Primary Study on Influence of Conventional Hydrochemical Components on Suspension of Endogenous Fine Loess Particles in Groundwater over Loess Regions
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogeological Background of the Case Study Area
2.2. Preparation and Characterization of the Endogenous Fine Loess Particles from Loess Sample
2.3. Designed Tests
2.4. Characterization and Data Analysis
3. Results and Discussion
3.1. Characterization of the Endogenous Fine Loess Particles
3.2. Effect of a Single Factor on the AS and Suspension of EFLPs
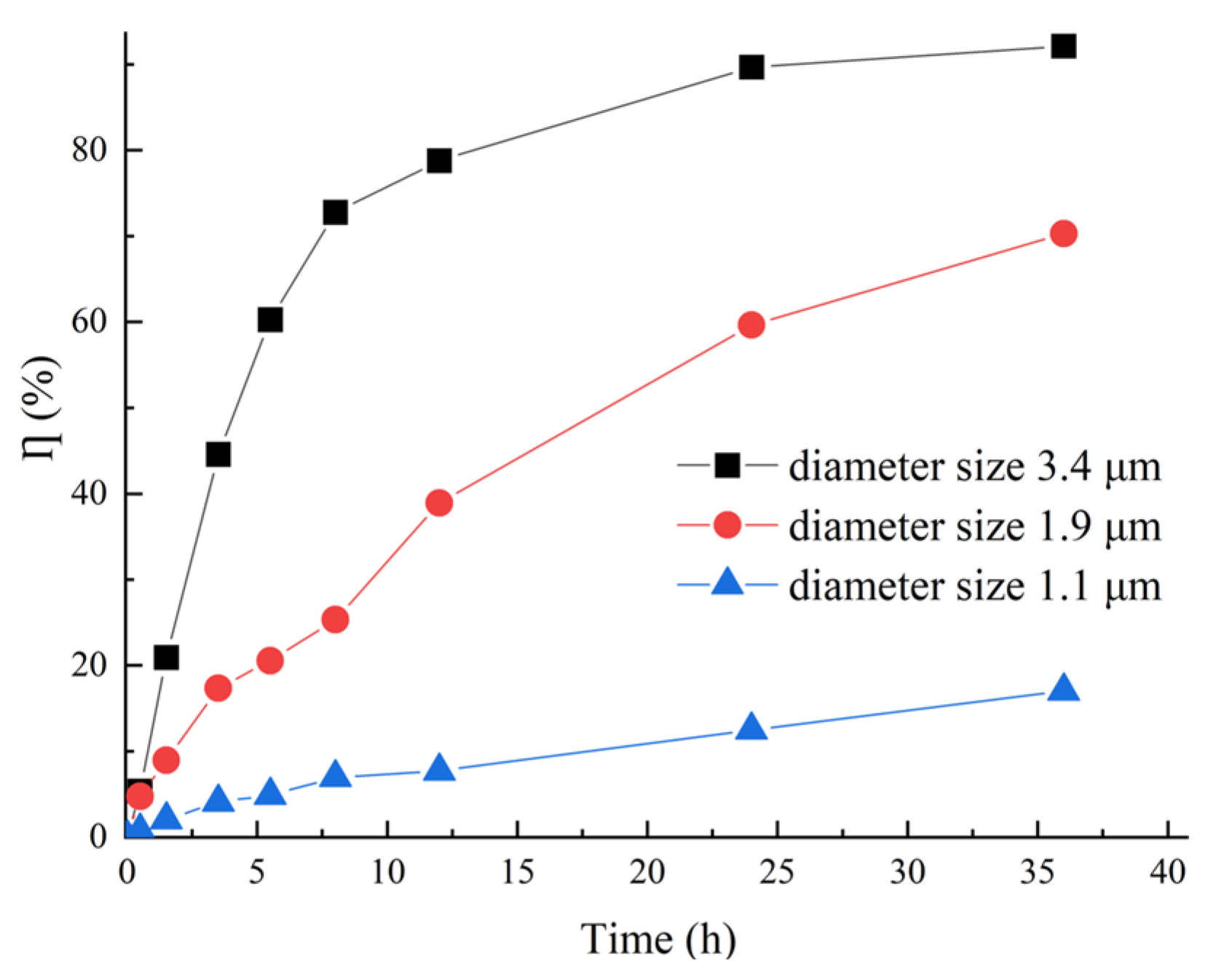
3.2.1. EFLP Diameter Size
3.2.2. Hydrochemical Component Concentration
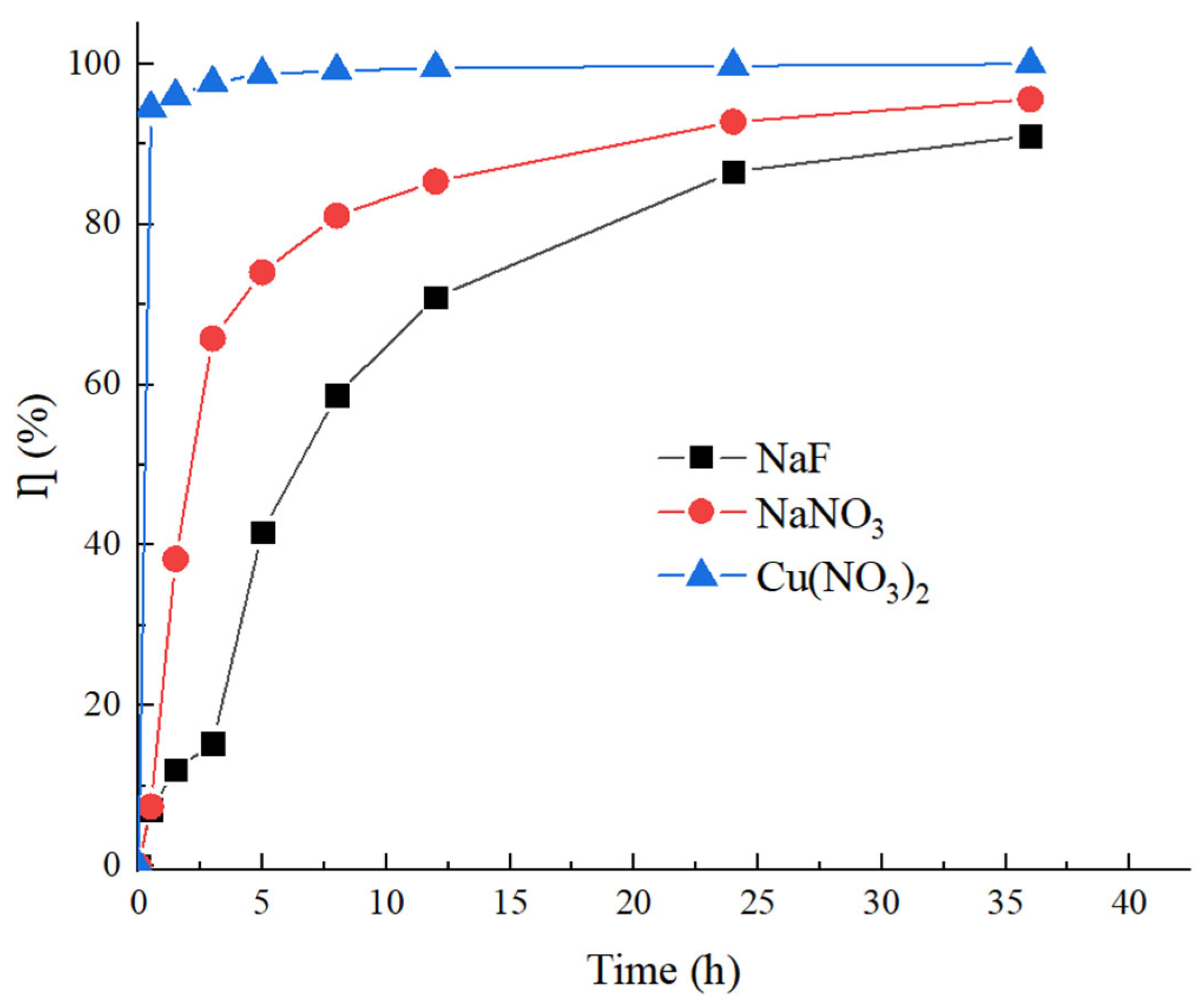
3.2.3. Hydrochemical Component
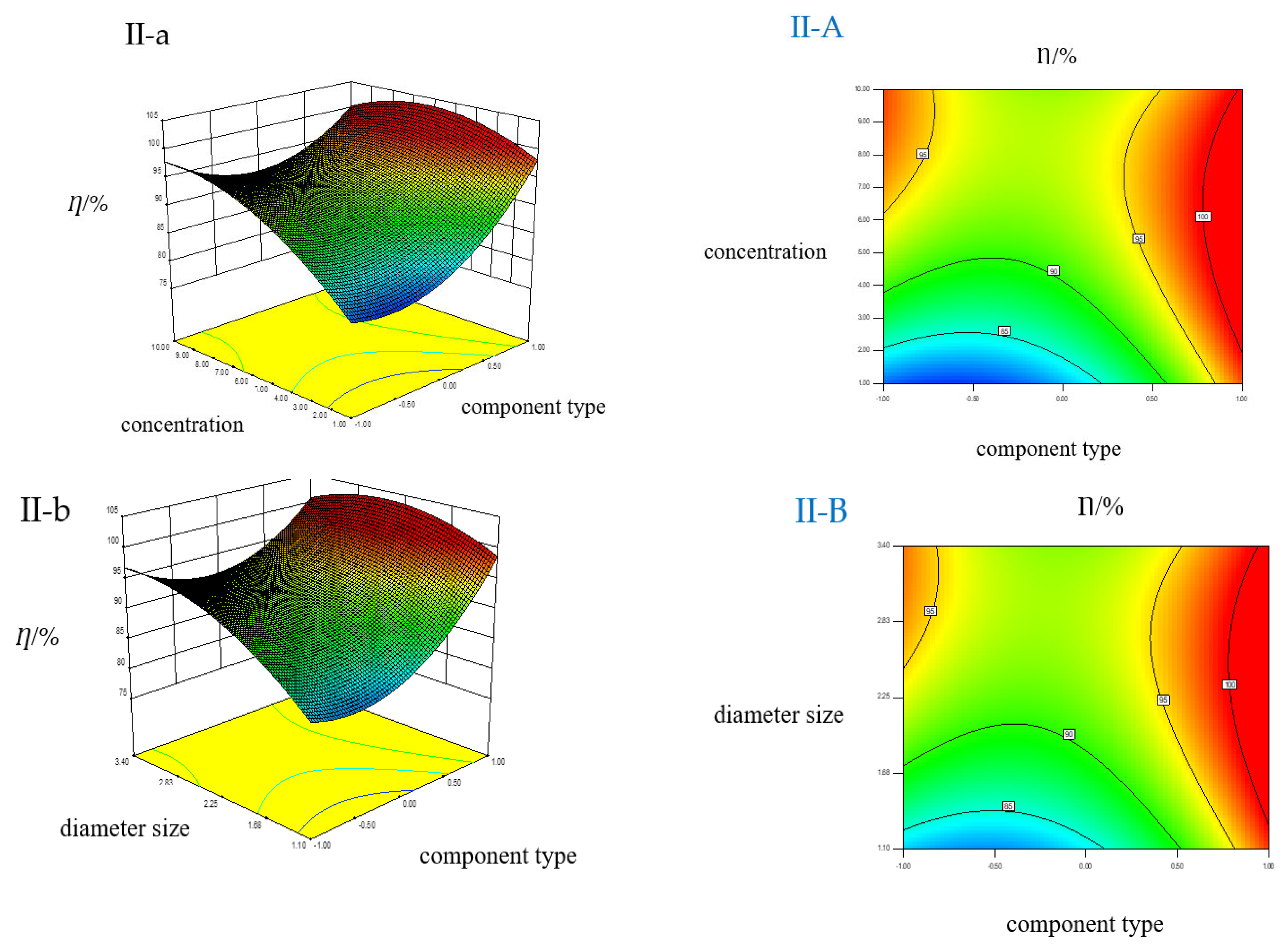
3.3. Multiple Factors’ Interaction in EFLP Suspension
3.3.1. Multiple Factors’ Interaction in EFLP Suspension at 3-h Reaction
3.3.2. Multiple Factors’ Interaction on EFLP Suspension at 36-h Reaction
3.4. CCC Value Determined Based on DLVO Theory
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, F.; Xu, B.L.; He, Y.; Brookes, P.C.; Tang, C.X.; Xu, J.M. Differences in transport behavior of natural soil colloids of contrasting sizes from nanometer to micron and the environmental implications. Sci. Total Environ. 2018, 634, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Cheng, J.H.; Xian, Q.S.; Cui, J.F.; Tang, X.Y.; Wang, G.X. Dynamics and sources of colloids in shallow groundwater in lowland wells and fracture flow in sloping farmland. Water Res. 2019, 156, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.N.; Wang, J.; Xue, W.; Zhao, J.B.; Wang, J.; Liu, X.H. Effect of the size of variable charge soil particles on cadmium accumulation and adsorption. J. Soil. Sediment 2017, 17, 2810–2821. [Google Scholar] [CrossRef]
- Liu, G.S.; Zhong, H.; Ahmad, Z.; Yang, X.; Huo, L. Transport of engineered nanoparticles in porous media and its enhancement for remediation of contaminated groundwater. Crit. Rev. Env. Sci. Technol. 2020, 50, 2301–2378. [Google Scholar] [CrossRef]
- Zhuang, J.; Flury, M.; Jin, Y. Colloid-facilitated Cs transport through water-saturated hanford sediment and ottawa sand. Environ. Sci. Technol. 2003, 37, 4905–4911. [Google Scholar] [CrossRef]
