A Novel Approach for Rapid Dewatering of Water-Based Ink Wastewater Sludge under Low Temperature and Its Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Sludge Source
2.2. Sludge Treatment and Dewatering Procedure
2.3. Sludge Dewaterability
2.4. Sludge Characterization
2.4.1. Differential Scanning Calorimetry Analysis
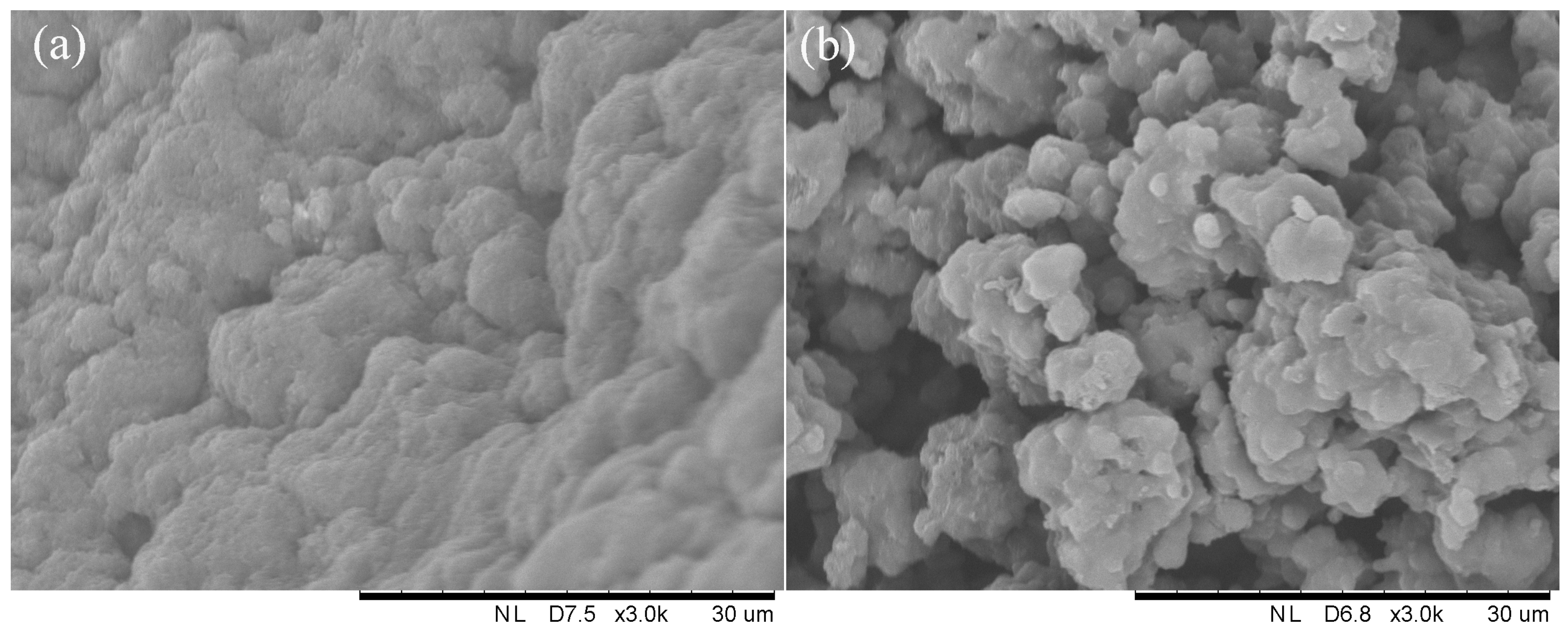
2.4.2. SEM Observation
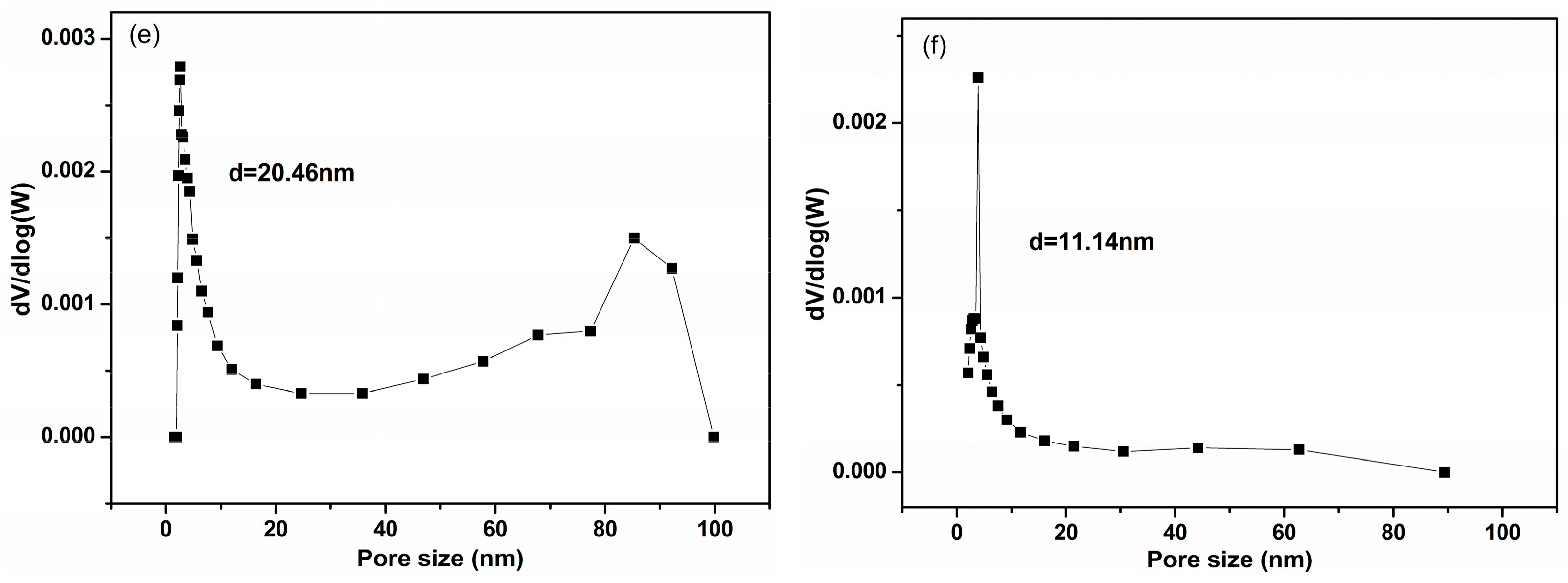
2.4.3. Specific Surface Area and Pore Size Distribution
2.4.4. XPS Test
2.5. Wastewater Analysis
3. Results and Discussion
