Development and Validation of a Simple Analytical Method to Quantify Tocopherol Isoforms in Food Matrices by HPLC–UV–Vis
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
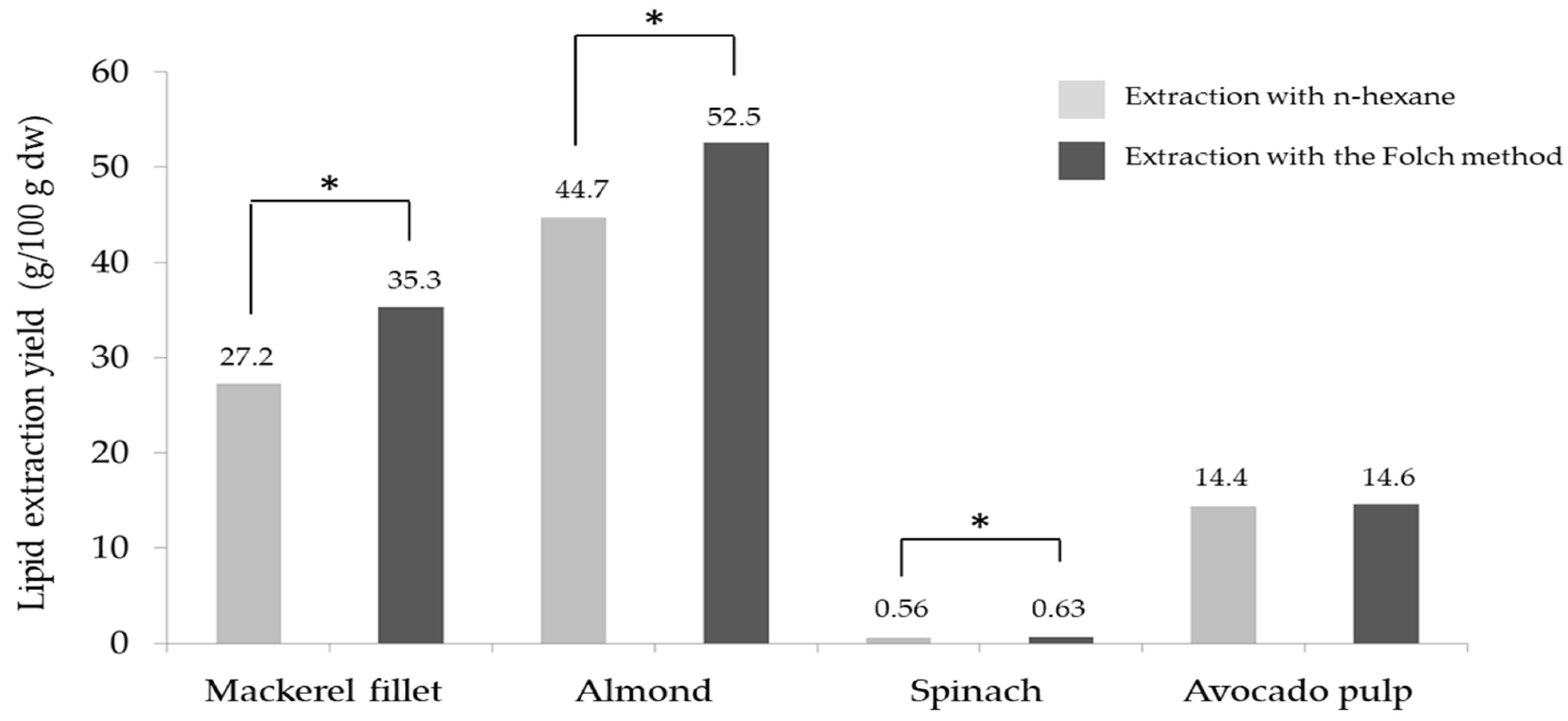
2.3. Lipid Extraction
2.4. Tocopherol Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Tocopherol Isoforms by NP-HPLC–UV–Vis
3.2. Tocopherol Quantification in Food Matrices
3.3. Assessment of Recovery of Tocopherol in Food Matrices
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, Y.; Zhang, Q.; Wang, J.; Li, Y.; Wang, X.; Bao, Y. Vitamin E synthesis and response in plants. Front. Plant Sci. 2022, 13, 994058. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, S.; Wang, X.; Jung, C. Recent advances in our understanding of tocopherol biosynthesis in plants: An overview of key genes, functions, and breeding of vitamin E improved crops. Antioxidants 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Khan, N.R.; Subhan, Z.; Mehmood, S.; Amin, A.; Rabbani, I.; -Rehman, F.; Basit, H.M.; Syed, H.K.; Khan, I.U.; et al. Novel curcumin-encapsulated α-tocopherol nanoemulsion system and its potential application for wound healing in diabetic animals. Biomed. Res. Int. 2022, 2022, 7669255. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Pinaffi-Langley, A.C.C.; Fuentes, J.; Speisky, H.; de Camargo, A.C. Vitamin E as an essential micronutrient for human health: Common, novel, and unexplored dietary sources. Free Radic. Biol. Med. 2021, 176, 312–321. [Google Scholar] [CrossRef]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of α-tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Tocopherols and tocotrienols in plants and their products: A review on methods of extraction, chromatographic separation, and detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed harmonized nutrient reference values for populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef]
- Aksoz, E.; Korkut, O.; Aksit, D.; Gokbulut, C. Vitamin E (A-, β + Γ- and Δ-tocopherol) levels in plant oils. Flavour. Fragr. J. 2020, 35, 504–510. [Google Scholar] [CrossRef]
- Zaaboul, F.; Liu, Y. Vitamin E in foodstuff: Nutritional, analytical, and food technology aspects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 964–998. [Google Scholar] [CrossRef] [PubMed]
- Claeys, E.; Vossen, E.; De Smet, S. Determination of α-tocopherol by reversed-phase HPLC in feed and animal-derived foods without saponification. J. Sci. Food Agric. 2016, 96, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, A.; La Torre, G.L.; Gervasi, T.; di Matteo, G.; Spano, M.; Ingallina, C.; Salvo, A. A Fast and efficient ultrasound-assisted extraction of tocopherols in cow milk followed by HPLC determination. Molecules 2021, 26, 4645. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, H.; Yu, Y.; Zhao, J.; Liu, L.; Zhao, S.; Xie, J.; Li, C.; Shen, M. Simultaneous determination of tocopherols, phytosterols, and squalene in vegetable oils by high performance liquid chromatography-tandem mass spectrometry. Food Anal. Methods 2021, 14, 1567–1576. [Google Scholar] [CrossRef]
