Improving Raman-Based Models for Real-Time Monitoring the CHO Cell Culture Process with Effective Variable Selection Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
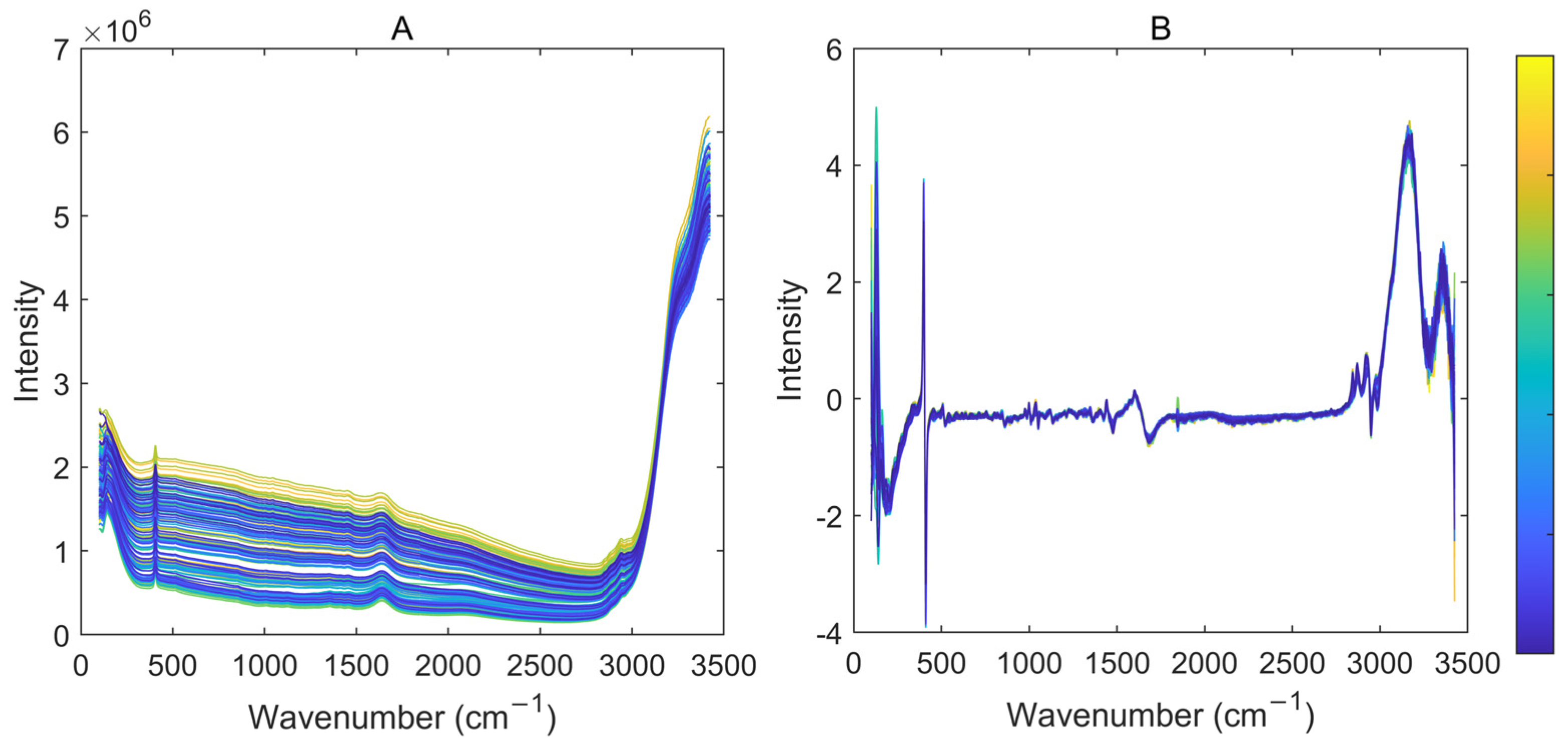
2.2. Data Acquisition
2.3. Variable Selection
2.3.1. Spectral Regions Selection
2.3.2. Variable Selection Algorithms
2.4. Model Calibration and Validation
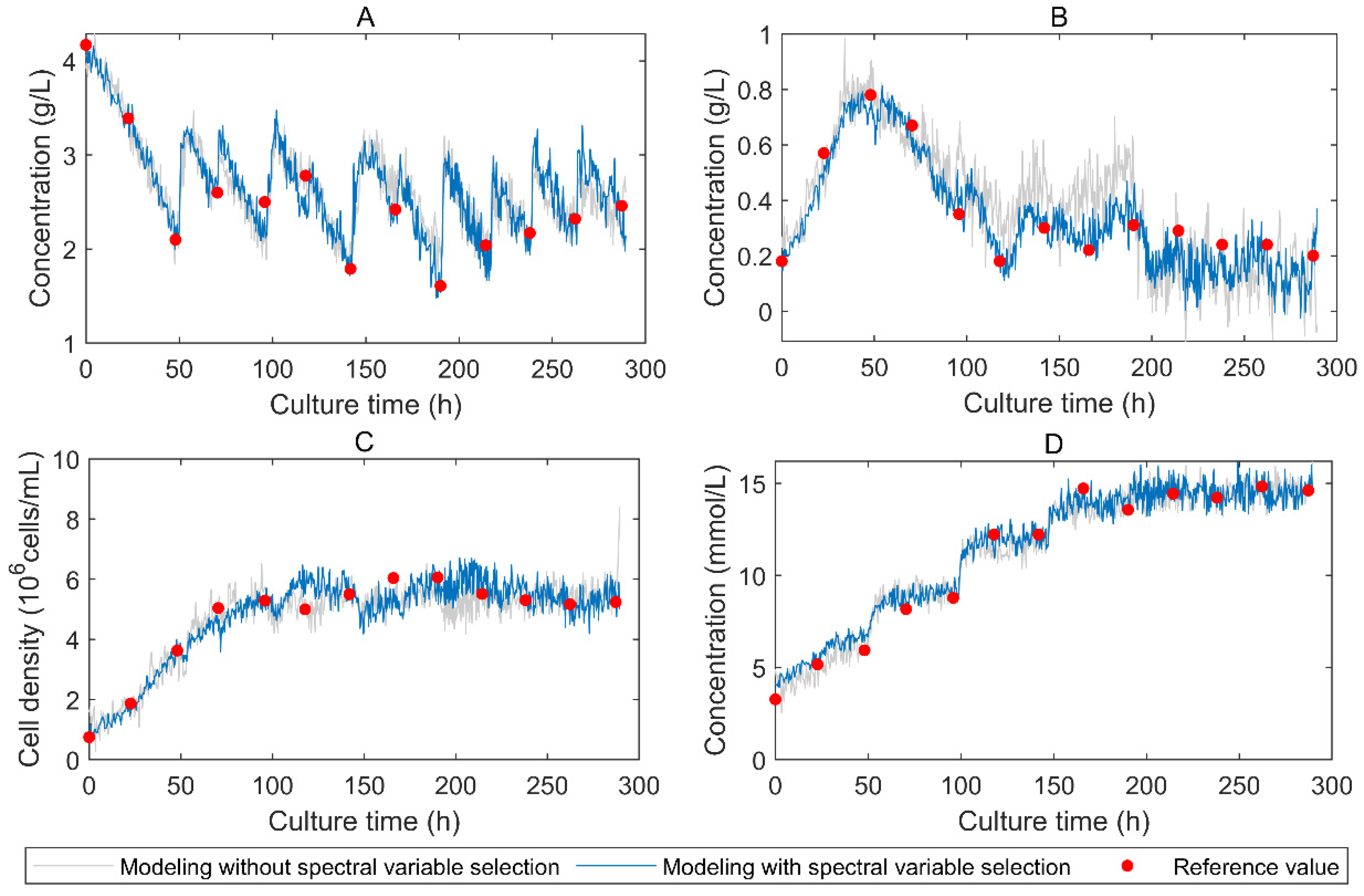
3. Results and Discussion
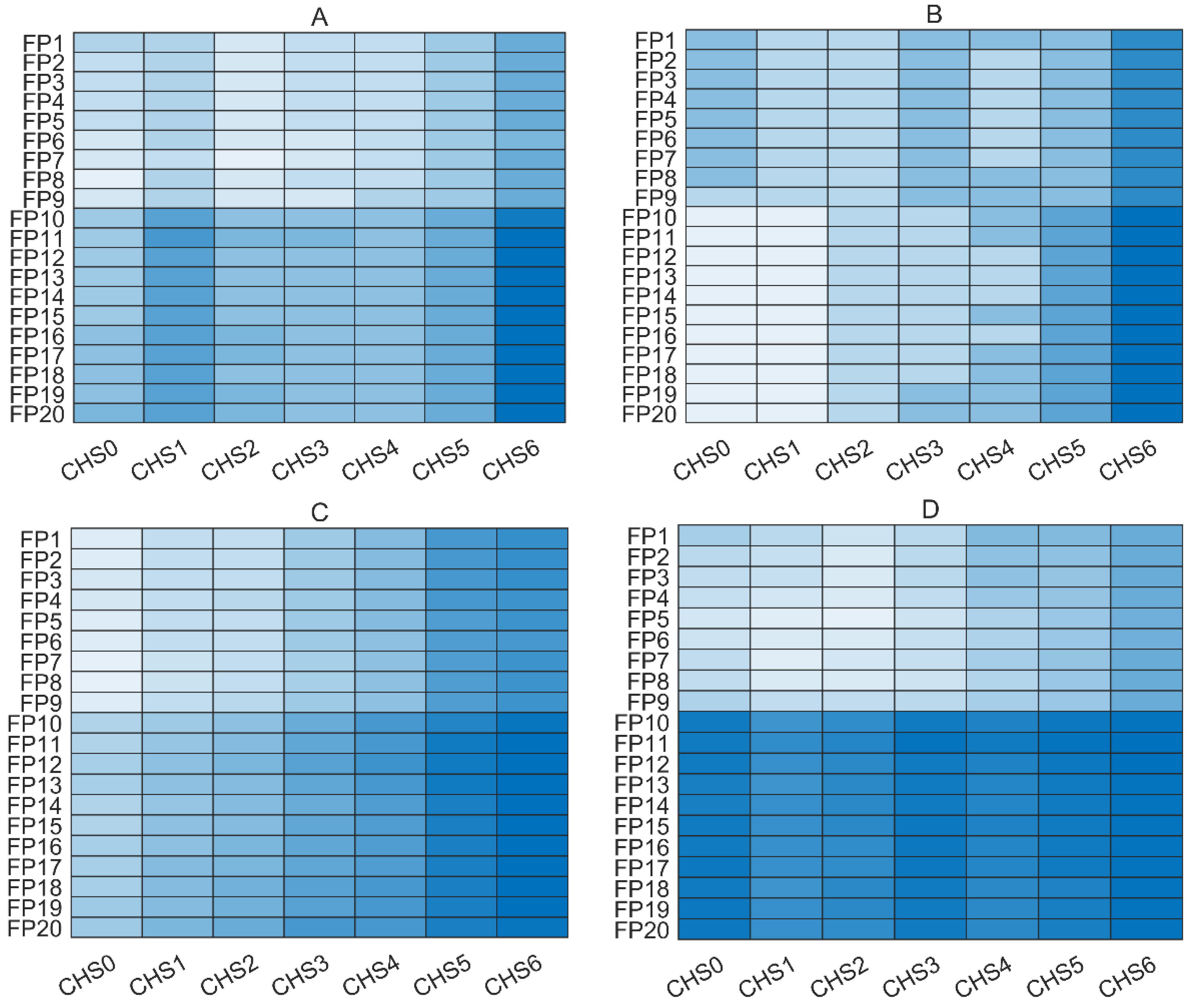
3.1. Comparison of Different Spectral Regions
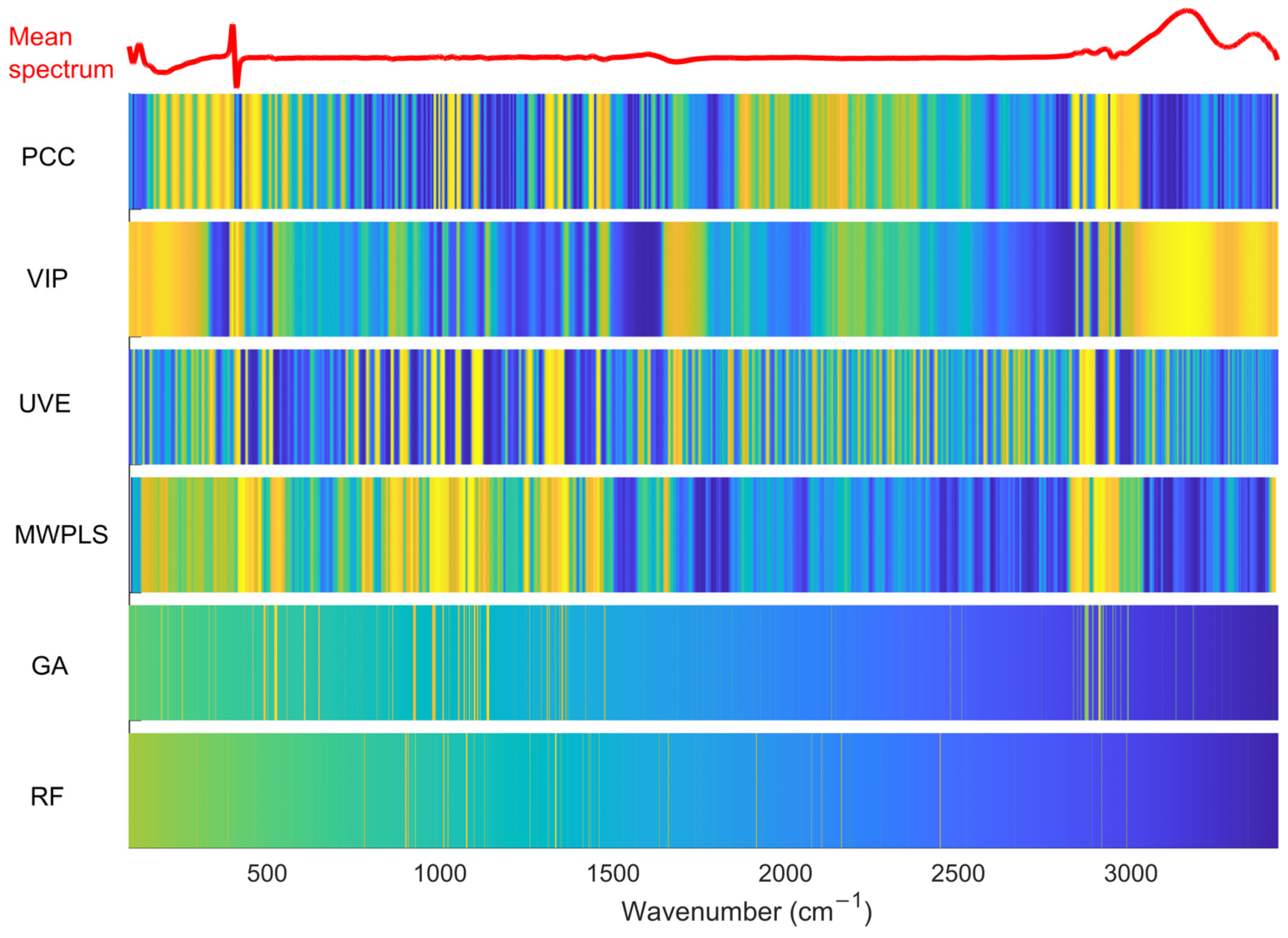
3.2. Comparison of Different Variable Selection Algorithms
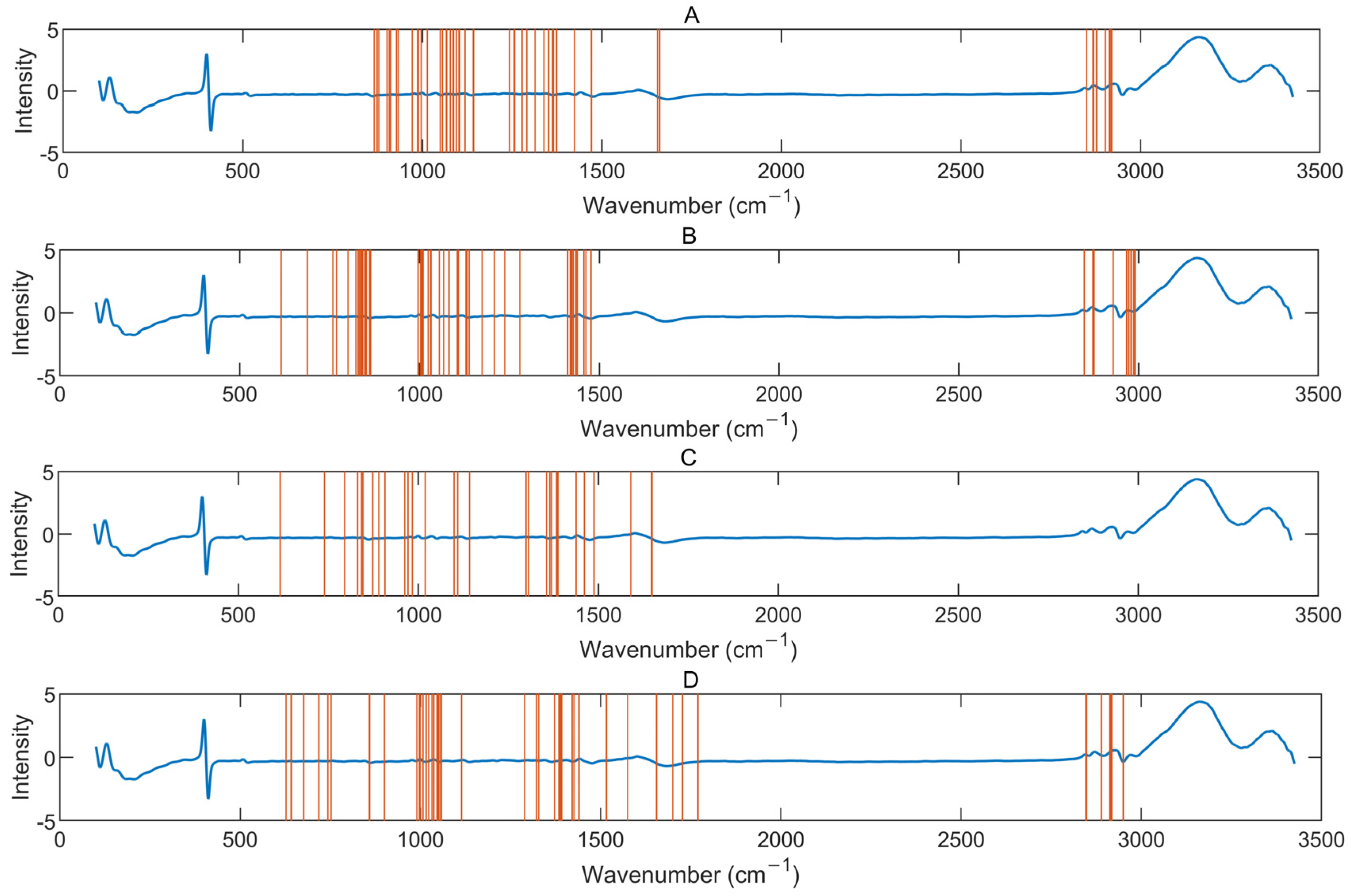
3.3. Combination of Variable Selection Algorithms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goh, J.B.; Ng, S.K. Impact of host cell line choice on glycan profile. Crit. Rev. Biotechnol. 2018, 38, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, F.R.; Mantalaris, A.; Asprey, S.P. Modelling of Mammalian Cells and Cell Culture Processes. Cytotechnology 2004, 44, 27–46. [Google Scholar] [CrossRef]
- Jin, S.; Sun, F.; Hu, Z.; Li, Y.; Zhao, Z.; Du, G.; Shi, G.; Chen, J. Online quantitative substrate, product, and cell concentration in citric acid fermentation using near-infrared spectroscopy combined with chemometrics. Spectrochim. Acta Part A 2023, 285, 121842. [Google Scholar] [CrossRef] [PubMed]
- Abu-Absi, N.R.; Kenty, B.M.; Cuellar, M.E.; Borys, M.C.; Sakhamuri, S.; Strachan, D.J.; Hausladen, M.C.; Li, Z.J. Real time monitoring of multiple parameters in mammalian cell culture bioreactors using an in-line Raman spectroscopy probe. Biotechnol. Bioeng. 2011, 108, 1215–1221. [Google Scholar] [CrossRef]
- Webster, T.A.; Hadley, B.C.; Hilliard, W.; Jaques, C.; Mason, C. Development of generic Raman models for a GS-KOTM CHO platform process. Biotechnol. Prog. 2018, 34, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, A.; Schorner, G.; Khodabandehlou, H.; Wan, T.; Coufal, M.; Undey, C. A machine-learning approach to calibrate generic Raman models for real-time monitoring of cell culture processes. Biotechnol. Bioeng. 2019, 116, 2575–2586. [Google Scholar] [CrossRef]
- Webster, T.A.; Hadley, B.C.; Dickson, M.; Busa, J.K.; Jaques, C.; Mason, C. Feedback control of two supplemental feeds during fed-batch culture on a platform process using inline Raman models for glucose and phenylalanine concentration. Bioprocess Biosyst. Eng. 2021, 44, 127–140. [Google Scholar] [CrossRef]
