Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies
Abstract
1. Introduction
2. Methodology
3. Determination of Pharmaceuticals in Environmental Samples
3.1. Sample Preparation Techniques
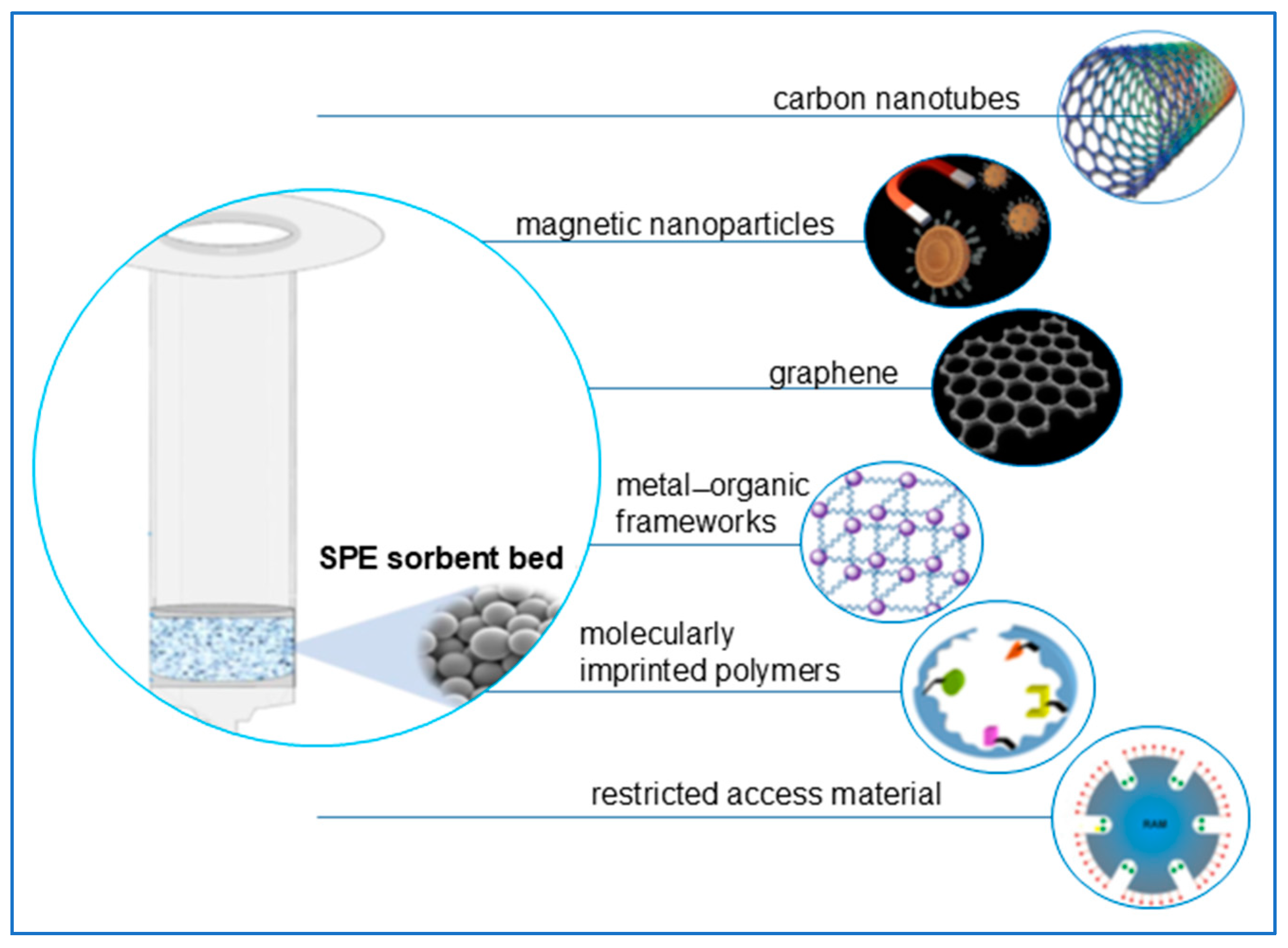
3.1.1. Solid-Phase Extraction (SPE)
Alternative Sorption Materials
3.1.2. QuEChERS
3.1.3. Microextraction Techniques
3.2. Instrumental Separation and Detection Technique
4. Conclusions and Future Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Toxicol. Environ. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- OECD. Pharmaceutical Residues: Freshwater: Hazards and Policy Responses. Available online: https://www.oecd-ilibrary.org/environment/pharmaceutical-residues-in-freshwater_c936f42d-en (accessed on 15 March 2024).