- Gachumi, G.; Demelenne, A.; Poudel, A.; Dallal Bashi, Z.; El-Aneed, A. Novel fast chromatography-tandem mass spectrometric quantitative approach for the determination of plant-extracted phytosterols and tocopherols. Molecules 2021, 26, 1402. [Google Scholar] [CrossRef]
- Dugo, L.; Russo, M.; Cacciola, F.; Mandolfino, F.; Salafia, F.; Vilmercati, A.; Fanali, C.; Casale, M.; De Gara, L.; Dugo, P.; et al. Determination of the phenol and tocopherol content in italian high-quality extra-virgin olive oils by using LC-MS and multivariate data analysis. Food Anal. Methods 2020, 13, 1027–1041. [Google Scholar] [CrossRef]
- Lyashenko, S.; Chileh-Chelh, T.; Rincón-Cervera, M.Á.; Lyashenko, S.P.; Ishenko, Z.; Denisenko, O.; Karpenko, V.; Torres-García, I.; Guil-Guerrero, J.L. Screening of lesser-known salted–dried fish species for fatty acids, tocols, and squalene. Foods 2023, 12, 1083. [Google Scholar] [CrossRef]
- Vincent, C.; Mesa, T.; Munne-Bosch, S. Identification of a new variety of avocados (Persea americana Mill. CV. Bacon) with high vitamin e and impact of cold storage on tocochromanols composition. Antioxidants 2020, 9, 403. [Google Scholar] [CrossRef]
- Çelik, F.; Balta, M.F.; Ercişli, S.; Gündoğdu, M.; Karakaya, O.; Yaviç, A. Tocopherol contents of almond genetic resources from eastern and western turkey. Erwerbs-Obstbau 2019, 61, 257–262. [Google Scholar] [CrossRef]
- Ayoub, M.; de Camargo, A.C.; Shahidi, F. Antioxidants and bioactivities of free, esterified and insoluble-bound phenolics from berry seed meals. Food Chem. 2016, 197, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Knecht, K.; Sandfuchs, K.; Kulling, S.E.; Bunzel, D. Tocopherol and tocotrienol analysis in raw and cooked vegetables: A validated method with emphasis on sample preparation. Food Chem. 2015, 169, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.A.; Galleguillos-Fernández, R.; González-Barriga, V.; Valenzuela, R.; Speisky, H.; Fuentes, J.; Valenzuela, A. Fatty acid profile and bioactive compound extraction in purple viper’s bugloss seed oil extracted with green solvents. J. Am. Oil Chem. Soc. 2020, 97, 319–327. [Google Scholar] [CrossRef]
- Mitsikaris, P.D.; Kokokiris, L.; Pritsa, A.; Papadopoulos, A.N.; Kalogiouri, N.P. Investigating the tocopherol contents of walnut seed oils produced in different european countries analyzed by HPLC-UV: A comparative study on the basis of geographical origin. Foods 2022, 11, 3719. [Google Scholar] [CrossRef]
- Urvaka, E.; Mišina, I.; Soliven, A.; Górnaś, P. Rapid Separation of all four tocopherol homologues in selected fruit seeds via supercritical fluid chromatography using a solid-core C18 column. J. Chem. 2019, 2019, 5307340. [Google Scholar] [CrossRef]
- Andrés, V.; Villanueva, M.J.; Tenorio, M.D. Simultaneous determination of tocopherols, retinol, ester derivatives and β-carotene in milk- and soy-juice based beverages by HPLC with diode-array detection. LWT 2014, 58, 557–562. [Google Scholar] [CrossRef]
