Physicochemical Properties of Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis)
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Rooibos and Reagents
2.2. Solid–Liquid Extraction of Green Rooibos
2.3. Moisture Content (MC), Water Activity (aw), and Sorption Isotherms
2.4. Colour Evaluation
2.5. Thermogravimetric Analysis (TGA)
2.6. Fourier Transform Infrared (FT-IR)
2.7. Statistical Analysis
3. Results and Discussions
3.1. Moisture Content (MC) and Water Activity (aw) of Green Rooibos Extracts
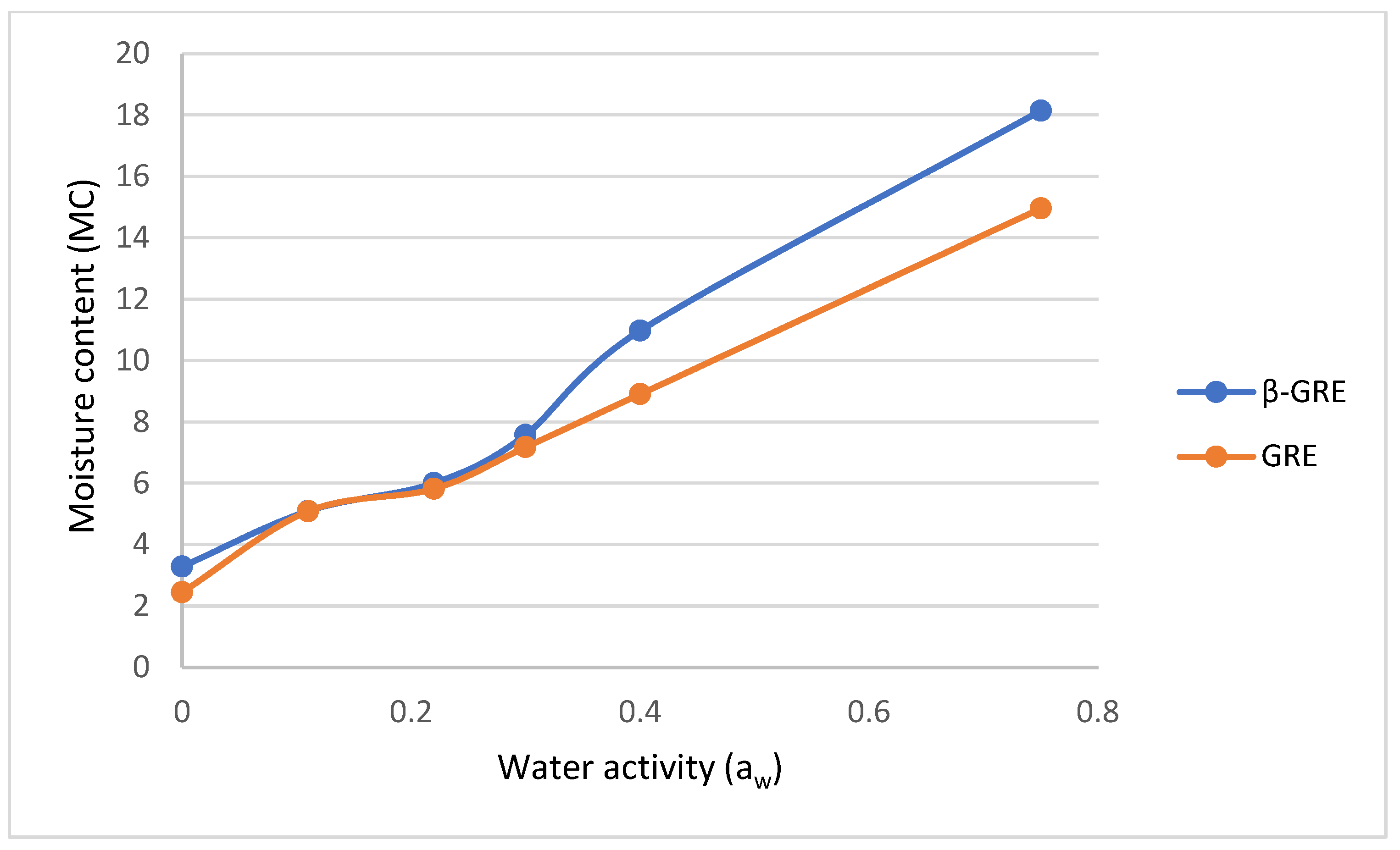
3.2. Moisture Content as a Function of Water Activity via Sorption Isotherms
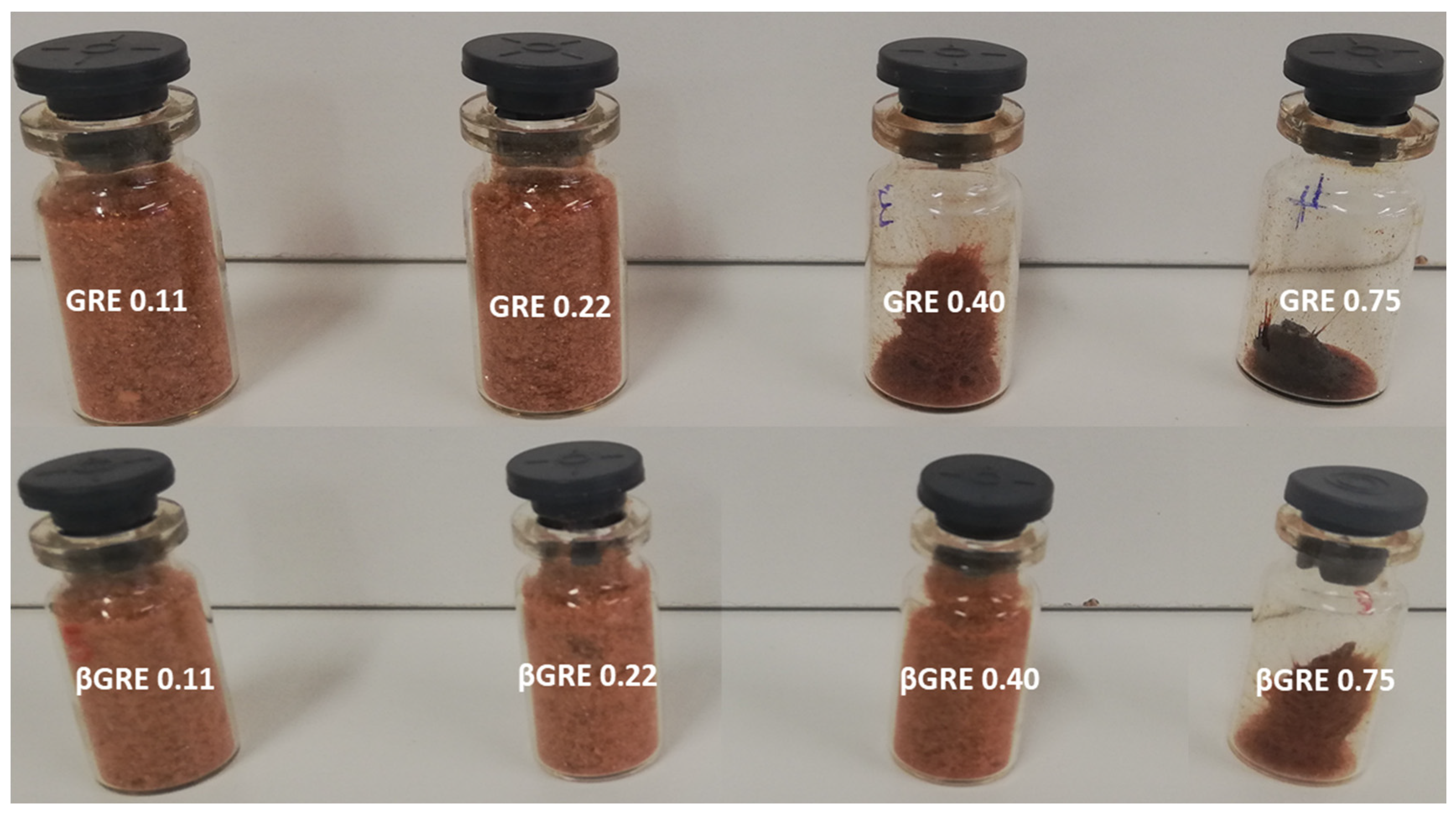
3.3. Colour (L*a*b*) of Green Rooibos Extracts
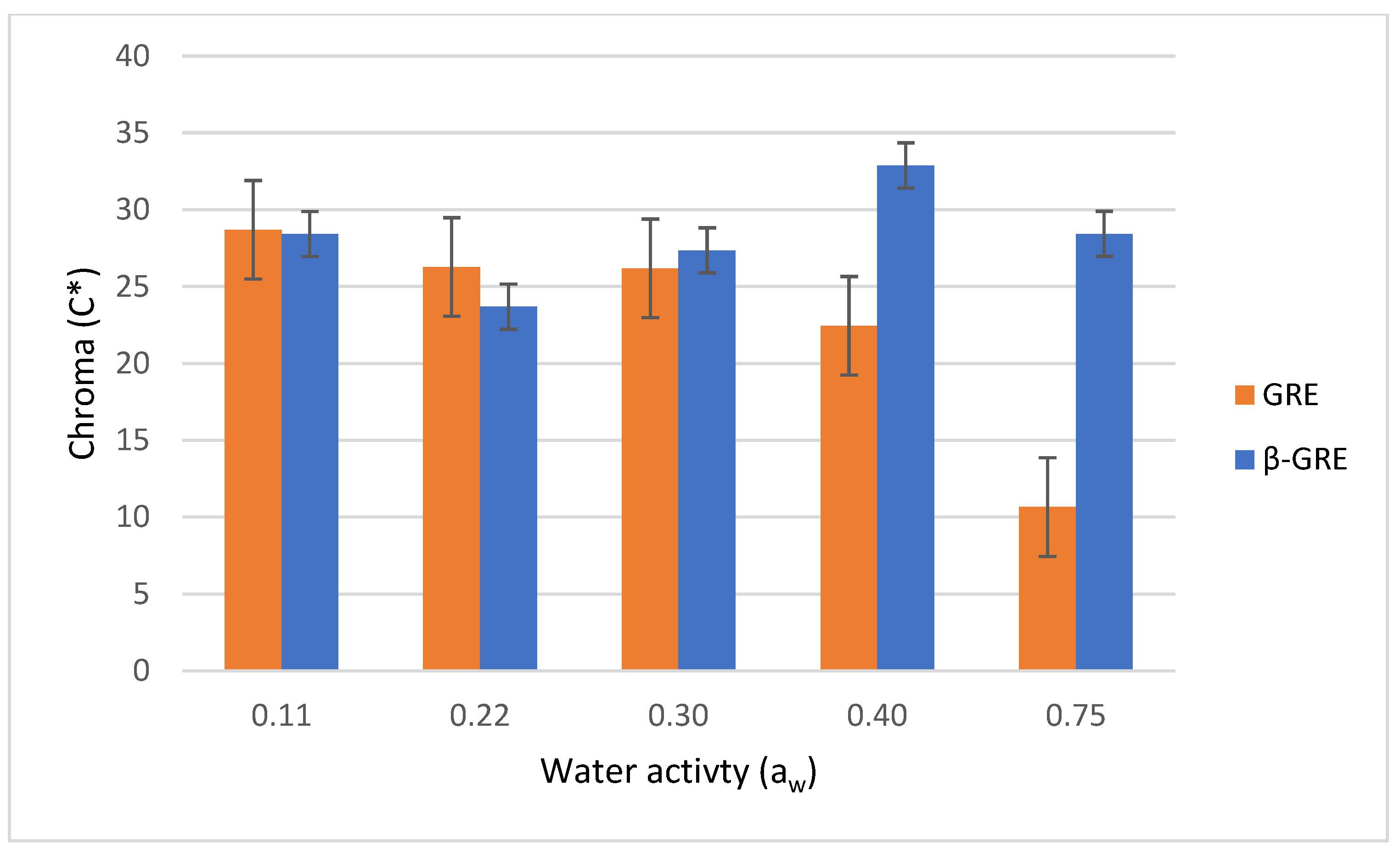
3.4. Colour (L*, a*, b*, and C*) of Green Rooibos Extracts as a Function of Water Activity
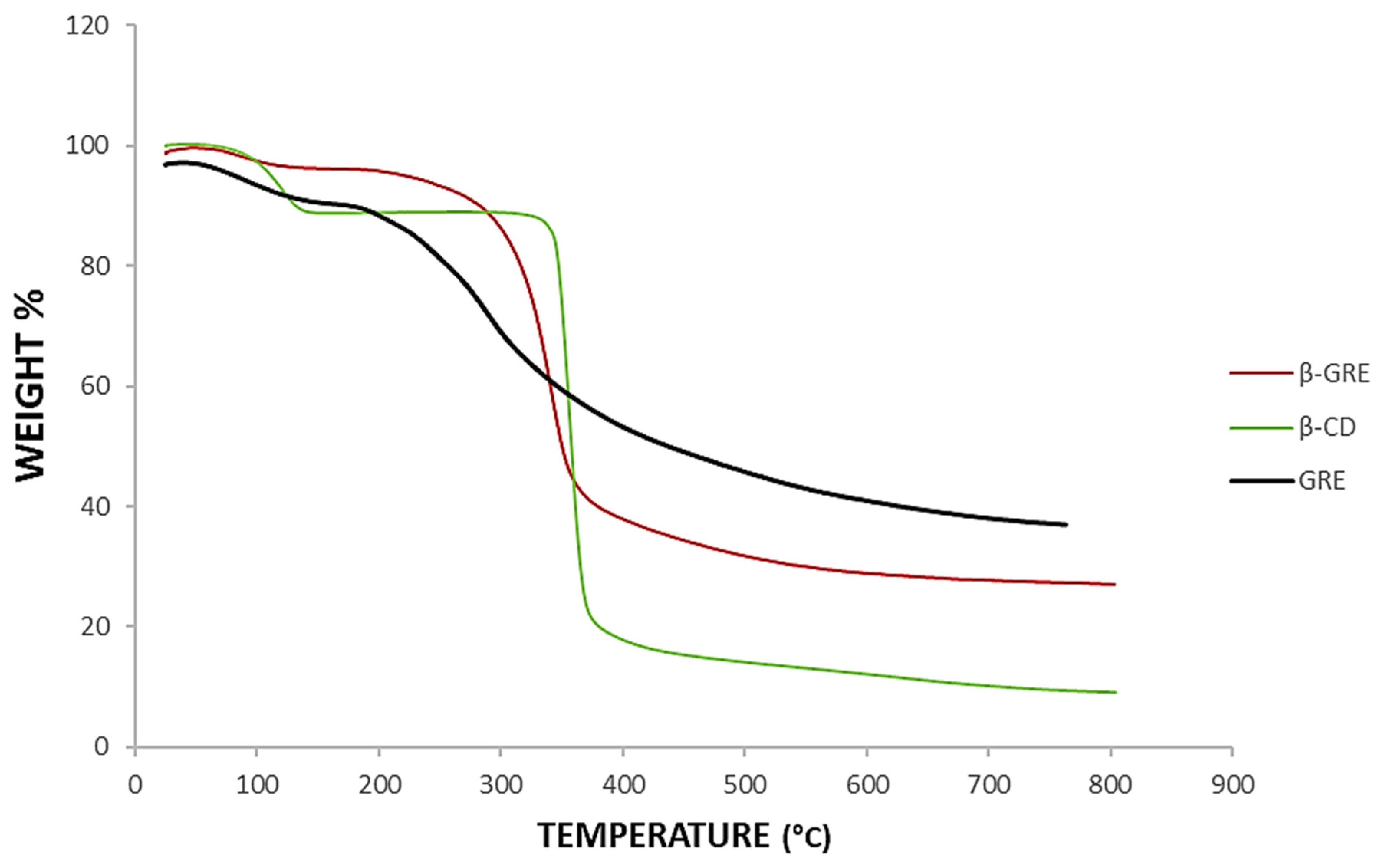
3.5. Thermogravimetric Analysis (TGA)
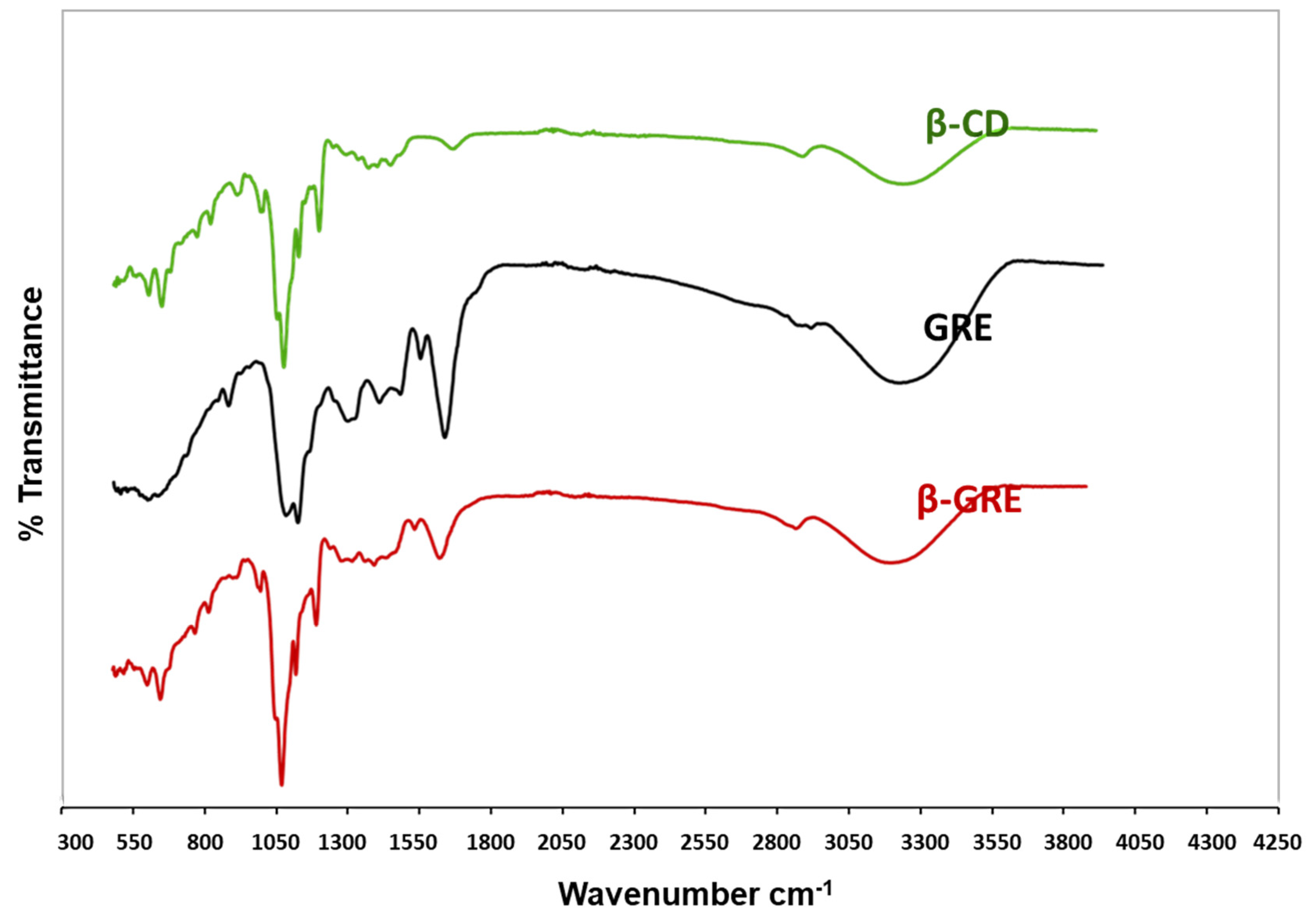
3.6. Fourier Transforms Infrared (FT-IR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oral, R.A.; Dogan, M.; Sarioglu, K. Effects of certain polyphenols and extracts on furans and acrylamide formation in model system, and total furans during storage. Food Chem. 2014, 142, 423–429. [Google Scholar] [CrossRef]
