The Influence of Processing on the Bioactive Compounds of Small Berries
Abstract
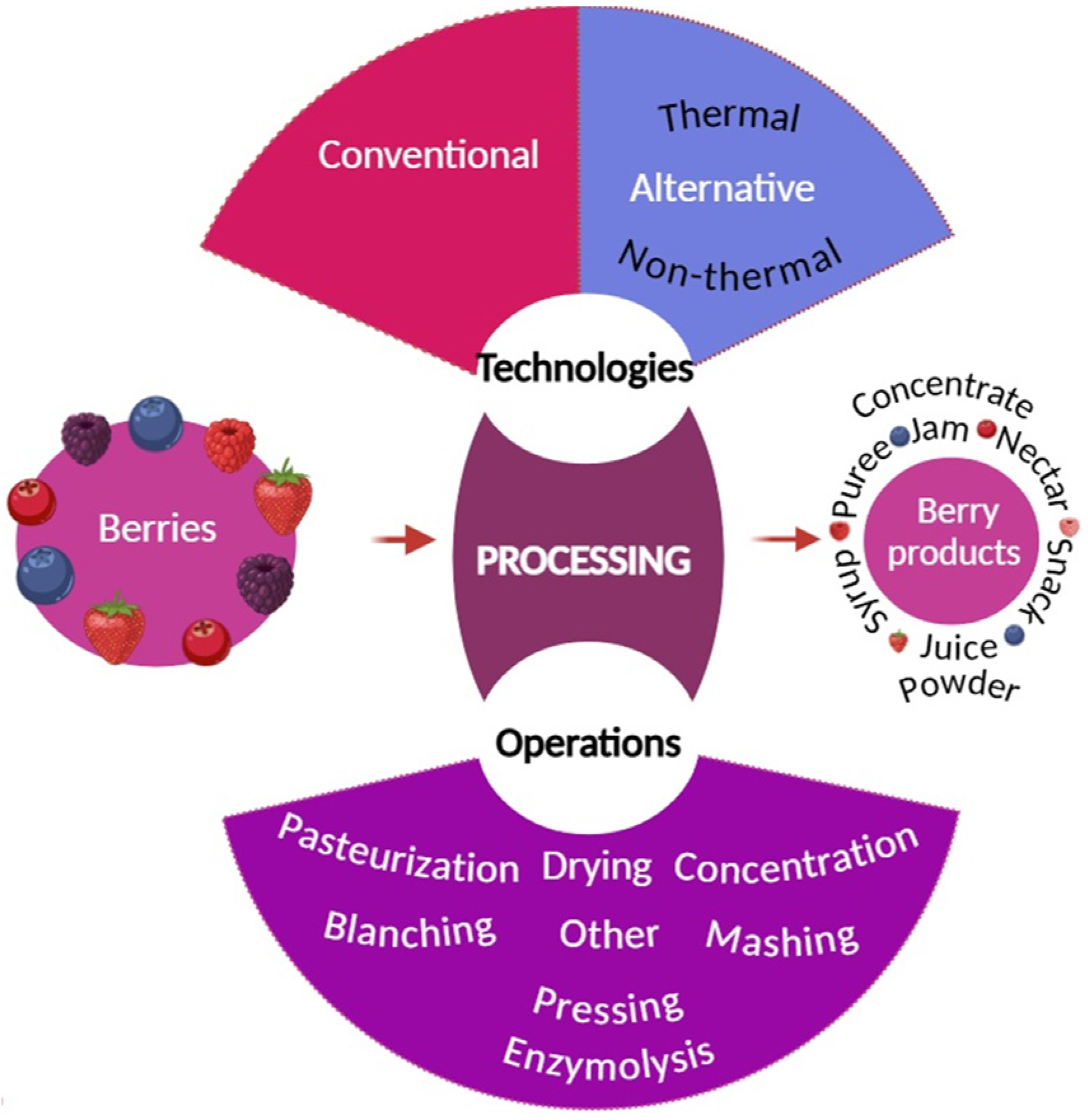
:1. Types of Berries
2. Bioactive Compounds from Small Berries
3. Comparative Analysis of Extraction and Analytical Methods for Determining Antioxidant Activity
3.1. Extraction Methods
3.2. Analytical Methods for Determining Antioxidant Activity
4. Processing of Small Berries and Their Effects on Bioactive Compounds
4.1. Enzymatic, Mashing, and Pressing Treatments
4.2. Blanching
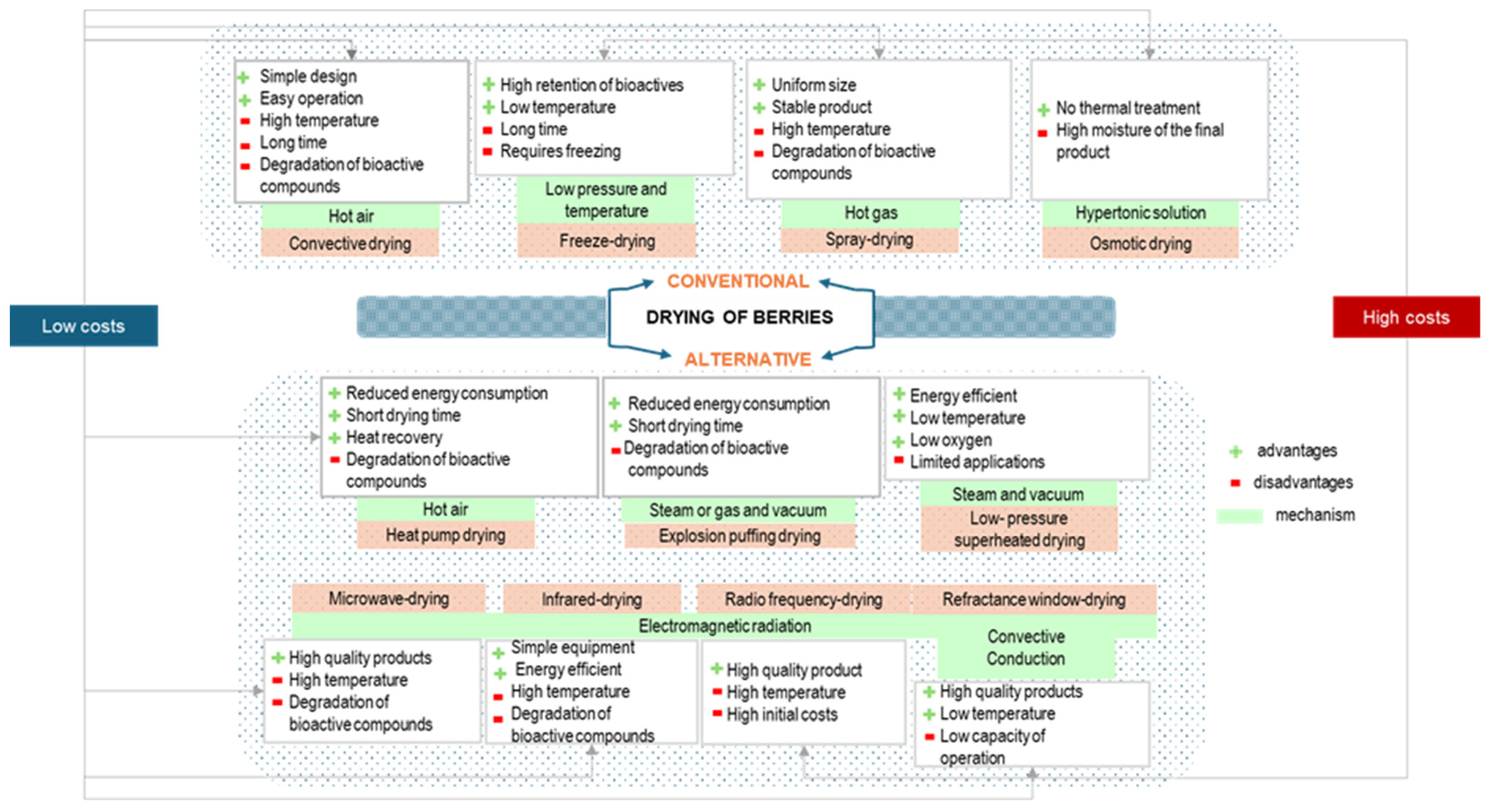
4.3. Drying
4.4. Pasteurization
4.5. Concentration
4.6. Storage and Shelf Life
5. Health Implications and Allergic Reactions to Small Berries
5.1. Anticancer Activity
5.2. Inflammatory Properties and Cardiovascular Protection
5.3. Diabetes Management
5.4. Neuroprotection
5.5. Antimicrobial Properties
5.6. Allergic Reactions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anderson, H.A. Berries: A Global History; Reaktion Books LTD.: London UK, 2018. [Google Scholar]
- Kumar, S.; Baghel, M.; Yadav, A.; Kumar Dhakar, M. Postharvest Biology and Technology of Berries. In Postharvest Biology and Technology of Temperate Fruits; Mir, S.A., Shah, M.A., Mir, M.M., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 349–370. [Google Scholar]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, S.N.; Guevara-Gonzalez, R.G.; Miranda-Lopez, R.; Feregrino-Perez, A.A.; Torres-Pacheco, I.; Vazquez-Cruz, M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013, 54, 1195–1207. [Google Scholar] [CrossRef]
- Manganaris, G.A.; Goulas, V.; Vicente, A.R.; Terry, L.A. Berry antioxidants: Small fruits providing large benefits. J. Sci. Food Agr. 2014, 94, 825–833. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Hidalgo, G.I.; Almajano, M.P. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants 2017, 6, 7. [Google Scholar] [CrossRef]
- Rodriquez De Luna, S.L.; Ramirez-Garza, R.E.; Serna Saldivar, S.O. Environmentally friendly methods for flavonoid extraction from plant material: Impact of their operating conditions on yield and antioxidant properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.J.; Jian, W.J.; Qian, B.J.; Dong, Y.; Pang, J. Quantitative studies on structure-DPPH• scavenging activity relationships of food phenolic acids. Molecules 2012, 17, 12910–12924. [Google Scholar] [CrossRef]
- Bilawal, A.; Ishfaq, M.; Gantumur, M.A.; Qayum, A.; Shi, R.; Fazilani, S.A.; Anwar, A.; Jiang, Z.; Hou, J. A review of the bioactive ingredients of berries and their applications in curing diseases. Food Biosci. 2021, 44, 101407. [Google Scholar] [CrossRef]
- Lidikova, J.; Ceryova, N.; Grygorieva, O.; Bobkova, A.; Bobko, M.; Arvay, J.; Snirc, M.; Brindza, J.; Norbova, M.; Harangozo, L.; et al. Cornelian cherry (Cornus mas L.) as a promising source of antioxidant phenolic substances and minerals. Eur. Food Res. Technol. 2024, 250, 1745–1754. [Google Scholar] [CrossRef]
- Martinovic, A.; Cavoski, I. The exploitation of cornelian cherry (Cornus mas L.) cultivars and genotypes from Montenegro as a source of natural bioactive compounds. Food Chem. 2020, 318, 126549. [Google Scholar] [CrossRef] [PubMed]
- Urbstaite, R.; Raudone, L.; Janulis, V. Phytogenotypic anthocyanin profiles and antioxidant activity variation in fruit samples of the American cranberry (Vaccinium macrocarpon Aiton). Antioxidants 2022, 11, 250. [Google Scholar] [CrossRef]
- Zannou, O.; Koca, Y. Greener extraction of anthocyanins and antioxidant activity from blackberry (Rubus spp) using natural deep eutectic solvents. LWT Food Sci. Technol. 2022, 158, 113184. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanogluu, E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-Based assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Gao, N.; Zang, Z.; Meng, X.; Lin, Y.; Yang, S.; Yang, Y.; Jin, Z.; Li, B. Classification and antioxidant assays of polyphenols: A review. J. Future Foods 2024, 4–3, 193–204. [Google Scholar] [CrossRef]
- Albert, C.; Codina, G.G.; Melinda Hejja, M.; Csaba Dezso, A.; Chetrariu, A.; Dabija, A. Study of antioxidant activity of aarden blackberries (Rubus fruticosus L.) extracts obtained with different extraction solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Enache, I.M.; Benito-Roman, O.; Coman, G.; Vizireanu, C.; Stanciuc, N.; Andronoiu, D.G.; Mihalcea, L.; Sanz, M.T. Extraction optimization and valorization of the cornelian cherry fruits extracts: Evidence on antioxidant activity and food applications. Appl. Sci. 2021, 11, 10729. [Google Scholar] [CrossRef]
- Wei, E.; Yang, R.; Zhao, H.; Wang, P.; Zhao, S.; Zhai, W.; Zhang, Y.; Zhou, H. Microwave-assisted extraction releases the antioxidant polysaccharides from seabuckthorn (Hippophae rhamnoides L.) berries. Int. J. Biol. Macromol. 2019, 123, 280–290. [Google Scholar] [CrossRef]
- Boeing, S.; Barizao, E.O.; Costa e Silva, B.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of solvent effect on the extraction of phenolic compounds and antioxidant capacities from the berries: Application of principal component analysis Joana. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Günal-Köroğlu, D.; Suyunc, G.; Yildirim, R.; Turan, S. Antioxidant potential of some fruit extracts prepared with different solvents. J. Inst. Sci. Technol. 2021, 11, 1127–1139. [Google Scholar] [CrossRef]
- Simic, V.M.; Rajkovicc, K.M.; Stojicevic, S.S.; Velickovic, D.T.; Nikolic, N.C.; Lazic, M.L.; Karabegovic, I.T. Optimization of microwave-assisted extraction of total polyphenolic compounds from chokeberries by response surface methodology and artificial neural network. Sep. Purif. Technol. 2016, 160, 89–97. [Google Scholar] [CrossRef]
- Gitea, M.A.; Bungau, S.G.; Gitea, D.; Pasca, B.M.; Purza, A.L.; Radu, A.-F. Evaluation of the phytochemistry–therapeutic activity relationship for grape seeds oil manuel. Life 2023, 13, 178. [Google Scholar] [CrossRef] [PubMed]
- Sepahpour, S.; Selamat, J.; Abdull Manap, M.Y.; Khatib, A.; Abdull Razis, A.F. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Jun Xue, S.; Forney, C.F.; Lim, L.-T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Hanula, M.; Wyrwisz, J.; Moczkowska, M.; Horbanczuk, O.K.; Pogorzelska-Nowicka, E.; Wierzbicka, A. Optimization of microwave and ultrasound extraction methods of Acai berries in terms of highest content of phenolic compounds and antioxidant activity. Appl. Sci. 2020, 10, 8325. [Google Scholar] [CrossRef]
- Lin, S.; Meng, X.; Tan, C.; Tong, Y.; Wan, M.; Wang, M.; Zhao, Y.; Deng, H.; Kong, Y.; Ma, Y. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa extracted using an ultrasonic-microwave-assisted natural deep eutectic solvent extraction method. Ultrason. Sonochem. 2022, 89, 106102. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Floares (Oarga), D.; Cocan, I.; Alexa, E.; Poiana, M.A.; Berbecea, A.; Boldea, M.V.; Negrea, M.; Obistioiu, D.; Radulov, I. Influence of extraction methods on the phytochemical profile of Sambucus nigra L. Agronomy 2023, 13, 3061. [Google Scholar] [CrossRef]
- Behrangi, N.; Ghafoori, H.; Farahmand, Z.; Khani, E.M.; Sanati, M.H. Comparison among Cornelian cherry and Prunus cerasus according to phenolic content and antioxidant capacity by three various methods of extraction. Food Nutri. Sci. 2015, 6, 1166–1173. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-Gonzaez, M.; Carrera, C.; Palma, M.; Alvarez, J.A.; Barbero, G.F.; Ayuso, J. Extraction of Antioxidants from Blackberry (Rubus ulmifolius L.): Comparison between ultrasound- and microwave-assisted extraction techniques. Agronomy 2019, 9, 745. [Google Scholar] [CrossRef]
