Aluminum Phosphate Nanoplates Synthesized via Green Method Using Cork Oak Somatic Embryo-Derived Phytates
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Determination of Phosphate Content
2.3. Green Synthesis of Metallic Nanoparticles
2.4. Characterization of Nanoparticles
2.4.1. UV–Visible Spectroscopic Analysis
2.4.2. X-ray Powder Diffraction (XRD)
2.4.3. Transmission Electron Microscopy (TEM)
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Energy-Dispersive X-ray Spectroscopy (EDX)
2.4.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3. Results
3.1. Phytochemical Yield: Phosphate and Phytate Contents
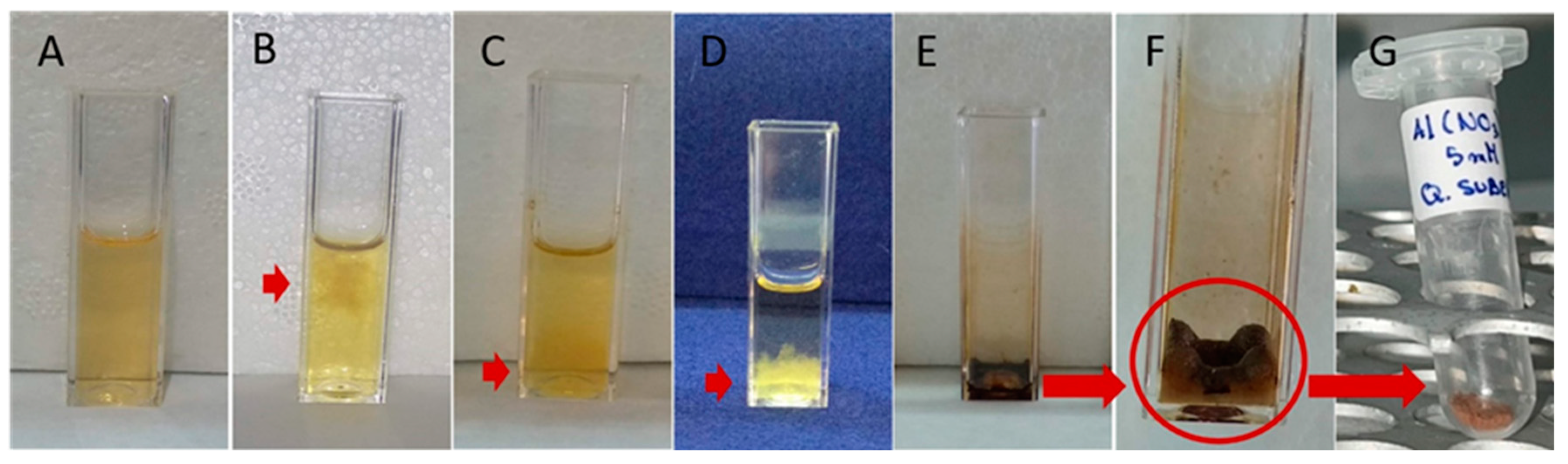
3.2. Synthesis of Metallic Nanoparticles
3.3. UV–Visible Spectroscopic Analysis
3.4. X-ray Powder Diffraction (XRD)
3.5. Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), and Energy-Dispersive X-ray Spectroscopy (EDX)
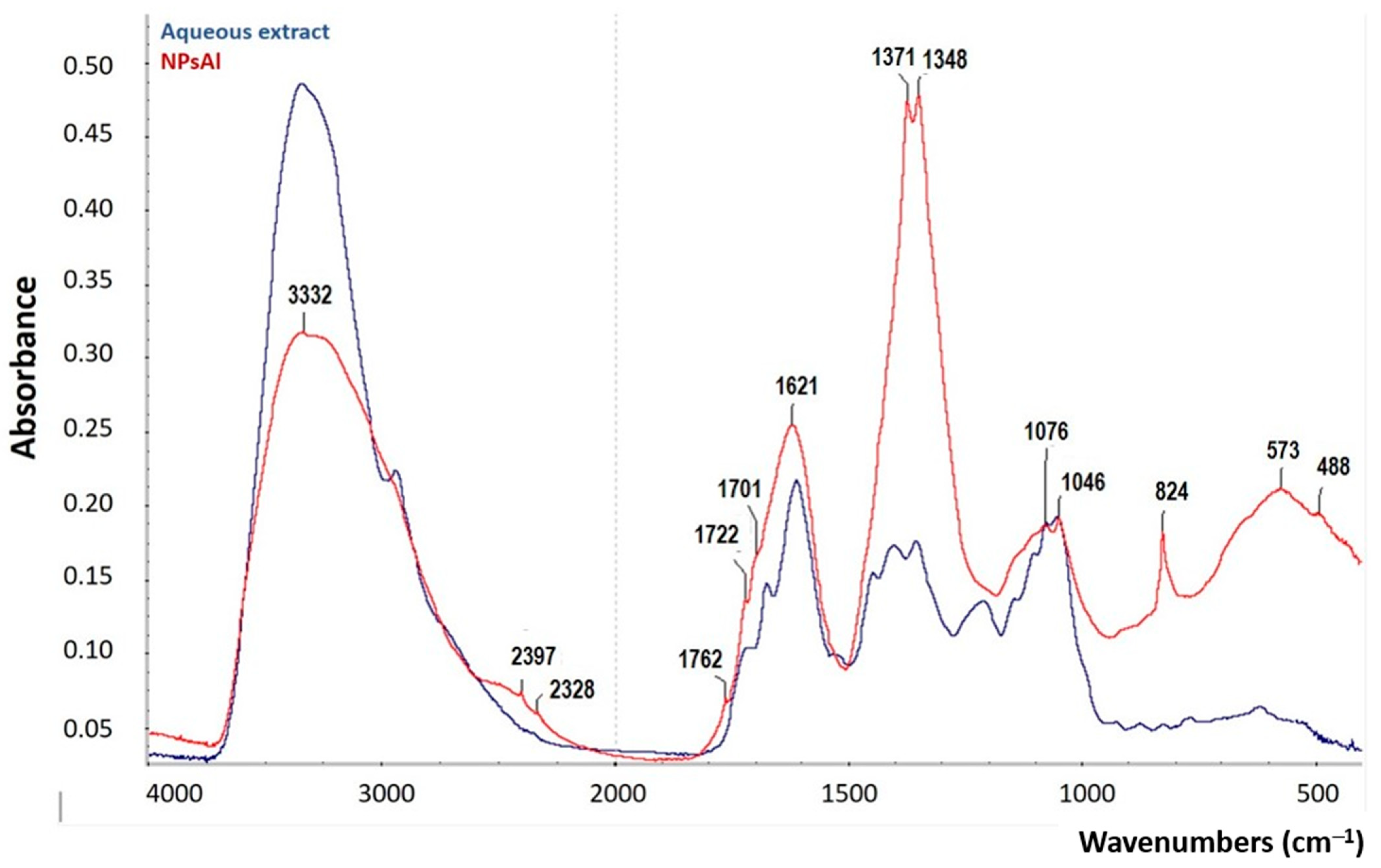
3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghorbani, H.R. A review of methods for synthesis of Al nanoparticles. Orient. J. Chem. 2014, 30, 1941–1949. [Google Scholar] [CrossRef]
- Iqbal, I.; Sharma, S.; Pathania, A.R. An insight into application and harmful effects of aluminum, aluminum complexes and aluminum nano-particles. Mater. Today Proc. 2022, 62, 4365–4369. [Google Scholar] [CrossRef]
- Elaiyappillai, E.; Arumugam, G.; Johnson, P.M.; Kogularasu, S.; Rajendran, R. Sonochemical assisted leaching of aluminum oxide nanoparticles from domestic aluminum wastes as non-toxic electrode material for energy storage application. J. Electrochem. Soc. 2020, 167, 110541. [Google Scholar] [CrossRef]
- AbdulKarim-Talaq, M.; Hassan, K.T.; Hameed, D.A. Improvement of thermal conductivity of novel asymmetric dimeric coumarin liquid crystal by doping with boron nitride and aluminum oxide nanoparticles. Mater. Chem. Phys. 2023, 297, 127367. [Google Scholar] [CrossRef]
- Hassanpour, P.; Panahi, Y.; Ebrahimi-Kalan, A.; Akbarzadeh, A.; Davaran, S.; Nasibova, A.N.; Kavetskyy, T. Biomedical applications of aluminum oxide nanoparticles. Micro Nano Lett. 2018, 13, 1227–1231. [Google Scholar] [CrossRef]
- Mukherjee, A.; Sadiq, I.M.; Prathna, T.C.; Chandrasekaran, N. Antimicrobial activity of aluminum oxide nanoparticles for potential clinical applications. Sci. Against Microb. Pathog. Commun. Curr. Res. Technol. Adv. 2011, 1, 245–251. [Google Scholar]
