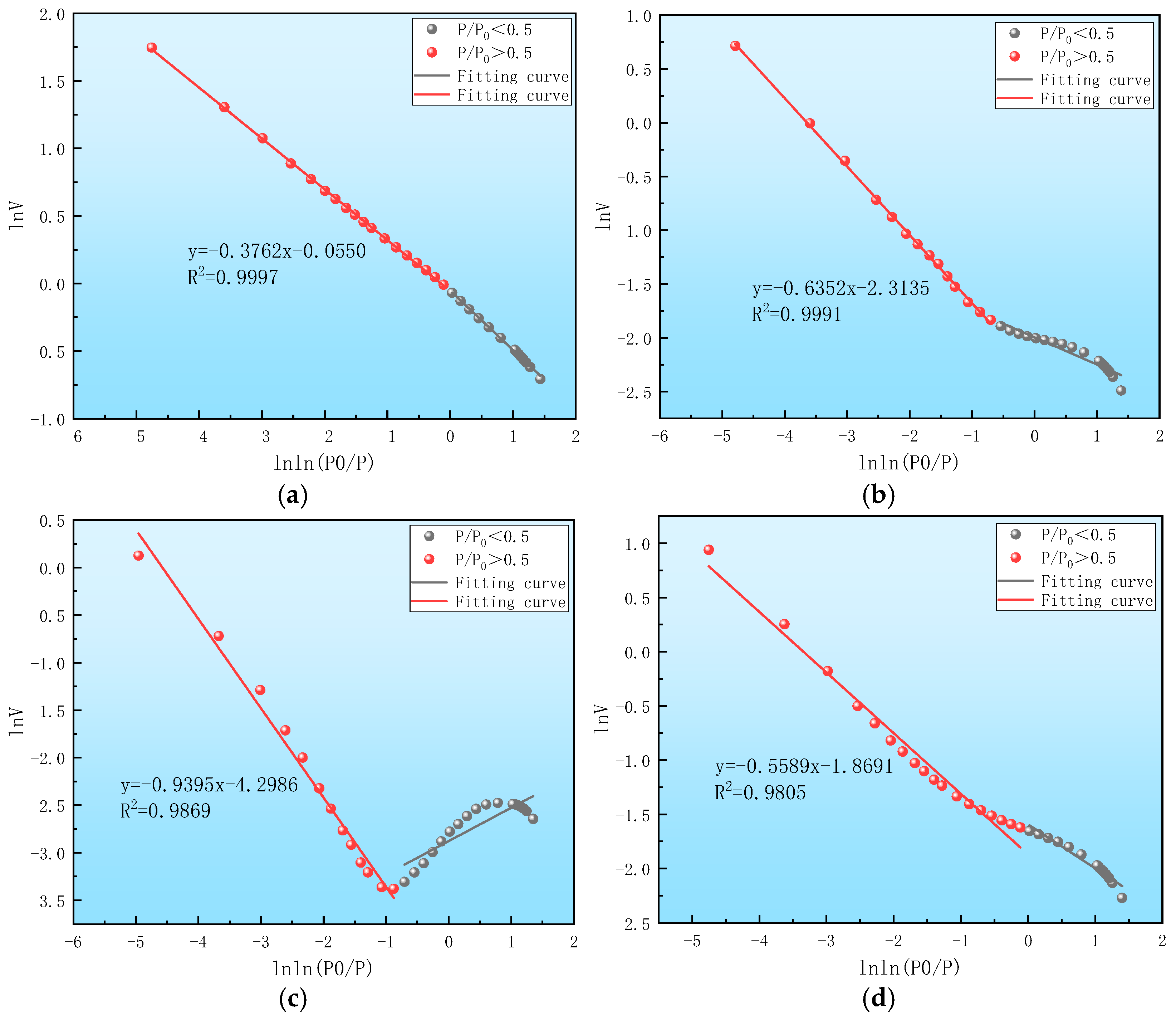
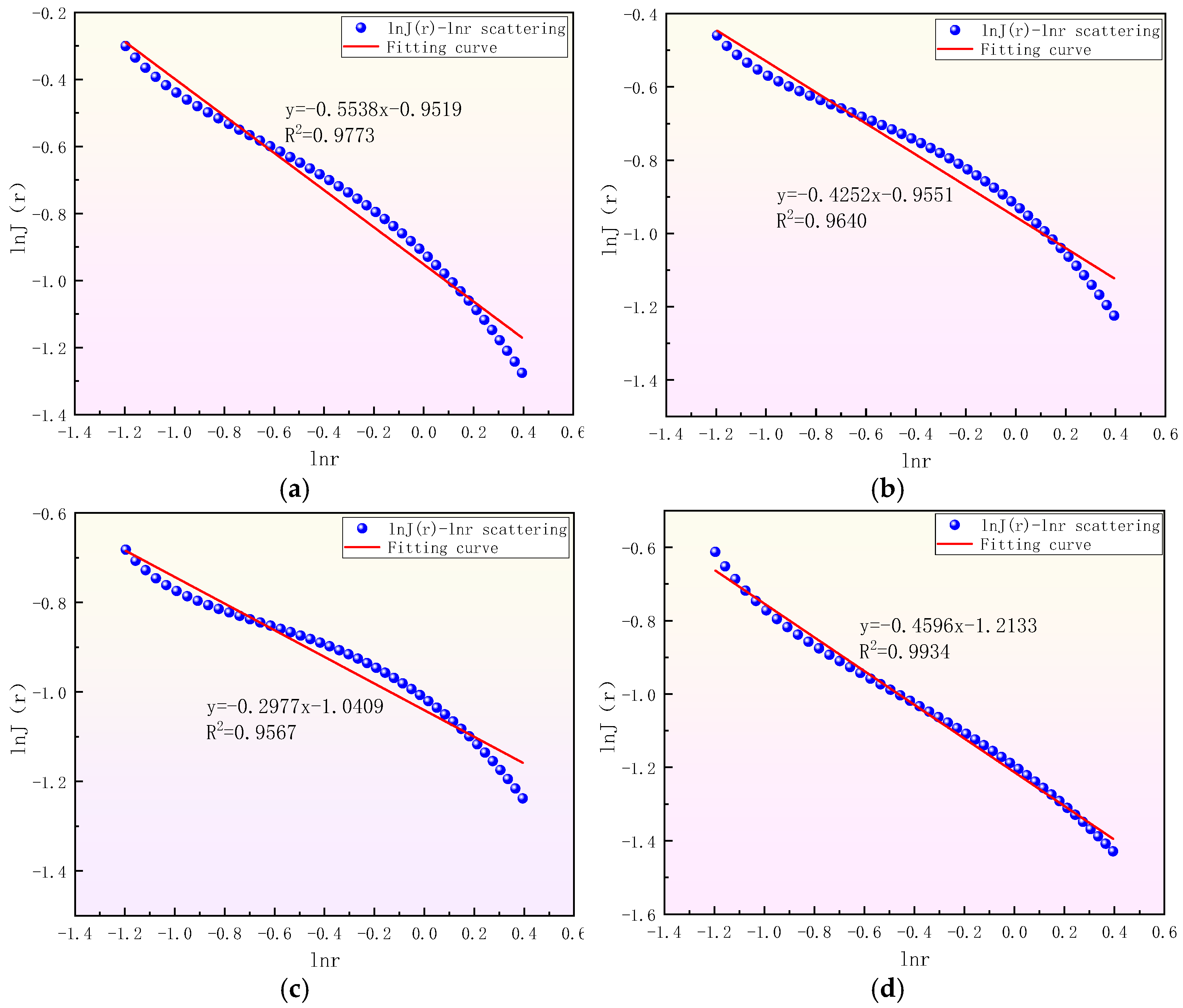

| Sample Number | Sample Source | Sampling Coal Seams | Depth/m | Industrial Analysis Indicators | Coal Type | |||
|---|---|---|---|---|---|---|---|---|
| Mad /% | Aad /% | Vad /% | FCad /% | |||||
| XJE | Xinjier mine | 9 Coal | 800 | 2.25 | 25.78 | 30.02 | 41.95 | Gas coal |
| PML | Pingmeiliu mine | Wu-8 Coal | 940 | 1.65 | 13.36 | 28.58 | 56.41 | 1/3 Coking coal |
| HYS | Hongyangsan mine | 7 Coal | 1100 | 0.76 | 9.81 | 12.26 | 77.17 | Thin Coal |
| LXK | Linxi mine | 12 Coal | 950 | 0.83 | 11.29 | 18.64 | 69.24 | Coking coal |
| Sample Number | Full Aperture Pore Volume (mL/g) | Full Aperture Specific Surface Area (m2/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Pore Volume | Micropore | Mesopore | Macropore | Total Surface Area | Micropore | Mesopore | Macropore | |
| XJE | 0.0589 | 0.0019 | 0.0187 | 0.0383 | 12.8713 | 5.3295 | 7.2445 | 0.2973 |
| PML | 0.0581 | 0.0019 | 0.0167 | 0.0395 | 12.5549 | 6.1451 | 6.2348 | 0.1750 |