- Chen, S.; Zenga, Z.; Hua, N.; Baia, B.; Wanga, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Espada-Bellido, E.; Ferreiro-Gonzalez, M.; Toledo-Dominguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-assisted extraction of two types of antioxidant compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the individual and simultaneous extraction methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef]
- Gentscheva, G.; Milkova-Tomova, I.; Nikolova, K.; Buhalova, D.; Velichka, A.; Gugleva, V.; Nadezhda Petkova, N.; Yotkovska, I.; Ivanova, N. Antioxidant activity and chemical characteristics of Sambucus nigra L. Blossom from different regions in Bulgaria. Horticulturae 2022, 8, 309. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Dabie, K.; Abassah-Oppong, S.; Peprah-Yamoah, E. Optimization of ultrasound-assisted extraction of phenolic antioxidants from turkey berry (Solanum torvum Sw) fruits using response surface methodology. J. Appl. Res. Med. Aromat Plants 2022, 30, 100387. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, H.; Zhou, X.; Deng, Q.; Zhao, Y.; Zhao, C.; Gong, X. Optimization of the microwave-assisted extraction and antioxidant activities of anthocyanins from blackberry using a response surface methodology. RSC Adv. 2015, 5, 19686–19695. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Staniszewska, I.; Liu, Z.-L.; Zielinska, D.; Xiao, H.-W.; Pan, Z.; Nowak, K.W.; Zielinska, M. Microwave-vacuum-assisted drying of pretreated cranberries: Drying kinetics, bioactive compounds and antioxidant activity. LWT Food Sci. Technol. 2021, 146, 111464. [Google Scholar] [CrossRef]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Capuzzo, A.; Maffei, M.E. Extraction of bilberry (Vaccinium myrtillus) antioxidants using supercritical/subcritical CO2 and ethanol as co-solvent. J. Supercrit. Fluids 2016, 107, 358–363. [Google Scholar] [CrossRef]
- Grunovaite, L.; Pukalskiene, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96. [Google Scholar] [CrossRef]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Kitryte, V.; Kavaliauskaite, A.; Tamkute, L.; Pukalskiene, M.; Syrpas, M.; Rimantas Venskutonis, P. Zero waste biorefining of lingonberry (Vaccinium vitis-idaea L.) pomace into functional ingredients by consecutive high pressure and enzyme assisted extractions with green solvents. Food Chem. 2020, 322, 126767. [Google Scholar] [CrossRef] [PubMed]
- Briones-Labarca, V.; Giovagnoli-Vicuna, C.; Chacana-Ojeda, M. High pressure extraction increases the antioxidant potential and in vitro bio-accessibility of bioactive compounds from discarded blueberries. Cyta J. Food 2019, 17, 622–631. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Da Pieve, S.; Butler, F. Impact of high pressure processing on total antioxidant activity, phenolic, ascorbic acid, anthocyanin content and colour of strawberry and blackberry purees. Innov. Food Sci. Emerg. Technol. 2009, 10, 308–313. [Google Scholar] [CrossRef]
- Rios-Corripio, G.; Morales-de la Pena, M.; Welti-Chanes, J.; Guerrero-Beltran, J.A. Pulsed electric field processing of a pomegranate (Punica granatum L.) fermented beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Dara, A.; Feizy, J.; Naji-Tabasi, S.; Fooladi, E.; Rafe, A. Intensifed extraction of anthocyanins from Berberis vulgaris L. by pulsed electric feld, vacuum-cold plasma, and enzymatic pretreatments: Modeling and optimization. Chem. Biol. Technol. Agric. 2023, 10, 93. [Google Scholar] [CrossRef]
- Loncaric, A.; Celeiro, M.; Jozinovic, A.; Jelinic, J.; Kovac, T.; Jokic, S.; Babic, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green extraction methods for extraction of polyphenolic compounds from Blueberry pomace. Foods 2020, 9, 1521. [Google Scholar] [CrossRef]
- Bebec Markovinovic, A.; Stulic, V.; Putnik, P.; Birkic, A.; Jambrovic, M.; Sasko, D.; Ljubicic, J.; Pavlic, B.; Herceg, Z.; Bursac, D.K. Pulsed electric field (PEF) and high-power ultrasound (HPU) in the hurdle concept for the preservation of antioxidant bioactive compounds of Strawberry juice—A chemometric evaluation—Part I. Foods 2023, 12, 3172. [Google Scholar] [CrossRef]
- Granato, D.; Fidelis, M.; Haapakoski, M.; dos Santos Lima, A.; Viil, J.; Hellstrom, J.; Ratsep, R.; Kaldmae, H.; Bleive, U.; Azevedo, L.; et al. Enzyme-assisted extraction of anthocyanins and other phenolic compounds from blackcurrant (Ribes nigrum L.) press cake: From processing to bioactivities. Food Chem. 2022, 391, 133240. [Google Scholar] [CrossRef]
- Syrpas, M.; Valanciene, E.; Augustiniene, E.; Malys, N. Valorization of Bilberry (Vaccinium myrtillus L.) Pomace by enzyme-assisted extraction: Process optimization and comparison with conventional solid-liquid extraction. Antioxidants 2021, 10, 773. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Lovric, V.; Putnik, P.; Bursac Kovacevic, D.; Jukic, M.; Dragovic-Uzelac, V. Effect of microwave-assisted extraction on the phenolic compounds and antioxidant capacity of Blackthorn flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.P.C.; Silva, E.K. Pulsed electric field technology in vegetable and fruit juice processing: A review. Food Res. Int. 2024, 184, 114207. [Google Scholar] [CrossRef] [PubMed]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; Donnell, C.O.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Lan, T.; Wang, J.; Bao, S.; Zhao, Q.; Sun, X.; Fang, Y.; Ma, T.; Liu, S. Effects and impacts of technical processing units on the nutrients and functional components of fruit and vegetable juice. Food Res. Int. 2023, 168, 112784. [Google Scholar] [CrossRef]
- Weber, F.; Larsen, L.R. Influence of fruit juice processing on anthocyanin stability. Food Res. Int. 2017, 100, 354–365. [Google Scholar] [CrossRef]
- Woodward, G.M.; McCarthy, D.; Pham-Thanh, D.; Kay, C.D. Anthocyanins remain stable during commercial blackcurrant juice processing. J. Food Sci. 2011, 76, S408–S414. [Google Scholar] [CrossRef]
- Hager, A.; Howard, L.R.; Prior, R.L.; Brownmiller, C. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed black raspberry products. J. Food Sci. 2008, 73, H134–H140. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Prior, R.L. Processing and storage effects on monomeric anthocyanins, percent polymeric color, and antioxidant capacity of processed blackberry products. J. Agric. Food Chem. 2008, 56, 689–695. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.; Beekwilder, J.; Capanoglu, E. The effects of juice processing on black mulberry antioxidants. Food Chem. 2015, 186, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Zhang, M.; Sun, Y.; Sun, J. How to improve bayberry (Myrica rubra Sieb. et Zucc.) juice color quality: Effect of juice processing on bayberry anthocyanins and polyphenolics. J. Agric. Food Chem. 2006, 54, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Pérez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, X.; Wang, Z.; Chen, H.F. Optimization of microwave-assisted extraction of anthocyanins in red raspberries and identification of anthocyanin of extracts using high-performance liquid chromatography mass spectrometry. Eur. Food Res. Technol. 2007, 225, 511–523. [Google Scholar] [CrossRef]
- Bei, X.; Yu, X.; Zhou, C.; Yagoub, A.E.G.A. Improvement of the drying quality of blueberries by catalytic infrared blanching combined with ultrasound pretreatment. Food Chem. 2024, 447, 138983. [Google Scholar] [CrossRef]