- Eyster, T.; Talwar, S.; Fernandez, J.; Foster, S.; Hayes, J.; Allen, R.; Reidinger, S.; Wan, B.; Ji, X.; Aon, J.; et al. Tuning monoclonal antibody galactosylation using Raman spectroscopy-controlled lactic acid feeding. Biotechnol. Progr. 2021, 37, e3085. [Google Scholar] [CrossRef]
- Chen, G.; Hu, J.; Qin, Y.; Zhou, W. Viable cell density on-line auto-control in perfusion cell culture aided by in-situ Raman spectroscopy. Biochem. Eng. J. 2021, 172, 108063. [Google Scholar] [CrossRef]
- Wasalathanthri, D.P.; Rehmann, M.S.; Song, Y.; Gu, Y.; Mi, L.; Shao, C.; Chemmalil, L.; Lee, J.; Ghose, S.; Borys, M.C.; et al. Technology outlook for real-time quality attribute and process parameter monitoring in biopharmaceutical development—A review. Biotechnol. Bioeng. 2020, 117, 3182–3198. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Suhr, C.; Roth, B.; Meinhardt-Wollweber, M. Iterative morphological and mollifier-based baseline correction for Raman spectra. J. Raman Spectrosc. 2017, 48, 336–342. [Google Scholar] [CrossRef]
- Lieber, C.A.; Mahadevan-Jansen, A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 2003, 57, 1363–1367. [Google Scholar] [CrossRef]
- Jiang, H.; Xu, W.; Ding, Y.; Chen, Q. Quantitative analysis of yeast fermentation process using Raman spectroscopy: Comparison of CARS and VCPA for variable selection. Spectrochim. Acta Part A 2020, 228, 117781. [Google Scholar] [CrossRef]
- Classen, J.; Aupert, F.; Reardon, K.F.; Solle, D.; Scheper, T. Spectroscopic sensors for in-line bioprocess monitoring in research and pharmaceutical industrial application. Anal. Bioanal. Chem. 2017, 409, 651–666. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Kalita, D.; Ahmed, M.T.; ElMasry, G.; Makino, Y. Effect of variable selection algorithms on model performance for predicting moisture content in biological materials using spectral data. Anal. Chim. Acta 2022, 1202, 339390. [Google Scholar] [CrossRef]
- Rafferty, C.; Johnson, K.; O’Mahony, J.; Burgoyne, B.; Rea, R.; Balss, K.M. Analysis of chemometric models applied to Raman spectroscopy for monitoring key metabolites of cell culture. Biotechnol. Progr. 2020, 36, e2977. [Google Scholar] [CrossRef]
- Schwarz, H.; Mäkinen, M.E.; Castan, A.; Chotteau, V. Monitoring of amino acids and antibody N-glycosylation in high cell density perfusion culture based on Raman spectroscopy. Biochem. Eng. J. 2022, 182, 108426. [Google Scholar] [CrossRef]
- Petillot, L.; Pewny, F.; Wolf, M.; Sanchez, C.; Thomas, F.; Sarrazin, J.; Fauland, K.; Katinger, H.; Javalet, C.; Bonneville, C. Calibration transfer for bioprocess Raman monitoring using Kennard Stone piecewise direct standardization and multivariate algorithms. Eng. Rep. 2020, 2, e12230. [Google Scholar] [CrossRef]
