PharmiTech: Addressing Polypharmacy Challenges through AI-Driven Solutions
Abstract
1. Introduction
2. Related Work
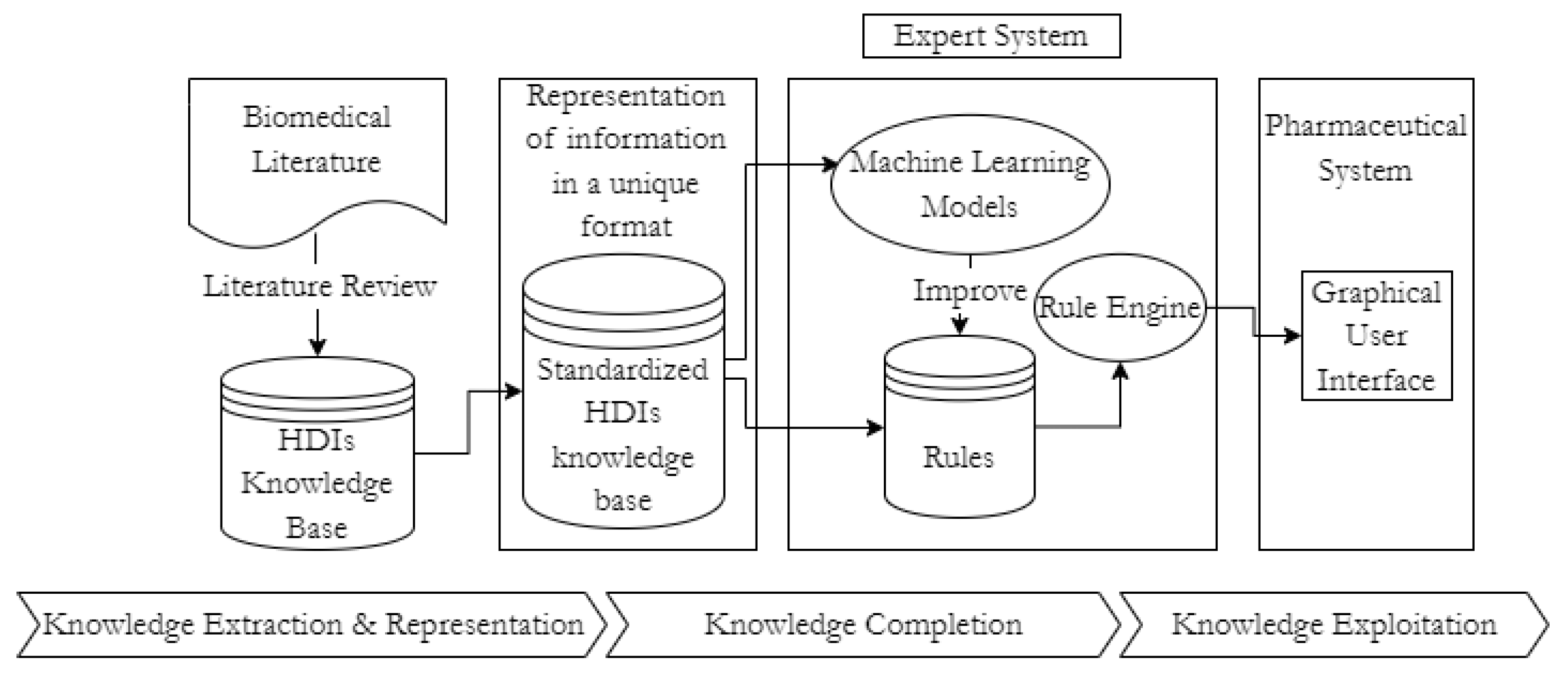
3. Proposed Solution
3.1. Herb-Drug Interaction Module
3.2. Drug Abuse Module
3.2.1. Patient Clustering
3.2.2. Cluster Analysis
- Type 1—Patient with a cardiovascular disease, diabetes, osteoporosis, hypothyroidism and hyperthyroidism, sleep or psychiatric disorder.