- Vhangani, L.N.; Favre, L.C.; Rolandelli, G.; Van Wyk, J.; del Pilar Buera, M. Optimising the Polyphenolic Content and Antioxidant Activity of Green Rooibos (Aspalathus linearis) Using Beta-Cyclodextrin Assisted Extraction. Molecules 2022, 27, 3556. [Google Scholar] [CrossRef]
- Ku, S.K.; Kwak, S.; Kim, Y.; Bae, J.S. Aspalathin and Nothofagin from Rooibos (Aspalathus linearis) Inhibits High Glucose-Induced Inflammation In Vitro and In Vivo. Inflammation 2014, 38, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Marnewick, J.; Joubert, E.; Joseph, S.; Swanevelder, S.; Swart, P.; Gelderblom, W. Inhibition of tumour promotion in mouse skin by extracts of rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia), unique South African herbal teas. Cancer Lett. 2005, 224, 193–202. [Google Scholar] [CrossRef]
- Beltrán-Debón, R.; Rull, A.; Rodríguez-Sanabria, F.; Iswaldi, I.; Herranz-López, M.; Aragonès, G.; Camps, J.; Alonso-Villaverde, C.; Menéndez, J.; Micol, V.; et al. Continuous administration of polyphenols from aqueous rooibos (Aspalathus linearis) extract ameliorates dietary-induced