- Matthews, T.E.; Berry, B.N.; Smelko, J.; Moretto, J.; Moore, B.; Wiltberger, K. Closed loop control of lactate concentration in mammalian cell culture by Raman spectroscopy leads to improved cell density, viability, and biopharmaceutical protein production. Biotechnol. Bioeng. 2016, 113, 2416–2424. [Google Scholar] [CrossRef]
- Santos, R.M.; Kaiser, P.; Menezes, J.C.; Peinado, A. Improving reliability of Raman spectroscopy for mAb production by upstream processes during bioprocess development stages. Talanta 2019, 199, 396–406. [Google Scholar] [CrossRef]
- André, S.; Lagresle, S.; Hannas, Z.; Calvosa, É.; Duponchel, L. Mammalian cell culture monitoring using in situ spectroscopy: Is your method really optimised? Biotechnol. Progr. 2017, 33, 308–316. [Google Scholar] [CrossRef]
- Domján, J.; Pantea, E.; Gyürkés, M.; Madarász, L.; Kozák, D.; Farkas, A.; Horváth, B.; Benkő, Z.; Nagy, Z.K.; Marosi, G.; et al. Real-time amino acid and glucose monitoring system for the automatic control of nutrient feeding in CHO cell culture using Raman spectroscopy. Biotechnol. J. 2022, 17, 2100395. [Google Scholar] [CrossRef] [PubMed]
- André, S.; Lagresle, S.; Da Sliva, A.; Heimendinger, P.; Hannas, Z.; Calvosa, É.; Duponchel, L. Developing global regression models for metabolite concentration prediction regardless of cell line: Developing global regression models. Biotechnol. Bioeng. 2017, 114, 2550–2559. [Google Scholar] [CrossRef]
- Rafferty, C.; O’Mahony, J.; Rea, R.; Burgoyne, B.; Balss, K.M.; Lyngberg, O.; O’Mahony-Hartnett, C.; Hill, D.; Schaefer, E. Raman spectroscopic based chemometric models to support a dynamic capacitance based cell culture feeding strategy. Bioprocess Biosyst. Eng. 2020, 43, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Tulsyan, A.; Wang, T.; Schorner, G.; Khodabandehlou, H.; Coufal, M.; Undey, C. Automatic real-time calibration, assessment, and maintenance of generic Raman models for online monitoring of cell culture processes. Biotechnol. Bioeng. 2020, 117, 406–416. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Qin, Y.; Chen, G.; Hu, J.; Wang, Q.; Zhou, W. The application of Raman spectroscopy for monitoring product quality attributes in perfusion cell culture. Biochem. Eng. J. 2021, 173, 108064. [Google Scholar] [CrossRef]