- Yuan, C.; Xie, Y.; Jin, R.; Ren, L.; Zhou, L.; Zhu, M.; Ju, Y. Simultaneous analysis of tocopherols, phytosterols, and squalene in vegetable oils by high-performance liquid chromatography. Food Anal. Methods 2017, 10, 3716–3722. [Google Scholar] [CrossRef]
- Cruz, R.; Casal, S. Direct analysis of vitamin A, vitamin E, carotenoids, chlorophylls and free sterols in animal and vegetable fats in a single normal-phase liquid chromatographic run. J. Chromatogr. A 2018, 1565, 81–88. [Google Scholar] [CrossRef]
- Pokkanta, P.; Sookwong, P.; Tanang, M.; Setchaiyan, S.; Boontakham, P.; Mahatheeranont, S. Simultaneous Determination of tocols, γ-oryzanols, phytosterols, squalene, cholecalciferol and phylloquinone in rice bran and vegetable oil samples. Food Chem. 2019, 271, 630–638. [Google Scholar] [CrossRef]
- Cortés-Herrera, C.; Artavia, G.; Leiva, A.; Granados-Chinchilla, F. Liquid chromatography analysis of common nutritional components, in feed and food. Foods 2018, 8, 1. [Google Scholar] [CrossRef] [PubMed]
- FDA; Center for Drug Evaluation and Research. Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. 2018. Available online: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed on 1 July 2024).
- U.S. Department of Agriculture Agricultural Research Service. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 July 2024).
- Awatif, I.I.; Shaker, M.A. Quality characteristics of high-oleic sunflower oil extracted from some hybrids cultivated under egyptian conditions. Helia 2014, 37, 113–126. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R. Frying stability of high oleic sunflower oils as affected by composition of tocopherol isomers and linoleic acid content. Food Chem. 2013, 141, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Nolasco, S.M.; Aguirrezábal, L.A.N.; Lúquez, J.; Mateo, C. Variability in oil tocopherol concentration and composition of traditional and high oleic sunflower hybrids (Helianthus annuus L.) in the pampean region (Argentina). Grasas Aceites 2006, 57, 260–269. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Palaiogiannis, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Evaluation of the efficacy and synergistic effect of α- and δ-tocopherol as natural antioxidants in the stabilization of sunflower oil and olive pomace oil during storage conditions. Int. J. Mol. Sci. 2023, 24, 1113. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Shang, X.; Keum, Y.-S. Advances in lipid extraction methods—A review. Int. J. Mol. Sci. 2021, 22, 13643. [Google Scholar] [CrossRef]
- Campidelli, M.L.L.; Carneiro, J.D.S.; Souza, E.C.; Magalhães, M.L.; Nunes, E.E.C.; Faria, P.B.; Franco, M.; Vilas Boas, E.V.B. Effects of the drying process on the fatty acid content, phenolic profile, tocopherols and antioxidant activity of baru almonds (Dipteryx alata Vog.). Grasas Aceites 2020, 71, 343. [Google Scholar] [CrossRef]
