Study of the Oxidation of Phenol in the Presence of a Magnetic Composite Catalyst CoFe2O4/Polyvinylpyrrolidone
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CoFe2O4 and CoFe2O4/Polyvinylpyrrolidone Magnetic Composite Catalyst
2.3. Determination of Structural Characteristics of the Obtained Magnetic Composites and Process Products
3. Results
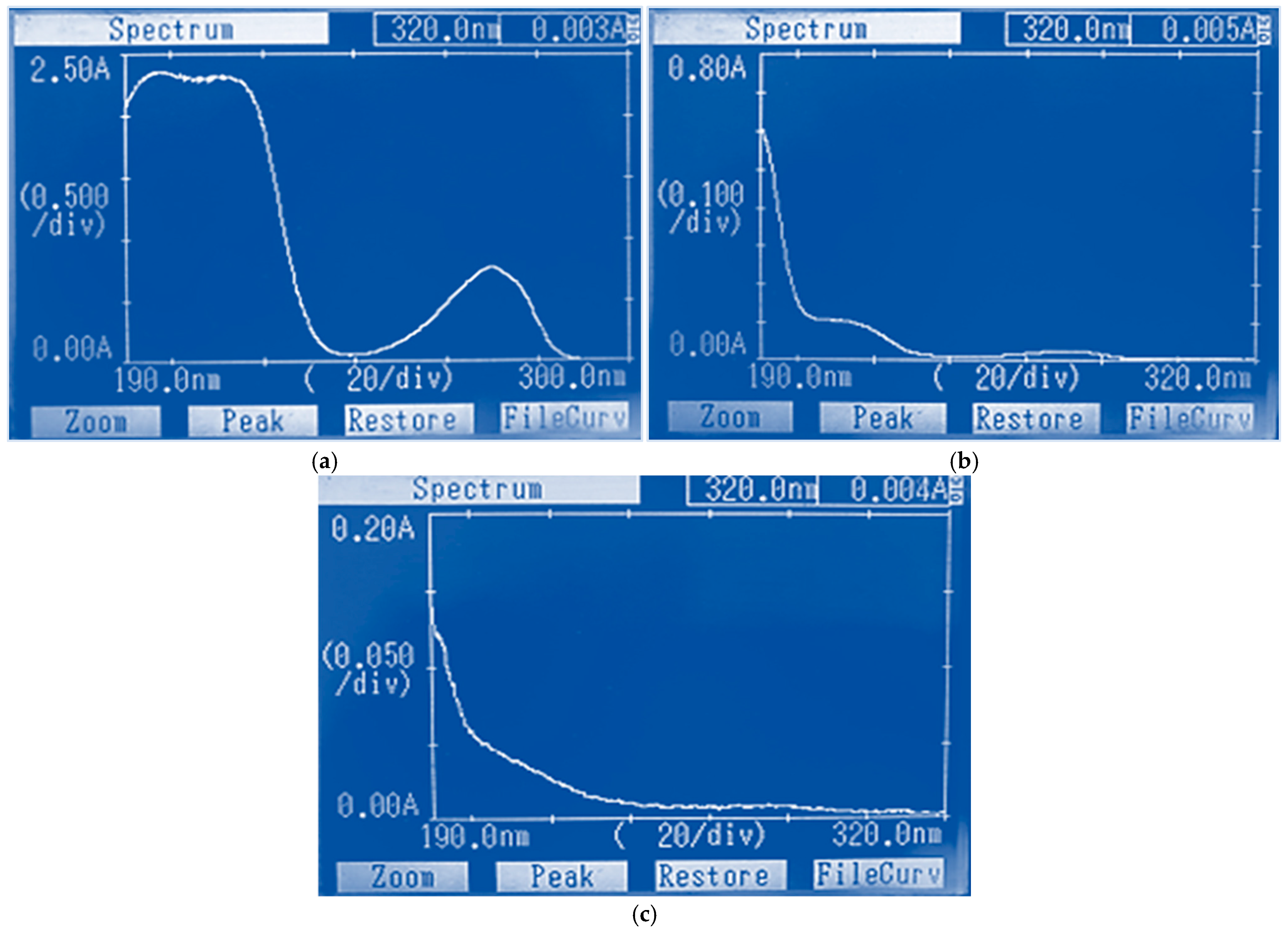
3.1. Studying the Properties of Nanocomposites Using Physico-Chemical Research Methods
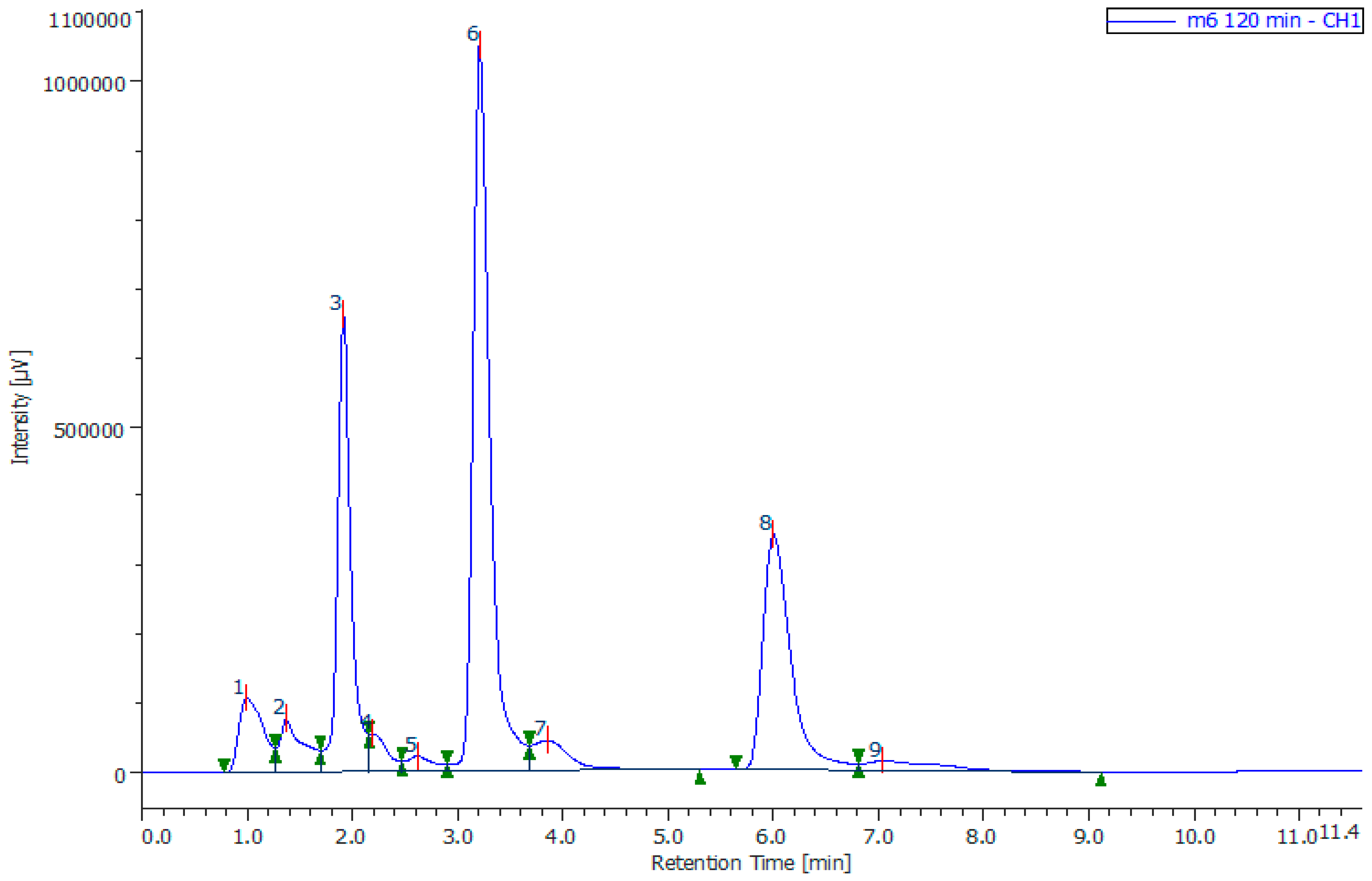
3.2. Testing of Synthesized CoFe2O4/PVP Nanocomposite during Phenol Oxidation
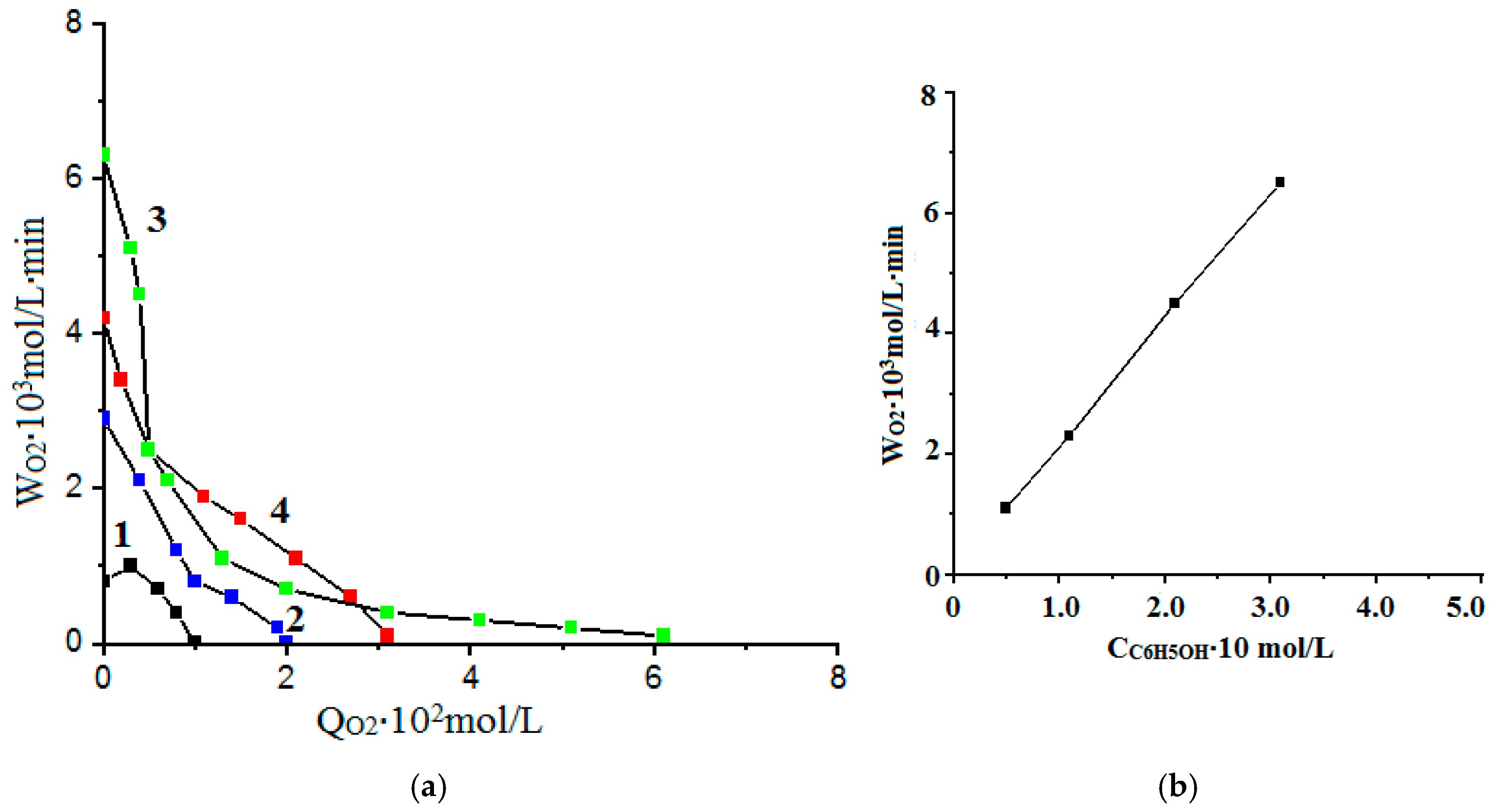
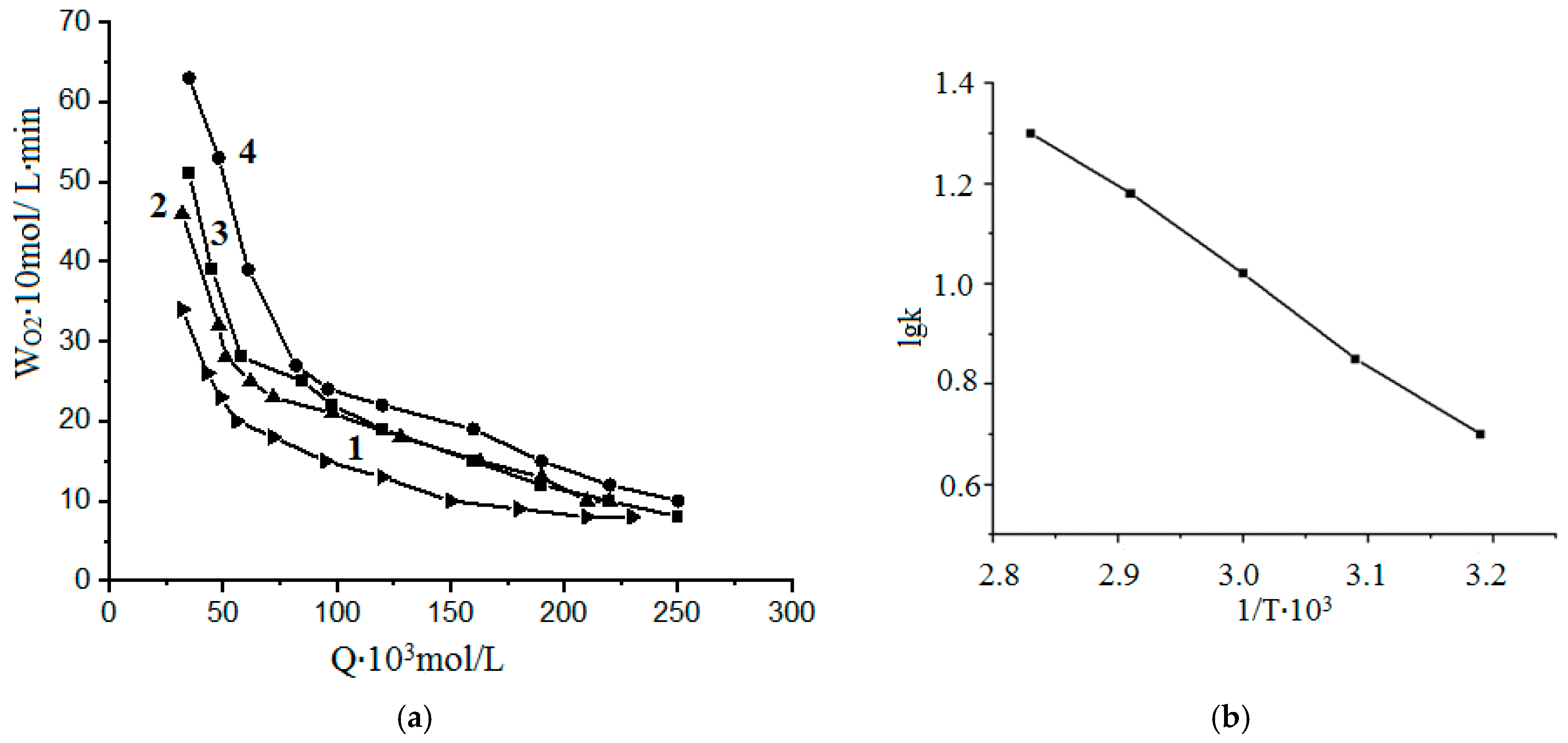
- The kinetic regularities of the oxidation reaction of phenol with hydrogen peroxide in the presence of CoFe2O4/PVP were studied at different pH values and concentrations of reagents (catalyst, hydrogen peroxide, and phenol).
- The results were processed in coordinates W = f (Q) and φ = f (Q),
- where
- W is the rate of oxygen absorption (mol/L∙min);
- Q is the amount of oxygen absorbed (mol/L).
- The reaction rate was calculated using the following formula:
- where f = (Plab∙T0)/(P0 (T0 + Texp,)),
- where Texp is the temperature of the experiment, °C.
- T0 is the standard temperature of 273.2 °C.
- P0 is the standard pressure of 101.3 Pa.
- Plab is the pressure in the laboratory, kPa.
- Then, W = ∆V/∆T∙f/(22.4∙60∙10),
- where 22.4 is the volume of 1 mole of gas, L/mol.
- ∆V/∆T is the volume of absorbed oxygen (mL) per unit time (min).
- 60 is 60 s in 1 min,
- 10 is the volume of the starting substance, mL.
- The amount of absorbed oxygen was calculated using the following formula:
- where Vt is a volume of absorbed oxygen (mL) at a certain point in time (min).
- V0 is a volume of absorbed oxygen (mL) at the beginning of the experiment (min).
- The calculation of the activation energy (Ea) of the reaction at different temperatures (T1 and T2) and at the rate constants for these reactions k1 and k2, respectively, was carried out using the following formula:
- where k1 and k2 are the rate constants at temperatures T1 and T2;
- 8.314 is the universal gas constant, J/mol.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Usov, N.A. Expert Opinion: Magnetic Nanoparticles: Theory and Modern Technological Applications. 5 February 2016. Available online: https://habr.com/ru/companies/misis/articles/390127/ (accessed on 29 July 2024).