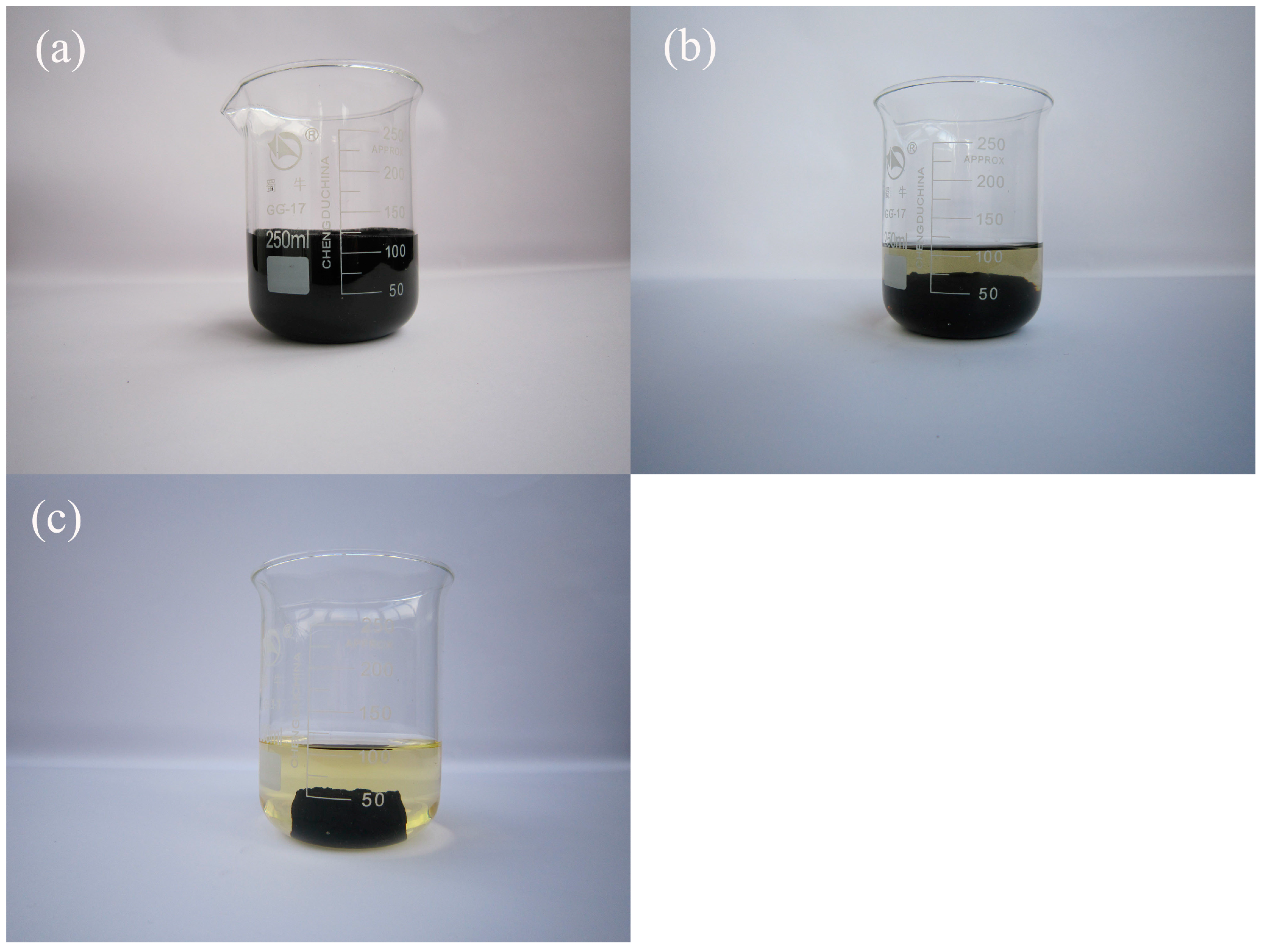
3.1. Dewatering Efficiency
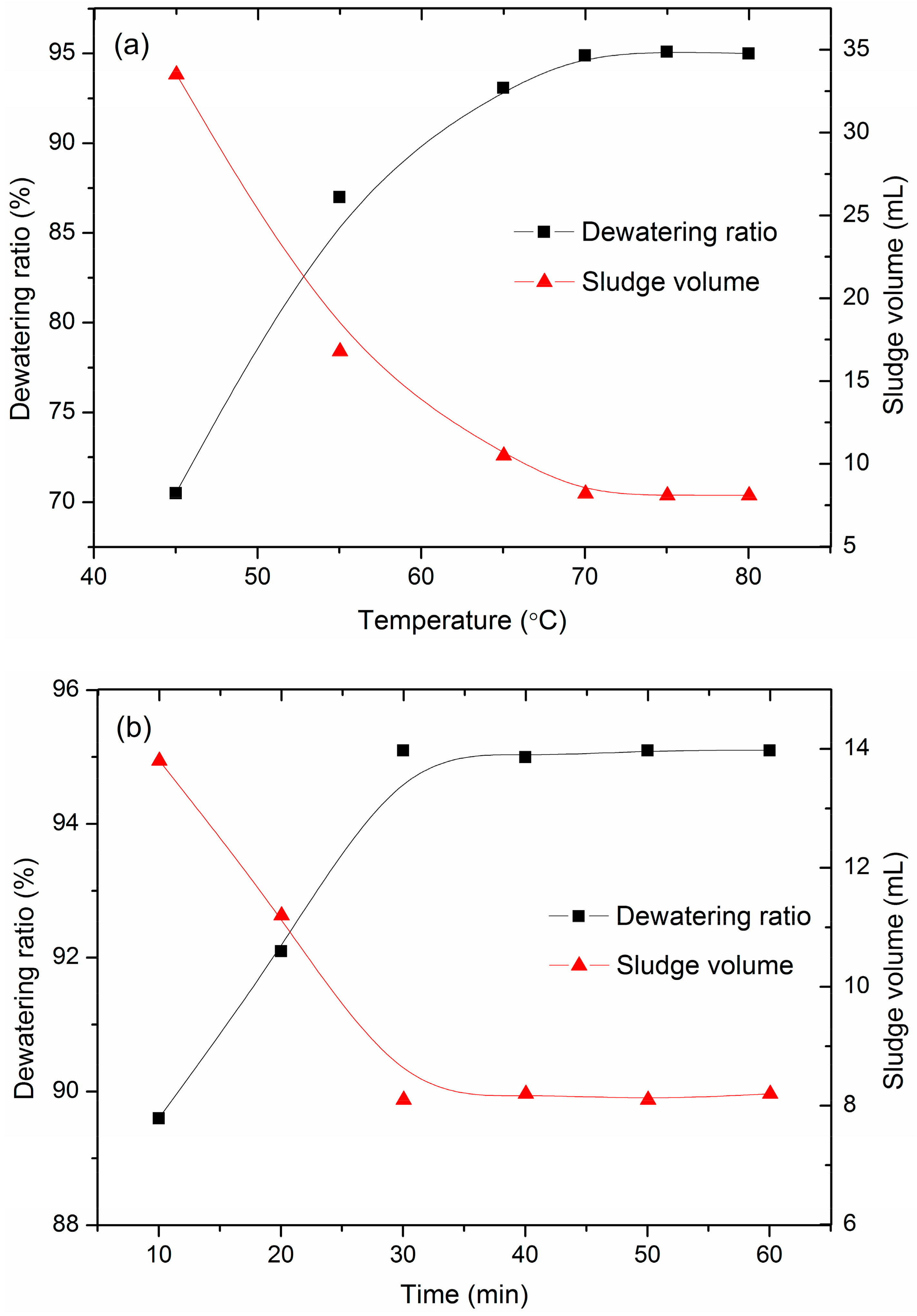
3.2. Effect of Temperature and Time on Sludge Dewatering
3.3. Characteristics of Sludge
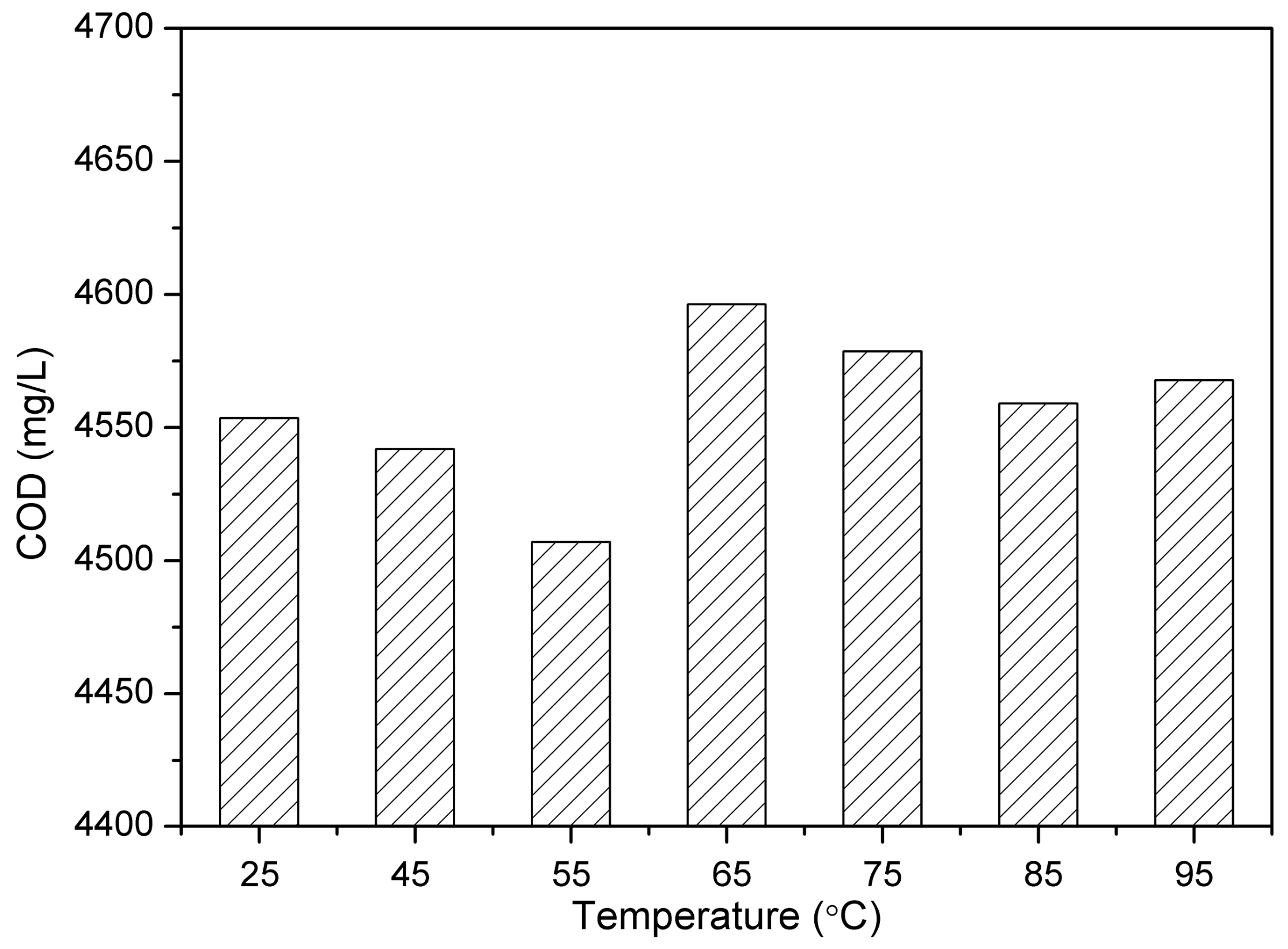