- Mehmood, T.; Liland, K.H.; Snipen, L.; Sæbø, S. A review of variable selection methods in Partial Least Squares Regression. Chemom. Intell. Lab. Syst. 2012, 118, 62–69. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Li, H.-D.; Deng, B.-C.; Cao, D.-S. An overview of variable selection methods in multivariate analysis of near-infrared spectra. TTrAC Trends Anal. Chem. 2019, 113, 102–115. [Google Scholar] [CrossRef]
- Santos, R.M.; Kessler, J.-M.; Salou, P.; Menezes, J.C.; Peinado, A. Monitoring mAb cultivations with in-situ raman spectroscopy: The influence of spectral selectivity on calibration models and industrial use as reliable PAT tool. Biotechnol. Progr. 2018, 34, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; He, J.; Zhang, S.; Li, L. Nondestructive prediction and visualization of total flavonoids content in Cerasus Humilis fruit during storage periods based on hyperspectral imaging technique. J. Food Process Eng. 2021, 44, e13807. [Google Scholar] [CrossRef]
- Yu, H.-D.; Yun, Y.-H.; Zhang, W.; Chen, H.; Liu, D.; Zhong, Q.; Chen, W.; Chen, W. Three-step hybrid strategy towards efficiently selecting variables in multivariate calibration of near-infrared spectra. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117376. [Google Scholar] [CrossRef] [PubMed]
- Variable selection in near-infrared spectroscopy: Benchmarking of feature selection methods on biodiesel data. Anal. Chim. Acta 2011, 692, 63–72. [CrossRef] [PubMed]
- Li, P.; Ma, J.; Zhong, N. Raman spectroscopy combined with support vector regression and variable selection method for accurately predicting salmon fillets storage time. Optik 2021, 247, 167879. [Google Scholar] [CrossRef]
- Zhao, F.; Wan, Y.; Nie, L.; Jiao, J.; Gao, D.; Sun, Y.; Chen, Z.; Shi, Y.; Yang, J.; Pan, J.; et al. 1 H NMR-based process understanding and biochemical marker identification methodology for monitoring CHO cell culture process during commercial-scale manufacturing. Biotechnol. J. 2023, 18, e2200616. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dong, X.; Wan, Y.; Gao, D.; Chen, Z.; Zhang, Y.; Zheng, Z.; Chen, K.; Jiao, J.; Sun, Y.; et al. Development of an in-line Raman analytical method for commercial-scale CHO cell culture process monitoring: Influence of measurement channels and batch number on model performance. Biotechnol. J. 2023, 19, e2300395. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Chen, Z.; Yi, S. Wavelength selection algorithm based on minium correlation coefficient for multivariate calibartion. Spectrosc. Spectr. Anal. 2022, 42, 719–725. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemom. