Industrial-Level Brewing Using Oenological Saccharomyces cerevisiae and Schizosaccharomyces pombe as Mixed-Inoculum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Equipment
2.2. Brewing Procedures
2.2.1. LSC Trials
2.2.2. CSC Trials
2.3. Chemical Analyses of Beer
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Chemical Characterization of Beers
3.2. Volatile Organic Compounds
3.3. Sensory Evaluation of Beers Fermented by Sequential Inoculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudolph, M.J. The food product development process. In Developing New Food Products for a Changing Marketplace; Brody, A.L., Lord, J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 75–90. [Google Scholar]
- Reisman, A.B. Problems in scale-up of biotechnology production processes. Critic. Rev. Biotechnol. 1993, 13, 195–253. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.D.; Bowser, T.J.; McGlynn, W.G. Scaling up Your Food Process. Available online: https://extension.okstate.edu/fact-sheets/scaling-up-your-food-process.html (accessed on 6 August 2024).
- Archambault, C.J.; Gerds, W.R.W.; Mills, A.M. Scale up in brewing: Factors in changing batch size from 5 gallons to 15 barrels. In Academia and Industrial Pilot Plant Operations and Safety; Moore, M.K., Ledesma, E.B., Eds.; ACS Publications: Washington, DC, USA, 2014; pp. 85–90. [Google Scholar]
- Du, Y.H.; Wang, M.Y.; Yang, L.H.; Tong, L.L.; Guo, D.S.; Ji, X.J. Optimization and scale-up of fermentation processes driven by models. Bioengineering 2022, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Albino, M.; Gargalo, C.L.; Nadal-Rey, G.; Albæk, M.O.; Krühne, U.; Gernaey, K.V. Hybrid Modeling for On-Line Fermentation Optimization and Scale-Up: A Review. Processes 2024, 12, 1635. [Google Scholar] [CrossRef]
- Naydenova, V.; Badova, M.; Vassilev, S.; Iliev, V.; Kaneva, M.; Kostov, G. Encapsulation of brewing yeast in alginate/chitosan matrix: Lab-scale optimization of lager beer fermentation. Biotechnol. Biotechnol. Equip. 2014, 28, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Romanini, E.; Rastelli, S.; Donadini, G.; Lambri, M.; Bertuzzi, T. Pyridoxine and folates during small and large scale brewing. J. Inst. Brew. 2021, 127, 135–139. [Google Scholar] [CrossRef]
- Cela, N.; Condelli, N.; Perretti, G.; Di Cairano, M.; De Clippeleer, J.; Galgano, F.; De Rouck, G. Scale-up of gluten free brewing processes: Differences in gluten reduction ability, analytical attributes and hedonic perception. In Proceedings of the 15th International Trends in Brewing Conference, Ghent, Belgium, 2–6 April 2023. [Google Scholar]
- Schnaitter, M.; Kell, A.; Kollmannsberger, H.; Schüll, F.; Gastl, M.; Becker, T. Scale-up of dry hopping trials: Importance of levelfor aroma and taste perceptions. Chem. Ing. Tech. 2016, 88, 1955–1965. [Google Scholar] [CrossRef]
- McCaig, R.; McKee, J.; Pfisterer, E.A.; Hysert, D.W.; Munoz, E.; Ingledew, W.M. Very high gravity brewing—Laboratory and pilot plant trials. J. Am. Soc. Brew. Chem. 1992, 50, 118–125. [Google Scholar] [CrossRef]
- Nienow, A.W.; Nordkvist, M.; Boulton, C.A. Scale-down/scale-up studies leading to improved commercial beer fermentation. Biotechnol. J. 2011, 6, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Tijerino, M.B.S.; San Martín-González, M.F.; Domingo, J.A.V.; Huang, J.Y. Life cycle assessment of industrial beer brewing at different scales on a unit operation basis. Sustainability 2023, 15, 11416. [Google Scholar] [CrossRef]
- Laseter, T.M.; Morris, J. Three Notch’D: Economies of Scale in Brewing. Darden Case No. UVA-OM-1750. 2021. Available online: https://ssrn.com/abstract=3970590 (accessed on 8 June 2024).