metabolic disturbances in hyperlipidemic mice. Phytomedicine 2011, 18, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; DeBeer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Wu, J.W.; Hsieh, C.L.; Wang, H.Y.; Chen, H.Y. Inhibitory effects of guava (Psidium guajava L.) leaf extracts and its active compounds on the glycation process of protein. Food Chem. 2009, 113, 78–84. [Google Scholar] [CrossRef]
- Favreau-farhadi, N.; Pecukonis, L.; Barrett, A. The Inhibition of Maillard Browning by Different Concentrations of Rosmarinic Acid and Epigallocatechin-3-Gallate in Model, Bakery, and Fruit Systems. J. Food Sci. 2015, 80, 2140–2146. [Google Scholar] [CrossRef]
- Albahari, P.; Jug, M.; Radić, K.; Jurmanović, S.; Brnčić, M.; Brnčić, S.R.; Čepo, D.V. Characterization of olive pomace extract obtained by cyclodextrin-enhanced pulsed ultrasound assisted extraction. LWT—Food Sci. Technol. 2018, 92, 22–31. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Human, C.; de Beer, D.; Aucamp, M.; Marx, I.J.; Malherbe, C.J.; Viljoen-Bloom, M.; van der Rijst, M.; Joubert, E. Preparation of rooibos extract-chitosan microparticles: Physicochemical characterisation and stability of aspalathin during accelerated storage. LWT—Food Sci. Technol. 