Green Method of Doping Photochromic TiO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Titania Sample Synthesis
2.2. Characterization
3. Results
3.1. Characteristics of Diaphragm Discharge
3.2. Sol Characteristics
3.3. Photochromic Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, C.; Ren, X.; Dai, Z.; Zhang, Y.; Qi, X.; Pan, C. Present perspectives of advanced characterization techniques in TiO2-based photocatalysts. ACS Appl. Mater. Interfaces 2017, 9, 23265–23286. [Google Scholar] [CrossRef] [PubMed]
- Basavarajappa, P.S.; Patil, S.B.; Ganganagappa, N.; Reddy, K.R.; Raghu, A.V.; Reddy, C.V. Recent progress in metal-doped TiO2, nonmetal doped/codoped TiO2 and TiO2 nanostructured hybrids for enhanced photocatalysis. Int. J. Hydrogen Energy 2020, 45, 7764–7778. [Google Scholar] [CrossRef]
- Joost, U.; Sutka, A.; Oja, M.; Smits, K.; Döbelin, N.; Loot, A.; Järvekülg, M.; Hirsimäki, M.; Valden, M.; Nõmmiste, E. Reversible photodoping of TiO2 nanoparticles for photochromic applications. Chem. Mater. 2018, 30, 8968–8974. [Google Scholar] [CrossRef]
- Kikuchi, E.; Iida, K.; Fujishima, A.; Itoh, K. Photochromism and photoelectrochemistry of amorphous and polycrystalline WO3 films. J. Electroanal. Chem. 1993, 351, 105–114. [Google Scholar] [CrossRef]
- Belhomme, L.; Duttine, M.; Labrugère, C.; Coicaud, E.; Rougier, A.; Penin, N.; Dandre, A.; Ravaine, S.; Gaudon, M. Investigation of the photochromism of WO3, TiO2, and composite WO3–TiO2 nanoparticles. Inorg. Chem. 2024, 63, 10079–10091. [Google Scholar] [CrossRef]
- Paul, N.; Patowary, M.; Pegu, B.; Mohanta, D. Physical properties of nanoscale TiO2 related to Ag-doping and photochromic behavior. Nanosci. Nanotechnol. Lett. 2013, 5, 452–456. [Google Scholar] [CrossRef]