- Dank, A.; van Mastrigt, O.; Yang, Z.; Dinesh, V.M.; Lillevang, S.K.; Weij, C.; Smid, E.J. The cross-over fermentation concept and its application in a novel food product: The dairy miso case study. LWT 2021, 142, 111041. [Google Scholar] [CrossRef]
- De Simone, N.; Russo, P.; Tufariello, M.; Fragasso, M.; Solimando, M.; Capozzi, V.; Grieco, F.; Spano, G. Autochthonous biological resources for the production of regional industrial beers: Exploring possible contributions of cereals, hops, microbes, and other ingredients. Foods 2021, 10, 1831. [Google Scholar] [PubMed]
- Siesto, G.; Pietrafesa, R.; Tufariello, M.; Gerardi, C.; Grieco, F.; Capece, A. Application of microbial cross-over for the production of Italian grape ale (IGA), a fruit beer obtained by grape must addition. Food Biosci. 2023, 52, 102487. [Google Scholar] [CrossRef]
- Peces-Pérez, R.; Vaquero, C.; Callejo, M.J.; Morata, A. Biomodulation of physico- chemical parameters, aromas, and sensory profile of industrial beers by using non-Saccharomyces yeasts. ACS Omega 2022, 7, 17822–17840. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.S. Application of the fission yeast Schizosaccharomyces pombe in human nutrition. FEMS Yeast Res. 2023, 23, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Tristezza, M.; Fantastico, L.; Vetrano, C.; Bleve, G.; Corallo, D.; Mita, G.; Grieco, F. Molecular and technological characterization of Saccharomyces cerevisiae strains isolated from natural fermentation of Susumaniello grape must in Apulia, Southern Italy. Int. J. Microbiol. 2014, 897428. [Google Scholar] [CrossRef]
- Baiano, A.; Fiore, A.; la Gatta, B.; Tufariello, M.; Gerardi, C.; Grieco, F. Single and interactive effects of unmalted cereals, hops, and yeasts on quality of white-inspired industrial beers. Beverages 2023, 9, 9. [Google Scholar]
- Tufariello, M.; Grieco, F.; Fiore, A.; Gerardi, C.; Capozzi, V.; Baiano, A. Effects of brewing procedures and oenological yeasts on chemical composition, antioxidant activity, and sensory properties of emmer-based industrial beers. LWT 2024, 199, 116044. [Google Scholar] [CrossRef]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef]