2020, 117, 108653. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Čanadanović-Brunet, J.; Ćetković, G.; Šaponjac, V.T. Microencapsulates and extracts from red beetroot pomace modify antioxidant capacity, heat damage and colour of pseudocereals-enriched einkorn water biscuits. Food Chem. 2018, 268, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.M. Room at the Top as well as at the Bottom: Structure of Functional Food Inclusion Compounds. In Cyclodextrin—A Versatile Ingredient; Arora, P., Dhingra, N., Eds.; Intechopen: London, UK, 2018; pp. 119–134. [Google Scholar] [CrossRef][Green Version]
- Haidong, L.; Fang, Y.; Zhihong, T.; Changle, R. Study on preparation of β-cyclodextrin encapsulation tea extract. Int. J. Biol. Macromol. 2011, 49, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Navarro-orcajada, S.; García-carmona, F.; Manuel, L. Trends in Food Science & Technology Applications of cyclodextrins in food science: A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Maraulo, G.E.; dos Santos Ferreira, C.; Mazzobre, M.F. Β-Cyclodextrin Enhanced Ultrasound-Assisted Extraction as a Green Method to Recover Olive Pomace Bioactive Compounds. J. Food Process. Preserv. 2021, 45, e15194. [Google Scholar] [CrossRef]