- Samal, K.; Mahapatra, S.; Ali, M.H. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Brausch, J.M.; Connors, K.A.; Brooks, B.W.; Rand, G.M. Human pharmaceuticals in the aquatic environment: A review of recent toxicological studies and considerations for toxicity testing. Rev. Environ. Contam. Toxicol. 2012, 218, 1–99. [Google Scholar] [PubMed]
- Patrolecco, L.; Ademollo, N.; Greni, P.; Tolomei, A.; Caraciollo, A.B.; Capri, S. Simultaneous determination of human pharmaceuticals in water samples by solid phase extraction and HPLC with UV-fluorescence detection. Microchem. J. 2013, 107, 165–171. [Google Scholar] [CrossRef]
- Hussain, A.; Ashique, S.; Hassan, M.Z.; Afzal, O.; Asiri, Y.I.; Kumar, P.; Dua, K.; Webster, T.J.; Altamimi, A.S.A.; Altamimi, M.A. Pharmaceutical contaminants in aquatic systems, conventional and green strategies, recent updates, challenges and policies, and potential outcomes. J. Mol. Liq. 2023, 389, 122905. [Google Scholar] [CrossRef]
- Stratulat, A.; Sousa, É.M.L.; Calisto, V.; Lima, D.L. Solid phase extraction using biomass-based sorbents for the quantification of pharmaceuticals in aquatic environments. Microchem. J. 2023, 188, 108465. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and removal of pharmaceuticals in wastewater treatment plants. Process Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Adeoye, J.B.; Tan, Y.H.; Lau, S.Y.; Tan, Y.Y.; Chiong, T.; Mubarak, N.M.; Khalid, M. Advanced oxidation and biological integrated processes for pharmaceutical wastewater treatment: A review. J. Environ. Manag. 2024, 353, 120170. [Google Scholar] [CrossRef]
- Horváth, G.; Szalay, Z.; Šimo, F.; Vidová, B.; Hlavanda, P.; Szarka, A.; Hrouzková, S.; Debnárová, S.; Zažímal, F.; Homola, T. Sustainable remediation of paint factory wastewater using electrocoagulation. Environ. Sci. Water Res. Technol. 2024, 10, 702–717. [Google Scholar] [CrossRef]
- Kyriakopoulos, G.; Xiarchos, I.; Doulia, D. Treatment of contaminated water with pesticides via adsorption. Int. J. Environ. Technol. Manag. 2006, 6, 515–524. [Google Scholar] [CrossRef]
- Feng, K.; Gong, J.; Qu, J.; Niu, R. Dual-Mode-Driven Micromotor Based on Foam-like Carbon Nitride and Fe3O4 with Improved Manipulation and Photocatalytic Performance. ACS Appl. Mater. Interfaces 2022, 14, 44271–44281. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, Z.; Li, Q.; Chen, L.; Gong, J.; Wang, H.; Li, Y.; Qu, J.; Niu, R. Harnessing Synchronous Photothermal and Photocatalytic Effects of Substoichiometric MoO3–x Nanoparticle-Decorated Membranes for Clean Water Generation. ACS Appl. Mater. Interfaces 2024, 16, 18855–18866. [Google Scholar] [CrossRef] [PubMed]
- Zamparas, M.; Kyriakopoulos, G.; Kapsalis, V.; Drosos, M.; Kalavrouziotis, I. Application of novel composite materials as sediment capping agents: Column experiments and modelling. Desalin. Water Treat. 2019, 170, 111–118. [Google Scholar] [CrossRef]
- Loos, R.; Gawlik, B.M.; Locoro, G.; Rimaviciute, E.; Contini, S.; Bidoglio, G. EU-wide survey of polar organic persistent pollutants in European river waters. Environ. Pol. 2009, 157, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Peake, B.M.; Braund, R.; Tong, A.Y.C.; Tremblay, L.A. Detection and presence of pharmaceuticals in the environment. In The Life-Cycle of Pharmaceuticals in the Environment; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–107. [Google Scholar] [CrossRef]
- Saleh, A.; Larsson, E.; Yamini, Y.; Jönsson, J.Ä. Hollow fibre liquid phase microextraction as a preconcentration and clean-up step after pressurized hot water extraction for the determination of non-steroidal anti-inflammatory drugs in sewage sludge. J. Chromatogr. A 2011, 1218, 1331–1339. [Google Scholar] [CrossRef]