3.4. Characteristics of Dehydrated Water
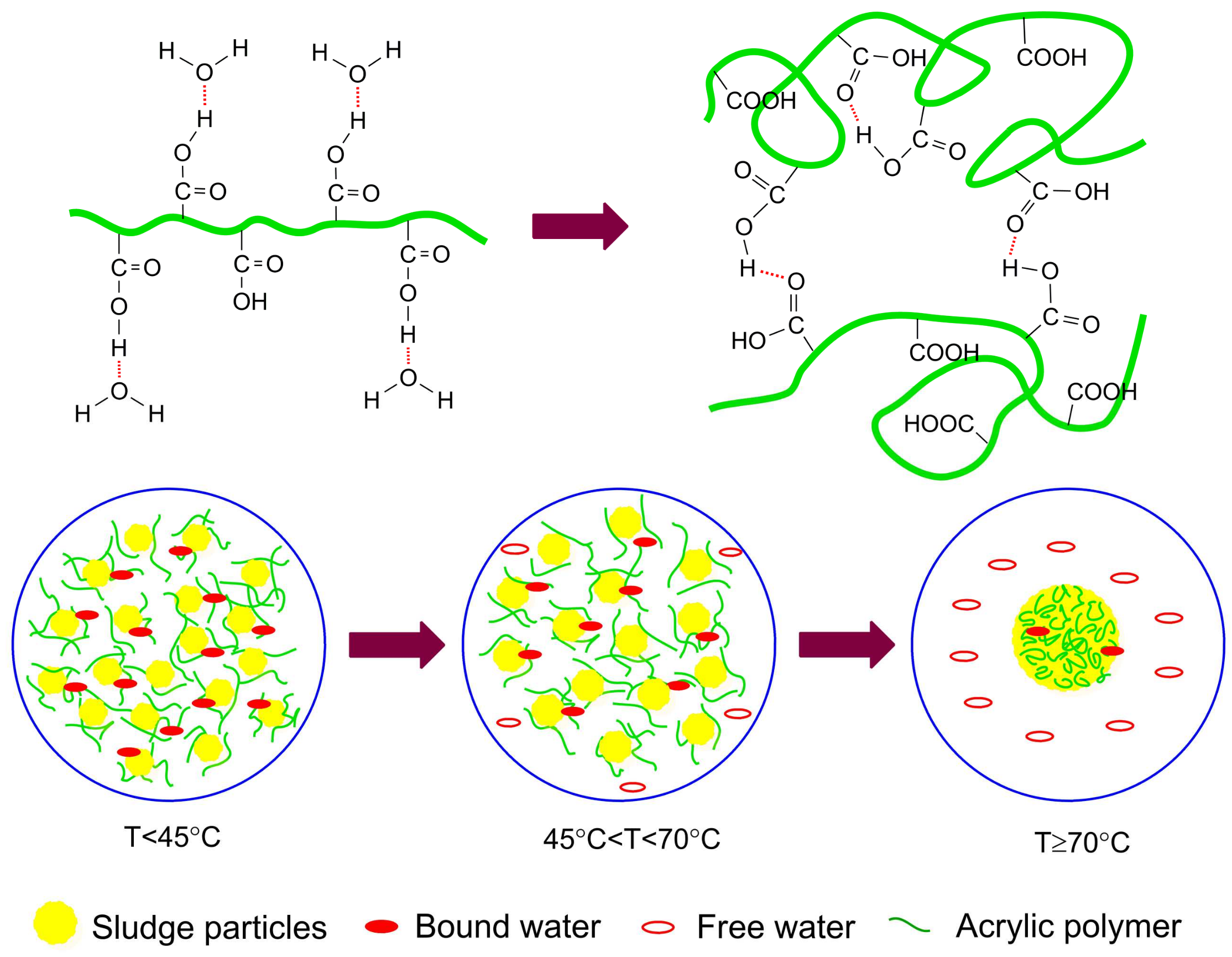
3.5. Dewatering Mechanism and Model
- (1)
- When the temperature increased to 45 °C, the particles aggregated and formed large flocs, resulting in better dewaterability. This is mainly because the hydrogen bonding between hydrophilic groups in the sludge (such as carboxyl and hydroxyl) and water molecules was destroyed due to temperature increases, which reduced the water-absorbing capacity of the sludge flocs. As the temperature continued increasing (below 70 °C), the thermal motion of water molecules increased, and part of the bound water within the sludge was transformed into free water and could be easily removed. Accompanied by the reunion of particles, the volume of sludge decreased, and a large, loosely clumped sludge was obtained.