- Rajha, H.N.; Chacar, S.; Afif, C.; Vorobiev, E.; Louka, N.; Maroun, R.G. β-Cyclodextrin-Assisted Extraction of Polyphenols from Vine Shoot Cultivars. J. Agric. Food Chem. 2015, 63, 3387–3393. [Google Scholar] [CrossRef]
- Miller, N.; DeBeer, D.; Aucamp, M.; Malherbe, C.J.; Joubert, E. Inulin as microencapsulating agent improves physicochemical properties of spray-dried aspalathin-rich green rooibos (Aspalathus linearis) extract with α-glucosidase inhibitory activity. J. Funct. Foods 2018, 48, 400–409. [Google Scholar] [CrossRef]
- Tutunchi, P.; Roufegarinejad, L.; Hamishehkar, H.; Alizadeh, A. Extraction of red beet extract with β-cyclodextrin-enhanced ultrasound assisted extraction: A strategy for enhancing the extraction efficacy of bioactive compounds and their stability in food models. Food Chem. 2019, 297, 124994. [Google Scholar] [CrossRef]
- Maraulo, G.E.; dos Santos Ferreira, C.; Beaufort, C.E.; Ugarte, M.G.; Mazzobre, M.F. Encapsulation of Bergamot Essential Oil Components in β-Cyclodextrin by Ultrasound-Assisted Co-precipitation Method: Optimization, Characterization, and Antibacterial Activity. Food Bioprocess Technol. 