- Degueldre, C.; Benedicto, A. Colloid generation during water flow transients. Appl. Geochem. 2012, 27, 1220–1225. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhong, L.R.; Song, X.M.; Adeel, M.; Yang, Y.S. Natural colloids facilitated transport of steroidal estrogens in saturated porous media: Mechanism and processes. Environ. Pollut. 2022, 315, 120315. [Google Scholar] [CrossRef]
- Saiers, J.E.; Hornberger, G.M. The role of colloidal kaolinite in the transport of cesium through laboratory sand columns. Water Resour. Res. 1996, 32, 33–41. [Google Scholar] [CrossRef]
- Sen, T.K.; Khilar, K.C. Review on subsurface colloids and colloid-associated contaminant transport in saturated porous media. Adv. Colloid. Interface Sci. 2006, 119, 71–96. [Google Scholar]
- Chen, H.; Gao, B.; Yang, L.Y.; Ma, L.Q. Montmorillonite enhanced ciprofloxacin transport in saturated porous media with sorbed ciprofloxacin showing antibiotic activity. J. Contam. Hydrol. 2015, 173, 1–7. [Google Scholar] [CrossRef]
- Dibyanshu, K.; Chhaya, T.; Raychoudhury, T. A Review on the fate and transport behavior of engineered nanoparticles: Possibility of becoming an emerging contaminant in the groundwater. Int. J. Environ. Sci. Technol. 2023, 20, 4649–4672. [Google Scholar] [CrossRef]
- Deb, D.; Chakma, S. Colloid and colloid-facilitated contaminant transport in subsurface ecosystem—A concise review. Int. J. Environ. Sci. Technol. 2022, 20, 6955–6988. [Google Scholar] [CrossRef]
- Missong, A.; Holzmann, S.; Bol, R.; Nischwitz, V.; Puhlmann, H.; von Wilpert, K.; Siemens, J.; Klumpp, E. Leaching of natural colloids from forest topsoils and their relevance for phosphorus mobility. Sci. Total Environ. 2018, 634, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles-formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sun, R.; Hu, S.H.; Zhong, Y.Q.W.; Wu, Y.G. Aromatic compounds releases aroused by sediment resuspension alter nitrate transformation rates and pathways during aerobic-anoxic transition. J. Hazard. Mater. 2022, 424, 127365. [Google Scholar] [CrossRef]
- Zhou, B.; Wu, Y.; Chan, J.; Hu, S.H. Batch adsorption and column transport studies of 2,4,6-trinitrotoluene in Chinese loess. B Environ. Contam. Tox. 2019, 103, 75–81. [Google Scholar] [CrossRef]
- Zhou, B.; Zhang, Z.H.; Wang, S.C.; Wu, Y.; Hu, S.H.; Sun, R. Batch adsorption and column leaching studies of aniline in Chinese loess under different hydrochemical conditions. B Environ. Contam. Tox. 2020, 104, 511–519. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.Y.; Duan, R.; He, X.D. Occurrence, Controlling Factors and Health Risks of Cr6+ in Groundwater in the Guanzhong Basin of China. Expo. Health 2022, 14, 239–251. [Google Scholar] [CrossRef]