- Maharjan, A.; Gautam, R.; Lee, G.; Kim, D.; Lee, D.; Acharya, M.; Kim, H.; Heo, Y.; Kim, C. Assessment of skin sensitization potential of zinc oxide, aluminum oxide, manganese oxide, and copper oxide nanoparticles through the local lymph node assay: 5-bromo-deoxyuridine flow cytometry method. J. Toxicol. Environ. Health Part A 2024, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Honda, J.; Sugawa, K.; Tahara, H.; Danno, M.; Suzuki, A.; Kurumi, S.; Kimura, T.; Kosuge, Y.; Ikake, H.; Hashimoto, T.; et al. Photothermal Therapeutic Nanomaterials Composed of Plasmonic Aluminum Nanostructures for Effective Killing of Cells. ACS Appl. Nano Mater. 2024, 7, 2889–2902. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of superparamagnetic nanoparticles coated with polyacrylic acid and aluminum hydroxide as an efficient contrast agent for multimodal imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef]
- Isitman, N.A.; Dogan, M.; Bayramli, E.; Kaynak, C. The role of nanoparticle geometry in flame retardancy of polylactide nanocomposites containing aluminum phosphinate. Polym. Degrad. Stab. 2012, 97, 1285–1296. [Google Scholar] [CrossRef]
- Cai, G.; Wu, J.; Guo, J.; Wan, Y.; Zhou, Q.; Zhang, P.; Yu, X.; Wang, M. A novel inorganic aluminum phosphate-based flame retardant and thermal insulation coating and performance analysis. Materials 2023, 16, 4498. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Jiang, P.; Liu, D.; Jia, X.; Wang, X.; Zhou, F. Direct ink writing of aluminum-phosphate-bonded Al2O3 ceramic with ultra-low dimensional shrinkage. Ceram. Int. 2022, 48, 864–871. [Google Scholar] [CrossRef]
- Gou, Y.; Xie, Y.; Shen, S.; Xing, H.; Jin, P.; Li, H.; Chao, X.; Hu, D. Enhancement of aluminum phosphate adhesion performance by nano-clay for terracotta figurine restoration. Int. J. Adhes. Adhes. 2024, 132, 103685. [Google Scholar] [CrossRef]
- Kumar, N.; Biswas, K. Cryomilling: An environment friendly approach of preparation large quantity ultra refined pure aluminum nanoparticles. J. Mater. Res. Technol. 2019, 8, 63–74. [Google Scholar] [CrossRef]
- Tran, G.T.; Nguyen, N.T.H.; Nguyen, N.T.T.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T.V. Plant extract-mediated synthesis of aluminum oxide nanoparticles for water treatment and biomedical applications: A review. Environ. Chem. Lett. 2023, 21, 2417–2439. [Google Scholar] [CrossRef]
- Al-Zahrani, S.; Astudillo-Calderón, S.; Pintos, B.; Pérez-Urria, E.; Manzanera, J.A.; Martín, L.; Gomez-Garay, A. Role of synthetic plant extracts on the production of silver-derived nanoparticles. Plants 2021, 10, 1671. [Google Scholar] [CrossRef]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Ravindran, V.; Ravindran, G.; Sivalogan, S. Total and phytate phosphorus contents of various foods and feedstuffs of plant origin. Food Chem. 1994, 50, 133–136. [Google Scholar] [CrossRef]
- Reinmuth, M.; Pramanik, S.; Douglas, J.T.; Day, V.W.; Bowman-James, K. Structural impact of chelation on phytate, a highly phosphorylated biomolecule. Eur. J. Inorg. Chem. 2019, 2019, 1870–1874. [Google Scholar] [CrossRef]