| HYS | 0.0665 | 0.0052 | 0.0198 | 0.0415 | 23.4356 | 15.6650 | 7.6147 | 0.1559 |
| LXK | 0.0553 | 0.0032 | 0.0152 | 0.0369 | 15.9192 | 10.0040 | 5.6801 | 0.2351 |
| Sample Number | Fitting Equation | Fitting Degree R2 | Equation Slope K | Fractal Dimension Di (i = 1,2,3) | Fractal Model |
|---|---|---|---|---|---|
| XJE | y = −1.1282x − 2.2951 | 0.9647 | −1.1282 | 2.8718 | Sponge |
| y = −0.9395x − 4.2986 | 0.9869 | −0.9395 | 2.0605 | FHH | |
| y = −0.5538x − 0.9519 | 0.9773 | −0.5538 | 2.5538 | Density function | |
| PML | y = −1.2147x − 2.1529 | 0.9684 | −1.2147 | 2.7853 | Sponge |
| y = −0.6352x − 2.3135 | 0.9991 | −0.6352 | 2.3648 | FHH | |
| y = −0.4252x − 0.9551 | 0.9640 | −0.4252 | 2.4252 | Density function | |
| HYS | y = −1.3565x − 2.0425 | 0.9458 | −1.3565 | 2.6435 | Sponge |
| y = −0.3762x − 0.0550 | 0.9997 | −0.3762 | 2.6238 | FHH | |
| y = −0.2977x − 1.0409 | 0.9567 | −0.2977 | 2.2977 | Density function | |
| LXK | y = −1.0609x − 2.4156 | 0.9493 | −1.0609 | 2.9391 | Sponge |
| y = −0.5589x − 1.8691 | 0.9805 | −0.5589 | 2.4411 | FHH | |
| y = −0.4596x − 1.2133 | 0.9934 | −0.4596 | 2.4596 | Density function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wu, Y.; Qiao, L. Comprehensive Characterization and Metamorphic Control Analysis of Full Apertures in Different Coal Ranks within Deep Coal Seams. Appl. Sci. 2024, 14, 8566. https://doi.org/10.3390/app14188566
Li Q, Wu Y, Qiao L. Comprehensive Characterization and Metamorphic Control Analysis of Full Apertures in Different Coal Ranks within Deep Coal Seams. Applied Sciences. 2024; 14(18):8566. https://doi.org/10.3390/app14188566
Chicago/Turabian StyleLi, Qi, Yong Wu, and Lei Qiao. 2024. "Comprehensive Characterization and Metamorphic Control Analysis of Full Apertures in Different Coal Ranks within Deep Coal Seams" Applied Sciences 14, no. 18: 8566. https://doi.org/10.3390/app14188566
APA StyleLi, Q., Wu, Y., & Qiao, L. (2024). Comprehensive Characterization and Metamorphic Control Analysis of Full Apertures in Different Coal Ranks within Deep Coal Seams. Applied Sciences, 14(18), 8566. https://doi.org/10.3390/app14188566