- Gao, X.; Wu, P.; Zhao, X.; Shi, Y.; Wang, J.; Zhang, B. Soil moisture variability along transects over a well-developed gully in the Loess Plateau, China. Catena 2011, 87, 357–367. [Google Scholar] [CrossRef]
- Buggle, B.; Hambach, U.; Müller, K.; Zöller, L.; Marković, S.B.; Glaser, B. Iron mineralogical proxies and quaternary climate change in SE-European loess–paleosol sequences. Catena 2014, 117, 4–22. [Google Scholar] [CrossRef]
- Cheng, P.; Burrf, G.S.; Zhou, W.J.; Chen, N.; Hou, Y.Y.; Du, H.; Fu, Y.C.; Lu, X.F. The deficiency of organic matter 14c dating in chinese loess-paleosol sample. Quat. Geochronol. 2020, 56, 101051. [Google Scholar] [CrossRef]
- Xiao, Y.Y.; Wu, S.Y.; Sui, P.S.; Niu, Y.L.; Sun, W.D.; Wang, G.D.; Kong, J.J.; Shao, F.L.; Wang, X.H.; Gong, H.M.; et al. Chemical variations of loess from the Chinese Loess Plateau and its implications. Int. Geol. Rev. 2023, 65, 1372–1387. [Google Scholar] [CrossRef]
- Fredlund, D.G.; Morgenstern, N.R. Stress State Variables for Unsaturated Soils. J. Geotech. Eng. Div. 1977, 103, 447–466. [Google Scholar] [CrossRef]
- Kato, S.; Kawai, K. Deformation characteristics of a compacted clay in collapse under isotropic and triaxial stress state. J. Jpn. Geotech. Soc. 2020, 40, 75–90. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, Y.; Li, X.; Pan, W.S. Microscopic structure changes of Malan loess after humidification in South Jingyang Plateau, China. Environ. Earth Sci. 2019, 78, 287. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, H.; Jiang, S.; Vandenberghe, J.; Wang, S.; Cosgrove, R. Provenance of loess deposits in the Eastern Qinling Mountains (central China) and their implications for the paleoenvironment. Quat. Sci. Rev. 2012, 43, 94–102. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, H.; Hou, K.; Zhang, Q.Y.; Zhang, Y.T. Vertical distribution characteristics of soil moisture with different strata in deep profile in Guanzhong Basin, China. Environ. Earth Sci. 2020, 79, 103. [Google Scholar] [CrossRef]
- Luo, K.; Zhang, S.; Tian, Y.; Gao, X. Arsenic distribution pattern in different sources of drinking water and their geological background in Guanzhong Basin, Shaanxi, China. ACTA Geol. Sin-Engl. 2014, 88, 984–994. [Google Scholar] [CrossRef]