- López-Serna, R.; Petrovic, M.; Barceló, D. Development of a fast instrumental method for the analysis of pharmaceuticals in environmental and wastewaters based on ultra high performance liquid chromatography (UHPLC)-tandem mass spectrometry (MS/MS). Chemosphere 2011, 85, 1390–1399. [Google Scholar] [CrossRef]
- Guan, J.; Zhan, C.; Wang, Y.; Guo, Y.; Huang, P.; Zhao, L. Simultaneous determination of 12 pharmaceuticals by ultrasound-assisted dispersive liquid-liquid microextraction coupled with ultra-high performance liquid chromatography with tandem mass spectrometry. J. Anal. Bioanal. Chem. 2016, 408, 8099–8109. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, H.; Adams, C.D.; Gamagedara, S.; Stayton, I.; Timmons, T.; Ma, Y. Investigation of pharmaceuticals in Missouri natural and drinking water using high performance liquid chromatography-tandem mass spectrometry. Water. Res. 2011, 45, 1818–1828. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Kónyová, Z.; Czifruszová, M.; Nikš, M.; Purgelová, A.; Horniačková, M.; Göböová, M.; Komjáthy, H.; Slimáková, L. Štandardný diagnostický a terapeutický postup pre implementáciu antimikrobiálnej politiky v ústavných zdravotníckych zariadeniach; Ministerstvo zdravotníctva Slovenskej republiky: Bratislava, Slovakia, 2019; Available online: https://www.mzsr.sk/Zdroje?/Sources/dokumenty/SDTP/standardy/1-6-2020/096_KM_Standardny_diagnosticky_a_terapeuticky_postup_pre_implementaciu_antimikrobialnej_politiky.pdf (accessed on 15 June 2024).
- Proctor, K.; Petrie, B.; Barden, R.; Arnot, T.; Kasparzyk-Hordern, B. Multi-residue ultra-performance liquid chromatography coupled with tandem mass spectrometry method for comprehensive multi-class anthropogenic compounds of emerging concern analysis in catchment-based exposure-driven study. J. Anal. Bioanal. Chem. 2019, 411, 7061–7086. [Google Scholar] [CrossRef]
- Idder, S.; Ley, L.; Mazellier, P.; Budzinski, H. Quantitative on-line preconcentration-liquid chromatography coupled with tandem mass spectrometry method for the determination of pharmaceutical compounds in water. Anal. Chim. Acta 2013, 805, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Klančar, A.; Trontelj, J.; Roškar, R. Development of a Multi-Residue Method for Monitoring 44 Pharmaceuticals in Slovene Surface Water by SPE-LC-MS/MS. Water Air Soil Poll. 2018, 229, 192. [Google Scholar] [CrossRef]
- Axel, M.; Kortesmäki, E.; Brozinski, J.M.; Kronberg, L. An online SPE LC-MS/MS method for the analysis of antibiotics in environmental water. Environ. Sci. Pollut. Res. 2017, 24, 8692–8699. [Google Scholar] [CrossRef] [PubMed]
- Avar, P.; Zrínyi, Z.; Maász, G.; Takátsy, A.; Lovas, S.; Tóth, L.; Pirger, Z. β-Estradiol and ethinyl-estradiol contamination in the rivers of the Carpathian Basin. Environ. Sci. Pollut. Res. 2016, 23, 11630–11638. [Google Scholar] [CrossRef] [PubMed]
- Uraipong, C.D.; Allan, R.; Chunchua, L.R.; Kennedy, I.; Wong, V.; Lee, N.A. A survey of 17α-ethinylestradiol and mestranol residues in Hawkesbury River, Australia, using a highly specific enzyme-linked immunosorbent assay (ELISA) demonstrates the levels of potential biological significance. Ecotox. Environ. Saf. 2017, 144, 585–592. [Google Scholar] [CrossRef]
- Spilsbury, F.D.; Inostroza, P.A.; Svedberg, P.; Cannata, C.; Ragas, A.M.J.; Backhaus, T. Defining the data gap: What do we know about environmental exposure, hazards and risks of pharmaceuticals in the European aquatic environment? Water Res. 2024, 251, 121002. [Google Scholar] [CrossRef]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R Soc. Lond. B Biol. Sci. 2014, 369, 1656. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Analysis, occurrence and removal of pharmaceuticals in African water resources: A current status. J. Environ. Manag. 2020, 253, 109741. [Google Scholar] [CrossRef]