- Palombi, L.; Tufariello, M.; Durante, M.; Fiore, A.; Baiano, A.; Grieco, F. Assessment of the impact of unmalted cereals, hops, and yeast strains on volatolomic and olfactory profiles of Blanche industrial beers: A chemometric approach. Food Chem. 2023, 416, 135783. [Google Scholar] [CrossRef] [PubMed]
- Gabriella, S.; Salvatore, M.; Anna, R.; Giovanni, C.; Floriana, B.; Grazia, V.M. Innovative technologies optimizing the production process of “Castagne del Prete”: Impact on microstructure and volatile compounds. LWT 2022, 168, 113881. [Google Scholar] [CrossRef]
- BJCP Beer Style Guidelines. 2015. Available online: https://www.bjcp.org/docs/2015_Guidelines_Beer.pdf (accessed on 10 August 2024).
- Liu, S.Q.; Hui Quek, A.Y. Evaluation of beer fermentation with a novel yeast Williopsis saturnus. Food Technol. Biotechnol. 2016, 54, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Guo, M.; Du, J.; Zhang, Z.A. Cloudy wheat beer enriched with okra [Abelmoschus esculentus (L.) Moench]: Effects on volatile compound and sensorial attributes. Int. J. Food Prop. 2018, 21, 289–300. [Google Scholar] [CrossRef]
- Jordán, M.J.; Margaría, C.A.; Shaw, P.E.; Goodner, K.L. Aroma active components in aqueous kiwi fruit essence and kiwi fruit puree by GC-MS and multidimensional GC/GC-O. J. Agric. Food Chem. 2002, 50, 5386–5390. [Google Scholar] [CrossRef] [PubMed]
- Castellari, L.; Magrini, A.; Passarelli, P.; Zambonelli, C. Effect of must fermentation temperature on minor products formed by cryo and noncryotolerant Saccharomyces cerevisiae strains. It. J. Food Sci. 1995, 7, 125–132. [Google Scholar]
- Estela-Escalante, W.; Rychtera, M.; Melzoch, K.; Hatta-Sakoda, B. Effect of aeration on the fermentative activity of Saccharomyces cerevisiae culture in apple juice. Rev. Mex. De Ing. Química 2012, 11, 211–226. [Google Scholar]
- Iorizzo, M.; Letizi, F.; Albanese, G.; Coppola, F.; Gambuti, A.; Testa, B.; Aversano, R.; Forino, M.; Coppola, R. Potential for lager beer production from Saccharomyces cerevisiae strains isolated from the vineyard environment. Processes 2021, 9, 1628. [Google Scholar] [CrossRef]