- Ren, X.F.; Li, P.Y.; He, X.D.; Su, F.M.; Elumalai, V. Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch. Environ. Contam. Toxicol. 2021, 80, 74–91. [Google Scholar] [CrossRef]
- Qiao, Z.X.; Sun, R.; Wu, Y.G.; Hu, S.H.; Liu, X.Y.; Chan, J.W.; Mi, X.H. Characteristics and metabolic pathway of the bacteria for heterotrophic nitrification and aerobic denitrification in aquatic ecosystems. Environ. Res. 2020, 191, 110069. [Google Scholar] [CrossRef]
- Xiao, W.D.; Meng, G.C.; Meng, C.Z.; Sun, R.; Hu, S.H.; Wu, Y. New insights into microbial community for simultaneous removal of carbon and nitrogen via heterotrophic nitrification aerobic denitrification process. J. Environ. Chem. Eng. 2024, 12, 112896. [Google Scholar] [CrossRef]
- Duan, L.; Wang, W.K.; Sun, Y.Q. Ammonium nitrogen adsorption-desorption characteristics and its hysteresis of typical soils from Guanzhong Basin, China. Asian J. Chem. 2013, 25, 3850–3854. [Google Scholar] [CrossRef]
- Shen, C.Y.; Jin, Y.; Zhuang, J.; Li, T.T.; Xing, B.S. Role and importance of surface heterogeneities in transport of particles in saturated porous media. Crit. Rev. Environ. Sci. Technol. 2020, 50, 244–329. [Google Scholar] [CrossRef]
- Bessho, K.; Degueldre, C. Generation and sedimentation of colloidal bentonite particles in water. Appl. Clay Sci. 2009, 43, 253–259. [Google Scholar] [CrossRef]
- Wang, W.K.; Duan, L.; Yang, X.T.; Tian, H. Shallow groundwater hydro-chemical evolution and simulation with special focus on Guanzhong Basin, China. Environ. Eng. Manag. J. 2013, 12, 1447–1455. [Google Scholar] [CrossRef]
- Wang, M.; Gao, B.; Tang, D. Review of key factors controlling engineered nanoparticle transport in porous media. J. Hazard. Mater. 2016, 318, 233–246. [Google Scholar] [CrossRef]
- Xu, C.Y.; Zhou, T.T.; Wang, C.L.; Liu, H.Y.; Zhang, C.T.; Hu, F.N. Aggregation of polydisperse soil colloidal particles: Dependence of hamaker constant on particle size. Geoderma 2020, 359, 113999. [Google Scholar] [CrossRef]
- Tang, X.W.; Li, Z.Z.; Chen, Y.M.; Wang, Z.Q. Removal of Zn(II) from aqueous solution with natural Chinese loess: Behaviors and affecting factors. Desalination 2009, 249, 49–57. [Google Scholar] [CrossRef]
- Tripathi, V.P.; Srivastava, V.C.; Tripathi, K.A. Optimization of an azo dye batch adsorption parameters using Box-Behnken design. Desalination 2009, 249, 1273–1279. [Google Scholar] [CrossRef]