- Ribarova, F.; Zanev, R.; Shishkov, S.; Rizov, N. α-Tocopherol, fatty acids and their correlations in Bulgarian foodstuffs. J. Food Comp. Anal. 2003, 16, 659–667. [Google Scholar] [CrossRef]
- Gouta, H.; Laaribi, I.; Ksia, E.; Juan, T.; Estopañan, G.; Martínez-Gómez, P. Physical properties, biochemical and antioxidant contents of new promising tunisian almond genotypes: Traits stability, quality aspects and post-harvest attributes. J. Food Comp. Anal. 2021, 98, 103840. [Google Scholar] [CrossRef]
- Özcan, M.M.; Motuk, F. Physico-chemical properties, tocopherol contents, fatty acid composition and phenolic compounds of olive oil as affected by papain and cellulase application. J. Food Meas. Charact. 2023, 17, 1021–1032. [Google Scholar] [CrossRef]
- Houmy, N.; Melhaoui, R.; Mansouri, F.; Moumen, A.B.; Fauconnier, M.L.; Sindic, M.; Serghini-Caid, H.; Elamram, A. Assessment of fatty acids profile, oil yield and tocopherol content of four almond cultivars grown in eastern Morocco. Mor. J. Chem. 2021, 9, 697–700. [Google Scholar] [CrossRef]
- Fratianni, A.; Vitone, C.; D’Agostino, A.; Trivisonno, M.C.; Falasca, L.; Panfili, G. Development of functional pasta enriched with green leafy vegetables: Impact on liposoluble compounds, nutritional, technological and sensorial quality. Int. J. Food Sci. Technol. 2024, 59, 1121–1128. [Google Scholar] [CrossRef]
- Lu, Q.-Y.; Zhang, Y.; Wang, Y.; Wang, D.; Lee, R.; Gao, K.; Byrns, R.; Heber, D. California Hass avocado: Profiling of carotenoids, tocopherol, fatty acid, and fat content during maturation and from different growing areas. J. Agric. Food Chem. 2009, 57, 10408–10413. [Google Scholar] [CrossRef] [PubMed]
- Yerlikaya, P.; Alp, A.C.; Tokay, F.G.; Aygun, T.; Kaya, A.; Topuz, O.K.; Yatmaz, H.A. Determination of fatty acids and vitamins A, D and E intake through fish consumption. Int. J. Food Sci. Technol. 2022, 57, 653–661. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.-S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards substitution of hexane as extraction solvent of food products and ingredients with no regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef]
- Aminullah Bhuiyan, A.K.M.; Ratnayake, W.M.N.; Ackman, R.G. Nutritional composition of raw and smoked atlantic mackerel (Scomber Scombrus): Oil- and water-soluble vitamins. J. Food Comp. Anal. 1993, 6, 172–184. [Google Scholar] [CrossRef]
- Devadason, C.; Jayasinghe, C.V.L.; Sivaganehsan, R.; Gotoh, N. Fatty acid composition and tocopherol content of processed marine fish and contribution of omega-3 fatty acids. Indian J. Fish. 2019, 66, 118–124. [Google Scholar] [CrossRef]
- Azrina, A.; Abd Aziz, N.; Khoo, H.E.; Amin, I.; Al-Sheraji, S.H.; Rizal, M. Cholesterol and alpha-tocopherol contents of fish and other seafood from the straits of Malacca. Int. Food Res. J. 2015, 22, 1494–1500. [Google Scholar]
- Kodad, O.; Socias i Company, R.; Alonso, J.M. Genotypic and environmental effects on tocopherol content in almond. Antioxidants 2018, 7, 6. [Google Scholar] [CrossRef]