- Durand, G.A.; Corazza, M.L.; Blanco, A.M.; Corazza, F.C. Dynamic optimization of the mashing process. Food Contr. 2009, 20, 1127–1140. [Google Scholar] [CrossRef]
- Zheng, X.; D’Amore, T.; Russell, I.; Stewart, G.G. Factors influencing maltotriose utilization during brewery wort fermentations. J. Am. Soc. Brew. Chem. 1994, 52, 41–47. [Google Scholar] [CrossRef]
- Alfeo, V.; De Francesco, G.; Sileoni, V.; Blangiforti, S.; Palmeri, R.; Aerts, G.; Perretti, G.; Todaro, A. Physicochemical properties, sugar profile, and non-starch polysaccharides characterization of old wheat malt landraces. J. Food Comp. Anal. 2021, 102, 103997. [Google Scholar] [CrossRef]
- Da Silva, G.A.; Augusto, F.; Poppi, R.J. Exploratory Analysis of the Volatile Profile of Beers by HS-SPME-GC. Food Chem. 2008, 111, 1057–1063. [Google Scholar] [CrossRef]
- Stewart, G. The Production of Secondary Metabolites with Flavour Potential during Brewing and Distilling Wort Fermentations. Fermentation 2017, 3, 63. [Google Scholar] [CrossRef]
- Tufariello, M.; Rizzuti, A.; Palombi, L.; Ragone, R.; Capozzi, V.; Gallo, V.; Grieco, F. Non-targeted metabolomic approach as a tool to evaluate the chemical profile of sparkling wines fermented with autochthonous yeast strains. Food Control 2021, 126, 108099. [Google Scholar] [CrossRef]
- Ocvirk, M.; Mlinarič, N.K.; Košir, I.J. Comparison of sensory and chemical evaluation of lager beer aroma by gas chromatography and gas chromatography/mass spectrometry. J. Sci. Food Agric. 2018, 98, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Pires, M.A.; Pastrana, L.M.; Fuciños, P.; Abreu, C.S.; Oliveira, S.M. Sensorial perception of astringency: Oral mechanisms and current analysis methods. Foods 2020, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Thompson-Witrick, K.A.; Pitts, E.R.; Nemenyi, J.L.; Budner, D. The impact packaging type has on the flavor of wine. Beverages 2021, 7, 36. [Google Scholar] [CrossRef]