- Sleiman, M.; Vildozo, D.; Ferronato, C.; Chovelon, J.M. Photocatalytic degradation of azo dye metanil yellow: Optimization and kinetic modeling using a chemometric approach. Appl. Catal. B Environ. 2007, 77, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, X.W.; Chen, Y.M.; Zhan, L.T.; Li, Z.Z.; Tang, Q. Adsorption behavior and mechanism of Cd(II) on loess soil from China. J. Hazard. Mater. 2009, 172, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.H.; Lu, C.; Zhang, C.J.; Zhang, Y.J.; Yao, H.R.; Wu, Y.G. Effects of fresh and degraded dissolved organic matter derived from maize straw on copper sorption onto farmland loess. J. Soil. Sediment 2016, 16, 327–338. [Google Scholar] [CrossRef]
- Daâssi, D.; Frikha, F.; Zouari-Mechichi, H.; Belbahri, L.; Woodward, S.; Mechichi, T. Application of response surface methodology to optimize decolourization of dyes by the laccase-mediator system. J. Environ. Manag. 2012, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.B.; Zhu, Y.J.; Jia, X.X.; Shao, M.A.; Niu, X.Q.; Liu, J.Y. Distributions of arsenic and other heavy metals, and health risk assessments for groundwater in the Guanzhong Plain region of China. Environ. Res. 2020, 181, 108957. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.Z.; Wang, X.J.; Jian, H.M.; Chen, H.T.; Yu, Z.Y. Behavior of suspended particles in the Changjiang Estuary: Size distribution and trace metal contamination. Mar. Pollut. Bull. 2016, 103, 159–167. [Google Scholar] [CrossRef]
- Hsu, J.P.; Yu, H.Y. Critical coagulation concentration of a salt-free colloidal dispersion. J. Phys. Chem. B 2006, 110, 7600–7604. [Google Scholar] [CrossRef]
- Gao, J.; Sugimoto, T.; Kobayashi, M. Effects of ionic valence on aggregation kinetics of colloidal particles with and without a mixing flow. J. Colloid. Interf. Sci. 2023, 638, 733–742. [Google Scholar] [CrossRef]
- Gao, X.D.; Ren, K.L.; Zhu, Z.H.; Zhang, L.; Li, S.; Wang, J.K.; Xu, Y.D. Specific ion effects: The role of anions in the aggregation of permanently charged clay mineral particles. J. Soil. Sedim. 2023, 23, 263–272. [Google Scholar] [CrossRef]
- Gao, B.; Cao, X.D.; Dong, Y.; Luo, Y.M.; Ma, L.Q. Colloid deposition and release in soils and their association with heavy metals. Crit. Rev. Environ. Sci. 2011, 41, 336–372. [Google Scholar]
- Gao, X.D.; Li, S.; Liu, X.M.; Hu, F.N.; Tian, R.; Li, H. The Effects of NO3− and Cl− on negatively charged clay aggregation. Soil Till Res. 2019, 186, 242–248. [Google Scholar] [CrossRef]