- Kumar, A.; Dash, G.K.; Sahoo, S.K.; Lal, M.K.; Sahoo, U.; Sah, R.P.; Ngangkham, U.; Kumar, S.; Baig, M.J.; Sharma, S.; et al. Phytic acid: A reservoir of phosphorus in seeds plays a dynamic role in plant and animal metabolism. Phytochem. Rev. 2023, 22, 1281–1304. [Google Scholar] [CrossRef]
- Reid, D.A.; Lott, J.N.; Attree, S.M.; Fowke, L.C. Mineral nutrition in white spruce (Picea glauca [Moench] Voss) seeds and somatic embryos. I. phosphorus, phytic acid, potassium, magnesium, calcium, iron and zinc. Plant Sci. 1999, 141, 11–18. [Google Scholar] [CrossRef]
- Gomez-Garay, A.; Manzanera, J.A.; Pintos-Lopez, B. Embryogenesis in Oak species. A review. For. Syst. 2014, 23, 191–198. [Google Scholar]
- Adewale, O.B.; Egbeyemi, K.A.; Onwuelu, J.O.; Potts-Johnson, S.S.; Anadozie, S.O.; Fadaka, A.O.; Osukoya, O.A.; Aluko, B.T.; Johnson, J.; Obafemi, T.O.; et al. Biological synthesis of gold and silver nanoparticles using leaf extracts of Crassocephalum rubens and their comparative in vitro antioxidant activities. Heliyon 2020, 6, e05501. [Google Scholar] [CrossRef] [PubMed]
- Callejas, F.R. Tablas de Espectroscopía Infrarroja; Departamento de Física y Química, UNAM (Universidad Nacional Autónoma de México): Mexico City, Mexico, 2000. [Google Scholar]
- Gomez-Garay, A.; Lopez, J.A.; Camafeita, E.; Bueno, M.A.; Pintos, B. Proteomic perspective of Quercus suber somatic embryogenesis. J. Proteom. 2013, 93, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Murthy, H.N.; Joseph, K.S.; Hahn, J.E.; Lee, H.S.; Paek, K.Y.; Park, S.Y. Suspension culture of somatic embryos for the production of high-value secondary metabolites. Physiol. Mol. Biol. Plants 2023, 29, 1153–1177. [Google Scholar] [CrossRef]
- Harborne, A.J. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Amini, S.M.; Akbari, A. Metal nanoparticles synthesis through natural phenolic acids. IET Nanobiotechnol. 2019, 13, 771–777. [Google Scholar] [CrossRef]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Castro, L.M.G.; Ribeiro, T.B.; Machado, M.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Unraveling the Effect of Dehulling Methods on the Nutritional Composition of Acorn Quercus spp. J. Food Compos. Anal. 2022, 106, 104354. [Google Scholar] [CrossRef]
- Nikolic, N.; Orlovic, S.; Krstic, B.; Kevrešan, Ž. Variability of acorn nutrient concentrations in pedunculate oak (Quercus robur L.) genotypes. J. For. Sci. 2006, 52, 51–60. [Google Scholar] [CrossRef]
- Graf, E. Applications of phytic acid. J. Am. Oil Chem. Soc. 1983, 60, 1861–1867. [Google Scholar] [CrossRef]