- Toshima, N.; Yonezawa, T. Bimetallic nanoparticles novel materials for chemical and physical applications. New J. Chem. 1998, 22, 1179–1201. [Google Scholar] [CrossRef]
- Pachón, L.D.; Rothenberg, G. Transition-metal nanoparticles: Synthesis, stability and the leaching issue. Appl. Organomet. Chem. 2008, 22, 288–299. [Google Scholar] [CrossRef]
- Pileni, M.P. Magnetic Fluids: Fabrication, Magnetic Properties, and Organization of Nanocrystals. Adv. Funct. Mater. 2001, 11, 323–336. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.W.; Choi, H.; Lee, J.H.; Hur, H.G. Synthesis of nanosized biogenic magnetite and comparison of its catalytic activity in ozonation. Appl. Catal. B Environ. 2008, 83, 208–213. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Kuznetsov, N.T.; Meshalkin, V.P.; Salerno, M.; Fabiano, B. Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor. Found. Chem. Eng. 2016, 50, 59–66. [Google Scholar] [CrossRef]
- Sassykova, L.; Shakiyeva, T.; Dossumova, B.; Ilmuratova, M.S.; Muktaly, D.; Zhaxibayeva, Z.M.; Sassykova, A.; Baizhomartov, B. Catalysts, magnetic composites for removal of phenol-containing compounds from wastewater. Rasayan J. Chem. 2023, 16, 1605–1612. [Google Scholar] [CrossRef]
- Reverberi, A.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of Copper Nanoparticles in Ethylene Glycol by Chemical Reduction with Vanadium (+2) Salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef]
- Khabibullin, V.R.; Stepanov, G.V. Effect of a low-frequency magnetic field on the release of heat by magnetic nanoparticles of different shapes. Russ. J. Phys. Chem. A 2020, 94, 439–444. [Google Scholar] [CrossRef]
- Prijic, S.; Sersa, G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol. Oncol. 2011, 45, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.S.A.; Kim, H.; Lee, H.; Ryu, C.; Lee, J.Y.; Yoon, J. Synthesis of Magnetic Ferrite Nanoparticles with High Hyperthermia Performance via a Controlled Co-Precipitation Method. Nanomaterials 2019, 9, 1176. [Google Scholar] [CrossRef] [PubMed]
- Fedosyuk, V.M.; Mirgorod, Y.A. Investigation of structure and magnetic properties of cobalt-nickel and manganese ferrites nanoparticles synthesized in direct micelles of sodium dodecyl sulphate system. Proc. Natl. Acad. Sci. Belarus Phys.-Tech. Ser. 2016, 1, 93–98. [Google Scholar]