- (2)
- As the temperature was elevated above the softening point of acrylic resins (70 °C), the polymer chains were curled up and folded rather than being stretched out. The softening and shrinking of the polymer chains can connect or wrap the suspended solids in the sludge by means of van der Waals forces, hydrogen bonding, or intermolecular cohesion, leaving more chances for the floccules to contact and aggregate. During this process, a large number of hydrophilic carboxyl groups of polymer chains might be enclosed within the sludge or form internal hydrogen bonding, which reduces the interaction with water molecules and increases the hydrophobicity of the sludge particles. Therefore, the bound water content of sludge with carboxyl groups was released. At the same time, the sludge particles were packed closely together, thus reducing the water-holding capacity and volume of sludge; a large amount of surface water was also released. Less water was absorbed on the sludge surfaces and held by the sludge particles, which released more water molecules. The released free water can diffuse from the inner portions of the flocs to the solution through the internal pore within the sludge due to the shrinkage of acrylic chains and the extrusion of sludge particles. Finally, the particles adhered to each other to form a clot of compacted cake with a large number of pores within the interior and high hardness, thereby improving the dewaterability of sludge.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robert, T. “Green ink in all colors”—Printing ink from renewable resources. Prog. Org. Coat. 2015, 78, 287–292. [Google Scholar] [CrossRef]
- Vishali, S.; Karthikeyan, R. A comparative study of strychnos potatorum and chemical coagulants in the treatment of paint and industrial effluents: An alternate solution. Sep. Sci. Technol. 2014, 49, 2510–2517. [Google Scholar] [CrossRef]
- Khannous, L.; Elleuch, A.; Fendri, I.; Jebahi, N.; Khlaf, H.; Gharsallah, N. Treatment of printing wastewater by a combined process of coagulation and biosorption for a possible reuse in agriculture. Desalin. Water Treat. 2016, 57, 5723–5729. [Google Scholar] [CrossRef]
- Fendri, I.; Khannous, L.; Timoumi, A.; Gharsallah, N.; Gdoura, R. Optimization of coagulation-flocculation process for printing ink industrial wastewater treatment using response surface methodology. Afr. J. Biotechnol. 2013, 12, 4819–4826. [Google Scholar]
- Yang, P.; Wang, Z.; Wang, J. Study on acid precipitation–electrocatalysis method for the treatment of water-soluble printing ink wastewater. Ind. Water Treat. 2013, 33, 51–54. [Google Scholar]
- Ma, X.; Xia, H. Coagulation combined with fenton process for the treatment of water-based printing ink wastewater. Environ. Eng. Manag. J. 2013, 12, 2169–2174. [Google Scholar] [CrossRef]
- Balea, A.; Monte, M.C.; Fuente, E.; Negro, C.; Blanco, A. Application of cellulose nanofibers to remove water-based flexographic inks from wastewaters. Environ. Sci. Pollut. Res. 2017, 24, 5049–5059. [Google Scholar] [CrossRef] [PubMed]
- Doke, S.M.; Yadav, G.D. Synthesis of novel titania membrane support via combustion synthesis route and its application in decolorization of aqueous effluent using microfiltration. Clean Technol. Environ. Policy 2016, 18, 139–149. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, W.; Wang, Q.; Huang, Y.; Meng, C.; Wang, D. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Cao, B.; Zhang, W.; Yang, P.; Xu, Q.; Wang, D.; Shen, X. Improvement of wastewater sludge dewatering properties using polymeric aluminum-silicon complex flocculants conditioning: Importance of aluminum/silicon ratio. Colloids Surf. A Physicochem. Eng. Asp. 2017, 530, 134–145. [Google Scholar] [CrossRef]