- Yadav, S.K.; Sehgal, S. Effect of domestic processing and cooking on selected antinutrient contents of some green leafy vegetables. Plant Foods Hum. Nutr. 2003, 58, 1–11. [Google Scholar] [CrossRef]
- Daneluti, A.L.M.; Velasco, M.V.R.; Baby, A.R.; Matos, J.D.R. Thermal behavior and free-radical-scavenging activity of phytic acid alone and incorporated in cosmetic emulsions. Cosmetics 2015, 2, 248–258. [Google Scholar] [CrossRef]
- Luttrell, B.M. The biological relevance of the binding of calcium ions by inositol phosphates. J. Biol. Chem. 1993, 268, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- Jung, N.C.; Tamai, Y. Polyphosphate (phytate) formation in Quercus acutissima-Scleroderma verrucosum ectomycorrhizae supplied with phosphate. J. Plant Interact. 2013, 8, 291–303. [Google Scholar] [CrossRef]
- Upadhyay, J.; Tiwari, N.; Durgapal, S.; Jantwal, A.; Kumar, A. Phytic acid: As a natural antioxidant. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 437–450. [Google Scholar]
- Khattab, R.; Goldberg, E.; Lin, L.; Thiyam, U. Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 2010, 122, 1266–1272. [Google Scholar] [CrossRef]
- Jimenez, B.C.; Sarmanho, G.; Murphy, K.E.; Bustos, A.R.M.; Baudrit, J.R.V. NanoUV-VIS: An interactive visualization tool for monitoring the evolution of optical properties of nanoparticles throughout synthesis reactions. J. Res. Natl. Inst. Stand. Technol. 2017, 122, 1. [Google Scholar]
- Qi, Y.; Qi, H.; Li, J.; Lu, C. Synthesis, microstructures and UV–vis absorption properties of β-Ni (OH) 2 nanoplates and NiO nanostructures. J. Cryst. Growth 2008, 310, 4221–4225. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, K.; Yang, M.; Han, Y.; Zhang, Q.; Conde, J.; Yang, Y.; Alfranca, G.; Wang, Y.; Ma, L.; et al. Gastric parietal cell and intestinal goblet cell secretion: A novel cell-mediated in vivo metal nanoparticle metabolic pathway enhanced with diarrhea via Chinese herbs. Nanoscale Res. Lett. 2019, 14, 1–13. [Google Scholar] [CrossRef]
- Martin, J.; Plain, J. Fabrication of aluminum nanostructures for plasmonics. J. Phys. D Appl. Phys. 2014, 48, 184002. [Google Scholar] [CrossRef]
- Mekky, W.; Nicholson, P.S. Nano-aluminum-phosphate via a polymerized organic-inorganic complex route. J. Mater. Process. Technol. 2007, 190, 393–396. [Google Scholar] [CrossRef]
- Devamani, R.H.P.; Alagar, M. Synthesis and characterization of aluminum phosphate nanoparticles. Int. J. Appl. Sci. Eng. Res. 2012, 1, 769–775. [Google Scholar] [CrossRef]
- Peplinski, B.; Adamczyk, B.; Formanek, P.; Meyer, C.; Krüger, O.; Scharf, H.; Reinsch, S.; Ostermann, M.; Nofz, M.; Jäger, C.; et al. Nanocrystalline and stacking-disordered β-cristobalite AlPO4 chemically stabilized at room temperature: Synthesis, physical characterization, and X-ray powder diffraction data. Powder Diffr. 2017, 32, S193–S200. [Google Scholar] [CrossRef]