- Ding, W.Q.; He, J.H.; Wang, L.; Liu, X.M. Effect of nonclassical polarization of Na+ and K+ on the stability of soil colloidal particles in suspension. Surf. Rev. Lett. 2017, 24, 1850019. [Google Scholar]
- Katz, A.L.; Xu, M.; Steiner, J.C.; Trusiak, A.; Alimova, A.; Gottlieb, P.; Block, K. Influence of cations on aggregation rates in mg-montmorillonite. Clay Clay Miner. 2013, 61, 105154. [Google Scholar] [CrossRef]
- Katana, B.; Takács, D.; Csapó, E.; SzabóJamnik, T.; Szilagyi, I. Ion specific effects on the stability of halloysite nanotube colloids-inorganic salts versus ionic liquids. J. Phys. Chem. B 2020, 124, 9757–9765. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Li, Y.; Ci, H.L.; Miao, L.Z.; You, G.X.; Wu, J.; Xu, Y. Influence of aggregation and sedimentation behavior of bare and modified zero-valent-iron nanoparticles on the Cr(VI) removal under various groundwater chemistry conditions. Chemosphere 2022, 296, 133905. [Google Scholar] [CrossRef] [PubMed]
- Grolimund, D.; Borkovec, M. Releaseof colloidal particles innatural porous media by monovalent and divalent cations. J. Contam. Hydrol. 2006, 8, 155–175. [Google Scholar] [CrossRef]
- Xu, Z.; Niu, Z.W.; Pan, D.Q.; Zhao, X.D.; Wei, X.Y.; Li, X.L.; Tan, Z.Y.; Chen, X.M.; Liu, C.L.; Wu, W.S. Mechanisms of bentonite colloid aggregation, retention, and release in saturated porous media: Role of counter ions and humic acid. Sci. Total Environ. 2021, 793, 148545. [Google Scholar] [CrossRef]
- Huang, X.X.; Yang, G. Charge reversal and anion effects during adsorption of metal ions at clay surfaces: Mechanistic aspects and influence factors. Chem. Phys. 2020, 529, 110575. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, H.; Li, W.; He, Y.; Brookes, P.C.; Xu, J. Aggregation kinetics of natural soil nanoparticles in different electrolytes. Eur. J. Soil Sci. 2014, 65, 206–217. [Google Scholar] [CrossRef]
- Park, J.A.; Kim, S.B. DLVO and XDLVO calculations for bacteriophage ms2 adhesion to iron oxide particles. J. Contam. Hydrol. 2015, 181, 131–140. [Google Scholar] [CrossRef]
- Al-Musawi, T.J.; Nezamaddin, M.; Waleed, M.S.K.; Mika, S.; Shaziya, H.S.; Saeideh, S.; Davoud, B. Sonophotocatalytic degradation and operational parameters optimization of diazinon using magnetic cobalt–graphene nanocomposite as a catalyst. J. Water Process Eng. 2022, 46, 102548. [Google Scholar] [CrossRef]