- Kulkarni, A.; Miller, S.E. Advances in Green and Sustainable Chemistry. In Contemporary Chemical Approaches for Green and Sustainable Drugs; Elsevier: Amsterdam, The Netherlands, 2022; pp. 27–45. [Google Scholar]
- Khan, H.K.; Rehman, M.Y.A.; Malik, R.N. Fate and toxicity of pharmaceuticals in water environment: An insight on their occurrence in South Asia. J. Environ. Manag. 2020, 271, 111030. [Google Scholar] [CrossRef]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic effects of NSAIDs in non-target species: A review from the perspective of the aquatic environment. Environ. Pollut. 2021, 273, 115891. [Google Scholar] [CrossRef]
- Fayaz, T.; Renuka, N.; Ratha, S.K. Antibiotic occurrence, environmental risks, and their removal from aquatic environments using microalgae: Advances and future perspectives. Chemosphere 2024, 349, 140822. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.C.; Stefan, M.I.; Parnis, J.M.; Metcalfe, C.H.D. Direct UV photolysis of selected pharmaceuticals, personal care products and endocirne disruptors in aqueos samples. Water Res. 2015, 84, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Brezovšek, P.; Eleršek, T.; Filipič, M. Toxicities of four anti-neoplastic drugs and their binary mixtures tested on the green alga Pseudokirchneriella subcapitata and the cyanobacterium Synechococcus leopoliensis. Water Res. 2014, 52, 168–177. [Google Scholar] [CrossRef]
- Kümmer, K. The presence of pharmaceuticals in the environment due to human use--present knowledge and future challenges. J. Environ. Manag. 2009, 90, 2354–2366. [Google Scholar] [CrossRef]
- Peng, Y.; Gautam, L.; Hall, S.W. The detection of drugs of abuse and pharmaceuticals in drinking water using solid-phase extraction and liquid chromatography-mass spectrometry. Chemosphere 2019, 223, 438–447. [Google Scholar] [CrossRef]
- Herrera-Herrera, A.V.; Hernánder-Borges, J.; Borges-Miquel, T.; Rodríguez-Delgado, M.Á. Dispersive liquid–liquid microextraction combined with ultra-high performance liquid chromatography for the simultaneous determination of 25 sulfonamide and quinolone antibiotics in water samples. J. Pharm. Biomed. Anal. 2013, 75, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Tenorio, R.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L. Determination of Pharmaceuticals Discharged in Wastewater from a Public Hospital Using LC-MS/MS Technique. J. Mex. Chem. Soc. 2021, 65, 1. [Google Scholar]
- Martínez-Piernas, A.B.; Plaza-Bolanos, P.; Gilabert, A.; Agüera, A. Application of a fast and sensitive method for the determination of contaminants of emerging concern in wastewater using a quick, easy, cheap, effective, rugged and safe-based extraction and liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2021, 1653, 462396. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Zioris, I.; Danis, T.; Bikiaris, D.; Lambropoulou, D. Comprehensive investigation of a wide range of pharmaceuticals and personal care products in urban and hospital wastewaters in Greece. Sci. Total Environ. 2019, 694, 133565. [Google Scholar] [CrossRef]
- Khulu, S.; Ncube, S.; Nuapia, Y.; Madikizela, L.M.; Tutu, H.; Richards, H.; Ndungu, K.; Mavhunga, E.; Chimuka, L. Multivariate optimization of a two-way technique for extraction of pharmaceuticals in surface water using a combination of membrane assisted solvent extraction and a molecularly imprinted polymer. Chemosphere 2022, 286, 131973. [Google Scholar] [CrossRef]
- Omotola, E.O.; Olatunji, O.S. Quantification of selected pharmaceutical compounds in water using liquid chromatography-electrospray ionisation mass spectrometry (LC-ESI-MS). Heliyon 2020, 6, e05787. [Google Scholar] [CrossRef] [PubMed]
- Kiszkiel-Taudul, I. Determination of Antihistaminic Pharmaceuticals in Surface Water Samples by SPE-LC-MS/MS Method. Microchem. J. 2021, 162, 105874. [Google Scholar] [CrossRef]