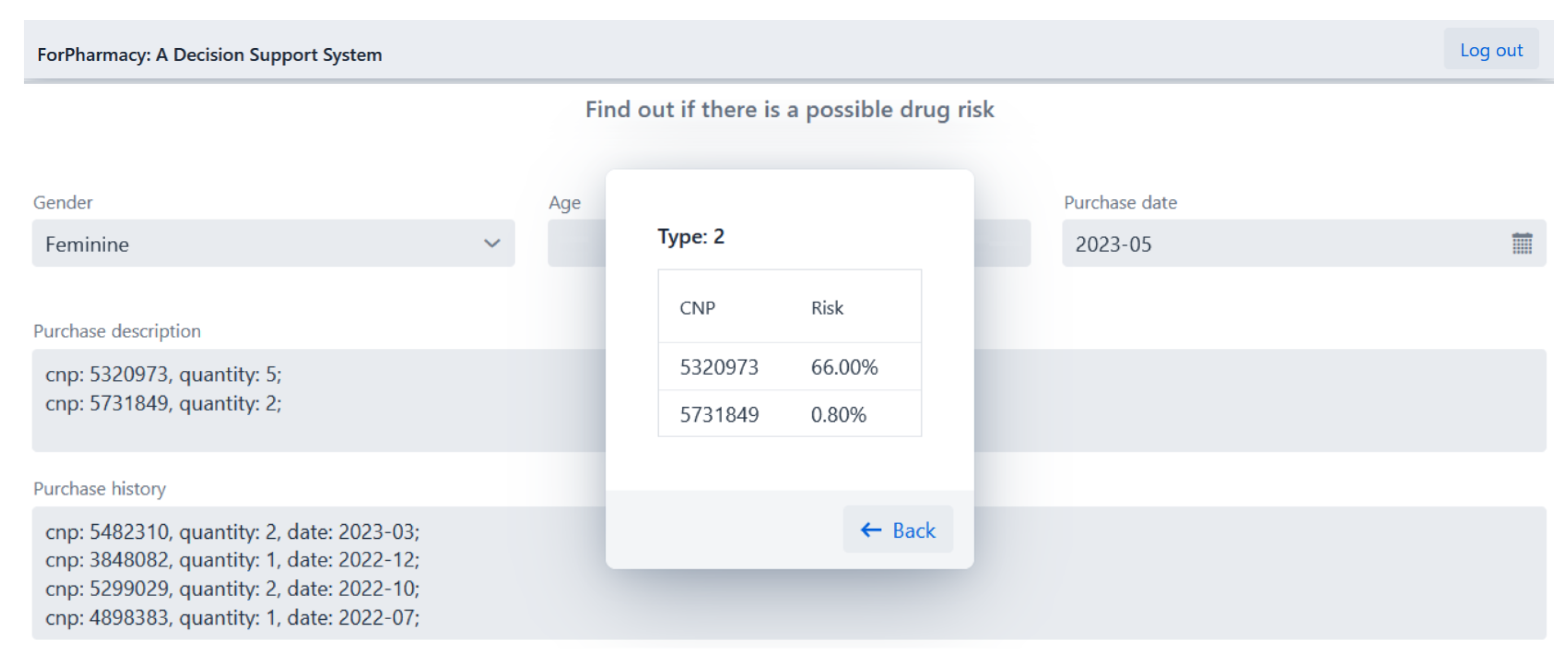
- Type 2—Patient with a cardiovascular disease, diabetes, sleep or psychiatric disorder.
- Type 3—Patient with a cardiovascular disease, diabetes, anemia or benign prostatic hyperplasia.
- Type 4—Patient with an alergic or non-alergic respiratory disease, autoimune disease, skin disease, infection, sleep or psychiatric disorders.