- Chekanova, A.E.; Sorkina, T.A.; Dubov, A.L.; Nikiforov, V.N.; Davydova, G.A.; Selezneva, I.I.; Goodilin, E.A.; Trusov, L.A.; Korolev, V.V.; Aref’ev, I.M.; et al. New environmental nontoxic agents for the preparation of core-shell magnetic nanoparticles. Mendeleev Commun. 2009, 19, 72–74. [Google Scholar] [CrossRef]
- Koksharov, Y.A.; Gubin, S.P.; Taranov, I.V.; Khomutov, G.B.; Gulyaev, Y.V. Magnetic Nanoparticles in Medicine: Progress, Problems, and Advances. J. Commun. Technol. Electron. 2022, 67, 101–116. [Google Scholar] [CrossRef]
- Petranovska, A.L.; Fedorenko, O.M.; Horbyk, P.P.; Chuyko, O.O.; Chehun, V.F.; Dubrovin, I.V.; Semko, L.S.; Storozhuk, L.P.; Abramov, M.V.; Revo, S.L. Development and properties of magnetically sensitive nanocomposites for directed transport of medicinal products. Nanosistemi Nanomater. Nanotehnol. 2005, 3, 812–823. [Google Scholar]
- Sassykova, L.; Nalibayeva, A.; Aubakirov, Y.; Tashmukhambetova, Z.; Otzhan, U.; Zhakirova, N.; Faizullaeva, M. Preparation and study of catalysts on metal blocks for neutralization of exhaust gases of the stationary diesel generator. Orient. J. Chem. 2017, 33, 1941–1948. [Google Scholar] [CrossRef]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; González-Carreño, T.; Serna, C.J. The preparation of magnetic nanoparticles for applications in biomedicine. J. Phys. D 2003, 36, R182–R197. [Google Scholar] [CrossRef]
- Zhang, W.X. Nanoscale iron particles for environmental remediation: An overview. J. Nanopart. Res. 2003, 5, 323–332. [Google Scholar] [CrossRef]
- Nehra, P.; Chauhan, R.P.; Garg, N.; Verma, K. Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. Br. J. Biomed. Sci. 2017, 75, 13–18. [Google Scholar] [CrossRef]
- Xenariou, S.; Griesenbach, U.; Ferrari, S.; Dean, P.; Scheule, R.K.; Cheng, S.H.; Geddes, D.M.; Plank, C.; Alton, E.W.F.W. Using magnetic forces to enhance non-viral gene transfer to airway epithelium in vivo. Gene Ther. 2006, 13, 1545–1552. [Google Scholar] [CrossRef] [PubMed]
- Baranov, D.A.; Gubin, S.P. Magnetic nanoparticles: Advances and problems of chemical synthesis. Radio Electron. Nanosyst. Inf. Technol. 2009, 1, 129–147. [Google Scholar]
- Khedr, M.H.; Omar, A.A.; Abdel-Moarty, S.A. Magnetic nanocomposites: Preparation and characterization of Co-ferrite nanoparticles. Colloids Surf. A 2006, 281, 8–14. [Google Scholar] [CrossRef]