- Crespo-Monteiro, N.; Destouches, N.; Epicier, T.; Balan, L.; Vocanson, F.; Lefkir, Y.; Michalon, J.Y. Changes in the chemical and structural properties of nanocomposite Ag: TiO2 films during photochromic transitions. J. Phys. Chem. C 2014, 118, 24055–24061. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Rozman, N.; Leoni, M.; Seabra, M.P.; Skapin, A.S.; Pullar, R.C.; Labrincha, J.A. Cu–TiO2 hybrid nanoparticles exhibiting tunable photochromic behavior. J. Phys. Chem. C 2015, 119, 23658–23668. [Google Scholar] [CrossRef]
- Morita, M.; Toyoda, S.; Kiuchi, H.; Abe, T.; Kumagai, K.; Saida, T.; Fukuda, K. Chromogenic amorphous MoO3−x nanosheets and their nanostructured films for smart window applications. ACS Appl. Nano Mater. 2021, 4, 8781–8788. [Google Scholar] [CrossRef]
- Miyazaki, H.; Matsuura, T.; Ota, T. Vanadium oxide-based photochromic composite film. RSC Adv. 2017, 7, 2388–2391. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.; Gao, X.; Sun, H.; Peng, D.; Zou, H.; Zhang, Q.; Hao, X. Fast self-bleaching Nb2O5-based photochromics for high security dynamic anti-counterfeiting and optical storage applications. Chem. Eng. J. 2022, 435, 134801. [Google Scholar] [CrossRef]
- Songara, S.; Patra, M.K.; Manoth, M.; Saini, L.; Gupta, V.; Gowd, G.S.; Vadera, S.R.; Kumar, N. Synthesis and studies on photochromic properties of vanadium doped TiO2 nanoparticles. J. Photochem. Photobiol. A Chem. 2010, 209, 68–73. [Google Scholar] [CrossRef]
- Songara, S.; Singh, J.; Patra, M.K.; Gupta, V.; Saini, L.; Gowd, G.S.; Kumar, N. Wet chemical synthesis of molybdenum-doped amorphous TiO2 nanoparticles and studies on their photochromic properties. Int. J. Green Nanotechnol. 2012, 4, 399–407. [Google Scholar] [CrossRef]
- Songara, S.; Saini, L.; Gowd, G.S.; Rajpurohit, J.S.; Gupta, V.; Patra, M.K.; Vadera, S.R.; Kumar, N. Improved photochromic properties of W6+ doped nanostructured TiO2 coatings. Res. Surf. Interfaces 2024, 14, 100201. [Google Scholar] [CrossRef]