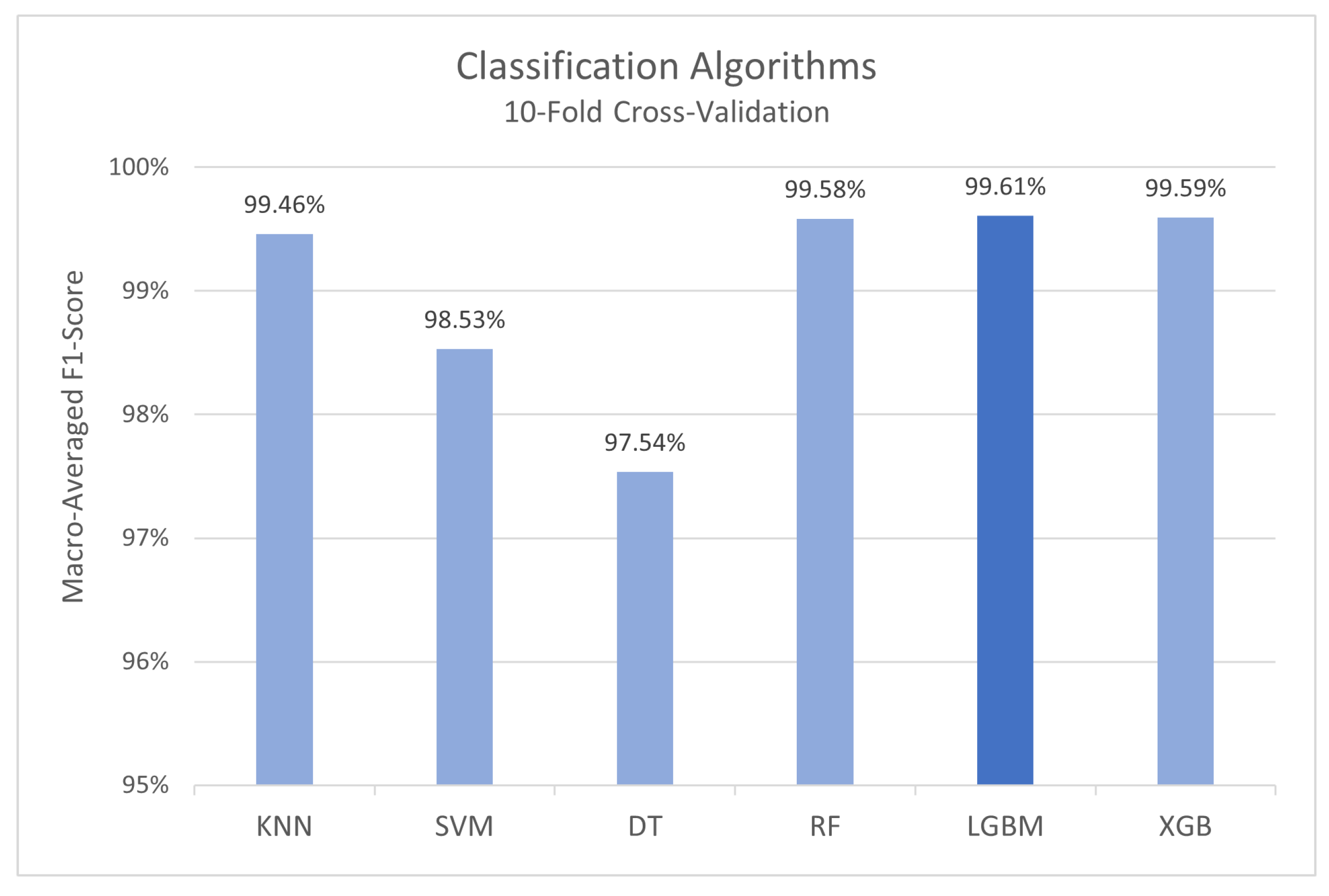
3.2.3. Model Cross-Validation
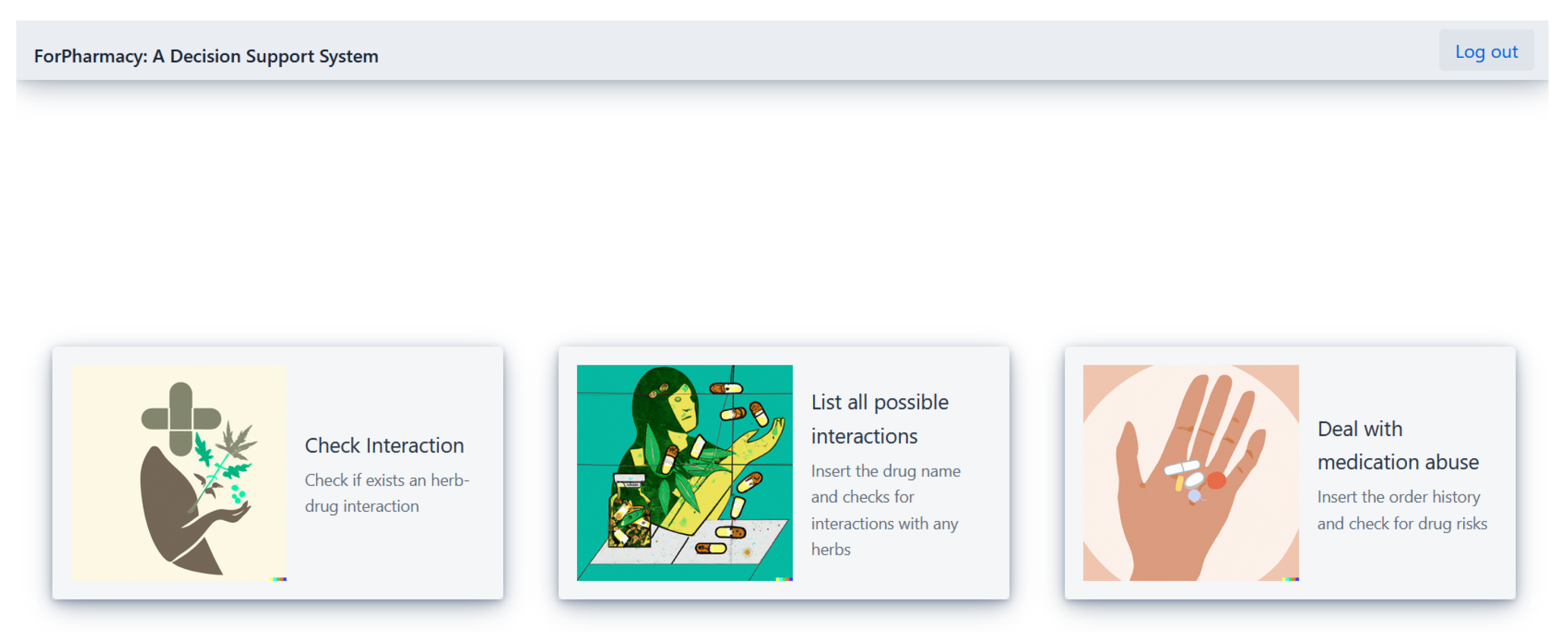
4. Case Study
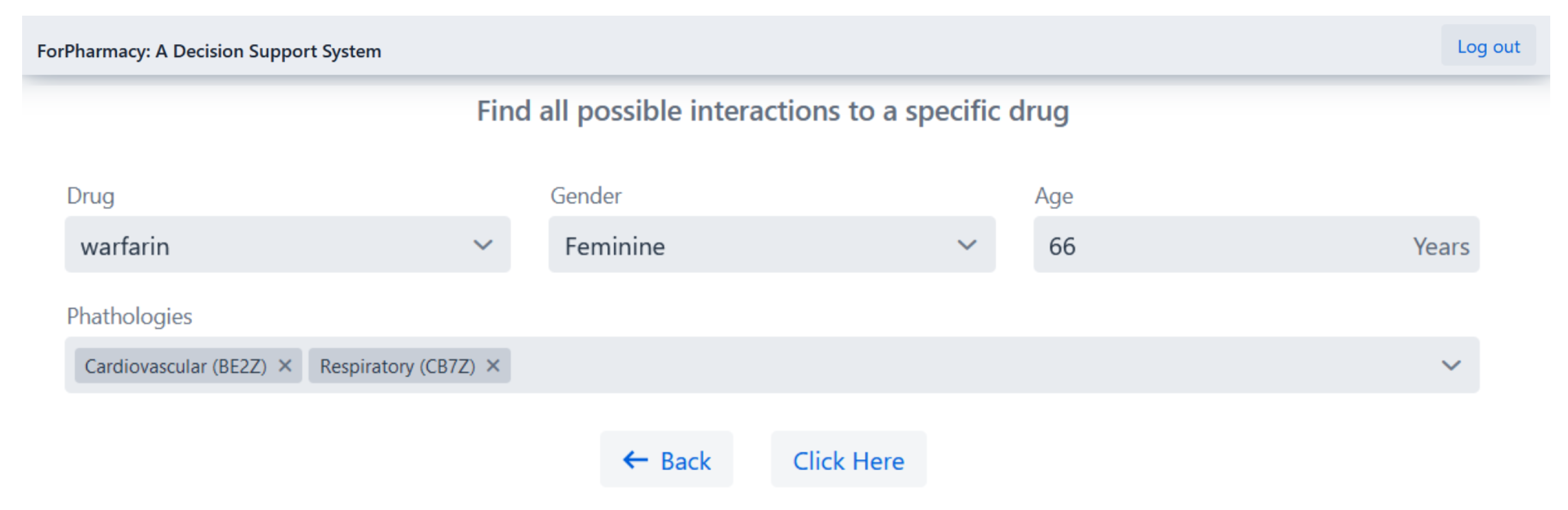
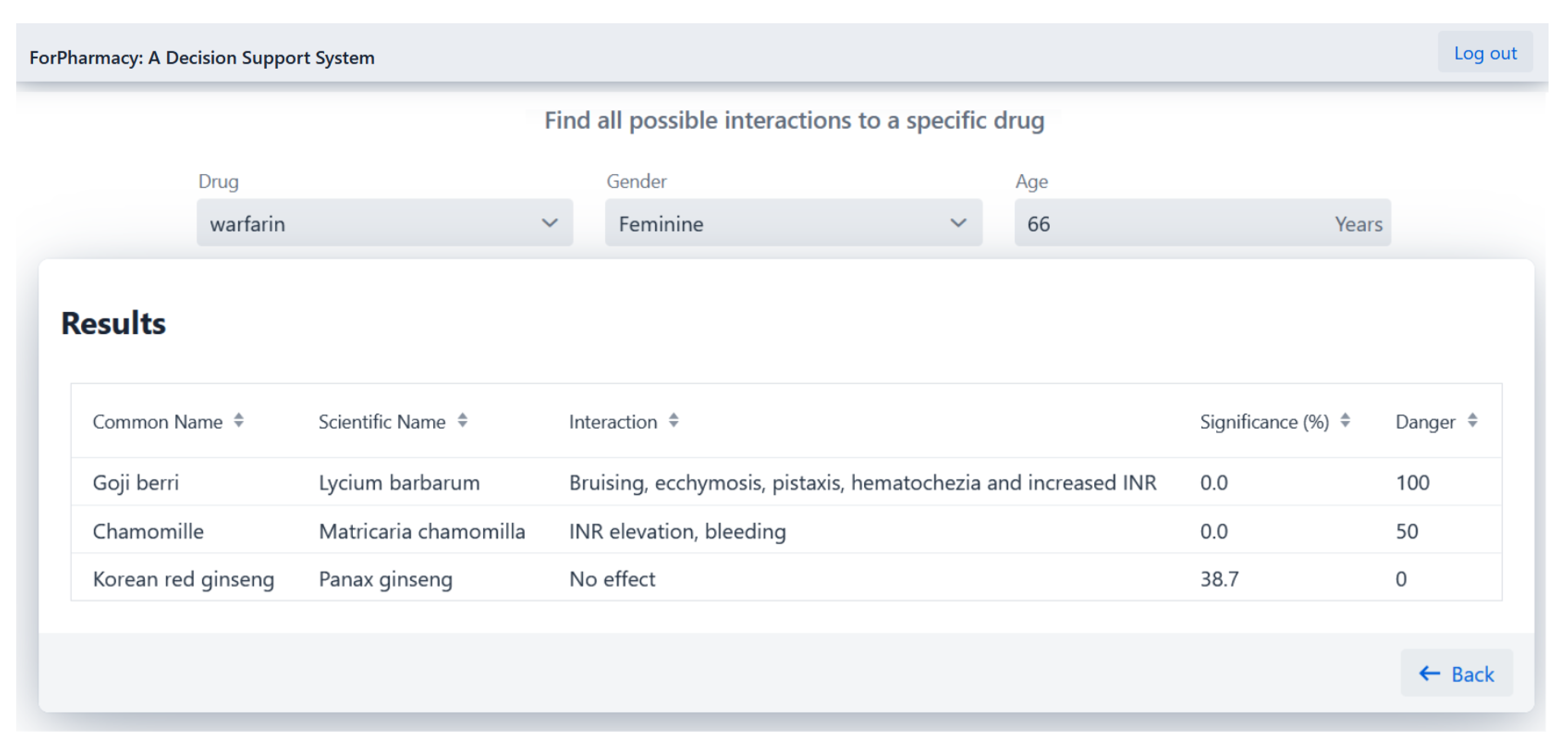
4.1. Herb-Drug Interaction Detection
4.2. Drug Abuse Detection
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Curtain, C.; Peterson, G.M. Review of computerized clinical decision support in community pharmacy. J. Clin. Pharm. Ther. 2014, 39, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sutton, R.T.; Pincock, D.; Baumgart, D.C.; Sadowski, D.C.; Fedorak, R.N.; Kroeker, K.I. An overview of clinical decision support systems: Benefits, risks, and