- Zi, Z.; Sun, Y.; Zhu, X.; Yang, Z.; Dai, J.; Song, W. Synthesis and magnetic properties of CoFe2O4 ferrite nanoparticles. J. Magn. Magn. Mater. 2009, 321, 1251–1255. [Google Scholar] [CrossRef]
- Stanislavov, A.S.; Yanovska, A.A.; Kuznetsov, V.N.; Sukhodub, L.B.; Sukhodub, L.F. The Comparison of Magnetite Nanospheres Formation in Polysaccharide Covers by Various Ways of Syntheses. J. Nano-Electron. Phys. 2015, 7, 02009-1. [Google Scholar]
- Mmelesi, O.K.; Masunga, N.; Kuvarega, A.; Nkambule, T.T.; Mamba, B.B.; Kefeni, K.K. Cobalt ferrite nanoparticles and nano-composites: Photocatalytic, antimicrobial activity and toxicity in water treatment. Mater. Sci. Semicond. 2021, 123, 105523. [Google Scholar] [CrossRef]
- Kritika, R.I. Therapeutic applications of magnetic nanoparticles: Recent advances. Mater. Adv. 2022, 3, 7425–7444. [Google Scholar] [CrossRef]
- Guélou, E.; Barrault, J.; Fournier, J.; Tatibouët, J.-M. Active iron species in the catalytic wet peroxide oxidation of phenol over pillared clays containing iron. Appl. Catal. B 2003, 44, 1–8. [Google Scholar] [CrossRef]
- Oliveira, R.V.M.; Costa, J.A.S.; Romão, L.P.C. Bifunctional green nanoferrites as catalysts for simultaneous organic pollutants reduction and hydrogen generation: Upcycling strategy. J. Environ. Manag. 2024, 351, 119994. [Google Scholar] [CrossRef]
- Iorio, E.D.; Colombo, C.; Cheng, Z.; Capitani, G.; Mele, D.; Ventruti, G.; Angelico, R. Characterization of magnetite nano-particles synthetized from Fe(II)/nitrate solutions for arsenic removal from water. J. Environ. Chem. Eng. 2019, 7, 102986. [Google Scholar] [CrossRef]
- Brussino, P.; Gross, M.S.; Ulla, M.A.; Banús, E.D. Copper and iron-based monolithic catalysts for phenol Catalytic Wet Per-oxide Oxidation (CWPO): Support and iron effects on the catalytic performance. J. Environ. Chem. Eng. 2023, 11, 110858. [Google Scholar] [CrossRef]
- Shapkin, N.P.; Panasenko, A.E.; Khal’chenko, I.G.; Pechnikov, V.S.; Maiorov, V.Y.; Maslova, N.V.; Razov, V.I.; Papynov, E.K. Magnetic Composites Based on Cobalt Ferrite, Vermiculite, and Rice Husks: Synthesis and Properties. Russ. J. Inorg. Chem. 2020, 65, 1614–1622. [Google Scholar] [CrossRef]