- Zielinska, M.; Zielinska, D. Effects of freezing, convective and microwave-vacuum drying on the content of bioactive compounds and color of cranberries. LWT 2019, 104, 202–209. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas-Ramella, M.; Franco, D.; Gomes da Cruz, A.; Zengin, G.; Kumar, M.; Dhama, K.; Lorenzo, J.M. The role of emerging technologies in the dehydration of berries: Quality, bioactive compounds, and shelf life. Food Chem. X 2022, 16, 100465. [Google Scholar] [CrossRef]
- Mendez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Mujumdar, A. Berry drying: Mechanism, pretreatment, drying technology, nutrient preservation, and mathematical models. Food Eng. Rev. 2019, 11, 61–77. [Google Scholar] [CrossRef]
- López, J.; Vega-Gálvez, A.; Bilbao-Sainz, C.; Chiou, B.S.; Uribe, E.; Quispe-Fuentes, I. Influence of vacuum drying temperature on: Physico-chemical composition and antioxidant properties of murta berries. J. Food Process Eng. 2017, 40, e12569. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Chen, H.J.; Gao, H.Y.; Song, L.L.; Mu, H.L. Effects of vacuum freeze-drying and hot-air drying on the quality of blue- berry fruits. Food Sci. 2014, 35, 64–68. [Google Scholar]
- Ozcelik, M.; Heigl, A.; Kulozik, U.; Ambros, S. Effect of hydrocolloid addition and microwave-assisted freeze drying on the characteristics of foamed raspberry puree. Innov. Food Sci. Emerg. Technol. 2019, 56, 102183. [Google Scholar] [CrossRef]
- Liu, Z.L.; Xie, L.; Zielinska, M.; Pan, Z.; Deng, L.Z.; Zhang, J.S.; Gao, L.; Wang, S.Y.; Zheng, Z.A.; Xiao, H.W. Improvement of drying efficiency and quality attributes of blueberries using innovative far-infrared radiation heating assisted pulsed vacuum drying (FIR-PVD). Innov. Food Sci. Emerg. Technol. 2022, 77, 102948. [Google Scholar] [CrossRef]
- Gagneten, M.; Corfield, R.; Mattson, M.G.; Sozzi, A.; Leiva, G.; Salvatori, D.; Schebor, C. Spray-dried powders from berries extracts obtained upon several processing steps to improve the bioactive components content. Powder Technol. 2019, 342, 1008–1015. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic dehydration of Honeoye strawberries in solutions enriched with natural bioactive molecules. LWT 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Zielinska, M.; Ropelewska, E.; Xiao, H.W.; Mujumdar, A.S.; Law, C.L. Review of recent applications and research progress in hybrid and combined microwave-assisted drying of food products: Quality properties. Crit. Rev. Food Sci. Nutr. 2020, 60, 2212–2264. [Google Scholar] [CrossRef]
- Zielinska, M.; Michalska, A. The influence of convective, microwave vacuum and microwave-assisted drying on blueberry pomace physicochemical properties. Int. J. Food Eng. 2018, 14, 0170332. [Google Scholar] [CrossRef]
- Wojdyło, A.; Figiel, A.; Oszmiański, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef]
- Jiang, H.; Shen, Y.; Zhen, L.; Li, W.; Zhang, Q. Evaluation of strawberries dried by radio frequency energy. Dry. Technol. 2019, 37, 312–321. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Z.; Bi, J.; Zhou, L.; Yi, J.; Wu, X. Effect of hybrid drying methods on physicochemical, nutritional and antioxidant properties of dried black mulberry. LWT Food Sci. Technol. 2017, 80, 178–184. [Google Scholar] [CrossRef]
- Si, X.; Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Li, Z. Comparison of different drying methods on the physical properties, bioactive compounds and antioxidant activity of raspberry powders. J. Sci. Food Agric. 2016, 96, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, C.; Shi, J.; Xue, S.; Mu, Y.; Lin, Z.; Liu, H. Analysis of volume expansion and dehydration rate of berry slab under microwave-vacuum puffing conditions. LWT Food Sci. Technol. 2013, 52, 39–48. [Google Scholar] [CrossRef]
- Celli, G.B.; Khattab, R.; Ghanem, A.; Brooks, M.S.L. Refractance WindowTM drying of haskap berry—Preliminary results on anthocyanin retention and physicochemical properties. Food Chem. 2016, 194, 218–221. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M.; Verkerk, R. Effect of thermal and non-thermal treatments on the color of citrus juice: A review. Food Rev. Int. 2023, 39, 3555–3577. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Review: Alternatives to Conventional Thermal Treatments in Fruit-juice Processing. Part 1: Techniques and Applications, Crit. Rev. Food Sci. 2015, 57, 501–523. [Google Scholar]
- Marszałek, K.; Mitek, M.; Skąpska, S. The effect of thermal pasteurization and high-pressure processing at cold and mild temperatures on the chemical composition, microbial and enzyme activity in strawberry purée. Innov. Food Sci. Emerg. Technol. 2015, 27, 48–56. [Google Scholar] [CrossRef]
- Azofeifa, G.; Quesada, S.; Pérez, A.M.; Vaillant, F.; Michel, A. Pasteurization of blackberry juice preserves polyphenol-dependent inhibition for lipid peroxidation and intracellular radicals. J. Food Compos. Anal. 2015, 42, 56–62. [Google Scholar] [CrossRef]
- Sójka, M.; Nowakowska, A.; Hejduk, A. Influence of enzymatic clarification, filtration, and pasteurization on ellagitannin and anthocyanin content in raspberry juices. Eur. Food Res. Technol. 2024, 250, 351–359. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Perricone, M.; Speranza, B.; Campaniello, D.; Sinigaglia, M.; Corbo, M.R. Nonthermal Technologies for Fruit and Vegetable Juices and Beverages: Overview and Advances. Compr. Rev. Food Sci. Food Saf. 2018, 17, 2–62. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- You, Y.; Li, N.; Han, X.; Guo, J.; Zhao, Y.; Liu, G.; Huang, W.; Zhan, J. Influence of different sterilization treatments on the color and anthocyanin contents of mulberry juice during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2018, 48, 1–10. [Google Scholar] [CrossRef]
- Karacam, C.H.; Sahin, S.; Oztop, M.H. Effect of high pressure homogenization (microfluidization) on the quality of Ottoman Strawberry (F. ananassa) juice. LWT 2015, 64, 932–937. [Google Scholar] [CrossRef]
- Engmann, F.N.; Ma, Y.; Zhang, H.; Yu, L.; Deng, N. The application of response surface methodology in studying the effect of heat and high hydrostatic pressure on anthocyanins, polyphenol oxidase, and peroxidase of mulberry (Morus nigra) juice. J. Sci. Food Agric. 2014, 94, 2345–2356. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf life eva- luation during cold storage. J. Food Sci. Technol. 2017, 54, 832–841. [Google Scholar] [CrossRef]
- Dolas, R.; Saravanan, C.; Kaur, B.P. Emergence and era of ultrasonic’s in fruit juice preservation: A review. Ultrason. Sonochem. 2019, 58, 104609. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, J.; Zhao, T.; Yang, X.; Zhang, J.; Yang, H. Bioavailability and mechanisms of dietary polyphenols affected by non-thermal processing technology in fruits and vegetables. Curr. Res. Food Sci. 2024, 8, 100715. [Google Scholar] [CrossRef]
- Cheng, X.F.; Zhang, M.; Adhikari, B. Changes in quality attributes of strawberry purees processed by power ultrasound or thermal treatments. Food Sci. Technol. Res. 2014, 20, 1033–1041. [Google Scholar] [CrossRef]
- Golmohamadi, A.; Möller, G.; Powers, J.; Nindo, C. Effect of ultrasound frequency on antioxidant activity, total phenolic and anthocyanin content of red raspberry puree. Ultrason. Sonochem. 2013, 20, 1316–1323. [Google Scholar] [CrossRef]