- Marasco Júnior, C.A.; Sartore, D.M.; Lamarca, R.S.; da Silva, B.F.; Santos-Neto, J.; Gomes, P.C.F.d.L. On-line solid-phase extraction of pharmaceutical compounds from wastewater treatment plant samples using restricted access media in column-switching liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2021, 1180, 122896. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Bianco, G.; Coviello, D.; Lafiosca, M.C.; Masi, S.; Mancini, I.M.; Bufo, S.A.; Scrano, L.; Caniani, D. Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 2019, 43, 886–895. [Google Scholar] [CrossRef]
- Althakafy, J.T.; Kulsing, C.; Grace, M.R.; Marriott, P.J. Liquid chromatography—Quadrupole Orbitrap mass spectrometry method for selected pharmaceuticals in water samples. J. Chromatogr. A 2017, 1515, 164–171. [Google Scholar] [CrossRef]
- Hassan, A.A.; Tanimu, A.; Alhooshani, K. Iron and cobalt-containing magnetic ionic liquids for dispersive micro-solid phase extraction coupled with HPLC-DAD for the preconcentration and quantification of carbamazepine drug in urine and environmental water samples. J. Mol. Liq. 2021, 336, 116370. [Google Scholar] [CrossRef]
- Qin, H.; Liu, H.; Liu, Y.; Di, S.; Bao, Y.; Zhai, Y.; Zhu, S. Recent advances in sample preparation and chromatographic analysis of pharmaceuticals and personal care products in environment. Trends Analyt. Chem. 2023, 164, 117112. [Google Scholar] [CrossRef]
- Midikizela, L.M.; Ncube, S.; Tutu, H.; Richards, H.; Newman, B.; Ndungu, K.; Chimuka, L. Pharmaceuticals and their metabolites in the marine environment: Sources, analytical methods and occurrence. Trends Environ. Anal. Chem. 2020, 28, e00104. [Google Scholar] [CrossRef]
- Jia, W.; Liu, H.; Ma, Y.; Huang, G.; Liu, Y.; Zhao, B.; Xie, D.; Huang, K.; Wang, R. Reproducibility in nontarget screening (NTS) of environmental emerging contaminants: Assessing different HLB SPE cartridges and instruments. Sci. Total Environ. 2024, 912, 168971. [Google Scholar]
- Qureshi, M.; Stecher, G.; Huck, C.; Bonn, G.K. Preparation of polymer based sorbents for solid phase extraction of polyphenolic compounds. Open Chem. 2011, 9, 206–212. [Google Scholar]
- Chen, L.; Yan, X.; Zhou, X.; Peng, P.; Sun, Q.; Zhao, F. Advances in the on-line solid-phase extraction-liquid chromatography-mass spectrometry analysis of emerging organic contaminants. Trends Anal. Chem. 2023, 160, 116976. [Google Scholar] [CrossRef]
- Camillieri, J.; Baudot, R.; Wiest, L.; Vulliet, E.; Cren-Olivé, C.; Daniele, G. Multiresidue fully automated online SPE-HPLC-MS/MS method for the quantification of endocrine-disrupting and pharmaceutical compounds at trace level in surface water. Int. J. Environ. Anal. Chem. 2014, 95, 67–81. [Google Scholar] [CrossRef]
- Hashemi, B.; Zohrabi, P.; Shamsipur, M. Recent developments and applications of different sorbents for SPE and SPME from biological samples. Talanta 2018, 187, 337–347. [Google Scholar] [CrossRef]
- Martinez-Sena, T.; Armenta, S.D.L.; Guardia, M.; Esteve-Turillas, F.A. Determination of non-steroidal anti-inflammatory drugs in water and urine using selective molecular imprinted polymer extraction and liquid chromatography. J. Pharm. Biomed. Anal. 2016, 131, 48–53. [Google Scholar] [CrossRef]
- Alinezhad, H.; Amiri, A.; Tarahomi, M.; Maleki, B. Magnetic solid-phase extraction of non-steroidal anti-inflammatory drugs from environmental water samples using polyamidoamine dendrimer functionalized with magnetite nanoparticles as a sorbent. Talanta 2018, 183, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Hemmati, S. Magnetic nanoparticles embedded with graphene quantum dots and multiwalled carbon nanotubes as a sensing platform for electrochemical detection of progesterone. Sens. Actuators B 2017, 238, 346–356. [Google Scholar] [CrossRef]