- Silva, A.S.; Roman, F.F.; Dias, A.V.; Diaz de Tuesta, J.L.; Narcizo, A.; Silva, A.P.F.; Çaha, I.; Deepak, F.L.; Bañobre-López, M.; Ferrari, A.M.C.; et al. Hybrid multi-core shell magnetic nanoparticles for wet peroxide oxidation of paracetamol: Application in synthetic and real matrices. J. Environ. Chem. Eng. 2023, 11, 110806. [Google Scholar] [CrossRef]
- Zambrzycki, C.; Shao, R.; Misra, A.; Streb, C.; Herr, U.; Güttel, R. Iron based core-shell structures as versatile materials: Mag-netic support and solid catalyst. Catalysts 2021, 11, 72. [Google Scholar] [CrossRef]
- Zhong, L.-S.; Hu, J.-S.; Liang, H.-P.; Cao, A.-M.; Song, W.-G.; Wan, L.-J. Self-Assembled 3D Flowerlike Iron Oxide Nanostructures and Their Application in Water Treatment. Adv. Mater. 2006, 18, 2426–2431. [Google Scholar] [CrossRef]
- Dossumova, B.T.; Sassykova, L.R.; Shakiyeva, T.V.; Muktaly, D.; Batyrbayeva, A.A.; Kozhaisakova, M.A. Catalysts Based on Iron Oxides for Wastewater Purification from Phenolic Compounds: Synthesis, Physicochemical Analysis, Determination of Catalytic Activity. ChemEngineering 2024, 8, 8. [Google Scholar] [CrossRef]
- Tomita, K.; Oshima, Y. Stability of manganese oxide in catalytic supercritical water oxidation of phenol. Ind. Eng. Chem. Res. 2004, 43, 7740–7743. [Google Scholar] [CrossRef]
- Tsuchida, E.; Nishide, H. Polymer-Metal Complexes and Their Catalytic Activity. Adv. Polym. Sci. 1977, 24, 1–87. [Google Scholar]
- Habibi, D.; Faraji, A.R.; Arshadi, M.; Veisi, H.; Gil, A. Manganese nanocatalyst and N-hydroxyphthalimide as an efficient catalytic system for selective oxidation of ethylbenzene, cyclohexene and oximes under aerobic condition. J. Mol. Catal. A Chem. 2014, 382, 41–54. [Google Scholar] [CrossRef]
- Wanna, W.H.; Janmanchi, D.; Thiyagarajan, N.; Ramu, R.; Tsai, Y.F.; Yu, S.S.F. Selective oxidation of simple aromatics catalyzed by nano-biomimetic metal oxide catalysts: A mini review. Front. Chem. 2020, 8, 589178. [Google Scholar] [CrossRef]
- Arefieva, O.D.; Vasilyeva, M.S.; Kuryavy, V.G.; Ustinov, A.Y.; Zemnukhova, L.A.; Gushchina, D.D. Oxidative destruction of phenol on Fe/SiO2 catalysts. Water Sci. Technol. 2020, 81, 2189–2201. [Google Scholar] [CrossRef]
- Tulepov, M.; Mansurov, Z.; Sassykova, L.; Baiseitov, D.; Dalelhanuly, O.; Ualiev, Z.; Gabdrashova, S.; Kudyarova, Z. Research of iron-containing concentrates of Balkhash deposit (Kazakhstan) for processing of low-grade coal. J. Chem. Technol. Metall. 2019, 54, 531–538. [Google Scholar]