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Centner, V.; Massart, D.-L.; De Noord, O.E.; De Jong, S.; Vandeginste, B.M.; Sterna, C. Elimination of Uninformative Variables for Multivariate Calibration. Anal. Chem. 1996, 68, 3851–3858. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.-J.; Wu, H.-L.; Cai, C.-B.; Xu, L.; Yu, R.-Q. An ensemble of Monte Carlo uninformative variable elimination for wavelength selection. Anal. Chim. Acta 2008, 612, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Zhao, Z.; Jia, K.; Li, X. A feasibility study on quantitative analysis of glucose and fructose in lotus root powder by FT-NIR spectroscopy and chemometrics. Food Chem. 2012, 133, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-H.; Berry, R.J.; Siesler, H.W.; Ozaki, Y. Wavelength Interval Selection in Multicomponent Spectral Analysis by Moving Window Partial Least-Squares Regression with Applications to Mid-Infrared and Near-Infrared Spectroscopic Data. Anal. Chem. 2002, 74, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Jouan-Rimbaud, D.; Massart, D.-L.; Leardi, R.; De Noord, O.E. Genetic Algorithms as a Tool for Wavelength Selection in Multivariate Calibration. Anal. Chem. 1995, 67, 4295–4301. [Google Scholar] [CrossRef]
- Leardi, R. Application of genetic algorithm-PLS for feature selection in spectral data sets. J. Chemom. 2000, 14, 643–655. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Li, H.-D.; Wood, L.R.E.; Fan, W.; Wang, J.-J.; Cao, D.-S.; Xu, Q.-S.; Liang, Y.-Z. An efficient method of wavelength interval selection based on random frog for multivariate spectral calibration. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 111, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, H.; Mehdizadeh, H.; Drapeau, D.; Yoon, S. In-line monitoring of amino acids in mammalian cell cultures using Raman spectroscopy and multivariate chemometrics models. Eng. Life Sci. 2018, 18, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; André, S.; Saint Cristau, L.; Lagresle, S.; Hannas, Z.; Calvosa, É.; Devos, O.; Duponchel, L. Multivariate statistical process control (MSPC) using Raman spectroscopy for in-line culture cell monitoring considering time-varying batches synchronized with correlation optimized warping (COW). Anal. Chim. Acta 2017, 952, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Berry, B.; Moretto, J.; Matthews, T.; Smelko, J.; Wiltberger, K. Cross-scale predictive modeling of CHO cell culture growth and metabolites using Raman spectroscopy and multivariate analysis. Biotechnol. Progr. 2015, 31, 566–577. [Google Scholar] [CrossRef]