strategies for success. NPJ Digit. Med. 2020, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Armando, L.G.; Miglio, G.; de Cosmo, P.; Cena, C. Clinical decision support systems to improve drug prescription and therapy optimisation in clinical practice: A scoping review. BMJ Health Care Inform. 2023, 30, e100683. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zheng, H.; Chen, X.; Zhao, Y.; Gao, T.; Dong, H.; Luo, H.; Weng, Z. DDI-GCN: Drug-drug interaction prediction via explainable graph convolutional networks. Artif. Intell. Med. 2023, 144, 102640. [Google Scholar] [CrossRef]
- Cai, R.; Liu, M.; Hu, Y.; Melton, B.L.; Matheny, M.E.; Xu, H.; Duan, L.; Waitman, L.R. Identification of adverse drug-drug interactions through causal association rule discovery from spontaneous adverse event reports. Artif. Intell. Med. 2017, 76, 7–15. [Google Scholar] [CrossRef]
- Cuvelier, E.; Robert, L.; Musy, E.; Rousselière, C.; Marcilly, R.; Gautier, S.; Odou, P.; Beuscart, J.B.; Décaudin, B. The clinical pharmacist’s role in enhancing the relevance of a clinical decision support system. Int. J. Med Inform. 2021, 155, 104568. [Google Scholar] [CrossRef]
- Hines, L.; Saverno, K.R.; Warholak, T.L.; Taylor, A.; Grizzle, A.J.; Murphy, J.E.; Malone, D.C. Pharmacists’ awareness of clinical decision support in pharmacy information systems: An exploratory evaluation. Res. Soc. Adm. Pharm. 2011, 7, 359–368. [Google Scholar] [CrossRef]
- Fugh-Berman, A. Herb-drug interactions. Lancet 2000, 355, 134–138. [Google Scholar] [CrossRef]
- Posadzki, P.; Watson, L.K.; Ernst, E. Herb—Drug interactions: An overview of systematic reviews. Br. J. Clin. Pharmacol. 2013, 75, 603–618. [Google Scholar] [CrossRef]
- Martins, A.; Costa, F.; Maia, E.; Praça, I.; Lages, M.; Pontes, C.; Guarino, M. A Clinical Decision Support System to Reduce Herb-Drug Interaction at Community Pharmacies: A Scoping Review. Available online: https://preprints.jmir.org/preprint/47649 (accessed on 1 January 2024).