- Eglītis, R.; Joost, U.; Zukuls, A.; Rubenis, K.; Ignatans, R.; Avotiņa, L.; Baumane, L.; Smits, K.; Hirsimaki, M.; Kaambre, T.; et al. Strong, rapid, and reversible photochromic response of Nb doped TiO2 nanocrystal colloids in hole scavenging media. ACS Appl. Mater. Interfaces 2020, 12, 57609–57618. [Google Scholar] [CrossRef]
- Xu, J.; Li, K.; Wu, S.; Shi, W.; Peng, T. Preparation of brookite titania quasi nanocubes and their application in dye-sensitized solar cells. J. Mater. Chem. A 2015, 3, 7453–7462. [Google Scholar] [CrossRef]
- Manzoli, M.; Freyria, F.S.; Blangetti, N.; Bonelli, B. Brookite, a sometimes under evaluated TiO2 polymorph. RSC Adv. 2022, 12, 3322–3334. [Google Scholar] [CrossRef]
- Zou, Y.; Yu, T.; Huang, X.; Li, Y.; Guo, L.; Yan, H.; Zhou, J.; Wang, Y. Hydrothermal synthesis of Zn-doped brookite TiO2 for enhanced visible-light-responsive photocatalytic performance. Mater. Res. Express 2023, 10, 085005. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol–gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Kusova, T.; Titov, V.; Agafonov, A. Mo-doped TiO2 using plasma in contact with liquids: Advantages and limitations. J. Chem. Technol. Biotechnol. 2021, 96, 1125. [Google Scholar] [CrossRef]
- Eisenberg, G. Colorimetric determination of hydrogen peroxide. Ind. Eng. Chem. Anal. Ed. 1943, 15, 327–328. [Google Scholar] [CrossRef]
- Shen, V.K.; Siderius, D.W.; Krekelberg, W.P.; Hatch, H. (Eds.) NIST Standard Reference Simulation Website, NIST Standard Reference Database Number 173; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2024. Available online: https://data.nist.gov/pdr/lps/FF429BC178798B3EE0431A570681E858232 (accessed on 13 January 2024).
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman spectrum of brookite, TiO2 (PBCA, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Gil, H.A.C.; De Queiroz, A.A.A. The interaction between polyvinylpyrrolidone and I2 as probed by Raman spectroscopy. J. Mol. Struct. 1999, 478, 93–98. [Google Scholar] [CrossRef]