- Zavala-Ortiz, D.A.; Denner, A.; Aguilar-Uscanga, M.G.; Marc, A.; Ebel, B.; Guedon, E. Comparison of partial least square, artificial neural network, and support vector regressions for real-time monitoring of CHO cell culture processes using in situ near-infrared spectroscopy. Biotechnol. Bioeng. 2022, 119, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Hubli, G.B.; Banerjee, S.; Rathore, A.S. Near-infrared spectroscopy based monitoring of all 20 amino acids in mammalian cell culture broth. Talanta 2023, 254, 124187. [Google Scholar] [CrossRef]
- Tanemura, H.; Kitamura, R.; Yamada, Y.; Hoshino, M.; Kakihara, H.; Nonaka, K. Comprehensive modeling of cell culture profile using Raman spectroscopy and machine learning. Sci. Rep. 2023, 13, 21805. [Google Scholar] [CrossRef]
- Li, H.-D.; Xu, Q.-S.; Liang, Y.-Z. libPLS: An integrated library for partial least squares regression and linear discriminant analysis. Chemom. Intell. Lab. Syst. 2018, 176, 34–43. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Q.; Ma, S.; Liu, H.; Dong, B.; Yang, J.; Liu, D. Prussian Blue as a Highly Sensitive and Background-Free Resonant Raman Reporter. Anal. Chem. 2017, 89, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, Y.; Lin, K.; Zhou, X.; Liu, S.; Sun, J. New spectral assignment of n-propanol in the C―H stretching region. J. Raman Spectrosc. 2016, 47, 1385–1393. [Google Scholar] [CrossRef]
- De Gelder, J.; De Gussem, K.; Vandenabeele, P.; Moens, L. Reference database of Raman spectra of biological molecules. J. Raman Spectrosc. 2007, 38, 1133–1147. [Google Scholar] [CrossRef]
- Pecul, M.; Rizzo, A.; Leszczynski, J. Vibrational Raman and Raman Optical Activity Spectra of d-Lactic Acid, d-Lactate, and d-Glyceraldehyde: Ab Initio Calculations. J. Phys. Chem. A 2002, 106, 11008–11016. [Google Scholar] [CrossRef]


| Spectral Range | N | Analyzed Components | Literature |
|---|---|---|---|
| 800–1800 cm−1 | 1001 | key biochemical values, VCD | [9] |
| 500–1700 cm−1 | 1201 | Gluc, Lac | [19] |
| 400–1600 cm−1 | 1201 | Gluc, Lac | [8] |
| 400–1800 cm−1 | 1401 | Gluc, Lac, titer | [20] |
| 440–1860 cm−1 | 1421 | VCD, Lac, NH4+, amino acids | [17] |
| 350–1775 cm−1 | 1426 | Gluc, Lac, VCD | [21] |
| 321–1890 cm−1 | 1570 | Gluc, Lac, amino acids | [22] |
| 350–1775 cm−1, 2800–3000 cm−1 | 1627 | Gluc, Lac | [23] |
| 415–1800 cm−1, 2800–3100 cm−1 | 1687 | VCD, viability, cell diameter | [24] |
| 300–1850 cm−1, 2900–3200 cm−1 | 1852 | Gluc, Lac, Glu, VCD | [25] |