2024. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, H.; Wu, G.; Zhang, H.; Wang, L.; Qian, H.; Qi, X. Reduction of 5-hydroxymethylfurfural formation by flavan-3-ols in Maillard reaction models and fried potato chips. J. Sci. Food Agric. 2018, 98, 5294–5301. [Google Scholar] [CrossRef]
- Li, D.; Zhu, M.; Liu, X.; Wang, Y.; Cheng, J. Insight into the effect of microcapsule technology on the processing stability of mulberry polyphenols. LWT—Food Sci. Technol. 2020, 126, 109144. [Google Scholar] [CrossRef]
- Koteswara, C.; Sung, E.; Young, S.; Hwan, C. Inclusion complexation of catechins-rich green tea extract by β-cyclodextrin: Preparation, physicochemical, thermal, and antioxidant properties. LWT—Food Sci. Technol. 2020, 131, 109723. [Google Scholar] [CrossRef]
- Akinfenwa, A.O.; Abdul, N.S.; Docrat, F.T.; Marnewick, J.L.; Luckay, R.C.; Hussein, A.A. Cytotoxic effects of phytomediated silver and gold nanoparticles synthesised from rooibos (Aspalathus linearis), and aspalathin. Plants 2021, 10, 2460. [Google Scholar] [CrossRef] [PubMed]