- Chattopadhyay, A. Wave reflection in triclinic crystalline medium. Arch. Appl. Mech. 2006, 76, 65–74. [Google Scholar] [CrossRef]
- Graetsch, H. Two forms of aluminum phosphate tridymite from X-ray powder data. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2000, 56, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Chang, Y.C. Fabrication of Al2O3 nanofluid by a plasma arc nanoparticles synthesis system. J. Mater. Process. Technol. 2008, 207, 193–199. [Google Scholar] [CrossRef]
- Nayak, S.S.; Wadhawa, G.C.; Shivankar, V.S.; Inamadar, R.F.; Sonawale, C. Green synthesis of nanostructured aluminum: Antibacterial activity and dye degradation. Eur. J. Mol. Clin. Med 2020, 7, 2640–2654. [Google Scholar]
- Rajapaksha, R.D.A.A. Self-assembling smart materials for biomaterials applications. In Polymer Nanocomposite-Based Smart Materials; Woodhead Publishing: Sawston, UK, 2020; pp. 121–147. [Google Scholar]
- Mustafa, T.; Aslam, M.M.A.; Ruiz, K.H.; Javed, M.; Gao, J.; Sharif, M.H.; Khan, S. Fabrication of mechanically strong Al2O3 nanoplates derived monolithic ceramic. Ceram. Int. 2023, 49, 40478–40485. [Google Scholar] [CrossRef]
- Martínez Domínguez, B.; Ibáñez Gómez, M.; Rincón León, F. Acido fítico: Aspectos nutricionales e implicaciones analíticas. Arch. Latinoam. Nutr. 2002, 52, 219–231. [Google Scholar]
- Quiñone, D.; Veiga, N.; Savastano, M.; Torres, J.; Bianchi, A.; Kremer, C.; Bazzicalupi, C. Supramolecular interaction of inositol phosphates with Cu (ii): Comparative study of Ins P 6–Ins P 3. CrystEngComm 2022, 24, 2126–2137. [Google Scholar] [CrossRef]
- Ishiguro, T.; Ono, T.; Nakasato, K.; Tsukamoto, C.; Shimada, S. Rapid measurement of phytate in raw soymilk by mid-infrared spectroscopy. Biosci. Biotechnol. Biochem. 2003, 67, 752–757. [Google Scholar] [CrossRef]
- Zając, A.; Dymińska, L.; Lorenc, J.; Kaczmarek, S.M.; Leniec, G.; Ptak, M.; Hanuza, J. Spectroscopic properties and molecular structure of copper phytate complexes: IR, Raman, UV–Vis, EPR studies and DFT calculations. JBIC J. Biol. Inorg. Chem. 2019, 24, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Ikemoto, Y.; Kinoshita, T.; Moriwaki, T.; Yoshida, K.T. Fourier-transform spectra of metal salts of phytic acid in the mid-to far-infrared spectral range. Vib. Spectrosc. 2017, 92, 215–219. [Google Scholar] [CrossRef]
- Goj, P.; Handke, B.; Stoch, P. Vibrational characteristics of aluminum–phosphate compounds by an experimental and theoretical approach. Sci. Rep. 2022, 12, 17495. [Google Scholar] [CrossRef] [PubMed]