| 415–1850 cm−1, 1860–2100 cm−1, 2800–3100 cm−1 | 1978 | Gluc, Lac, NH4+ | [16] |
| 500–3100 cm−1 | 2601 | Gluc, phenylalanine | [7] |
| 400–3200 cm−1 | 2801 | Gluc, VCD, Lac, NH4+ | [26] |
| Component (Unit) | Batches 1–15 | Batch 16 | ||||
|---|---|---|---|---|---|---|
| n | Range | Mean ± SD | n | Range | Mean ± SD | |
| Gluc (g/L) | 173 | 1.50–4.18 | 2.42 ± 0.61 | 13 | 1.61–4.17 | 2.49 ± 0.68 |
| Lac (g/L) | 168 | 0.13–1.51 | 0.54 ± 0.36 | 13 | 0.18–0.78 | 0.35 ± 0.20 |
| VCD (×106 cells/mL) | 173 | 0.60–6.40 | 4.57 ± 1.59 | 13 | 0.75–6.06 | 4.65 ± 1.61 |
| NH4+ (mmol/L) | 169 | 2.67–15.39 | 9.91 ± 3.50 | 13 | 3.28–14.83 | 10.94 ± 4.14 |
| Component | Spectral Region | N | LV | Calibration | Prediction | PI (%) | ||
|---|---|---|---|---|---|---|---|---|
| RMSEC | RC | RMSEP | RP | |||||
| Gluc | FP8 + CHS0 | 1500 | 9 | 0.11 | 0.98 | 0.18 | 0.97 | 17 |
| FP17 + CHS0 | 2400 | 14 | 0.09 | 0.99 | 0.17 | 0.97 | 20 | |
| FP5 + CHS2 | 1400 | 7 | 0.13 | 0.98 | 0.14 | 0.98 | 34 | |
| Lac | FP8 + CHS0 | 1500 | 9 | 0.06 | 0.99 | 0.07 | 0.95 | 64 |
| FP17 + CHS0 | 2400 | 15 | 0.03 | 0.99 | 0.07 | 0.95 | 64 | |
| FP5 + CHS2 | 1400 | 5 | 0.06 | 0.98 | 0.07 | 0.94 | 61 | |
| VCD | FP8 + CHS0 | 1500 | 8 | 0.28 | 0.98 | 0.25 | 0.99 | 48 |
| FP17 + CHS0 | 2400 | 12 | 0.29 | 0.98 | 0.41 | 0.98 | 13 | |
| FP5 + CHS2 | 1400 | 9 | 0.27 | 0.99 | 0.26 | 0.99 | 44 | |
| NH4+ | FP8 + CHS0 | 1500 | 7 | 0.55 | 0.99 | 0.61 | 0.99 | 30 |
| FP17 + CHS0 | 2400 | 12 | 0.47 | 0.99 | 0.66 | 0.99 | 24 | |
| FP5 + CHS2 | 1400 | 7 | 0.50 | 0.99 | 0.52 | 0.99 | 39 | |
| Component | Algorithm | N | LV | Calibration | Prediction | PI (%) | ||
|---|---|---|---|---|---|---|---|---|
| RMSEC | RC | RMSEP | RP | |||||
| Gluc | MWPLS | 800 | 15 | 0.10 | 0.98 | 0.15 | 0.98 | 27 |
| GA | 100 | 8 | 0.16 | 0.97 | 0.18 | 0.97 | 16 | |
| RF | 80 | 9 | 0.09 | 0.99 | 0.23 | 0.95 | −8 | |
| Lac | MWPLS | 2900 | 14 | 0.05 | 0.99 | 0.11 | 0.88 | 41 |
| GA | 200 | 9 | 0.06 | 0.98 | 0.06 | 0.96 | 66 | |
| RF | 200 | 15 | 0.02 | 0.99 | 0.08 | 0.93 | 54 | |
| VCD | MWPLS | 800 | 10 | 0.36 | 0.97 | 0.44 | 0.96 | 8 |
| GA | 300 | 13 | 0.28 | 0.98 | 0.34 | 0.98 | 29 | |
| RF | 60 | 10 | 0.21 | 0.99 | 0.57 | 0.95 | −21 | |
| NH4+ | MWPLS | 100 | 7 | 0.77 | 0.98 | 1.17 | 0.98 | −36 |
| GA | 90 | 13 | 0.56 | 0.99 | 0.69 | 0.99 | 20 | |
| RF | 100 | 10 | 0.29 | 0.99 | 0.70 | 0.99 | 19 | |
| Component | Algorithm | N | LV | Calibration | Prediction | PI(%) | ||
|---|---|---|---|---|---|---|---|---|
| RMSEC | RC | RMSEP | RP | |||||