- Paczkowska, M.; Mizera, M.; Piotrowska, H.; Szymanowska-Powałowska, D.; Lewandowska, K.; Goscianska, J.; Pietrzak, R.; Bednarski, W.; Majka, Z.; Cielecka-Piontek, J. Complex of rutin with β-cyclodextrin as potential delivery system. PLoS ONE 2015, 10, e0120858. [Google Scholar] [CrossRef]
| Sample | aw | MC | L* | a* | b* |
|---|---|---|---|---|---|
| GRE | 0.181 ± 0.00 b | 3.29 ± 0.18 a | 47.95 ± 1.43 a | 16.12 ± 0.51 b | 23.01 ± 0.59 a |
| β-GRE | 0.111 ± 0.01 a | 2.45 ± 0.12 a | 53.70 ± 0.80 b | 15.96 ± 0.51 a | 24.46 ± 1.01 a |
| Sample | Sorption Isotherm | BET | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.11 | 0.22 | 0.30 | 0.4 | 0.75 | A | B | C | m0 | aw | |
| GRE | 5.10 ± 0.20 a | 6.00 ± 0.17 a | 7.57 ± 1.06 b | 10.98± 0.82 b | 18.14 ± 0.62 b | −0.0910 | −0.0222 | 0.0140 | 7.93 ± 0.11 b | 0.289 ± 1.21 b |
| β-GRE | 5.09 ± 0.01 a | 5.82 ± 0.10 a | 7.18 ± 0.42 a | 8.90 ± 0.24 a | 14.96 ± 1.24 a | −0.1168 | −0.0144 | 0.0092 | 6.40 ± 0.52 a | 0.226 ± 0.89 a |
| Sample | RH | L* | C* |
|---|---|---|---|
| GRE | 0.11 | 45.57 ± 0.20 d | 29.75 ± 0.53 f |
| GRE | 0.22 | 32.69 ± 3.02 c | 17.64 ±1.53 b |
| GRE | 0.30 | 44.80 ± 0.35 d | 28.03 ± 0.43 e |
| GRE | 0.40 | 47.79 ± 0.11 e | 23.23 ± 0.25 c |
| GRE | 0.75 | 07.17 ± 0.35 a | 06.91 ± 0.56 a |