- Olaniran, A.O.; Hiralal, L.; Mokoena, M.P.; Pillay, B. Flavour-active volatile compounds in beer: Production, regulation and control. J. Inst. Brew. 2017, 123, 13–23. [Google Scholar] [CrossRef]
- Schwarz, K.J.; Stübner, R.; Methner, F.J. Formation of styrene dependent on fermentation management during wheat beer production. Food Chem. 2012, 134, 2121–2125. [Google Scholar] [CrossRef] [PubMed]
- Satora, P.; Pater, A. The influence of different non-conventional yeasts on the odour-active compounds of produced beers. Appl. Sci. 2023, 13, 2872. [Google Scholar] [CrossRef]
- Tufariello, M.; Palombi, L.; Baiano, A.; Grieco, F. In-depth analysis of volatolomic and odorous profiles of novel industrial beer by permutation test features selection and multivariate correlation analysis. Food Chem. 2024, 453, 139702. [Google Scholar] [CrossRef]
- Twomey, P.J.; Kroll, M.H. How to use linear regression and correlation in quantitative method comparison studies. Int. J. Clinic. Pract. 2008, 62, 529–538. [Google Scholar] [CrossRef]
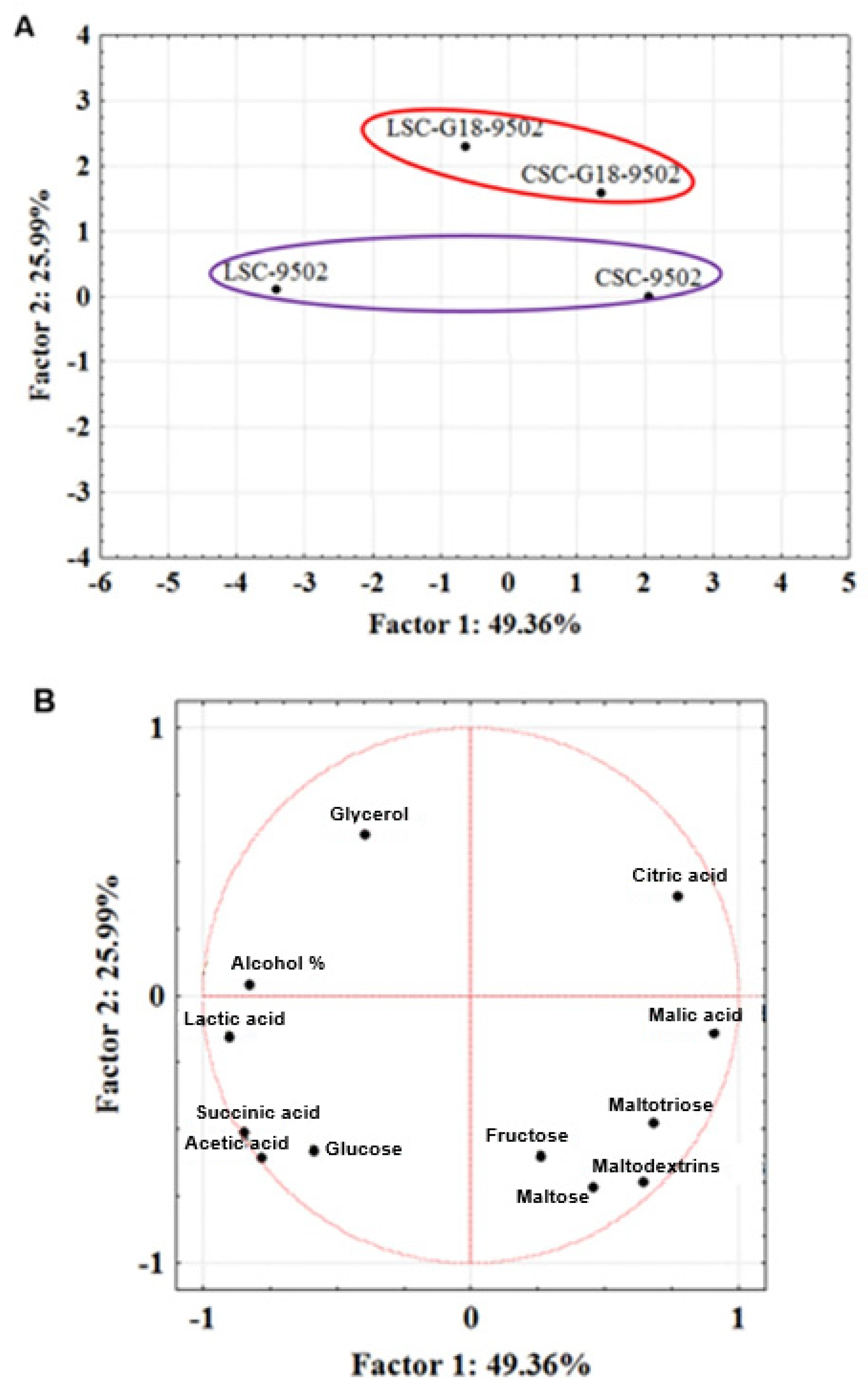
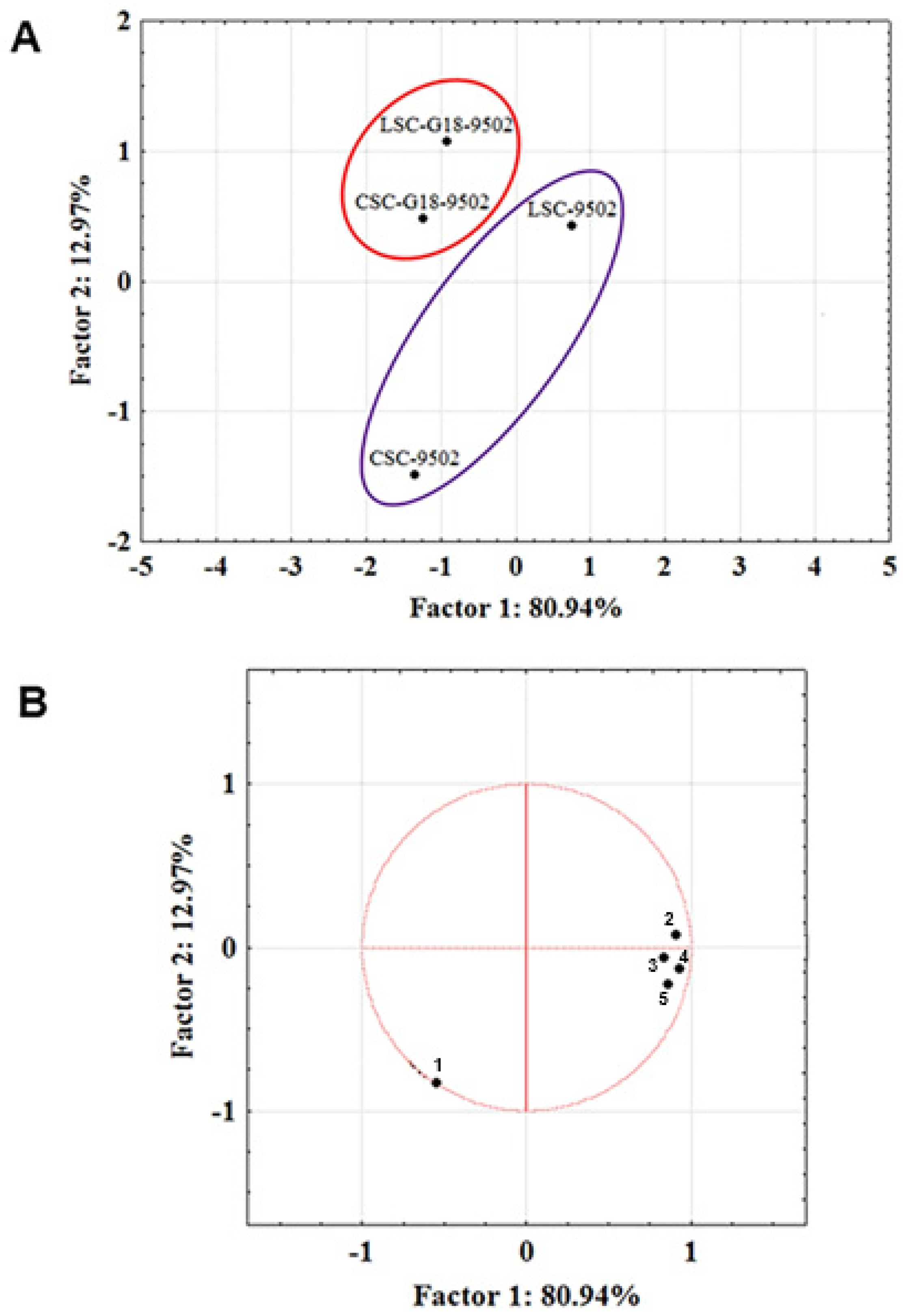
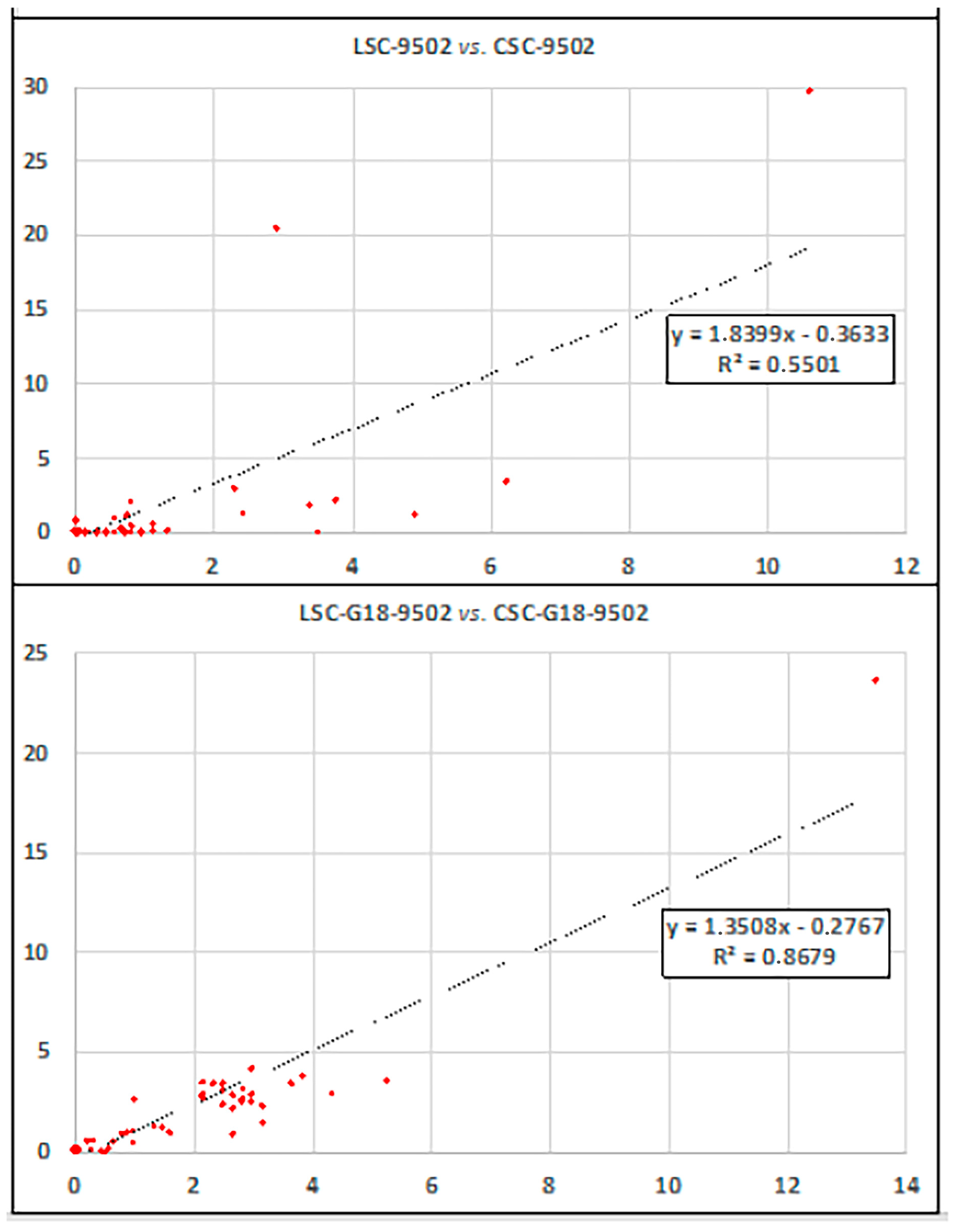
| Beers | Alcohol Content (%) | Organic Acids (g/L) | Sugars (g/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citric | Malic | Succinic | Lactic | Acetic | Maltodextrin | Maltotriose | Maltose | Glucose | Fructose | Glycerol | ||
| LSC-9502 | 6.23 ± 0.04 b | 0.75 ± 0.03 a | 0.81 ± 0.09 a | 4.91 ± 0.15 b | 1.14 ± 0.04 b | 2.43 ± 0.17 b | 10.60 ± 1.69 a | 2.91 ± 0.14 a | 2.32 ± 0.08 a | 3.53 ± 0.21 b | 0.83 ± 0.09 b | 3.39 ± 0.21 b |
| CSC-9502 | 4.36 ± 0.25 a | 1.15 ± 0.15 b | 2.03 ± 0.15 b | 1.13 ± 0.07 a | 0.49 ± 0.04 a | 1.28 ± 0.12 a | 29.64 ± 2.30 b | 20.42 ± 1.68 b | 2.97 ± 0.23 b | nd a | 0.41 ± 0.05 a | 1.76 ± 0.15 a |
| Significance | s | s | s | s | s | s | s | s | s | s | s | s |
| LSC-G18-9502 | 5.25 ± 0.05 b | 1.34 ± 0.21 a | 1.01 ± 0.08 a | 1.61 ± 0.09 b | 0.65 ± 007 a | 1.49 ± 0.17 a | 13.5 ± 0.14 a | 0.54 ± 0.10 b | 0.90 ± 0.09 a | 0.32 ± 0.06 a | 1.00 ± 0.09 b | 4.34 ± 0.33 b |