- Bhat, R.; Goh, K.M. Sonication treatment convalesce the overall quality of hand- pressed strawberry juice. Food Chem. 2017, 215, 470–476. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Rico-Alderete, I.A.; Rostro-Alanís, M.d.J.; Welti-Chanes, J.; Escobedo-Avellaneda, Z.J.; Soto-Caballero, M.C. Effect of High Hydrostatic Pressure and Pulsed Electric Fields Processes on Microbial Safety and Quality of Black/Red Raspberry Juice. Foods 2022, 11, 2342. [Google Scholar] [CrossRef] [PubMed]
- Caminiti, I.M.; Noci, F.; Muñoz, A.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. Impact of selected combinations of non-thermal processing technologies on the quality of an apple and cranberry juice blend. Food Chem. 2011, 124, 1387–1392. [Google Scholar] [CrossRef]
- Silva Amorim, D.; Silva Amorim, I.; Campos Chisté, R.; André Narciso Fernandes, F.; Regina Barros Mariutti, L.; Teixeira Godoy, H.; Rosane Barboza Mendonça, C. Non-thermal technologies for the conservation of açai pulp and derived products: A comprehensive review. Food Res. Int. 2023, 174, 113575. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, T.; Nayak, B.; Amirbahman, A.; Tripp, C.P.; Mukhopadhyay, S. Application of ultraviolet light assisted titanium dioxide photocatalysis for food safety: A review. Innov. Food Sci. Emerg. Technol. 2016, 38, 105–115. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Cyganowski, P.; Jamroz, P. Do we need cold plasma treated fruit and vegetable juices? A case study of positive and negative changes occurred in these daily beverages. Food Chem. 2022, 375, 131831. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Gan, Z.; Feng, X.; Hou, Y.; Sun, A.; Wang, R. Cold plasma jet with dielectric barrier configuration: Investigating its effect on the cell membrane of E. coli and S. cerevisiae and its impact on the quality of chokeberry juice. LWT 2021, 136, 110223. [Google Scholar] [CrossRef]
- Darvishi, H.; Salami, P.; Fadavi, A.; Saba, M.K. Processing kinetics, quality and thermodynamic evaluation of mulberry juice concentration process using Ohmic heating. Food Bioprod. Process. 2020, 123, 102–110. [Google Scholar] [CrossRef]
- Adorno, W.T.; Rezzadori, K.; Arend, G.D.; Chaves, V.C.; Reginatto, F.H.; Di Luccio, M.; Petrus, J.C.C. Enhancement of phenolic compounds content and antioxidant activity of strawberry (Fragaria × ananassa) juice by block freeze concentration technology. Int. J. Food Sci. Technol. 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Gunathilake, K.D.P.P.; Yu, L.J.; Rupasinghe, H.P.V. Reverse osmosis as a potential technique to improve antioxidant properties of fruit juices used for functional beverages. Food Chem. 2014, 148, 335–341. [Google Scholar] [CrossRef]
- Buve, C.; Kebede, B.T.; De Batselier, C.; Carrillo, C.; Pham, H.T.T.; Hendrickx, M.; Grauwet, T.; Van Loey, A. Kinetics of colour changes in pasteurised strawberry juice during storage. J. Food Eng. 2018, 216, 42–51. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Schell, J.; Betts, N.M.; Foster, M.; Scofield, R.H.; Basu, A. Cranberries improve postprandial glucose excursions in type 2 diabetes. Food Funct. 2017, 8, 3083–3090. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Carradori, S.; Casadei, M.A.; Paolicelli, P.; Petralito, S.; Ragno, R.; Cesa, S. Lycium barbarum polysaccharides: Extraction, purification, structural characterisation and evidence about hypoglycaemic and hypolipidaemic effects. A review. Food Chem. 2018, 254, 377–389. [Google Scholar] [CrossRef]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Zheng, R.X.; Xu, X.D.; Tian, Z.; Yang, J.S. Chemical constituents from the fruits of Hippophae rhamnoides. Nat. Prod. Res. 2009, 23, 1451–1456. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Bayram, H.M.; Ozturkcan, S.A. Bioactive components and biological properties of cornelian cherry (Cornus mas L.): A comprehensive review. J. Funct. Foods 2020, 75, 104252. [Google Scholar] [CrossRef]
- Turan, I.; Demir, S.; Kilinc, K.; Burnaz, N.A.; Yaman, S.O.; Akbulut, K.; Mentese, A.; Aliyazicioglu, Y.; Deger, O. Antiproliferative and apoptotic effect of Morus nigra extract on human prostate cancer cells. Saudi. Pharm. J. 2017, 25, 241–248. [Google Scholar] [CrossRef]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef]
- Mirmalek, S.A.; Faraji, S.; Ranjbaran, S.; Aryan, H.; Arani, H.Z.; Jangholi, E.; Marzouni, H.Z.; Salimi-Tabatabaee, S.A. Cyanidin 3-glycoside induced apoptosis in MCF-7 breast cancer cell line. Arch. Med. Sci. 2023, 19, 1092. [Google Scholar] [CrossRef] [PubMed]
- Veljkovic, B.; Djordjevic, N.; Dolicanin, Z.; Licina, B.; Topuzovic, M.; Stankovic, M.; Zlatic, N.; Dajic-Stevanovic, Z. Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not. Bot. Horti. Agrobo. 2019, 47, 359–367. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Fernández-Castillejo, S.; Pedret, A.; Llauradó, E.; Sola, R. Cyanidin-3-glucoside as a possible biomarker of anthocyanin-rich berry intake in body fluids of healthy humans: A systematic review of clinical trials. Nutr. Rev. 2020, 78, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Li, X.; Wood, J.W.; Ma, J.X. Mitochondrial dysfunctions, endothelial progenitor cells and diabetic retinopathy. J. Diabetes. Complicat. 2018, 32, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.C.; Jang, S.W.; Kim, T.H.; Kwon, C.H.; Kim, Y.K. Mulberry fruit (Moris fructus) extracts induce human glioma cell death in vitro through ROS-dependent mitochondrial pathway and inhibits glioma tumor growth in vivo. Nutr. Cancer 2010, 62, 402–412. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, M.; El-Kholie, E.; El-Garawani, I.; Sherif, N. Anticancer activity of Morus nigra on human breast cancer cell line (MCF-7): The role of fresh and dry fruit extracts. J. Biosci. Appl. Res 2016, 2, 352–361. [Google Scholar]
- Radbeh, Z.; Asefi, N.; Hamishehkar, H.; Roufegarinejad, L.; Pezeshki, A. Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (cornelian cherry) bioactive compounds. Biomed. Pharmacother. 2020, 125, 109906. [Google Scholar] [CrossRef]
- Agudelo, C.D.; Luzardo-Ocampo, I.; Hernández-Arriaga, A.M.; Rendón, J.C.; Campos-Vega, R.; Maldonado-Celis, M.E. Fermented non-digestible fraction of andean berry (Vaccinium meridionale Swartz) juice induces apoptosis in colon adenocarcinoma cells. Prev. Nutr. Food Sci. 2020, 25, 272. [Google Scholar] [CrossRef]
- Teleszko, M.; Wojdyło, A. Comparison of phenolic compounds and antioxidant potential between selected edible fruits and their leaves. J. Funct. Foods 2015, 14, 736–746. [Google Scholar] [CrossRef]
- Tian, Y.; Liimatainen, J.; Alanne, A.L.; Lindstedt, A.; Liu, P.; Sinkkonen, J.; Kallio, H.; Yang, B. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017, 220, 266–281. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Berries: Anti-inflammatory effects in humans. J. Agric. Food Chem. 2014, 62, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Rhone, M.; Lyons, T.J. Berries: Emerging impact on cardiovascular health. Nutr. Rev. 2010, 68, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Kocabas, S.; Sanlier, N. The power of berries against cardiovascular diseases. Nutr. Rev. 2024, 82, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, G.; Liao, D.; Zhu, Y.; Xue, X. Effects of berries consumption on cardiovascular risk factors: A meta-analysis with trial sequential analysis of randomized controlled trials. Sci. Rep. 2016, 6, 23625. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, H.; Shweta; Singh, D.; Singh, S.B.; Ganju, L. Anti-inflammatory activity of the functional groups present in Hippophae rhamnoides (Seabuckthorn) leaf extract. Inflammopharmacology 2018, 26, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Edirisinghe, I.; Burton-Freeman, B. Anti-diabetic actions of Berry polyphenols—Review on proposed mechanisms of action. J. Berry Res. 2016, 6, 237–250. [Google Scholar] [CrossRef]