- Stefanov, P.; Shipochka, M.; Stefchev, P.; Raicheva, Z.; Lazarova, V.; Spassov, L. XPS characterization of TiO2 layers deposited on quartz plates. J. Phys. Conf. Ser. 2008, 100, 012039. [Google Scholar] [CrossRef]
- Tandy, L.M.F.; Bueno, J.P.; Vong, Y.M.; Torres, I.Z.; Rojas, L.L.; Torres, C.J.R.; Gutierrez, H.M.; López, M.M. Reversible photochromic effect in the TiO2—Polymer hybrid system. J. Sol-Gel Sci. Technol. 2017, 82, 51–58. [Google Scholar] [CrossRef]
- Van Pham, D.; Patil, R.A.; Lin, J.H.; Lai, C.C.; Liou, Y.; Ma, Y.R. Doping-free bandgap tuning in one-dimensional Magnéli-phase nanorods of Mo4O11. Nanoscale 2016, 8, 5559–5566. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, W.; Qin, J.; Zhao, D.; Mao, B.; Xiao, Y.; Cao, M. Oxygen vacancy-rich mesoporous W18O49 nanobelts with ultrahigh initial Coulombic efficiency toward high-performance lithium storage. Electrochim. Acta 2016, 187, 329–339. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Glazkova, N.I.; Mikhaylov, R.V.; Serpone, N. In situ study of photo- and thermos-induced color centers in photochromic rutile TiO2 in the temperature range 90–720 K. Photochem. Photobiol. Sci. 2016, 15, 1289–1298. [Google Scholar] [CrossRef]
- Kuznetsov, V.N.; Serpone, N. Photoinduced coloration and photobleaching of titanium dioxide in TiO2/polymer compositions upon UV-and visible-light excitation of color centers absorption bands: Direct experimental evidence negating band-gap narrowing in anion-/cation-doped TiO2s. J. Phys. Chem. C 2007, 111, 15277–15288. [Google Scholar] [CrossRef]
- Khlyustova, A.; Evdokimova, A.; Shibaeva, V.; Sirotkin, N. Photochromic properties of novel composites on the base of biopolymers and plasma-modified Mo4O11 and W18O49 nanostructures. J. Vinyl Addit. Technol. 2024, 30, 585–595. [Google Scholar] [CrossRef]