| Gluc | MWPLS | 600 | 8 | 0.12 | 0.98 | 0.12 | 0.99 | 42 |
| GA | 200 | 7 | 0.13 | 0.98 | 0.16 | 0.97 | 25 | |
| RF | 200 | 12 | 0.06 | 0.99 | 0.19 | 0.95 | 12 | |
| Lac | MWPLS | 1300 | 5 | 0.08 | 0.98 | 0.07 | 0.93 | 60 |
| GA | 100 | 7 | 0.06 | 0.98 | 0.05 | 0.97 | 73 | |
| RF | 300 | 12 | 0.03 | 0.99 | 0.06 | 0.95 | 65 | |
| VCD | MWPLS | 1100 | 8 | 0.29 | 0.98 | 0.28 | 0.99 | 41 |
| GA | 400 | 7 | 0.30 | 0.98 | 0.33 | 0.99 | 31 | |
| RF | 100 | 10 | 0.18 | 0.99 | 0.36 | 0.98 | 25 | |
| NH4+ | MWPLS | 1300 | 7 | 0.51 | 0.99 | 0.54 | 0.99 | 37 |
| GA | 400 | 7 | 0.52 | 0.99 | 0.49 | 0.99 | 43 | |
| RF | 100 | 14 | 0.29 | 0.99 | 0.62 | 0.99 | 29 | |
| Component | Combination | N | LV | Calibration | Prediction | PI(%) | ||
|---|---|---|---|---|---|---|---|---|
| RMSEC | RC | RMSEP | RP | |||||
| Gluc | MWPLS-GA | 200 | 5 | 0.16 | 0.97 | 0.19 | 0.96 | 10 |
| MWPLS-RF | 70 | 12 | 0.09 | 0.99 | 0.12 | 0.99 | 43 | |
| MWPLS-GA-RF | 50 | 6 | 0.13 | 0.98 | 0.16 | 0.98 | 26 | |
| Lac | MWPLS-GA | 100 | 5 | 0.07 | 0.98 | 0.06 | 0.95 | 66 |
| MWPLS-RF | 90 | 14 | 0.04 | 0.99 | 0.10 | 0.87 | 47 | |
| MWPLS-GA-RF | 60 | 5 | 0.07 | 0.98 | 0.05 | 0.96 | 70 | |
| VCD | MWPLS-GA | 100 | 6 | 0.34 | 0.98 | 0.26 | 0.99 | 46 |
| MWPLS-RF | 200 | 10 | 0.17 | 0.99 | 0.44 | 0.96 | 7 | |
| MWPLS-GA-RF | 30 | 9 | 0.30 | 0.98 | 0.40 | 0.97 | 16 | |
| NH4+ | MWPLS-GA | 200 | 7 | 0.52 | 0.99 | 0.52 | 0.99 | 40 |
| MWPLS-RF | 200 | 12 | 0.25 | 0.99 | 0.79 | 0.99 | 8 | |
| MWPLS-GA-RF | 50 | 8 | 0.47 | 0.99 | 0.58 | 0.99 | 33 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Yan, X.; Qu, H. Improving Raman-Based Models for Real-Time Monitoring the CHO Cell Culture Process with Effective Variable Selection Strategies. Appl. Sci. 2024, 14, 8890. https://doi.org/10.3390/app14198890
Dong X, Yan X, Qu H. Improving Raman-Based Models for Real-Time Monitoring the CHO Cell Culture Process with Effective Variable Selection Strategies. Applied Sciences. 2024; 14(19):8890. https://doi.org/10.3390/app14198890
Chicago/Turabian StyleDong, Xiaoxiao, Xu Yan, and Haibin Qu. 2024. "Improving Raman-Based Models for Real-Time Monitoring the CHO Cell Culture Process with Effective Variable Selection Strategies" Applied Sciences 14, no. 19: 8890. https://doi.org/10.3390/app14198890
APA StyleDong, X., Yan, X., & Qu, H. (2024). Improving Raman-Based Models for Real-Time Monitoring the CHO Cell Culture Process with Effective Variable Selection Strategies. Applied Sciences, 14(19), 8890. https://doi.org/10.3390/app14198890