- Cabot, A.; Puntes, V.F.; Shevchenko, E.; Yin, Y.; Balcells, L.; Marcus, M.A.; Hughes, S.M.; Alivisatos, A.P. Vacancy Coalescence during Oxidation of Iron Nanoparticles. J. Am. Chem. Soc. 2007, 129, 10358–10360. [Google Scholar] [CrossRef]
- Yi, D.K.; Lee, S.S.; Ying, J.Y. Synthesis and applications of magnetic nanocomposite catalysts. Chem. Mater. 2006, 18, 2459–2461. [Google Scholar] [CrossRef]
- Sashko, N.; Vaitulevich, E.; Yurmazova, T. Synthesis and Properties of Iron-Based Magnetic Nanoparticles. Key Eng. Mater. 2016, 712, 282–287. [Google Scholar] [CrossRef]
- Sassykova, L.R.; Dossumova, B.T.; Ilmuratova, M.S.; Shakiyeva, T.V.; Baizhomartov, B.B.; Sassykova, A.R.; Zhaxibayeva, Z.M.; Abildin, T.S. Development of nanostructured catalysts for catalytic oxidative water purification from organic impuri-ties, including phenolic compounds. Chim. Techno Acta 2023, 10, 202310309. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, H.; Yan, Y. Preparation of novel catalyst-free Fe3C nanocrystals encapsulated NCNT structured catalyst for continuous catalytic wet peroxide oxidation of phenol. J. Hazard. Mater. 2021, 407, 124371. [Google Scholar] [CrossRef]
- Ovejero, G.; Sotelo, J.L.; Martínez, F.; Gordo, L. Novel heterogeneous catalysts in the wet peroxide oxidation of phenol. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef]
- Huth, S.; Lausier, J.; Gersting, S.W.; Rudolph, C.; Plank, C.; Welsch, U.; Rosenecker, J. Insights into the mechanism of magnetofection using PEI-based magnetofectins for gene transfer. J. Genet. Med. 2004, 6, 923–936. [Google Scholar] [CrossRef]
- Baabu, P.R.S.; Kumar, H.K.; Gumpu, M.B.; Babu, K.J.; Kulandaisamy, A.J.; Rayappan, J.B.B. Iron Oxide Nanoparticles: A Review on the Province of Its Compounds, Properties and Biological Applications. Materials 2023, 16, 59. [Google Scholar] [CrossRef]
- Dutta, S.K.; Akhter, M.; Ahmed, J.; Amin, M.K.; Dhar, P.K. Synthesis and Catalytic Activity of Spinel Ferrites: A Brief Review. Biointerface Res. Appl. Chem. 2022, 12, 4399–4416. [Google Scholar]
- Egorova, M.A.; Shabelskaya, N.P.; Radjabov, A.M.; Chernysheva, G.M.; Taranushich, V.A.; Zababurin, V.M.; Vyaltsev, A.V.; Ulyanova, V.A. Obtaining and properties of ferrite and chromite of copper (II). Izv. Vuzov Bull. High. Educ. Inst. North Caucasus Region Tech. Sci. 2021, 2, 69–74. [Google Scholar] [CrossRef]