- Rojo, L.E.; Ribnicky, D.; Logendra, S.; Poulev, A.; Rojas-Silva, P.; Kuhn, P.; Dorn, R.; Grace, M.H.; Ann Lila, M.; Raskin, I. In vitro and in vivo anti-diabetic effects of anthocyanins from Maqui Berry (Aristotelia chilensis). Food chem. 2012, 131, 387–396. [Google Scholar] [CrossRef]
- Banjari, I.; Misir, A.; Šavikin, K.; Jokić, S.; Molnar, M.; De Zoysa, H.K.S.; Waisundara, V.Y. Antidiabetic effects of Aronia melanocarpa and its other therapeutic properties. Front. Nutr. 2017, 4, 53. [Google Scholar] [CrossRef]
- Szot, I.; Zhurba, M.; Klymenko, S. Pro-health and functional properties of goji berry (Lycium spp). Agrobiodiversity Improv. Nutr. Health Life Qual. 2020, 4, 134–145. [Google Scholar]
- Singh, M.; Arseneault, M.; Sanderson, T.; Murthy, V.; Ramassamy, C. Challenges for research on polyphenols from foods in Alzheimer’s disease: Bioavailability, metabolism, and cellular and molecular mechanisms. J. Agric. Food Chem. 2008, 56, 4855–4873. [Google Scholar] [CrossRef]
- Yang, W.; Cui, K.; Li, X.; Zhao, J.; Zeng, Z.; Song, R.; Qi, X.; Xu, W. Effect of polyphenols on cognitive function: Evidence from population-based studies and clinical trials. J. Nutr. Health Aging. 2021, 25, 1190–1204. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L.; Proust-Lima, C.; Le Gouge, A.; Dartigues, J.F.; Barberger-Gateau, P. Flavonoid intake and cognitive decline over a 10-year period. Am. J. Epidemiol. 2007, 165, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprom, S.; Mosoni, P.; Leroy, S.; Kaewkod, T.; Desvaux, M.; Tragoolpua, Y. Antioxidants of fruit extracts as antimicrobial agents against pathogenic bacteria. Antioxidants 2022, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Aurori, M.; Niculae, M.; Hanganu, D.; Pall, E.; Cenariu, M.; Vodnar, D.C.; Bunea, A.; Fit, N.; Andrei, S. Phytochemical profile, antioxidant, antimicrobial and cytoprotective effects of cornelian cherry (Cornus mas L.) fruit extracts. Pharmaceuticals 2023, 16, 420. [Google Scholar] [CrossRef]
- Marzban, G.; Maghuly, F.; Herndl, A.; Katinger, H.; Laimer, M. Screening and identification of putative allergens in berry fruits of the Rosaceae family: Technical challenges. Biofactors 2008, 34, 37–46. [Google Scholar] [CrossRef]
- Krikeerati, T.; Rodsaward, P.; Nawiboonwong, J.; Pinyopornpanish, K.; Phusawang, S.; Sompornrattanaphan, M. Revisiting fruit allergy: Prevalence across the globe, diagnosis, and current management. Foods 2023, 12, 4083. [Google Scholar] [CrossRef]
- Costa, J.; Mafra, I. Rosaceae food allergy: A review. Crit. Rev. Food Sci. Nutr. 2023, 63, 7423–7460. [Google Scholar] [CrossRef]
- Larramendi, C.H.; García-Abujeta, J.L.; Vicario, S.; García-Endrino, A.; López-Matas, M.A.; García-Sedeño, M.D.; Carnés, J. Goji berries (Lycium barbarum): Risk of allergic reactions in individuals with food allergy. J. Investig. Allerg. Clin. 2012, 22, 345. [Google Scholar]
- Carnés, J.; de Larramendi, C.H.; López-Matas, M.A.; Ferrer, A.; Huertas, J. Allergenic sensitisation mediated by Wolfberry. In Lycium barbarum and Human Health; Chang, R.C.-C., So, K.-F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 179–198. [Google Scholar]
- Ponder, A.; Najman, K.; Aninowski, M.; Leszczyńska, J.; Głowacka, A.; Bielarska, A.M.; Lasinskas, M.; Hallmann, E. Polyphenols content, antioxidant properties and allergenic potency of organic and conventional blue honeysuckle berries. Molecules 2022, 27, 6083. [Google Scholar] [CrossRef]
- Mlcek, J.; Jurikova, T.; Skrovankova, S.; Sochor, J. Quercetin and its anti-allergic immune response. Molecules 2016, 21, 623. [Google Scholar] [CrossRef]
- Juríková, T.; Balla, S.; Sochor, J.; Pohanka, M.; Mlcek, J.; Baron, M. Flavonoid profile of saskatoon berries (Amelanchier alnifolia Nutt.) and their health promoting effects. Molecules 2013, 18, 12571–12586. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Jurikova, T.; Ercisli, S.; Mlcek, J.; Baron, M.; Balla, S.; Yilmaz, S.O.; Necas, T. Characterization of cornelian cherry (Cornus mas L.) genotypes-genetic resources for food production in Czech Republic. Genetika 2014, 46, 915–924. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, T.; Vasiljevic, T.; Ramchandran, L. Effect of processing on conformational changes of food proteins related to allergenicity. Trends Food Sci. Technol. 2016, 49, 24–34. [Google Scholar] [CrossRef]
| Berries | Extraction Conditions | Other Experimental Conditions | Antioxidant Capacity Assay |
|---|---|---|---|
| Solvent extraction | |||
| Cornelian cherry (Cornus mas L.) [32] | 100% methanol, S:L 1:10 | shaking 150 rpm, 40 °C, 24 h | TPC (mg GAE/kg): 1650 ± 330 DPPH (IC50) (mg/mL): 3.95 ± 0.18 FRAP (μmol/g): 190 ± 20 |
| Soxhlet extractor, 80 °C; evaporation, 40 °C | TPC (mg GAE/kg): 870 ± 64 DPPH (IC50) (mg/mL): 9.67 ± 2.8 FRAP (μmol/g): 200 ± 50 | ||
| Sour cherry (Prunus cerasus) [32] | 100% methanol, S:L 1:10 | shaking incubator 150 rpm, 40 °C, 24 h | TPC (mg GAE/kg): 1260 ± 310 DPPH (IC50) (mg/mL): 5.85 ± 1.18 FRAP (μmol/g): 170 ± 20 |
| Soxhlet extractor, 80 °C; rotator evaporator, 75 rpm, 40 °C | TPC (mg GAE/kg): 1080 ± 180 DPPH (IC50) (mg/mL): 8.13 ± 0.89 FRAP (μmol/g): 170 ± 40 | ||
| Black mulberry (Morus nigra) [21] | 70% acetone + 30% water, S:L 1:10 | magnetic stirring, 1 h, dark, room temperature | TPC (g GAE/kg d.w): 57.44 ± 0.21 |
| 70% methanol + 29.5% water + 0.5% acetic acid, S:L 1:10 | TAC (g CGE/kg d.w): 36.92 ± 0.08 | ||
| 50% ethanol + 50% water, S:L 1:10 | FRAP (mmol Fe2+/kg d.w): 1247.27 ± 2.59 DPPH: (mmol TE/kg d.w): 364.98 ± 9.10 ORAC (mmol TE/kg d.w): 819.21 ± 9.22 | ||
| 70% acetone + 29.5% water + 0.5% acetic acid, S:L 1:10 | FRAP: 1226.37 ± 0.48 DPPH: 318.63 ± 4.22 ORAC: 845.35 ± 50.84 | ||
| 50% acetone + 50% water | FRAP: 1282.60 ± 17.37 DPPH: 331.47 ± 1.06 ORAC: 960.29 ± 51.37 | ||
| Blackberry (Rubus ulmifolius) [21] | 70% acetone + 30% water, S:L 1:10 | TPC: 42.81 ± 0.28 | |
| 70% methanol + 29.5% water + 0.5% acetic acid, S:L 1:10 | TAC: 7.55 ± 0.10 | ||
| 50% ethanol + 50% water, S:L 1:10 | FRAP: 669.91 ± 8.84 DPPH: 217.73 ± 2.56 ORAC: 503.03 ± 6.67 | ||
| 70% acetone + 29.5% water + 0.5% acetic acid, S:L 1:10 | FRAP: 922.28 ± 10.98 DPPH: 313.08 ± 3.12 ORAC: 572.69 ± 20.06 | ||
| 50% acetone + 50% water, S:L 1:10 | FRAP: 879.04 ± 2.10 DPPH: 297.37 ± 5.14 ORAC: 474.77 ± 3.08 | ||
| Strawberry (Fragaria × ananassa) [21] | 70% acetone + 30% water | TPC: 29.55 ± 0.07 | |
| 70% methanol + 29.5% water + 0.5% acetic acid | TAC: 3.49 ± 0.02 | ||
| 50% ethanol + 50% water | FRAP: 435.69 ± 1.37 DPPH: 121.62 ± 1.97 ORAC: 225.93 ± 3.97 | ||
| 70% acetone + 29.5% water + 0.5% acetic acid | FRAP: 444.14 ± 5.12 DPPH: 126.41 ± 1.20 ORAC: 269.79 ± 11.29 | ||
| 50% acetone + 50% water | FRAP: 499.11 ± 0.66 DPPH: 140.50 ± 0.71 ORAC: 241.48 ± 1.12 | ||
| Black elder (Sambucus nigra L.) [27] | 50% ethanol + 50% water; pH 2, S:L 1:20 (g/mL) | magnetic stirring, 150 rpm, 5 h, 60 °C | TPC (mg GAE/g d.w): 2857 ± 205 TAC (mg/g d.w): 437.7 ± 43.3 Carotenoids (mg/g d.w): 86.46 ± 3.72 ORAC (mg TE/g d.w): 193.4 ± 10.1 DPPH (mg TE/g d.w): 56.0 ± 0.92 FRAP (μmol Fe2+/100 g d.w): 9786 ± 553 ABTS (mg AAE/g d.w): 5840 ± 162 |