- Brantley, S.; Argikar, A.; Lin, Y.; Nagar, S.; Paine, M. Herb-Drug Interactions: Challenges and Opportunities for Improved Predictions. Drug Metab. Dispos. Biol. Fate Chem. 2013, 42, 301–317. [Google Scholar] [CrossRef]
- Trinh, K.; Pham, D.; Le, L. Semantic Relation Extraction for Herb-Drug Interactions from the Biomedical Literature Using an Unsupervised Learning Approach. In Proceedings of the 2018 IEEE 18th International Conference on Bioinformatics and Bioengineering (BIBE), Taichung, Taiwan, 29–31 October 2018; pp. 334–337. [Google Scholar] [CrossRef]
- Qiao, Z.; Chai, T.; Zhang, Q.; Zhou, X.; Chu, Z. Predicting potential drug abusers using machine learning techniques. In Proceedings of the 2019 International Conference on Intelligent Informatics and Biomedical Sciences (ICIIBMS), Shanghai, China, 21–24 November 2019; pp. 283–286. [Google Scholar] [CrossRef]
- Deo, R.C. Machine Learning in Medicine. Circulation 2015, 132, 1920–1930. [Google Scholar] [CrossRef] [PubMed]
- Harnett, J.; Ung, C.; Hu, H.; Sultani, M.; Desselle, S. Advancing the pharmacist’s role in promoting the appropriate and safe use of dietary supplements. Complement. Ther. Med. 2019, 44, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Barenholtz, E.; Fitzgerald, N.D.; Hahn, W.E. Machine-learning approaches to substance-abuse research: Emerging trends and their implications. Curr. Opin. Psychiatry 2020, 33, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ip, C.M.; Lai, Y.S.; Zuo, Z. Overview of Current Herb–Drug Interaction Databases. Drug Metab. Dispos. 2022, 50, 86–94. [Google Scholar] [CrossRef]
- Wang, L.L.; Tafjord, O.; Cohan, A.; Jain, S.; Skjonsberg, S.; Schoenick, C.; Botner, N.; Ammar, W. SUPP.AI: Finding evidence for supplement-drug interactions. In Proceedings of the 58th Annual Meeting of the Association for Computational Linguistics: System Demonstrations, Online, 5–10 July 2020; pp. 362–371. [Google Scholar] [CrossRef]
- Liu, Y.; Ott, M.; Goyal, N.; Du, J.; Joshi, M.; Chen, D.; Levy, O.; Lewis, M.; Zettlemoyer, L.; Stoyanov, V. Roberta: A robustly optimized bert pretraining approach. arXiv 2019. [Google Scholar] [CrossRef]
- Lin, K.; Friedman, C.; Finkelstein, J. An automated system for retrieving herb-drug interaction related articles from MEDLINE. AMIA Jt. Summits Transl. Sci. Proceedings. AMIA Summit Transl. Sci. 2016, 2016, 140–149. [Google Scholar]
- Haynes, B.; McKibbon, K.; Wilczynski, N.; Walter, S.; Werre, S. Optimal Search Strategies for Retrieving Scientifically Strong Studies of Treatment from MEDLINE. BMJ 2005, 330, 1179. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Principal component analysis. WIREs Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- Cnudde, A.; Watrin, P.; Souard, F. HDI Highlighter, The First Intelligent Tool to Screen the Literature on Herb-Drug Interactions. Clin. Pharmacokinet. 2022, 61, 761–788. [Google Scholar] [CrossRef]