- Zhu, Y.; Wilkinson, K.L.; Wirthensohn, M. Changes in fatty acid and tocopherol content during almond (Prunus dulcis. cv. Nonpareil) kernel development. Sci. Hortic. 2017, 225, 150–155. [Google Scholar] [CrossRef]
- Zeb, A. A simple, sensitive HPLC-DAD method for simultaneous determination of carotenoids, chlorophylls and α-tocopherol in leafy vegetables. J. Food Meas. Charact. 2017, 11, 979–986. [Google Scholar] [CrossRef]
- Chun, J.; Lee, J.; Ye, L.; Exler, J.; Eitenmiller, R.R. Tocopherol and tocotrienol contents of raw and processed fruits and vegetables in the united states diet. J. Food Comp. Anal. 2006, 19, 196–204. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Tocopherol content and isomers’ composition in selected plant species. Plant Physiol. Biochem. 2008, 46, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Nemzer, B.; Al-Taher, F.; Abshiru, N. Extraction and natural bioactive molecules characterization in spinach, kale and purslane: A comparative study. Molecules 2021, 26, 2515. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Rodrígues, H.; Santos, D.; Campos, D.A.; Guerreiro, S.; Ratinho, M.; Rodrigues, I.M.; Pintado, M.E. Impact of processing approach and storage time on bioactive and biological properties of rocket, spinach and watercress byproducts. Foods 2021, 10, 2301. [Google Scholar] [CrossRef]
- Peraza-Magallanes, A.Y.; Pereyra-Camacho, M.A.; Sandoval-Castro, E.; Medina-Godoy, S.; Valdez-Morales, M.; Clegg, M.T.; Calderón-Vázquez, C.L. Exploring genetic variation, oil and α-tocopherol content in avocado (Persea americana) from Northwestern Mexico. Genet. Resour. Crop Evol. 2017, 64, 443–449. [Google Scholar] [CrossRef]
- Ford, N.A.; Spagnuolo, P.; Kraft, J.; Bauer, E. Nutritional composition of Hass avocado pulp. Foods 2023, 12, 2516. [Google Scholar] [CrossRef]


| Compound | Regression Equation | Linearity Coefficient (R2) | LOD (ppm) | LOQ (ppm) | Intra-Day Precision (RSD%) | Inter-Day Precision (RSD%) |
|---|---|---|---|---|---|---|
| α-tocopherol | y = 7.549x − 61.183 | 0.9988 | 0.38 | 1.28 | 1.79 (at 10 ppm) 1.50 (at 100 ppm) 1.87 (at 250 ppm) | 3.06 (at 10 ppm) 1.81 (at 100 ppm) 1.54 (at 250 ppm) |
| β-tocopherol | y = 4.206x − 34.324 | 0.9985 | 0.32 | 1.08 | 2.84 (at 10 ppm) 2.59 (at 100 ppm) 1.66 (at 250 ppm) | 4.43 (at 10 ppm) 2.51 (at 100 ppm) 4.32 (at 250 ppm) |
| γ-tocopherol | y = 8.144x − 75.855 | 0.9984 | 0.62 | 2.06 | 2.56 (at 10 ppm) 2.82 (at 100 ppm) 1.44 (at 250 ppm) | 7.80 (at 10 ppm) 7.77 (at 100 ppm) 5.05 (at 250 ppm) |
| δ-tocopherol | y = 6.361x − 56.739 | 0.9988 | 0.63 | 2.11 | 3.63 (at 10 ppm) 1.39 (at 100 ppm) 2.15 (at 250 ppm) | 7.51(at 10 ppm) 7.40 (at 100 ppm) 6.05 (at 250 ppm) |
| Food Matrix | Extraction Method | α-T | β-T | γ-T | δ-T |
|---|---|---|---|---|---|
| High Oleic Sunflower Oil | - | 163.5 ± 2.3 | n.d. | n.d. | n.d. |
| Mackerel Fillet | Folch n-hexane | 0.79 ± 0.05 a 1.33 ± 0.05 b | n.d. n.d. | n.d. 0.61 ± 0.02 | n.d. n.d. |