- Viswanadham, N.; Saxena, S.K.; Sreenivasulu, P. Facile synthesis of bio-fuel from glycerol over zinc aluminium phosphate nanoplates. Sustain. Energy Fuels 2017, 1, 1018–1022. [Google Scholar] [CrossRef]
- Pramanik, M.; Salunkhe, R.R.; Imura, M.; Yamauchi, Y. Phosphonate-derived nanoporous metal phosphates and their superior energy storage application. ACS Appl. Mater. Interfaces 2016, 8, 9790–9797. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Kaneti, Y.V.; Fathoni, K.B.; Kani, K.; Allah, A.E.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Alothman, Z.A.; Golberg, D.; et al. Self-assembly of two-dimensional bimetallic nickel–cobalt phosphate nanoplates into one-dimensional porous chainlike architecture for efficient oxygen evolution reaction. Chem. Mater. 2020, 32, 7005–7018. [Google Scholar] [CrossRef]
- Vrieling, H.; Ballestas, M.; Hamzink, M.; Willems, G.; Soema, P.; Jiskoot, W.; Kersten, G.; Metz, B. Stabilised aluminium phosphate nanoparticles used as vaccine adjuvant. Colloids Surf. B Biointerfaces 2019, 181, 648–656. [Google Scholar] [CrossRef]
- Reardon, P.; Huang, J.; Tang, J. Morphology Controlled Porous Calcium Phosphate Nanoplates and Nanorods with Enhanced Protein Loading and Release Functionality. Adv. Healthc. Mater. 2013, 2, 682–686. [Google Scholar] [CrossRef]
- Palacios, E.; Leret, P.; Mata, M.; Fernández, J.; Aza, A.; Rodríguez, M.; Rubio-Marcos, F. Self-Forming 3D Core-Shell Ceramic Nanostructures for Halogen-Free Flame Retardant Materials. ACS Appl. Mater. Interfaces 2016, 8, 9462–9471. [Google Scholar] [CrossRef]
- Eshchenko, L.S.; Korobko, E.V.; Paniatouski, A.V. Preparation and electrorheological properties of anhydrous aluminum orthophosphate. Inorg. Mater. 2023, 59, 75–80. [Google Scholar] [CrossRef]
- Luque, N.B.; Mujika, J.I.; Rezabal, E.; Ugalde, J.M.; Lopez, X. Mapping the affinity of aluminum (III) for biophosphates: Interaction mode and binding affinity in 1: 1 complexes. Phys. Chem. Chem. Phys. 2014, 16, 20107–20119. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintos, B.; Gomez-Garay, A. Aluminum Phosphate Nanoplates Synthesized via Green Method Using Cork Oak Somatic Embryo-Derived Phytates. Appl. Sci. 2024, 14, 8681. https://doi.org/10.3390/app14198681
Pintos B, Gomez-Garay A. Aluminum Phosphate Nanoplates Synthesized via Green Method Using Cork Oak Somatic Embryo-Derived Phytates. Applied Sciences. 2024; 14(19):8681. https://doi.org/10.3390/app14198681
Chicago/Turabian StylePintos, Beatriz, and Arancha Gomez-Garay. 2024. "Aluminum Phosphate Nanoplates Synthesized via Green Method Using Cork Oak Somatic Embryo-Derived Phytates" Applied Sciences 14, no. 19: 8681. https://doi.org/10.3390/app14198681