| β-GRE | 0.11 | 53.08 ± 0.43 f | 28.03 ± 0.57 e |
| β-GRE | 0.22 | 54.27 ± 0.48 f | 30.22 ± 0.16 f |
| β-GRE | 0.30 | 48.52 ± 0.36 e | 30.55 ± 0.33 f |
| β-GRE | 0.40 | 52.82 ± 0.63 f | 31.80 ± 0.46 g |
| β-GRE | 0.75 | 28.39 ± 0.83 b | 26.37 ± 0.86 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mogashoa, L.; Vhangani, L.N.; Van Wyk, J. Physicochemical Properties of Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Appl. Sci. 2024, 14, 8832. https://doi.org/10.3390/app14198832
Mogashoa L, Vhangani LN, Van Wyk J. Physicochemical Properties of Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Applied Sciences. 2024; 14(19):8832. https://doi.org/10.3390/app14198832
Chicago/Turabian StyleMogashoa, Letlhogonolo, Lusani Norah Vhangani, and Jessy Van Wyk. 2024. "Physicochemical Properties of Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis)" Applied Sciences 14, no. 19: 8832. https://doi.org/10.3390/app14198832
APA StyleMogashoa, L., Vhangani, L. N., & Van Wyk, J. (2024). Physicochemical Properties of Betacyclodextrin-Assisted Extracts of Green Rooibos (Aspalathus linearis). Applied Sciences, 14(19), 8832. https://doi.org/10.3390/app14198832