- Mohammadi, F.; Samaei, M.R.; Azhdarpoor, A.; Teiri, H.; Badeenezhad, A.; Rostami, S. Modelling and optimizing pyrene removal from the soil by phytoremediation using response surface methodology, artificial neural networks, and genetic algorithm. Chemosphere 2019, 237, 124486. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.J.W. Theory of the stability of lyophobic colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.P.; Liu, B.T. Stability of colloidal dispersions: Charge regulation/adsorption model. Langmuir 1998, 15, 5219–5226. [Google Scholar] [CrossRef]
- Wagner, C.; Green, F.B.M.; Maiworm, M.; Leinen, P.; Esat, T.; Ferri, N.; Friedrich, N.; Findeisen, R.; Tkatchenko, A.; Temirov, R. Quantitative imaging of electric surface potentials with single-atom sensitivity. Nat. Mater. 2019, 18, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.; Chen, K.L.; Zhou, R. Mechanism of divalent-ion-induced charge inversion of bacterial membranes. J. Phys. Chem. Lett. 2016, 7, 2434–2438. [Google Scholar] [CrossRef]
- Trefalt, G. Derivation of the inverse schulze-hardy rule. Phys. Rev. E 2016, 93, 1–5. [Google Scholar] [CrossRef]
- Trefalt, G.; Szilagyi, I.; Téllez, G.; Borkovec, M. Colloidal stability in asymmetric electrolytes: Modifications of the schulze-hardy rule. Langmuir 2017, 33, 1695–1704. [Google Scholar] [CrossRef]
- Tomba’cz, E.; Szekeres, M. Colloidal behavior of aqueous montmorillonite suspensions: The specific role of pH in the presence of indifferent electrolytes. Appl. Clay Sci. 2004, 27, 75–94. [Google Scholar] [CrossRef]
- Smith, G.N.; Finlayson, S.D.; Rogers, S.E.; Bartlett, P.; Eastoe, J. Electrolyte- induced instability of colloidal dispersions in nonpolar solvents. J. Phys. Chem. Lett. 2017, 8, 4668–4672. [Google Scholar] [CrossRef]




| −1 | 0 | 1 | ||
|---|---|---|---|---|
| Factor Levels | ||||
| particle size (A, µm) | 1.1 | 1.9 | 3.4 | |
| hydrochemical component (B) | NaF | NaNO3 | Cu(NO3)2 | |
| component concentration (C, mmol/L) | 1 | 5 | 10 | |
| Run | B | C | A | Ƞ (at 3 h) | Ƞ (at 36 h) |
|---|---|---|---|---|---|
| 1 | NaNO3 | 10 | 1.9 | 54.77 | 94.34 |
| 2 | NaNO3 | 5 | 3.4 | 65.71 | 94.82 |
| 3 | NaNO3 | 5 | 1.1 | 28.39 | 84.02 |
| 4 | NaF | 1 | 3.4 | 15.27 | 80.13 |
| 5 | Cu(NO3)2 | 10 | 1.9 | 94.27 | 99.18 |
| 6 | Cu(NO3)2 | 5 | 3.4 | 97.63 | 99.14 |
| 7 | NaNO3 | 1 | 1.9 | 16.09 | 79.15 |
| 8 | Cu(NO3)2 | 1 | 1.9 | 83.97 | 98.69 |
| 9 | NaF | 10 | 3.4 | 87.65 | 97.16 |
| 10 | NaF | 10 | 1.1 | 12.44 | 78.98 |
| 11 | Cu(NO3)2 | 5 | 1.1 | 90.54 | 99.37 |