| Cornelian cherry (Cornus mas L.) [19] | 60% ethanol + 40% water, S:L 1:10 | magnetic stirring, 150 rpm, 15 min, 40 °C | TPC (mg GAE/g d.w): 33.1 ± 1.19 TFC (mg QE/g d.w): 1.65 ± 0.07 TAA (mg TE/g d.w): 33.2 ± 0.5 |
| Strawberry (Fragaria recsa) [22] | 80% methanol + 20% water | stirring, 150 rpm, 1 h | DPPH (EC50) (mg/mL extract): 5.08 TPC (mg GAE/g extract): 33.32 ± 0.71 |
| 100% methanol | DPPH (EC50): 5.35 TPC: 33.94 ± 0.87 | ||
| 80% ethanol + 20% water | DPPH (EC50): 4.59 TPC: 32.11 ± 0.83 | ||
| 100% ethanol | DPPH (EC50): 6.55 TPC: 31.75 ± 0.61 | ||
| Raspberry (Rubus idaeus) [22] | 80% methanol + 20% water, S:L 1:2.5 | DPPH (EC50): 7.16 TPC: 17.31 ± 0.4 | |
| 100% methanol, S:L 1:2.5 | DPPH (EC50): 8.43 TPC: 20.31 ± 0.27 | ||
| 80% ethanol + 20% water, S:L 1:2.5 | DPPH (EC50): 8.43 TPC: 13.25 ± 0.47 | ||
| 100% ethanol, S:L 1:2.5 | DPPH (EC50): 13.31 TPC: 21.45 ± 0.79 | ||
| Sour cherry (Prunus cerasus) [22] | 80% methanol + 20% water, S:L 1:2.5 | DPPH (EC50): 13.07 TPC: 18.63 ± 0.17 | |
| 100% methanol, S:L 1:2.5 | DPPH (EC50): 11.78 TPC: 21.56 ± 0.12 | ||
| 80% ethanol + 20% water, S:L 1:2.5 | DPPH (EC50): 14.41 TPC: 18.9 ± 0.57 | ||
| 100% ethanol, S:L 1:2.5 | DPPH (EC50): 28.11 TPC: 20.96 ± 0.12 | ||
| Cornelian cherry (Cornus mas) [22] | 80% methanol + 20% water | DPPH (EC50): 11.90 TPC: 18.02 ± 0.38 | |
| 100% methanol | DPPH (EC50): 12.21 TPC: 16.18 ± 0.25 | ||
| 80% ethanol + 20% water | DPPH (EC50): 11.30 TPC: 17.69 ± 0.18 | ||
| 100% ethanol | DPPH (EC50): 15.51 TPC: 15.37 ± 0.16 | ||
| Cornelian cherry (Cornus mas L.) [12] | 80% methanol + 20% water, S:L 1:2 | stirring 12 h | TPC (mg GAE/kg d.w): 583.1 ± 11.7 (Nizhnyi cultivar)–1876.8 ± 16.6 (Svitliachok cultivar) TAC (mg/kg d.w): 50.1 ± 5.9 (Koralovyi cultivar)–593.4 ± 28.4 (Ekzotychnyi cultivar) DPPH (mmoli TE/kg d.w): 2.44 ± 0.02 (Nizhnyi cultivar)–2.81 ± 0.03 (Cm 01 cultivar) ABTS (mmoli TE/kg d.w): 5.53 ± 0.09 (Nizhnyi cultivar)–6.25 ± 0.07 (Cm 01 cultivar) FRAP (mmoli TE/kg d.w): 3.31 ± 0.09 (Nizhnyi cultivar)–4.89 ± 0.04 (Svitliachok cultivar) |
| Acai berries (Euterpe oleracea Mart.) [28] | 50% ethanol + 50% water, S:L 25:1 | water bath, 25 °C, 25 min | TPC (mg GAE/g d.w): 33.87 ± 0.11 TFC (mg QE/g d.w): 5.13 ± 0.10 TAC (mg CGE/g d.w): 4.60 ± 0.02 |
| water bath, 45 °C, 25 min | TPC: 35.26 ± 0.02 TFC: 5.66 ± 0.08 TAC: 4.50 ± 0.03 | ||
| Blackcurrant (Ribes nigrum L.) [29] | Choline chloride/glycerol 1:2, S:L 1:26 | water bath 51 °C, 51 min | FRAP (mmol TE/mg): 4.371 ABTS (mmol TE/mg): 0.401 TAC (U/mg): 44.329 |
| 60% ethanol + 40% water, S:L 1:36 | water bath 60 °C, 72 min, | FRAP: 2.618 ABTS: 0.291 TAC: 19.861 | |
| Ultrasound-assisted extraction | |||
| Blueberry (Vaccinium angustifolium) (pomace) [26] | Water: 100% S:L 1:20, 40 °C, pH 5 | sonication 30 vs. 90 min, 35 kHz | TPC (mg GAE/g d.w): 5.84 ± 0.03 vs. 6.31 ± 0.15 TFC (mg CE/g d.w): 2.45 ± 0.25 vs. 2.85 ± 0.11 TAC (mg ME/g d.w): 10.04 ± 0.10 vs. 14.1 ± 0.15 |
| 50% ethanol + 50% water S:L 1:10 vs. 1:15 vs. 1:20, 40 °C, pH 3.3 | sonication 60 min, 35 kHz | TPC: 22.57 ± 0.53 vs. 24.16 ± 0.25 vs. 35.95 ± 0.12 DPPH (mg TE/g d.w): 41.39 ± 0.61 vs. 51.75 ± 1.21 vs. 64.25 ± 0.39 | |
| 50% ethanol + 50% water S:L 1:15, 40 °C, pH 3.3 | sonication 40 min, 35 kHz | TPC: 22.23 ± 0.15 TFC: 19.41 ± 0.33 TAC: 31.32 ± 0.73 DPPH: 41.79 ± 0.92 | |
| 90% ethanol + 10% water S:L 1:15, 40 °C, pH 3.3 | TPC: 5.02 ± 0.09 TFC: 9.71 ± 0.19 TAC: 12.75 ± 0.17 DPPH: 10.95 ± 0.28 | ||
| 50% ethanol + 50% water S:L 1:15, 40 °C, pH 3.3 vs. 8.3 | sonication 40 min, 35 kHz | TPC: 22.23 ± 0.15 vs. 24.28 ± 0.27 TFC: 19.41 ± 0.33 vs. 20.50 ± 1.20 TAC: 31.31 ± 0.44 vs. 29.58 ± 0.27 DPPH: 41.78 ± 0.98 vs. 45.65 ± 1.74 | |
| 50% ethanol + 50% water S:L 1:15, 40 vs. 60 °C, pH 3.3 | sonication 40 min, 35 kHz | TPC: 30.33 ± 0.27 vs. 18.74 ± 0.13 TFC: 17.05 ± 1.23 vs. 45.45 ± 2.46 TAC: 35.59 ± 0.67 vs. 54.44 ± 1.36 DPPH: 30.82 ± 0.79 vs. 30.12 ± 0.60 | |
| Garden blackberries (Rubus fruticosus L.) [18] | 80% ethanol, solid/liquid ratio (S:L) 1:20 | sonication 30 min | TAC (mg CGE/100 g d.w): 885.08 ± 59.81 TFC (mg QE/100 g d.w): 240.93 ± 16.96 TPC (mg GAE/100 g d.w): 3311.84 ± 58.84 DPPH (%): 76.4078 ± 1.43 FRAP (mg AAE/100 g d.w): 3547.68 ± 154.76 |
| 70% acetone + 2% acetic acid, S:L 1:20 | TAC: 773.30 ± 68.34 TFC: 129.75 ± 9.51 TPC: 2789.97 ± 211.79 DPPH: 63.1294 ± 3.45 FRAP: 2455 ± 141.31 | ||
| 60% methanol + 3% formic acid, S:L 1:20 | TAC: 819.18 ± 194.8 TFC: 199.85 ± 19.45 TPC: 3245.97 ± 338.77 DPPH: 71.9624 ± 3.04 FRAP: 2853.13 ± 413.98 | ||
| 90% acetonitrile + 10% 6 molar HCl, S:L 1:20 | TAC: 642.96 ± 71.24 TFC: 163.01 ± 13.57 TPC: 2143.00 ± 321.21 DPPH: 47.2017 ± 2.47 FRAP: 1682.22 ± 357.37 | ||
| Blackberry (Rubus ulmifolius L.) [33] | 46% methanol + 54% water, 60.4 °C, pH 4.97, S:L 0.3:20 | sonication 200 W, 24 kHz, amplitude 30%, cycle 0.7 s | TPC (μg GAE/g): 15,697.76 ± 603.21 |
| 63.7% methanol + 54% water, 68.3 °C, pH 4.81, S:L 0.3:20 | sonication 200 W, 24 kHz, amplitude 70%, cycle 0.7 s | TAC (μ CGE/g): 1792.73 ± 68.84 | |
| 46.5% methanol + 53.5% water, 67.5 °C, pH 4.16, S:L 0.3:20 | sonication 200 W, 24 kHz, amplitude 66.8%, cycle 0.7 s | TPC: 14,809.73 ± 455.78 TAC: 1687.77 ± 50.08 | |
| Black goji berry (Lycium ruthenicum Murray) [34] | 33% ethanol + 67% water, solvent-to-sample ratio 40 mL/g | sonication 100 W, extraction time 30 min | TPC (mg GAE/g): 11.05 ± 0.30 TFC (mg RE/g): 19.77 ± 0.23 TAC (mg CGE/g): 88.74 ± 1.99 FRAP (mmol Fe2+/100 g): 8.04 ± 0.04 ABTS (mmol TE/100 g): 4.89 ± 0.046 |
| 50% ethanol + 50% water, solvent-to-sample ratio 30 mL/g | TPC: 9.60 TFC: 24.29 TAC: 76.94 FRAP: 6.33 ABTS: 4.54 | ||
| 50% ethanol + 50% water, solvent-to-sample ratio 40 mL/g | sonication 100 W, extraction time 60 min | TPC: 10.40 TFC: 26.22 TAC: 83.83 FRAP: 7.46 ABTS: 5.32 | |
| sonication 60 W, extraction time 60 min | TPC: 6.99 TFC: 23.25 TAC: 79.50 FRAP: 6.04 ABTS: 4.80 | ||
| Black chokeberry (Aronia melanocarpa L.) [35] | 58% methanol + 42% water, 70 °C, pH 3.87, S:L 0.5:17 | sonication amplitude 70%, cycle 0.7 s | TPC (mg GAE/g d.w): 38.0572 |
| 34% methanol + 66% water, 70 °C, pH 2, S:L 0.5:13.5 | TAC ( mg CGE/g d.w): 0.3055 | ||
| 54% methanol + 46% water, 70 °C, pH 2.72, S:L 0.5:18.2 | TPC: 37.8231 TAC: 0.2834 | ||
| Black elderberry (Sambucus nigra L.) [36] | Water: 100%, 40 °C, S:L 1:10 | sonication 40 kHz, extraction time 20 min | DPPH (mmol TE/g d.w): 135.1 ± 7 (“Dobrich” region)–236.5 ± 5 (“Rhodopes” region) FRAP (mmol TE/g d.w): 101.7 ± 2–193.0 ± 2 ABTS (mmol TE/g d.w): 199.1 ± 7–324.5 ± 5, TPC (mg GAE/g d.w): 29.3 ± 1.0–49.2 ± 1.0, TFC (mg QE/g d.w): 6.4 ± 0.5–18.6 ± 0.5 |
| Blackcurrant (Ribes nigrum L.) [29] | 60% ethanol + 40% water, pH 2.5, 45 °C, S:L 1:25 | sonication 50 kHz, microwave 226 W extraction time 385 s | FRAP (mmol TE/mg): 4.371 ABTS (mmol TE/mg): 0.401 TAC (U/mg): 44.329 Extraction rate anthocyanins: 97.05 ± 0.85% |
| microwave 226 W extraction time 385 s | FRAP: 3.832 ABTS: 0.339 TAC: 38.135 Extraction rate anthocyanins: 93.09 ± 0.75% | ||
| Turkey berry (Solanum torvum Sw) [37] | 56.7% ethanol + 43.3% water, 80 °C, 17.3 min, liquid/solid ratio 47.7 mL/g | sonication 40 kHz | TPC (mg GAE/g): 189.67 ± 4.21 DPPH (μg AAE/g): 44.87 ± 1.06 ABTS (μg TE/g): 112 ± 3.48 |
| Acai berries (Euterpe oleracea Mart.) [28] | 50% ethanol + 50% water, solvent/solid ratio 25:1 | sonication, 37 kHz, 100% amplitude, 25 °C, 2 vs. 25 vs. 45 min | TPC (mg GAE/g d.w): 34.15 ± 0.09 vs. 34.18 ± 0.49 vs. 34.12 ± 0.20 TFC (mg QE/g d.w): 5.16 ± 0.03 vs. 5.26 ± 0.01 vs. 5.16 ± 0.01 TAC (mg CGE/g d.w): 4.68 ± 0.07 vs. 4.94 ± 0.12 vs. 4.83 ± 0.05 |