| CSC-G18-9502 | 4.52 ± 0.29 a | 1.24 ± 0.11 a | 2.57 ± 0.20 b | 0.93 ± 0.10 a | 0.49 ± 0.05 a | 1.18 ± 0.11 a | 23.59 ± 1.5 b | nd a | 0.94 ± 0.07 a | 0.54 ± 0.02 b | 0.38 ± 0.03 a | 2.85 ± 0.09 a |
| Significance | s | ns | s | s | ns | ns | s | s | ns | s | s | s |
| Compounds | Sign | LSC-9502 | CSC-9502 | Sign. | LSC-G18-9502 | CSC-G18-9502 | Sign | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| ESTERS | |||||||||||
| Ethyl Acetate | s | 0.22 b | 0.06 | 0.02 a | 0 | s | 0.05 a | 0.01 | 0.01 a | 0.01 | ns |
| Isoamyl Acetate | s | 0.09 a | 0.04 | 0.06 a | 0.01 | ns | 0.02 a | 0 | 0.02 a | 0 | ns |
| Ethyl Hexanoate | s | 1.36 b | 0.18 | 0.10 a | 0.02 | s | 0.06 a | 0.01 | 0.21 b | 0.02 | s |
| Ethyl Heptanoate | ns | 0.01 a | 0 | 0.01 a | 0 | ns | nd a | nd a | ns | ||
| Methyl Octanoate | s | 0.01 a | 0 | 0.01 a | 0 | ns | nd a | nd a | ns | ||
| Ethyl Octanoate | ns | 0.58 a | 0.31 | 0.98 a | 0.34 | ns | 0.23 a | 0.03 | 0.51 a | 0.20 | ns |
| Methyl Decanoate | ns | 0.05 a | 0 | 0.03 a | 0.01 | ns | nd a | nd a | ns | ||
| Ethyl Decanoate | ns | 0.02 a | 0 | 0.77 b | 0.31 | s | 0.28 b | 0.05 | 0.07 a | 0.03 | s |
| Diethyl Succinate | s | 0.97 b | 0.18 | nd a | s | 0.07 b | 0.01 | nd a | s | ||
| Ethyl-9-Decenoate | ns | 0.07 a | 0.06 | 0.01 a | 0 | ns | 0.04 a | 0.03 | nd a | ns | |
| Phenyl Acetate | s | 0.09 a | 0.06 | 0.07 a | 0.02 | ns | 0.01 a | 0.00 | 0.03 a | 0.02 | ns |
| Ethyl Dodecanoate | s | 0.32 b | 0.01 | 0.07 a | 0 | s | nd a | 0.01 a | 0 | ns | |
| Hexyl Acetate | s | nd a | nd a | ns | nd a | nd a | ns | ||||
| Ethyl Lactate | ns | nd a | nd a | ns | 0.06 b | 0 | nd a | s | |||
| TOTAL | s | 3.78 a | 0.43 | 2.12 a | 0.68 | ns | 0.82 a | 0.06 | 0.87 a | 0.28 | ns |
| ALCOHOLS | |||||||||||
| 2-Methyl-1-Propanol | ns | 0.04 a | 0.02 | 0.01 a | 0 | ns | 0.02 b | 0 | nd a | s | |
| 1-Propanol | ns | nd a | nd a | ns | 0.01 b | 0 | nd a | s | |||
| 2 + 3 Methyl-1-Butanol | s | 0.57 b | 0.07 | nd a | s | 0.47 b | 0.06 | nd a | s | ||
| 1-Hexanol | ns | nd a | nd a | ns | 0.02 b | 0 | nd a | s | |||
| 2-Ethyl-1-Hexanol | ns | nd a | nd a | ns | nd a | nd a | ns | ||||