- Onur Şahin, E.; Dai, Y.; Chan, C.K.; Tüysüz, H.; Schmidt, W.; Lim, J.; Zhang, S.; Scheu, C.; Weidenthaler, C. Monitoring the structure evolution of Titanium oxide photocatalysts: From the molecular form via the amorphous state to the crystalline phase. Chem. A Eur. J. 2021, 27, 11600–11608. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Bozon-Verduraz, F. Niobium pentoxide prepared by soft chemical routes: Morphology, structure, defects and quantum size effect. Phys. Chem. Chem. Phys. 2003, 5, 1457–1466. [Google Scholar] [CrossRef]
- Zhang, L.; Mohamed, H.H.; Dillert, R.; Bahnemann, D. Kinetics and mechanisms of charge transfer processes in photocatalytic systems: A review. J. Photochem. Photobiol. C 2012, 13, 263–276. [Google Scholar] [CrossRef]
- Miller, L.E.; Hamm, F.A. Macromolecular properties of polyvinylpyrrolidone: Molecular weight distribution. J. Phys. Chem. 1953, 57, 110–122. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Kamińska, A.; Światek, M.; Rabek, J.F. Photo-oxidative degradation of some water-soluble polymers in the presence of accelerating agents. Angew. Makromol. Chem. 1998, 261, 109–121. [Google Scholar] [CrossRef]
- Kirkpatrick, M.J.; Locke, B.R. Effects of platinum electrode on hydrogen, oxygen, and hydrogen peroxide formation in aqueous phase pulsed corona electrical discharge. Ind. Eng. Chem. Res. 2006, 45, 2138–2142. [Google Scholar] [CrossRef]
- Kuzimicheva, L.; Titova, Y.; Maksimov, A. Generation of chemically active oxidative particles in electrolyte solutions under the action of glow and diaphragm discharges. Surf. Eng. Appl. Electrochem. 2007, 43, 90–93. [Google Scholar] [CrossRef]
- Holzer, F.; Locke, B.R. Influence of high voltage needle electrode material on hydrogen peroxide formation and electrode erosion in a hybrid gas–liquid series electrical discharge reactor. Plasma Chem. Plasma Process 2008, 28, 1–13. [Google Scholar] [CrossRef]