| sonication, 37 kHz, 100% amplitude, 45 °C, 2 vs. 25 vs. 45 min | TPC: 34.35 ± 0.09 vs. 35.95 ± 0.06 vs. 35.76 ± 0.07 TFC: 5.15 ± 0.07 vs. 5.19 ± 0.06 vs. 5.22 ± 0.08 TAC: 4.92 ± 0.13 vs. 4.88 ± 0.10 vs. 4.72 ± 0.33 | ||
| Cornelian cherry (Cornus mas L.) [32] | 100% methanol, S:L 1:10 | sonication, 40 °C, 30 min | TPC (mg GAE/kg): 1420 ± 119 DPPH (IC50) (mg/mL): 6.43 ± 0.34 FRAP (μmol/g): 190 ± 20 |
| Sour cherry (Prunus cerasus) [32] | 100% methanol, S:L 1:10 | sonication, 40 °C, 30 min | TPC (mg GAE/kg): 1470 ± 70 DPPH (IC50) (mg/mL): 4.70 ± 1.23 FRAP (μmol/g): 200 ± 20 |
| American cranberry (Vaccinium macrocarpon Aiton) [14] | 70% ethanol + 19% water + 1% HCl, S:L 1:20 | sonication, 80 kHz, 565 W, 15 min, room temperature | TAC (mg/g): 1.95 ± 0.11 (Early black cultivar)–8.13 ± 0.09 (Woolman cultivar) ABTS (μmol TE/g): 203.20 ± 9.19 (Baifay cultivar)–849.75 ± 10.88 (Woolman cultivar) FRAP (μmol TE/g): 215.23 ± 3.24 (Prolific cultivar)–528.05 ± 12.16 (Le Munyon cultivar) |
| Cornelian cherry (Cornus mas L.) [13] | 80% methanol + 20% water, S:L 1:20 | sonication, 400 W, 24 Hz, 15 min, 50 °C | TPC (mg GAE/100 g): 158 ± 2 (Krupnoplodni NS cultivar)–591 ± 10 (Kosten 1 cultivar) DPPH (mmoli TE/100 g): 623 ± 5 (Krupnoplodni NS cultivar)–1757 ± 4 (CT 02 cultivar) ABTS (mmoli TE/100 g): 441 ± 9 (Lukyanovsky cultivar)–1475 ± 25 (CT 02 cultivar) FRAP (μmol Fe2+/100 g): 1509 ± 1 (Krupnoplodni NS cultivar)–5954 ± 32 (CT 02 cultivar) |
| Blackberry (Rubus spp.) [15] | 100% water | sonication 20 min, 25 °C | DPPH (mmoli TE/g): 16.47 ± 0.3 FRAP (mmol Fe2+/g): 20.22 ± 1.02 FCA (mgTE/g): 17.47 ± 0.4 |
| 100% methanol, S:L 1:20 | DPPH: 23.10 ± 2.32 FRAP: 26.61 ± 2.01 FCA: 23.4 ± 1.14 | ||
| 100% ethanol, S:L 1:10 | DPPH: 17.47 ± 0.4 FRAP: 23.4 ± 1.14 FCA: 21.35 ± 2.93 | ||
| Choline chloride/citric acid ratio 1:2, 20% water, S:L 1:10 | DPPH: 68.77 ± 2.29 FRAP: 23.90 ± 0.86 FCA: 1.97 ± 0.21 | ||
| Malic acid/xylitol ratio 1:2, 20% water, S:L 1:10 | DPPH: 13.84 ± 1.39 FRAP: 6.20 ± 0.40 FCA: 94.21 ± 2.09 | ||
| Tartaric acid/xylitol ratio 1:2, 20% water, S:L 1:10 | DPPH: 18.00 ± 0.50 FRAP: 83.08 ± 3.78 FCA: 1.99 ± 0.10 | ||
| Acetic acid/sorbitol ratio 1:2, 20% water, S:L 1:10 | DPPH: 43.56 ± 6.92 FRAP: 73.95 ± 4.36 FCA: 4687.67 ± 83.58 | ||
| Microwave-assisted extraction | |||
| Blackberry (Rubus ulmifolius L.) [33] | 64% methanol + 36% water, 50.3 °C, pH 2, S:L 0.3:12 | microwaved 800 W, 5 min | TPC (μg GAE/g): 16,277.29 ± 688.42 |
| 26.3% methanol + 73.7% water, 100 °C, pH 2, S:L 0.3:20 | TAC (μ CGE/g): 2454.12 ± 65.90 | ||
| 51% methanol + 49% water, 100 °C, pH 2, S:L 0.3:15 | TPC: 15,677.97 ± 729.73 TAC: 2409.11 ± 69.16 | ||
| Acai berries (Euterpe oleracea Mart.) [28] | 50% ethanol + 50% water, S:L 25:1 | microwaved 360 W, 25 °C, 2 vs. 3.16 vs. 4.33 min, 1–9 cycles | TPC (mg GAE/g d.w): 34.36 ± 0.19 vs. 34.22 ± 0.20 vs. 33.42 ± 0.30 TFC (mg QE/g d.w): 5.04 ± 0.12 vs. 5.10 ± 0.07 vs. 4.92 ± 0.09 TAC (mg CGE/g d.w): 5.09 ± 0.07 vs. 4.94 ± 0.12 vs. 4.83 ± 0.05 |
| microwaved 360 W, 45 °C, 2 vs. 3.16 vs. 4.33 min, 1–9 cycles | TPC: 34.26 ± 0.30 vs. 35.33 ± 0.11 vs. 34.52 ± 0.77 TFC: 5.15 ± 0.07 vs. 5.19 ± 0.06 vs. 5.22 ± 0.08 TAC: 4.92 ± 0.13 vs. 4.88 ± 0.10 vs. 4.72 ± 0.33 | ||
| Black Chokeberry (Aronia melanocarpa L.) [23] | 53.6% ethanol + 46.4% water | microwaved 300 W, 5 min | TFC (mg GAE/100 g): 420.1 (optimum condition) |
| 40.3% ethanol + 59.7% water | TFC (mg GAE/100 g): 411.7 (economic condition) | ||
| 53.8% ethanol + 46.2% water | TFC (mg GAE/100 g): 448.3 (maximum yield) | ||
| Seabuckthorn berries (Hippophae rhamnoides L.) [20] | Water: 100% after degreasing with petroleum ether, S:L 10:1, polysaccharides (PBS) precipitated with 95% ethanol | microwaved 600 W, 6 min, 80 °C | PBS extraction yield (%): 0.264 ± 0.005 Hydroxyl radical (IC50) (mg/mL): 0.016 ± 0.001 DPPH (IC50) (mg/mL): 0.016 ± 0.001 Reducing power (EC50) (mg/mL): 0.148 ± 0.004 |
| heat reflux extraction, 6 min, 80 °C | PBS extraction yield: 0.207 ± 0.006 Hydroxyl radical (IC50): 2.691 ± 0.048 DPPH (IC50): 0.239 ± 0.0016 Reducing power (EC50): 1.868 ± 0.028 | ||
| Blackberry (Rubus spp.) [38] | 52% ethanol + 48% water, liquid/solid ratio 25 g/mL | microwaved 469 W, 4 min | Extraction yield (mg/g): 2.18 ± 0.06 ABTS (μmol TE/g): 32.18 ± 1.54 DPPH (μmol TE/g): 27.18 ± 1.33 |
| 60 min | Extraction yield: 1.81 ± 0.04 ABTS: 20.84 ± 1.49 DPPH: 17.01 ± 0.19 | ||
| Cranberries (Vaccinium macrocarpon L.) [39] | 80% methanol + 19.9% water + 0.1% HCl, liquid/solid ratio 2:1, ultrasound 30 s | microwaved 150 W, 5.33 W/g, pressure 5 ± 1 kPa | TPC (mg GAE/g d.w): 27.51 ± 0.56 TFC (mg GAE/100 g): 3.79 ± 0.11 TAC (mg CGE/g d.w): 2.22 ± 0.10 FRAP (mg TE/g d.w): 47.67 ± 0.39 |
| 80 °C, air velocity 1.5 m/s | TPC: 22.14 ± 0.49 TFC: 2.65 ± 0.05 TAC: 0.75 ± 0.10 FRAP: 33.03 ± 0.72 | ||
| Blueberry (Vaccinium corymbosum L.) [40] | 80% methanol + 19.9% water + 0.1% HCl, liquid/solid ratio 5:1 | microwaved 1.3 W/g, pressure 4–6 kPa; 60 °C | TPC (mg GAE/100 g d.w): 0.71 ± 0.01 ABTS (mmol TE/100 g d.w): 2.95 ± 0.21 |
| microwaved 1.3 W/g, pressure 4–6 kPa; 90 °C | TPC: 1.19 ± 0.03 ABTS: 6.37 ± 0.08 | ||
| Supercritical and subcritical CO2 extraction (SC-CO2) | |||
| Vilberry (Vaccinium myrtillus) [41] | SC-CO2, 25 MPa, 45 °C, 4 h SC-CO2: flow rate 8 kg/h CO2 with 6% co-solvent (30% water + 70% ethanol), SubC-CO2: flow rate 6 kg/h CO2 with 6% co-solvent (50% water + 50% ethanol) at 6 mL/min and 9% co-solvent (90% water + 10% ethanol) | DPPH (IC50) (μg/g d.w): 102.66 ± 2.64 ABTS (IC50) (μg/g d.w): 8.49 ± 0.41 Reducing power (EC50) (μg/g d.w): 10.30 ± 0.10 | |
| Black chokeberry (Aronia melanocarpa) (pomace) [42] | SC-CO2: flow rate 2 L/min CO2 | SC-CO2, 40 MPa, 40 °C, 149 min | Extraction yield (g/100 g d.w): 2.95 ± 0.85 ABTS (mmol TE/g): 0.011 ± 1.64 DPPH (mmol TE/g) 0.007 ± 2.51 ORAC (mmmol TE/g): 0.65 ± 2.93 TPC (mg GAE/g): 23.90 ± 0.05 |
| SC-CO2: flow rate 2 L/min CO2 with 2% co-solvent ethanol | Extraction yield: 3.32 ± 0.23 ABTS: 0.010 ± 3.11 DPPH: 0.009 ± 2.12 ORAC: 0.93 ± 3.03 TPC: 25.30 ± 0.05 | ||
| SC-CO2: flow rate 2 L/min CO2 with 5% co-solvent ethanol | Extraction yield: 4.88 ± 0.26 ABTS: 0.034 ± 2.44 DPPH: 0.011 ± 1.11 ORAC: 1.49 ± 0.41 TPC: 31.60 ± 0.05 | ||
| SC-CO2: flow rate 2 L/min CO2 with 10% co-solvent ethanol | Extraction yield: 7.08 ± 0.99 ABTS: 0.058 ± 3.34 DPPH: 0.025 ± 2.01 ORAC: 1.94 ± 0.75 TPC: 34.30 ± 0.05 | ||
| Pressurized liquid extraction (PLE) | |||
| Black elderberry (Sambucus nigra L.) [43] | 80% ethanol + 20% water | PLE, 150 p.s.i, 10 min, 20 °C | DPPH (IC50) (%): 50.25 ± 0.080 TFP (g/100 g × 10−2): 13.6968 TAC(g/100 g × 10−2): 48.4568 |
| PLE, 150 p.s.i, 10 min, 200 °C | DPPH: 67.69 ± 1.85 TFP: 20.1836 TAC: 52.8869 | ||
| Black chokeberry (Aronia melanocarpa) (pomace) [42] | 100% methanol | PLE, 10.3 MPa, 45 min, 40 °C | Extraction yield (g/100 g d.w): 34.11 ± 1.25 ABTS (mmol TE/g): 1.73 ± 1.39 DPPH (mmol TE/g): 1.00 ± 3.93 ORAC (mmmol TE/g): 8.79 ± 0.08 TPC (mg GAE/g): 401.75 ± 0.01 |
| PLE, 10.3 MPa, 45 min, 130 °C | Extraction yield: 48.13 ± 0.81 ABTS: 2.17 ± 1.57 DPPH: 1.29 ± 0.99 ORAC: 9.26 ± 0.11 TPC: 410 ± 0.01 | ||
| 100% water | PLE, 10.3 MPa, 45 min, 40 °C | Extraction yield: 21.38 ± 1.12 ABTS: 1.71 ± 1.65 DPPH: 0.55 ± 1.51 ORAC: 10.75 ± 0.08 TPC: 203.92 ± 0.05 | |
| 100% water | PLE, 10.3 MPa, 45 min, 130 °C | Extraction yield: 17.67 ± 1.31 ABTS: 1.44 ± 3.90 DPPH: 0.50 ± 1.75 ORAC: 6.57 ± 0.08 TPC: 182.89 ± 0.01 | |