| Phenyl Ethanol | s | 0.06 a | 0.01 | 0.08 a | 0.02 | ns | 0.05 a | 0 | 0.06 b | 0 | s |
| 1-Butanol | s | nd a | nd a | ns | nd a | nd a | ns | ||||
| 3-Methyl-1-Butanol | ns | nd a | 0.19 b | 0.02 | s | nd a | 0.05 b | 0.02 | s | ||
| TOTAL | s | 0.67 b | 0.04 | 0.28 a | 0.05 | s | 0.58 b | 0.08 | 0.12 a | 0.01 | s |
| TERPENES/NORISOPRENOIDS | |||||||||||
| β-Mircene | s | nd a | nd a | ns | nd a | nd a | ns | ||||
| D-Limonene | ns | 0.01 b | 0 | nd a | s | 0.02 b | 0 | nd a | s | ||
| 2-Bornanone | s | 0.05 a | 0.02 | nd a | ns | nd a | nd a | ns | |||
| Terpinen-4-Ol | s | 0.04 b | 0 | 0.01 a | 0 | s | nd a | nd a | ns | ||
| α-Terpineol | s | 0.47 b | 0.02 | nd a | s | nd a | nd a | ns | |||
| Citronellol | s | 0.16 b | 0.03 | nd a | s | nd a | nd a | ns | |||
| β-Damascenone | s | 0.09 b | 0 | nd a | s | nd a | nd a | ns | |||
| p-Cymene | ns | nd a | nd a | ns | 0.03 b | 0 | nd a | s | |||
| Linalool | ns | nd a | nd a | ns | nd a | 0.13 a | 0.06 | ns | |||
| TOTAL | s | 0.81 b | 0.03 | 0.02 a | 0 | s | 0.05 a | 0.01 | 0.15 a | 0.06 | ns |
| ALDEHYDES/KETONS | |||||||||||
| 2-Octanone | s | 0.03 a | 0.01 | 0.01 a | 0 | ns | 0.01 a | 0 | 0.01 a | 0 | ns |
| Nonanal | s | 0.03 b | 0.01 | nd a | s | nd a | 0.01 a | 0 | ns | ||
| Furfural | ns | nd a | nd a | ns | 0.01 b | 0 | nd a | s | |||
| Benzaldehyde | ns | 0.01 a | 0 | 0.01 a | 0 | ns | nd a | 0.01 a | 0 | ns | |
| TOTAL | s | 0.07 b | 0 | 0.02 a | 0 | s | 0.02 a | 0.01 | 0.03 a | 0 | ns |
| HYDROCARBONS | |||||||||||
| Styrene | s | nd a | 0.04 b | 0 | s | nd a | 0.01 b | 0 | s | ||
| TOTAL | s | nd a | 0.04 b | 0 | s | nd a | 0.01 b | 0 | s | ||
| ACIDS | |||||||||||
| Acetic Acid | s | 0.04 b | 0.01 | nd a | s | nd a | nd a | ns | |||
| Hexanoic Acid | s | nd a | nd a | ns | nd a | 0.02 b | 0 | s | |||
| Octanoic Acid | s | 0.04 a | 0 | 0.10 a | 0.02 | ns | nd a | 0.06 a | 0.02 | ns | |
| Nonanoic Acid | s | 0.73 b | 0.25 | nd a | s | nd a | nd a | ns | |||
| n-Decanoic Acid | ns | 0.33 b | 0.02 | nd a | s | nd a | nd a | ns | |||
| TOTAL ACIDS | s | 1.13 b | 0.24 | 0.10 a | 0.02 | s | nd a | 0.07 b | 0.02 | s | |