- Lukes, P.; Clupek, M.; Babicky, V.; Sisrova, I.; Janda, V. The catalytic role of tungsten electrode material in the plasmachemical activity of a pulsed corona discharge in water. Plasma Source Sci. Technol. 2011, 20, 034011. [Google Scholar] [CrossRef]
- Kuz’micheva, L.A.; Titova, Y.V.; Maksimov, A.I.; Vashurin, A.S.; Pukhovskaya, S.G. Effect of the cathode material on the accumulation of hydrogen peroxide in a plasma-solution system. Surf. Eng. Appl. Electrochem. 2013, 49, 485–487. [Google Scholar] [CrossRef]
- Kang, J.S.; Lee, Y.M. Effects of molecular weight of polyvinylpyrrolidone on precipitation kinetics during the formation of asymmetric polyacrylonitrile membrane. J. Appl. Polym. Sci. 2002, 85, 57–68. [Google Scholar] [CrossRef]



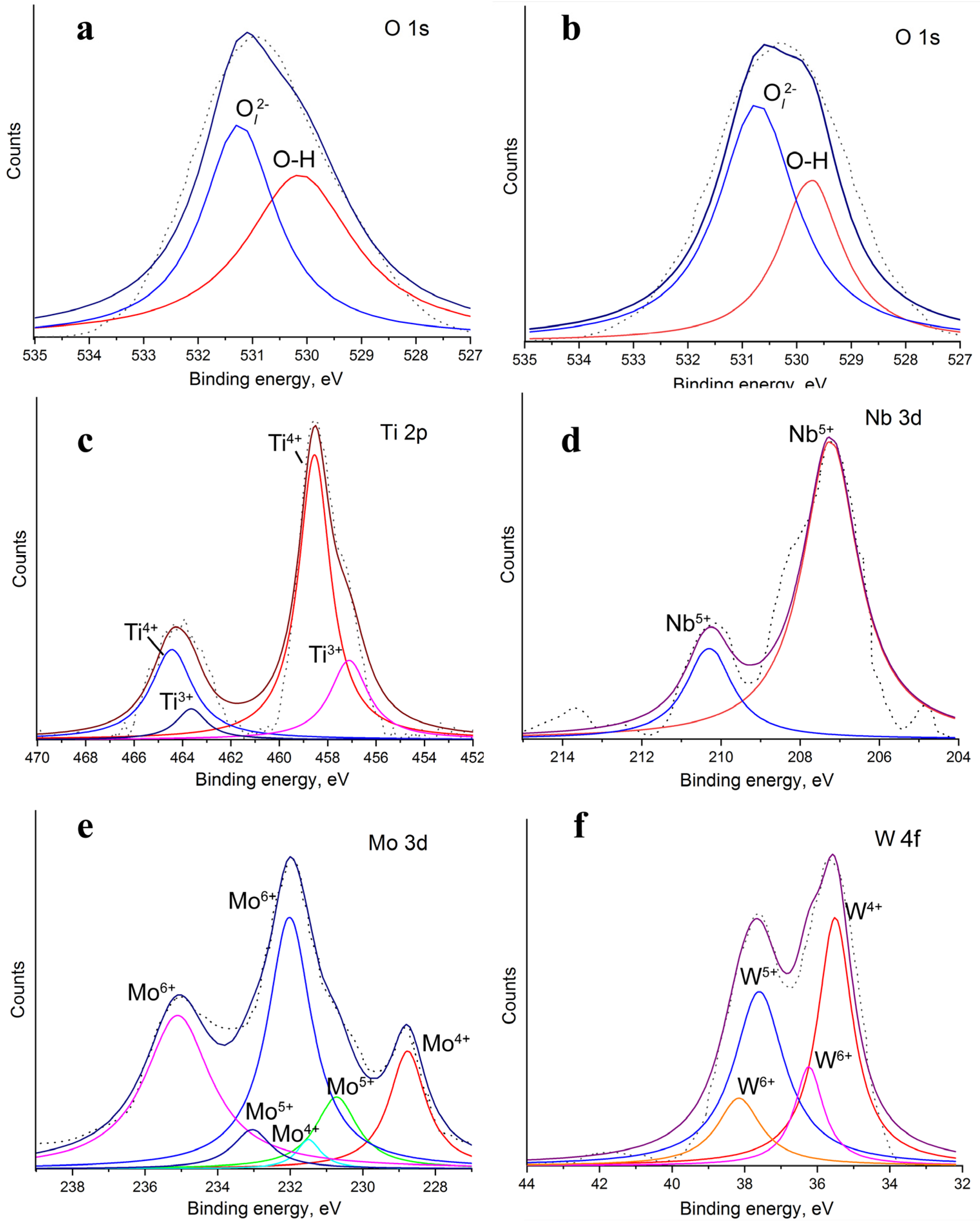
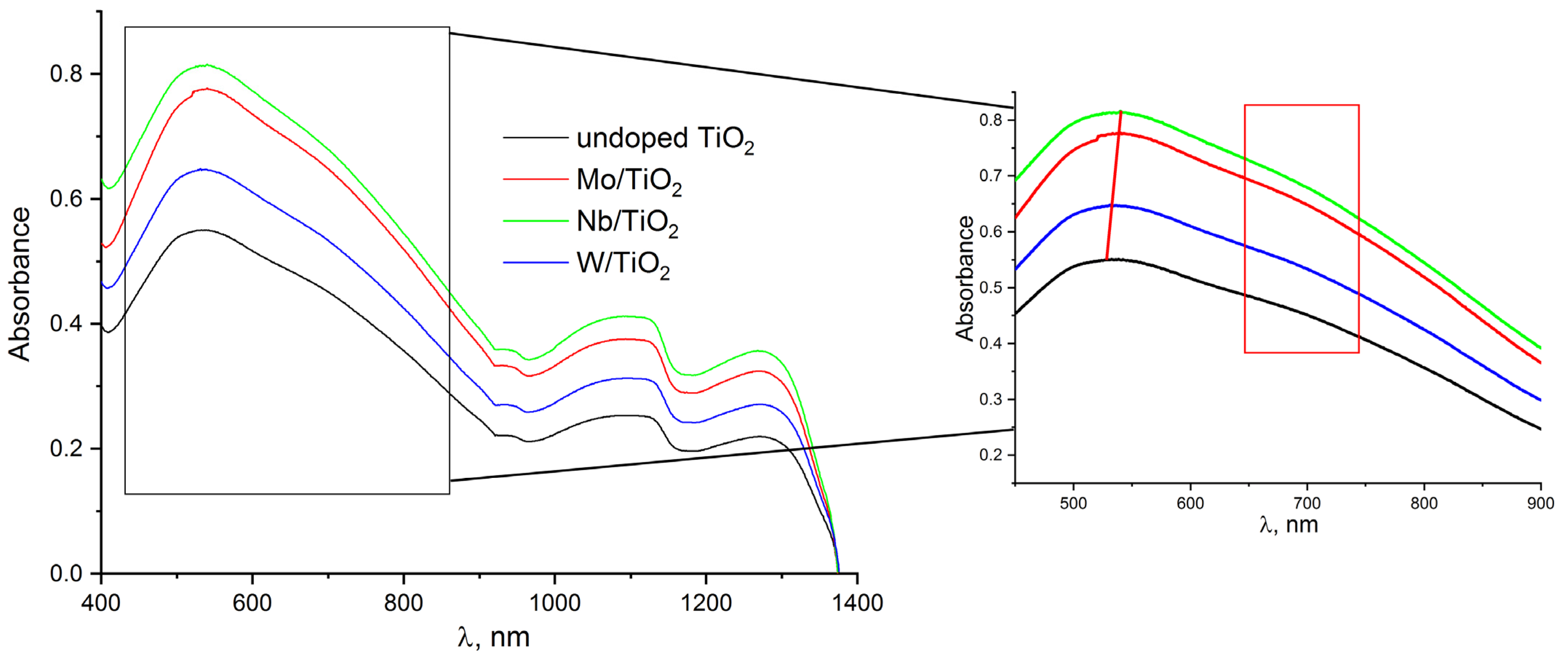
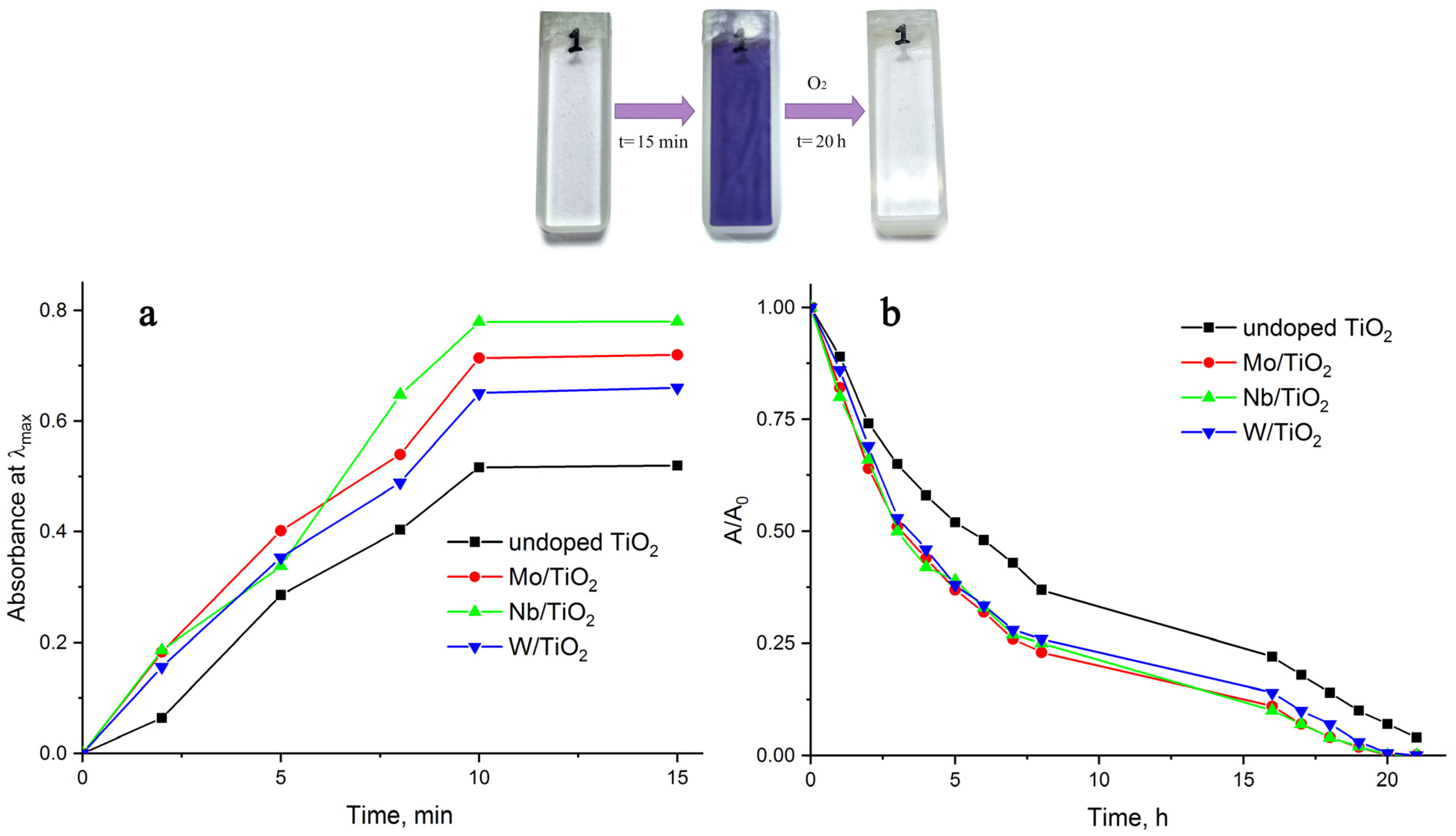
| Material of Electrode | MW(PVP), gol−1 | C(H2O2), mM |
|---|---|---|
| Mo | 27,724 | 1.40 |
| Nb | 29,256 | 0.91 |
| W | 28,281 | 1.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlyustova, A.; Evdokimova, A.; Sirotkin, N.; Shibaeva, V. Green Method of Doping Photochromic TiO2. Appl. Sci. 2024, 14, 8877. https://doi.org/10.3390/app14198877
Khlyustova A, Evdokimova A, Sirotkin N, Shibaeva V. Green Method of Doping Photochromic TiO2. Applied Sciences. 2024; 14(19):8877. https://doi.org/10.3390/app14198877
Chicago/Turabian StyleKhlyustova, Anna, Anastasia Evdokimova, Nikolay Sirotkin, and Valeriya Shibaeva. 2024. "Green Method of Doping Photochromic TiO2" Applied Sciences 14, no. 19: 8877. https://doi.org/10.3390/app14198877
APA StyleKhlyustova, A., Evdokimova, A., Sirotkin, N., & Shibaeva, V. (2024). Green Method of Doping Photochromic TiO2. Applied Sciences, 14(19), 8877. https://doi.org/10.3390/app14198877