- Abujaber, F.; Zougagh, M.; Jodeh, S.; Rios, Á.; Guzmán, B.F.J.; Martin-Doimeadios, R.C.R. Magnetic cellulose nanoparticles coated with ionic liquid as a new material for the simple and fast monitoring of emerging pollutants in waters by magnetic solid phase extraction. Microchem. J. 2018, 137, 490–495. [Google Scholar] [CrossRef]
- Abd Wahib, S.M.; Wan Ibrahim, W.A.; Marsin Sanagi, M.; Afzal Kamboh, M.; Abdul Keyon, A.S. Magnetic sporopollenin-cyanopropyltriethooxysilane-dispersive micro-solid phase extraction coupled with high performance liquid chromatography for the determination of selected non-steroidal anti-inflammatory drugs in water samples. J. Chromatogr. A 2018, 1532, 50–57. [Google Scholar] [CrossRef]
- Song, X.Y.; Chen, J.; Shi, Y.P. Different configurations of carbon nanotubes reinforced solid-phase microextraction techniques and their applications in the environmental analysis. Trends Anal. Chem. 2017, 86, 263–275. [Google Scholar] [CrossRef]
- Abujaber, F.; Ahmad, S.M.; Neng, N.R.; Rodríguez Martin-Doimeadios, R.C.; Guzmán Bernardo, F.J.; Nogueira, J.M.F. Bar adsorptive microextraction coated with multi-walled carbon nanotube phases—Application for trace analysis of pharmaceuticals in environmental waters. J. Chromatogr. A 2019, 1600, 17–22. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.; Hong, Y.; Lee, W.; Lee, S.; Chung, H.; Kim, H.; Jeong, D.-H. Determination of pharmaceuticals in solid samples in municipal wastewater treatment plants by online SPE LC–MS/MS using QuEChERS extraction. Environ. Monit. Assess. 2021, 193, 279. [Google Scholar] [CrossRef] [PubMed]
- Kachhawaha, A.S.; Nagarnaik, P.M.; Jadhav, M.; Pudale, A.; Labhasetwar, P.K.; Banerjee, K. Optimization of a modified QuEChERS method for multiresidue analysis of pharmaceuticals and personal care products in sewage and surface water by LC-MS/MS. J. AOAC Int. 2017, 100, 592–597. [Google Scholar] [CrossRef]
- Hrouzková, S.; Szarka, A.; Zichová, S. Pokroky a využitie mikroextrakčných techník na analýzu rezíduí pesticídov v potravinách. Chem. Listy 2018, 112, 165–174. [Google Scholar]
- Carasek, E.; Mores, L.; Merib, J. Basic principles, recent trends and future directions of microextraction technique for the analysis of aqueous environmental samples. Trends Environ. Anal. Chem. 2018, 19, e00060. [Google Scholar] [CrossRef]
- Rezai, F.; Yamini, Y.; Moradi, M.; Daraei, B. Supramolecular solvent-based hollow fiber liquid phase microextraction of benzodiazepines. Anal. Chim. Acta 2013, 804, 135–142. [Google Scholar] [CrossRef]
- Tong, A.Y.C.; Peake, B.M.; Braund, R. Disposal practices for unused medications around the world. Environ. Int. 2011, 37, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, S.; Di Carro, M.; Magi, E. Innovative sampling and extraction methods for the determination of nonsteroidal anti-inflammatory drugs in water. J. Pharm. Biomed. Anal. 2015, 106, 100–106. [Google Scholar] [CrossRef]
- Aparicio, I.; Martin, J.; Santos, J.L.; Malvar, J.L.; Alonso, E. Stir bar sorptive extraction and liquid chromatography–tandem mass spectrometry determination of polar and non-polar emerging and priority pollutants in environmental waters. J. Chromatogr. A 2017, 1500, 43–52. [Google Scholar] [CrossRef]
- Manzo, V.; Ulisse, K.; Rodríguez, I.; Pereir, E.; Richter, P. A moleculary imprinted polymer as a sorptive phase imobilized in a rotating disk extraction device for the determination of diclofenac and mefenamic acid in wastewater. Anal. Chim. Acta 2015, 889, 130–137. [Google Scholar] [CrossRef]
- Landmark, C.J.; Johannessen, S.I. Chapter 10.7—Therapeutic monitoring of antiepileptic drugs. In Handbook of Analytical Separations; Elsevier: Amsterdam, The Netherlands, 2020; pp. 225–256. [Google Scholar]