- Sassykova, L.; Kubekova, S.N.; Batyrbayeva, A.; Azhigulova, R.N.; Zhaxibayeva, Z.M.; Kozhaisakova, M.A.; Zhusupova, L.A.; Sendilvelan, S.; Ponomarenko, O. Hydrogenation of aromatic nitro compounds to amines on nickel and iron-containing catalysts. Rasayan J. Chem. 2021, 14, 1223–1229. [Google Scholar] [CrossRef]
- Naĭden, E.P.; Zhuravlev, V.A.; Itin, V.I.; Terekhova, O.G.; Magaeva, A.A.; Ivanov, Y.F. Magnetic properties and structural parameters of nanosized oxide ferrimagnet powders produced by mechanochemical synthesis from salt solutions. Phys. Solid State 2008, 50, 894–900. [Google Scholar] [CrossRef]
- Bolm, C.; Legros, J.; Le Paih, J.; Zani, L. Iron-catalyzed reactions in organic synthesis. Chem. Rev. 2004, 104, 6217–6254. [Google Scholar]
- Chen, J.P.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Magnetic Properties of nanophase cobalt particles synthesized in inversed micelles. J. Appl. Phys. 1994, 76, 6316–6318. [Google Scholar] [CrossRef]
- Dossumova, B.T.; Shakiyeva, T.V.; Muktaly, D.; Sassykova, L.R.; Baizhomartov, B.B.; Subramanian, S. Synthesis, Characteriza-tion of Magnetic Composites and Testing of Their Activity in Liquid-Phase Oxidation of Phenol with Oxygen. ChemEngineering 2022, 6, 68. [Google Scholar] [CrossRef]
- Narasimharao, K.; Ali, T.T.; Abu-Zied, B.M.; Alfaifi, S.Y. Combustion synthesis of nanocrystalline porous CoFexAl2-xO4 spinels: Structural, textural, magnetic, and electrical properties. Ceram. Int. 2023, 49, 13238–13248. [Google Scholar] [CrossRef]
- Sijo, A.K. Magnetic and structural properties of CoCrxFe2−xO4 spinels prepared by solution self combustion method. Ceram. Int. 2017, 43, 2288–2290. [Google Scholar] [CrossRef]
- Pileni, M.P. The Role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nat. Mater. 2003, 2, 145–150. [Google Scholar] [PubMed]
- Timofeeva, M.; Khankhasaeva, S.; Chesalov, Y.; Tsybulya, S.; Panchenko, V.; Dashinamzhilova, E. Synthesis of Fe, Al-pillared clays starting from the Al, Fe-polymeric precursor: Effect of synthesis parameters on textural and catalytic properties. Appl. Catal. B Environ. 2009, 88, 127–134. [Google Scholar] [CrossRef]
- Catrinescu, C.; Teodosiu, C.; Macoveanu, M.; Miehe-Brendlé, J.; Dred, R.L. Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res. 2003, 37, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Sapir, L.; Stanley, C.B.; Harries, D. Properties of Polyvinylpyrrolidone in a Deep Eutectic Solvent. J. Phys. Chem. A 2016, 120, 3253–3259. [Google Scholar] [CrossRef] [PubMed]