| TOTAL VOLATILE COMPOUNDS | 6.46 | 2.58 | 1.47 | 1.25 | |||||||
| Beers | Colour | Foam | Turbidity | Flavor | Gustatory Characteristics | Tactile Characteristics | OSQ | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Foam | Liquid | Amount | Persistence | OOI | OF | Malty | Hoppy | Floral | Fruity | Spicy | Yeast | Sweetness | Bitterness | Saltiness | Sourness | Alcoholic | Effervescence | Body/Fullness | |||
| LSC-G18-9502 | 1.0 ± 0 a | 2.7 ± 0.5 a | 2.3 ± 0.5 a | 2.2 ± 0.4 a | 3.7 ± 0.5 a | 2.5 ± 0.5 a | 3.8 ± 0.8 a | 2.8 ± 0.4 a | 2.8 ± 0.4 a | 2.2 ± 0.4 a | 2.5 ± 0.5 a | 2.7 ± 0.5 a | 2.5 ± 0.5 a | 2.2 ± 0.4 a | 3.2 ± 0.8 a | 2.7 ± 1.0 b | 3.2 ± 0.4 b | 2.8 ± 0.8 a | 3.0 ± 0.8 a | 3.0 ± 0.9 a | 3.0 ± 0.8 a |
| CSC-G18-9502 | 1.0 ± 0 a | 2.8 ± 1.4 a | 3.4 ± 0.8 b | 3.5 ± 1.0 b | 3.4 ± 1.1 a | 3.4 ± 1.0 b | 3.8 ± 0.9 a | 3.2 ± 1.0 a | 2.6 ± 1.4 a | 2.7 ± 1.4 a | 3.1 ± 1.5 a | 2.2 ± 1.5 a | 2.3 ± 1.5 a | 2.8 ± 0.6 a | 2.2 ± 0.8 a | 0.8 ± 0.4 a | 1.4 ± 0.4 a | 2.5 ± 1.2 a | 2.8 ± 0.6 a | 2.5 ± 0.8 a | 4.2 ± 0.7 a |
| Significance | ns | ns | s | s | ns | s | ns | ns | ns | ns | ns | ns | ns | ns | ns | s | s | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baiano, A.; Fiore, A.; Maruccia, F.; Gerardi, C.; Povero, M.; Grieco, F.; Tufariello, M. Industrial-Level Brewing Using Oenological Saccharomyces cerevisiae and Schizosaccharomyces pombe as Mixed-Inoculum. Appl. Sci. 2024, 14, 8609. https://doi.org/10.3390/app14198609
Baiano A, Fiore A, Maruccia F, Gerardi C, Povero M, Grieco F, Tufariello M. Industrial-Level Brewing Using Oenological Saccharomyces cerevisiae and Schizosaccharomyces pombe as Mixed-Inoculum. Applied Sciences. 2024; 14(19):8609. https://doi.org/10.3390/app14198609
Chicago/Turabian StyleBaiano, Antonietta, Anna Fiore, Francesco Maruccia, Carmela Gerardi, Marco Povero, Francesco Grieco, and Maria Tufariello. 2024. "Industrial-Level Brewing Using Oenological Saccharomyces cerevisiae and Schizosaccharomyces pombe as Mixed-Inoculum" Applied Sciences 14, no. 19: 8609. https://doi.org/10.3390/app14198609