| 80% methanol + 20% water | PLE, 10.3 MPa, 45 min, 130 °C | Extraction yield: 28.29 ± 1.99 ABTS: 2.05 ± 2.00 DPPH: 1.52 ± 1.14 ORAC: 10.89 ± 0.04 TPC: 453.68 ± 0.01 | |
| 80% acetone + 20% water | Extraction yield: 45.55 ± 2.21 ABTS: 1.94 ± 1.49 DPPH: 0.24 ± 3.51 ORAC: 9.23 ± 0.04 TPC: 490.38 ± 0.02 | ||
| Blackberry (Rubus fruticosus L.) (pomace) [44] | 100% water | PLE, 10.3 MPa, 130 °C, 3 cycles, 10 min | Extraction yield (g/100 g d.w): 5.09 ± 0.28 TPC (mg GAE/g d.w): 7.81 ± 0.03 ABTS (mg TE/g d.w): 48.73 ± 0.69 ORAC (mg TE/g d.w): 17.29 ± 1.04 |
| 100% ethanol | PLE, 10.3 MPa, 50–90 °C, 3 cycles, 5–15 min | Extraction yield: 26.34 ± 0.47 TPC: 29.14 ± 0.67 ABTS: 168.73 ± 3.53 ORAC: 90.97 ± 3.04 | |
| High-hydrostatic-pressure extraction (HHPE) | |||
| Blueberries (Vaccinium) [45] | 70% acetone + 29.5% water + 0.5% acetic acid | HHPE, 500 MPa, 20 °C, 5 min with pulses of 1 min | DPPH (μg TE/g): 1811 ± 1 FRAP (μg TE/g): 2001 ± 29 |
| HHPE, 500 MPa, 20 °C, 10 min with pulses of 1 min | DPPH: 1834 ± 8 FRAP: 2013 ± 13 | ||
| HHPE, 500 MPa, 20 °C, 15 min with pulses of 1 min | DPPH: 1854 ± 11 FRAP: 2060 ± 27 | ||
| Strawberries (Fragaria × ananassa cv, EI Santa) (puree) [46] | 100% water | HHPE, 400 MPa, 20 °C, 15 min | ARP (g/L): 1.25 ± 0.05 TPC (mg GAE/100 g d.w): 859.03 ± 6.56 TAC (mg/100 g d.w): 173.34 ± 6.51 |
| HHPE, 500 MPa, 20 °C, 15 min | ARP: 1.30 ± 0.02 TPC: 926.00 ± 5.93 TAC: 202.53 ± 5.40 | ||
| HHPE, 600 MPa, 20 °C, 15 min | ARP: 1.33 ± 0.02 TPC: 939.01 ± 0.99 TAC: 204.30 ± 1.60 | ||
| Blackberries (Rubus fruticosus cv, Loughness) (puree) [46] | 100% water | HHPE, 400 MPa, 20 °C, 15 min | ARP: 3.87 ± 1.11 TPC: 1546.26 ± 8.0 TAC: 1039.21 ± 4.51 |
| HHPE, 500 MPa, 20 °C, 15 min | ARP: 3.70 ± 0.57 TPC: 1724.65 ± 0.7 TAC: 1014.21 ± 0.10 | ||
| HHPE, 600 MPa, 20 °C, 15 min | ARP: 4.80 ± 1.79 TPC: 1778.44 ± 6.0 TAC: 1014.47 ± 1.00 | ||
| Pulsed electric field (PEF) extraction | |||
| Pomegranate (Punica granatum) (fermented beverage) [47] | PEF: flow rate 70 L/h, bipolar square-wave pulses, frequency 200 Hz | field strength 11.7 kV/cm, pulse width 15 μs | TAA (mg TE/100 mL): 264.66 ± 0.46 TFC (mg QE/100 mL): 115.00 ± 1.07 TAC (mg CGE/100 mL): 5.15 ± 0.03 |
| field strength 18.8 kV/cm, pulse width 20 μs | TAA: 279.41 ± 0.47 TFC: 120.23 ± 1.83 TAC: 5.41 ± 0.01 | ||
| Barberry (Berberis vulgaris L.) [48] | PEF: 100 pulses, 1 Hz | 7000 A | TAC (mg CGE/100 g d.w): 260.28 TPC (mg GAE/100 d.w): 462.75 |
| 5000 A | TAC: 248.90 TPC: 456.39 | ||
| PEF: 5000 A, 1 Hz | 75 pulses | TAC: 233.60 TPC: 428.91 | |
| 50 pulses | TAC: 224.78 TPC: 417.93 | ||
| EAE: 1.5% pectinase, 60 °C | TAC: 279.64 TPC: 484.93 | ||
| Blueberries (Vaccinium) (pomace) [49] | 100 pulses, 20 kV/cm, energy input 41.03 kJ/kg 50% ethanol + 49% water + 1% HCl | TPC (mg GAE/g d.w): 10.52 TAA (mmol TE/g d.w): 0.83 | |
| 100 pulses, 20 kV/cm, energy input 41.03 kJ/kg 50% methanol + 49% water + 1% HCl | TAC (µg/g d.w): 1757.32 TFC (µg/g d.w): 297.86 | ||
| Strawberry (Fragaria × ananassa Duch.) (Juice) [50] | PEF: 30 kV/cm, 100 Hz | 3 min | TPC (mg GAE/100 g): 118.85 ± 1.22 DPPH (µmol TE/100 g): 294.69 ± 0.15 FRAP (µmol TE/100 g): 879.81 ± 9.38 |
| 4.5 min | TPC: 116.75 ± 1.22 DPPH: 294.34 ± 0.15 FRAP: 846.92 ± 9.38 | ||
| Ultrasound: amplitude 25%, pulse 50% | 5 min | TPC: 120.24 ± 1.22 DPPH: 293.62 ± 0.15 FRAP: 877.46 ± 9.38 | |
| 7.5 min | TPC: 115.04 ± 1.22 DPPH: 294.64 ± 0.15 FRAP: 824.96 ± 9.38 | ||
| PEF + ultrasound | 3 min + 7.5 min | TPC: 121.37 ± 2.15 DPPH: 295.48 ± 0.25 FRAP: 907.78 ± 19.66 | |
| 4.5 min + 5 min | TPC: 127.80 ± 2.26 DPPH: 294.13 ± 0.32 FRAP: 911.25 ± 9.99 | ||
| Enzyme-assisted extraction | |||
| Blackberry (Rubus fruticosus L.) (pomace) [44] | SC-CO2: 25–55 MPa, 50–80 °C, 60–180 min, respectively | Extraction yield (g/100 g d.w): 9.93 ± 0.13 TPC (mg GAE/g d.w): 2.91 ± 0.14 ABTS (mg TE/g d.w): 0.55 ± 0.00 | |
| EAE–Viscozyme: pH 4.8, 50 °C, 360 min, 5 g substrate + 50 mL buffer + 500 μL Viscozyme L | Extraction yield: 7.83 ± 0.30 TPC: 2.28 ± 0.02 ABTS: 9.94 ± 0.04 | ||
| Blackcurrant (Ribes nigrum L.) (press cake) [51] | β-glucanase (Trichoderma reesei), solid/liquid 1:10, 200 ppm, 40 °C, 1 → 4 h, inactivation 60–80 °C | TAC (mg CGE/100 g d.w): 319 ± 24–396 ± 10 TPC (mg GAE/100 g d.w): 848 ± 97–1142 ± 51 DPPH (mg AAE/100 g d.w): 494 ± 2–527 ± 2 CUPRAC (mg AAE/100 g d.w): 1835 ± 158–1640 ± 60 | |
| Cellulase (Trichoderma reesei), solid/liquid 1:10, 200 ppm, 60 °C, 1 → 4 h, inactivation 60–80 °C | TAC: 319 ± 7–405 ± 9 TPC: 791 ± 22–938 ± 14 DPPH: 470 ± 2–552 ± 2 CUPRAC: 2200 ± 69–3531 ± 284 | ||
| Pectinase (Aspergillus niger), solid/liquid 1:10, 200 ppm, 60 °C, 1 → 4 h, inactivation 60–80 °C | TAC: 324 ± 23–386 ± 22 TPC: 836 ± 2–913 ± 11 DPPH: 495 ± 3–557 ± 4 CUPRAC: 2411 ± 26–3697 ± 205 | ||
| Pectinase (pectinesterase and polygalacturonase) (Aspergillus oryzae), solid/liquid 1:10, 200 ppm, 60 °C, 1 → 4 h, inactivation 60–80 °C | TAC: 336 ± 8–401 ± 19 TPC: 787 ± 11–898 ± 2 DPPH: 808 ± 83–1080 ± 33 CUPRAC: 1971 ± 34–3155 ± 150 | ||
| Pectinase (pectinesterase and polygalacturonase) (Aspergillus oryzae), solid/liquid 1:10, 200 ppm, 60 °C, 1 → 4 h, inactivation 60–80 °C | TAC: 334 ± 23–407 ± 15 TPC: 817 ± 11–998 ± 4 DPPH: 475 ± 3–537 ± 3 CUPRAC: 2418 ± 158–3148 ± 91 | ||
| Cellulase (Trichoderma reesei) + pectinase (Aspergillus oryzae), solid/liquid 1:10, 200 ppm, 60 °C, 1 → 4 h, inactivation 60–80 °C | TAC: 312 ± 11–383 ± 11 TPC: 891 ± 5–911 ± 51 DPPH: 457 ± 3–524 ± 5 CUPRAC: 2065 ± 69–3103 ± 107 | ||
| Pectin lyase, solid/liquid 1:4, 100 ppm, 40 °C, pH < 5.5, 1 → 4 h, inactivation 75 °C | TAC: 391 ± 19–394 ± 16 TPC: 739 ± 16–736 ± 3 DPPH: 545 ± 11–536 ± 3 CUPRAC: 1459 ± 138–1715 ± 69 | ||
| Cellulase, solid/liquid 1:4, 100 ppm, 50 °C, pH < 5.5, 1 → 4 h, inactivation 75 °C | TAC: 430 ± 21–382 ± 23 TPC: 665 ± 3–816 ± 13 DPPH: 459 ± 8–576 ± 25 CUPRAC: 1384 ± 90–2648 ± 119 | ||
| Cellulase + pectin lyase, solid/liquid 1:4, 100 ppm, 40–50 °C, pH < 5.5, 1 → 4 h, inactivation 75 °C | TAC: 376 ± 9–392 ± 21 TPC: 376 ± 2–495 ± 2 DPPH: 117 ± 3–149 ± 3 CUPRAC: 323 ± 45–970 ± 69 | ||
| Bilberry (Vaccinium myrtillus L.) (pomace) [52] | EAE–Viscozyme L (Aspergillus aculeatus), solid/liquid 1:10, pH 5, 50 °C, 20 min (optimization) | Extract yield (g/100 g d.w) EAE vs. SLE: 56.1 EAE vs. SLE: 0.7 vs. 43.1 EAE vs. SLE: 0.6 ABTS (mg TE/g d.w) EAE vs. SLE: 21.2 ± 0.6 vs. 15.8 ± 0.2 TPC (mg GAE/g d.w) EAE vs. SLE: 6.8 ± 0.1 vs. 5.3 ± 0.3 ORAC (mg TE/g d.w) EAE vs. SLE: 23.78 ± 0.2 vs. 21.6 ± 0.2 CUPRAC (mg TE/g d.w) EAE vs. SLE: 10.94 ± 0.1 vs. 8.8 ± 0.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitrașcu, L.; Banu, I.; Patraşcu, L.; Vasilean, I.; Aprodu, I. The Influence of Processing on the Bioactive Compounds of Small Berries. Appl. Sci. 2024, 14, 8713. https://doi.org/10.3390/app14198713
Dumitrașcu L, Banu I, Patraşcu L, Vasilean I, Aprodu I. The Influence of Processing on the Bioactive Compounds of Small Berries. Applied Sciences. 2024; 14(19):8713. https://doi.org/10.3390/app14198713
Chicago/Turabian StyleDumitrașcu, Loredana, Iuliana Banu, Livia Patraşcu, Ina Vasilean, and Iuliana Aprodu. 2024. "The Influence of Processing on the Bioactive Compounds of Small Berries" Applied Sciences 14, no. 19: 8713. https://doi.org/10.3390/app14198713
APA StyleDumitrașcu, L., Banu, I., Patraşcu, L., Vasilean, I., & Aprodu, I. (2024). The Influence of Processing on the Bioactive Compounds of Small Berries. Applied Sciences, 14(19), 8713. https://doi.org/10.3390/app14198713