- Diak, J.; Örmeci, B. Freeze-thaw treatment of RBC sludge from a remote mining exploration facility in subarctic Canada. Water Sci. Technol. 2017, 75, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Guo, M.; Xu, X.; Guan, B. The role of temperature and CaCl2 in activated sludge dewatering under hydrothermal treatment. Water Res. 2014, 50, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Wu, Z.; Wang, H.; Xiao, Z.; Wu, Y.; Chen, X.; Zeng, G. Enhancing the sludge dewaterability by electrolysis/electrocoagulation combined with zero-valent iron activated persulfate process. Chem. Eng. J. 2016, 303, 636–645. [Google Scholar] [CrossRef]
- Wang, L.; Li, A. Hydrothermal treatment coupled with mechanical expression at increased temperature for excess sludge dewatering: The dewatering performance and the characteristics of products. Water Res. 2015, 68, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Lee, S.F. Measurement of bound water content in sludge: The use of differential scanning calorimetry (DSC). J. Chem. Technol. Biotechnol. 1995, 62, 359–365. [Google Scholar] [CrossRef]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Laurent, J.; Casellas, M.; Carrère, H.; Dagot, C. Effects of thermal hydrolysis on activated sludge solubilization, surface properties and heavy metals biosorption. Chem. Eng. J. 2011, 166, 841–849. [Google Scholar] [CrossRef]
- Wang, R.; Liu, J.; Yu, Y.; Zhou, J.; Cen, K. Effects of calcium oxide on the surface properties of municipal wastewater sludge and its co-slurrying ability with coal. Sci. Total Environ. 2013, 456–457, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bougrier, C.; Albasi, C.; Delgenès, J.P.; Carrère, H. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. 2006, 45, 711–718. [Google Scholar] [CrossRef]
- Neyens, E.; Baeyens, J. A review of thermal sludge pre-treatment processes to improve dewaterability. J. Hazard. Mater. 2003, 98, 51–67. [Google Scholar] [CrossRef] [PubMed]


| Sludge Sample | O/C | C 1s (%Amount) | |||
|---|---|---|---|---|---|
| Peak 1 284.7–284.8 eV | Peak 2 286.3 eV | Peak 3 288.9–289.1 eV | Peak 2 + Peak 3 | ||
| C–C and C–H | C–O and C–OH | O=C–O | Hydrophilic Groups | ||
| Raw sludge | 0.244 | 84.0 | 11.2 | 4.8 | 16.0 |
| Thermal solidified sludge | 0.231 | 88.5 | 7.8 | 3.7 | 11.5 |
| Parameter | Value |
|---|---|
| Wet weight of sludge cake (g) | 9.318 |
| Dry weight of sludge cake (g) | 5.911 |
| The moisture content (%) | 36.6 |
| The amount of free water (g/g dry solid) | 0.393 |
| The free water proportion (%) | 68.2 |
| The bound water content (g/g dry solid) | 0.183 |
| The bound water proportion (%) | 31.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, R.; Pan, Y.; Yu, M.; Zou, Y. A Novel Approach for Rapid Dewatering of Water-Based Ink Wastewater Sludge under Low Temperature and Its Mechanism. Appl. Sci. 2024, 14, 8743. https://doi.org/10.3390/app14198743
Zhang B, Liu R, Pan Y, Yu M, Zou Y. A Novel Approach for Rapid Dewatering of Water-Based Ink Wastewater Sludge under Low Temperature and Its Mechanism. Applied Sciences. 2024; 14(19):8743. https://doi.org/10.3390/app14198743
Chicago/Turabian StyleZhang, Bin, Rongzhan Liu, Ying Pan, Mengnan Yu, and Yihui Zou. 2024. "A Novel Approach for Rapid Dewatering of Water-Based Ink Wastewater Sludge under Low Temperature and Its Mechanism" Applied Sciences 14, no. 19: 8743. https://doi.org/10.3390/app14198743
APA StyleZhang, B., Liu, R., Pan, Y., Yu, M., & Zou, Y. (2024). A Novel Approach for Rapid Dewatering of Water-Based Ink Wastewater Sludge under Low Temperature and Its Mechanism. Applied Sciences, 14(19), 8743. https://doi.org/10.3390/app14198743