| Almond | Folch n-hexane | 10.1 ± 0.5 9.50 ± 0.15 | n.d. n.d. | 1.44 ± 0.04 a 1.19 ± 0.05 b | n.d. n.d. |
| Spinach | Folch n-hexane | 0.16 ± 0.01 0.17 ± 0.01 | n.d. n.d. | n.d. 0.09 ± 0.01 | n.d. n.d. |
| Avocado | Folch n-hexane | 1.67 ± 0.02 a 1.47 ± 0.01 b | n.d. n.d. | 0.46 ± 0.01 a 0.04 ± 0.01 b | n.d. n.d. |
| Food Matrix | Extraction Method | Fortification Level (mg/100 g fw) | Recovery (%) | |||
|---|---|---|---|---|---|---|
| α-T | β-T | γ-T | δ-T | |||
| High Oleic Sunflower Oil | - | 160 | 100.3 ± 1.2 | 108.2 ± 2.6 | 105.5 ± 2.2 | 105.6 ± 2.4 |
| - | 320 | 100.3 ± 1.4 | 106.2 ± 2.5 | 105.8 ± 2.3 | 106.3 ± 1.7 | |
| Mackerel fillet | Folch | 0.80 | 87.8 ± 2.9 | 88.6 ± 4.1 | 104.4 ± 4.3 | 98.0 ± 3.9 |
| 1.60 | 86.8 ± 3.6 | 90.7 ± 4.7 | 86.0 ± 3.5 | 89.1 ± 1.9 | ||
| n-hexane | 1.30 | 93.3 ± 3.2 | 85.9 ± 6.9 | 95.4 ± 3.6 | 82.8 ± 6.5 | |
| 2.60 | 94.2 ± 1.9 | 82.9 ± 3.4 | 90.5 ± 1.8 | 79.5 ± 3.3 | ||
| Almond | Folch | 10.0 | 81.9 ± 2.6 | 77.8 ± 3.4 | 82.9 ± 0.9 | 80.3 ± 4.5 |
| 20.0 | 85.8 ± 0.7 | 83.9 ± 2.2 | 81.3 ± 2.5 | 88.8 ± 1.2 | ||
| n-hexane | 10.0 | 89.1 ± 2.0 | 78.0 ± 2.1 | 84.5 ± 2.4 | 86.2 ± 0.9 | |
| 20.0 | 92.6 ± 1.3 | 87.4 ± 1.6 | 86.2 ± 2.2 | 91.8 ± 0.6 | ||
| Spinach | Folch | 0.16 | 81.8 ± 2.4 | 89.2 ± 2.3 | 86.6 ± 5.7 | 88.4 ± 1.6 |
| 0.32 | 87.0 ± 1.0 | 85.1 ± 1.4 | 87.7 ± 3.4 | 86.2 ± 2.9 | ||
| n-hexane | 0.16 | 85.7 ± 1.9 | 89.2 ± 3.8 | 84.9 ± 2.4 | 80.6 ± 3.3 | |
| 0.32 | 87.0 ± 1.0 | 85.1 ± 1.4 | 87.6 ± 2.6 | 86.2 ± 2.9 | ||
| Avocado | Folch | 1.50 | 82.4 ± 0.7 | 85.4 ± 3.1 | 78.3 ± 1.6 | 82.3 ± 2.5 |
| 3.00 | 81.3 ± 0.5 | 84.1 ± 1.5 | 80.4 ± 0.4 | 88.7 ± 3.4 | ||
| n-hexane | 1.50 | 89.2 ± 2.1 | 89.0 ± 3.2 | 83.3 ± 4.0 | 86.2 ± 2.9 | |
| 3.00 | 89.3 ± 0.5 | 79.0 ± 4.0 | 86.8 ± 1.7 | 90.0 ± 2.9 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Santé, M.F.; López-Puebla, S.; de Camargo, A.C.; Guil-Guerrero, J.L.; Rincón-Cervera, M.Á. Development and Validation of a Simple Analytical Method to Quantify Tocopherol Isoforms in Food Matrices by HPLC–UV–Vis. Appl. Sci. 2024, 14, 8750. https://doi.org/10.3390/app14198750
Arias-Santé MF, López-Puebla S, de Camargo AC, Guil-Guerrero JL, Rincón-Cervera MÁ. Development and Validation of a Simple Analytical Method to Quantify Tocopherol Isoforms in Food Matrices by HPLC–UV–Vis. Applied Sciences. 2024; 14(19):8750. https://doi.org/10.3390/app14198750
Chicago/Turabian StyleArias-Santé, María Fernanda, Sussi López-Puebla, Adriano Costa de Camargo, José Luis Guil-Guerrero, and Miguel Ángel Rincón-Cervera. 2024. "Development and Validation of a Simple Analytical Method to Quantify Tocopherol Isoforms in Food Matrices by HPLC–UV–Vis" Applied Sciences 14, no. 19: 8750. https://doi.org/10.3390/app14198750
APA StyleArias-Santé, M. F., López-Puebla, S., de Camargo, A. C., Guil-Guerrero, J. L., & Rincón-Cervera, M. Á. (2024). Development and Validation of a Simple Analytical Method to Quantify Tocopherol Isoforms in Food Matrices by HPLC–UV–Vis. Applied Sciences, 14(19), 8750. https://doi.org/10.3390/app14198750