- Choi, Y.H.; Chin, Y.W. Multifaceted Factors Causing Conflicting Outcomes in Herb-Drug Interactions. Pharmaceutics 2020, 13, 43. [Google Scholar] [CrossRef]
- Prely, H.; Herledan, C.; Caffin, A.G.; Baudouin, A.; Larbre, V.; Maire, M.; Schwiertz, V.; Vantard, N.; Ranchon, F.; Rioufol, C. Real-life drug–drug and herb–drug interactions in outpatients taking oral anticancer drugs: Comparison with databases. J. Cancer Res. Clin. Oncol. 2022, 148, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Maia, E.; Praça, I. Herb–Drug Interactions: A Holistic Decision Support System in Healthcare. In Proceedings of the 2022 IEEE International Conference on E-health Networking, Application & Services (HealthCom), Genoa, Italy, 17–19 October 2022; pp. 1–6. [Google Scholar] [CrossRef]
- European Health Data Space Regulation Proposal. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:52022PC0197 (accessed on 1 January 2024).
- Infarmed - National Authority of Medicines and Health Products. Available online: https://www.infarmed.pt/web/infarmed/servicos-on-line/pesquisa-do-medicamento (accessed on 1 January 2024).
- Anatomical Therapeutic Chemical ATC Classification. Available online: https://www.who.int/tools/atc-ddd-toolkit/atc-classification (accessed on 1 January 2024).
- MacQueen, J. Some methods for classification and analysis of multivariate observations. In Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability, Oakland, CA, USA, 1 January 1967; Volume 1, pp. 281–297. [Google Scholar]
- Pearson, K. LIII. On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Milić, N.; Milošević, N.; Kon, S.G.; Božić, T.; Abenavoli, L.; Borrelli, F. Warfarin Interactions with Medicinal Herbs. Nat. Prod. Commun. 2014, 9, 1934578X1400900835. [Google Scholar] [CrossRef]
- Segal, R.; Pilote, L. Warfarin interaction with Matricaria chamomilla. Cmaj 2006, 174, 1281–1282. [Google Scholar] [CrossRef]
- International Normalised Ratio INR Test. Available online: https://www.healthdirect.gov.au/international-normalised-ratio-INR-test (accessed on 1 January 2024).



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.; Vitorino, J.; Maia, E.; Praça, I. PharmiTech: Addressing Polypharmacy Challenges through AI-Driven Solutions. Appl. Sci. 2024, 14, 8838. https://doi.org/10.3390/app14198838
Martins A, Vitorino J, Maia E, Praça I. PharmiTech: Addressing Polypharmacy Challenges through AI-Driven Solutions. Applied Sciences. 2024; 14(19):8838. https://doi.org/10.3390/app14198838
Chicago/Turabian StyleMartins, Andreia, João Vitorino, Eva Maia, and Isabel Praça. 2024. "PharmiTech: Addressing Polypharmacy Challenges through AI-Driven Solutions" Applied Sciences 14, no. 19: 8838. https://doi.org/10.3390/app14198838
APA StyleMartins, A., Vitorino, J., Maia, E., & Praça, I. (2024). PharmiTech: Addressing Polypharmacy Challenges through AI-Driven Solutions. Applied Sciences, 14(19), 8838. https://doi.org/10.3390/app14198838