- Petrović, M.; Gros, M.; Barceló, D. Chapter 2.4—Multi-residue analysis of pharmaceuticals using LC-tandem MS and LC-hybrid MS. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 157–183. [Google Scholar]
- Miao, X.S.; Metcalfe, C.D. Chapter 2.3—Analysis of neutral and acidic pharmaceuticals by liquid chromatography mass spectrometry. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2007; pp. 133–156. [Google Scholar]
- Sabourian, R.; Mirjalili, S.Z.; Namini, N.; Chavoshy, F.; Hajimahmoodi, M.; Safavi, M. HPLC methods for quantifying anticancer drugs in human samples: A systematic review. Anal. Biochem. 2020, 610, 113891. [Google Scholar] [CrossRef]
- Togunde, O.P.; Cudjoe, E.; Oakes, K.D.; Mirnaghi, F.S.; Servos, M.R.; Pawliszyn, J. Determination of selected pharmaceutical residues in wastewater using an automated open bed solid phase microextraction system. J. Chromatogr. A 2012, 1262, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Kosma, C.I.; Nannou, C.I.; Boti, V.I.; Albanis, T.A. Psychiatrics and selected metabolites in hospital and urban wastewaters: Occurrence, removal, mass loading, seasonal influence and risk assessment. Sci. Total Environ. 2019, 659, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lu, D.; Liu, C.; Hower, D. Analytical challenges and recent advances in the identification and quantitation of extractables and leachables in pharmaceutical and medical products. Trends Analyt. Chem. 2021, 141, 116286. [Google Scholar] [CrossRef]

| Sample | Analyte | Extraction Method | Instrumentation | Separation Conditions | LOD µg/L | Real Samples Finding µg/L | Ref. |
|---|---|---|---|---|---|---|---|
| Drinking water | NPS (15) Illegal drugs (3) Antidepressants (2) | SPE Strata-X-Drug B cartridge (60 mg/6 mL) Conditioning with 2 mL MeOH equilibration with 2 mL of 0.1 M HCl volume samples 200 mL acidified with 0.1 M HCl (pH = 2) washing with 0.1 M HCl elution 15% isopropanol/85% ethyl acetate and 2× 10% NH4OH/20% isopropanol/70% ethyl acetate evaporation and reconstitution with 0.1 mL LC–MS solvent (0.5% formic acid/5% acetonitrile/94.5% water) | LC–MS | A: 0.5% formic acid in water B: 0.5% formic acid in ACN | 0.00001–0.0011 | - | [39] |
| Drainage water | Antibiotics (25) | DLLME 5 mL samples 20% (w/v) NaCl pH = 7.6 685 μL/1250 μL ACN Vortex 30 min Centrifuge 4000 rpm 10 min Evaporate the solvent with N2 and dissolve in 150 μL of mobile phase | UHPLC–DAD | A: 0.3% CH3COOH in water B: ACN | 0.35–10.5 | - | [40] |
| Groundwater | NSAIDs (6) Antibiotics (6) | DLLME | UPLC–ESI–MS/MS | A: 0.1% formic acid B: ACN | 0.006–0.091 | [19] | |
| Hospital wastewater | Antibiotics (3) Pharmaceuticals (13) | SPE Oasis HLB cartridge (500 mg/6 cm3) pre-conditioning with MeOH and pure water volume of samples 200 mL (pH = 8 NH4OH) evaporation under vacuum elution MeOH | LC–MS/MS | A: 5 mM CH3COONH4 B: ACN | 0.02–0.59 | 0.63–3.29 | [41] |
| Municipal and hospital wastewater | Pharmaceuticals (58) Antibiotics (16) Pesticides (33) | QuEChERS d-SPE ACN:MeOH (85:15, v/v) vortex citric acid monohydrate trisodium citrate dihydrate vortex centrifuge supernatant 5 mL Z-Sep vortex centrifuge evaporation of 150 µL extract using N2 reconstitution to 150 µL H2O:ACN (90:10, v/v) | LC–QqLIT–MS/MS | A: 0.1% HCOOH in Milli-Q water B: MeOH | 0.002–0.2 | 0.005–0.677 | [42] |
| Municipal and hospital wastewater | Pharmaceuticals and personal care products (138) | SPE Oasis HLB cartridges (200 mg/cm3) Conditioning with Mili-Q water and MeOH sample with 5% Na2EDTA with a final concentration of 0.1% elution (×2) MeOH evaporation with N2 and reconstitution to 0.5 mL with MeOH | LC–MS/MS | A: 5 mM NH4COOH in Mili-Q water B: 5 mM NH4COOH in MeOH | 0.0002–0.00506 | <LOD–81.491 | [43] |