| 12 | NaF | 5 | 1.9 | 9.04 | 85.96 |
| 13 | NaF | 5 | 1.9 | 9.52 | 89.03 |
| 14 | NaNO3 | 5 | 1.9 | 14.25 | 90.98 |
| 15 | NaNO3 | 5 | 1.9 | 17.26 | 89.25 |
| 16 | NaNO3 | 5 | 1.9 | 17.96 | 94.02 |
| 17 | NaF | 1 | 1.1 | 5.61 | 78.92 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F-Value | p-Value | Significant/Non-Significant |
|---|---|---|---|---|---|---|
| Model | 20,397.73 | 9 | 2266.41 | 70.37 | <0.0001 | significant |
| A | 4240.13 | 1 | 4240.13 | 131.65 | <0.0001 | significant |
| B | 2662.29 | 1 | 2662.29 | 82.66 | <0.0001 | significant |
| C | 2329.83 | 1 | 2329.83 | 72.34 | <0.0001 | significant |
| AB | 196.98 | 1 | 196.98 | 6.12 | 0.0426 | significant |
| AC | 255.84 | 1 | 255.84 | 7.94 | 0.0258 | significant |
| BC | 1245.07 | 1 | 1245.07 | 38.66 | 0.0004 | significant |
| A2 | 8444.89 | 1 | 8444.89 | 262.21 | <0.0001 | significant |
| B2 | 23.98 | 1 | 24.04 | 0.75 | 0.4167 | non-significant |
| C2 | 228.67 | 1 | 228.67 | 7.13 | 0.0323 | significant |
| Residual | 225.46 | 7 | 32.21 | |||
| Lack of Fit | 154.31 | 3 | 51.44 | 2.89 | 0.1657 | non-significant |
| Pure Error | 71.15 | 4 | 17.79 | |||
| Cor Total | 20,623.18 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wang, X.; Wang, Z.; Lan, H.; Sun, R.; Hu, S.; Sun, X.; Wu, Y. Primary Study on Influence of Conventional Hydrochemical Components on Suspension of Endogenous Fine Loess Particles in Groundwater over Loess Regions. Appl. Sci. 2024, 14, 8809. https://doi.org/10.3390/app14198809
Zhang Z, Wang X, Wang Z, Lan H, Sun R, Hu S, Sun X, Wu Y. Primary Study on Influence of Conventional Hydrochemical Components on Suspension of Endogenous Fine Loess Particles in Groundwater over Loess Regions. Applied Sciences. 2024; 14(19):8809. https://doi.org/10.3390/app14198809
Chicago/Turabian StyleZhang, Zherui, Xinshuo Wang, Zuoyi Wang, Haiqiang Lan, Ran Sun, Sihai Hu, Xiaofeng Sun, and Yaoguo Wu. 2024. "Primary Study on Influence of Conventional Hydrochemical Components on Suspension of Endogenous Fine Loess Particles in Groundwater over Loess Regions" Applied Sciences 14, no. 19: 8809. https://doi.org/10.3390/app14198809
APA StyleZhang, Z., Wang, X., Wang, Z., Lan, H., Sun, R., Hu, S., Sun, X., & Wu, Y. (2024). Primary Study on Influence of Conventional Hydrochemical Components on Suspension of Endogenous Fine Loess Particles in Groundwater over Loess Regions. Applied Sciences, 14(19), 8809. https://doi.org/10.3390/app14198809