| River water | Anticonvulsants (1) Pacemakers (1) Muscle relaxants (1) Antiretroviral medicines (1) Antidepressants (1) | MASE-MIP (50 mg) Sample volume 18 mL 5 g NaCl mixing extraction time 60 min 1 mL CHCl3 (acceptor solvent) MeOH (eluent) | LC–q-TOF/MS | A: 0.1% HCOOH in deionized water B: 0.1% HCOOH in ACN | 0.09–0.20 | 0.31–2.48 | [44] |
| River water | Antiretroviral drugs (1) Antipyretics (1) Antibiotics (3) NSAIDs (1) Antiparasitics (1) | SPE Strata cartridges (200 mg/6 mL) Conditioning with MeOH elution 50% and 30% MeOH/distilled water sample volume 300 mL isolation 0.5% formic acid/MeOH evaporation reconstitution to 1 mL in 0.5% buffered MeOH concentration with compressed air and reconstitution in 1 mL of acidified MeOH | LC–MS/MS | A: 0.1% HCOOH in water B: 0.1% HCOOH in ACN | 0.0439–0.0219 | Up to 398.98 | [45] |
| River water | Antibiotics (1) Anticonvulsants (1) | SPE 1 L samples 25 mg AC-PPS/AC-SBG Conditioning with 10 mL of ultrapure water Elution 5 mL + 5 mL acetone | HPLC–UV HPLC–FLD | A: 1% CH3COOH in water B: ACN | Carbamazepine 0.69 Sulfamethoxazole 0.015 | - | [7] |
| Surface water | Antihistamine (4) | SPE Oasis HLB (500 mg) conditioning with distilled water volume 100 mL of equimolar mixture of samples (pH = 6.8) washing with distilled water elution with MeOH | LC–MS/MS | A: 0.1% HCOOH in water B: MeOH | 5.3 × 10−8 mol/L–7.8 × 10−8 mol/L | - | [46] |
| Wastewater | Anticonvulsants (1) Antidepressants (2) Antibiotics (2) Stimulators (1) | Online SPE RAM-BSA column (664.5 mg Luna C18) conditioning with MeOH elution with 0.05 mmol/L phosphate (pH = 6) elution 1.0 mg/mL BSA elution with ultrapure water and 25% (v/v) glutaraldehyde solution washing with 1 mg/mL sodium borohydride solution conditioning with ultrapure water | LC–MS/MS | A: 0.1% HCOOH ultrapure water B: 0.1% HCOOH in ACN C: ultrapure water | 0.01–3 | <9.60 | [47] |
| Wastewater | Antibiotics (3) Anticonvulsants (1) Antidiabetic drugs (1) Gastroprotectors (1) | SPE OASIS HLB cartridges (200 mg/6 mL) conditioning with MeOH and deionized water sample volume 20 mL washing with deionized water elution (×2) MeOH filtration 0.2 µm PTFE | LC–MS/MS | A: water without additives B: 0.1% HCOOH in water C: ACN | 0.0001–0.5114 | 0.00811–4.9859 | [48] |
| Wastewater | Pharmaceuticals and personal care products (13) | SPE Oasis HLB cartridge (60 mg/3 cm3) conditioning with MeOH and Mili-Q water sample volume 30 mL elution MeOH adjunction of 300 µL Mili-Q waters evaporation with N2 to 1.0 mL vortex | UHPLC–Q–Orbitrap–MS | A: 0.1% CH3COOH in water B: ACN | 0.01–0.61 | - | [49] |
| Wastewater | Anticonvulsants (1) | D-μ-SPE 40 mg MIL to 10 mL samples with 300 ug/L 10% NaCl w/v mixing 700 rpm pH 2.8 desorption 500 µL with ACN ultrasound 45 min | HPLC–DAD | A: ACN B: deionized water C: 50 mM phosphate buffer solution with pH 3.5 | Carbamazepine 0.51 | - | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szarka, A.; Vnuková, L.; Keršňáková, Z.; Viktoryová, N.; Hrouzková, S. Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies. Appl. Sci. 2024, 14, 8645. https://doi.org/10.3390/app14198645
Szarka A, Vnuková L, Keršňáková Z, Viktoryová N, Hrouzková S. Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies. Applied Sciences. 2024; 14(19):8645. https://doi.org/10.3390/app14198645
Chicago/Turabian StyleSzarka, Agneša, Lucia Vnuková, Zuzana Keršňáková, Nicolette Viktoryová, and Svetlana Hrouzková. 2024. "Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies" Applied Sciences 14, no. 19: 8645. https://doi.org/10.3390/app14198645
APA StyleSzarka, A., Vnuková, L., Keršňáková, Z., Viktoryová, N., & Hrouzková, S. (2024). Contamination with Pharmaceuticals in Aquatic Environment: Focus on Analytical Methodologies. Applied Sciences, 14(19), 8645. https://doi.org/10.3390/app14198645