- Dossumova, B.T.; Sassykova, L.R.; Shakiyeva, T.V.; Ilmuratova, M.S.; Sassykova, A.R.; Batyrbayeva, A.A.; Zhaxibayeva, Z.M.; Dzhatkambayeva, U.N.; Baizhomartov, B.B. Catalysts Based on Nanoscale Iron and Cobalt Immobilized on Polymers for Catalytic Oxidation of Aromatic Hydrocarbons: Synthesis, Physico-Chemical Studies, and Tests of Catalytic Activity. Processes 2024, 12, 29. [Google Scholar] [CrossRef]
- López-Ortega, A.; Lottini, E.; Fernández, C.D.J.; Sangregorio, C. Exploring the Magnetic Properties of Cobalt-Ferrite Nano-particles for the Development of a Rare-Earth-Free Permanent Magnet. Chem. Mater. 2015, 27, 4048–4056. [Google Scholar] [CrossRef]
- Xiong, G.; Joly, A.G.; Holtom, G.P.; Wang, C.; McCready, D.E.; Beck, K.M.; Hess, W.P. Excited Carrier Dynamics of α-Cr2O3/α-Fe2O3 Core−Shell Nanostructures. J. Phys. Chem. B 2006, 110, 16937–16940. [Google Scholar] [CrossRef]
- Chithra, M.; Anumol, C.N.; Argish, V.; Sahu, B.N.; Sahoo, S.C. Magnetic properties of co-ferrite nanoparticles prepared by co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 806. [Google Scholar] [CrossRef]
- Nguyen, T.K.C.; Nguyen, A.T. Structural, optical and magnetic properties of Y-doped CoFe2O4 nanoparticles prepared by a simple co-precipitation method. J. Mater. Sci. Mater. Electron. 2023, 34, 448. [Google Scholar] [CrossRef]
- Kakati, S.; Rendale, M.K.; Mathad, S.N. Synthesis, Characterization, and Applications of CoFe2O4 and M-CoFe2O4 (M = Ni, Zn, Mg, Cd, Cu, RE) Ferrites: A Review. Int. J. Self-Propagating High-Temp. Synth. 2021, 30, 189–2019. [Google Scholar] [CrossRef]
- Maaz, K.; Mumtaz, A.; Hasanain, S.K.; Ceylan, A. Synthesis and magnetic properties of cobalt ferrite (CoFe2O4) nanoparticles prepared by wet chemical route. J. Magn. Magn. Mater. 2007, 308, 289–295. [Google Scholar] [CrossRef]
- Siva, K.V.; Kumar, A.; Arockiarajan, A. Structural, magnetic and magnetoelectric investigations on CoFe2O4 prepared via various wet chemical synthesis route: A Comparative Study. J. Magn. Magn. Mater. 2021, 535, 168065. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Feng, Y.; Zhou, Y.; Wen, L.; Shih, K. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 2020, 383, 123056. [Google Scholar] [CrossRef]
- Alaerts, L.; Wahlen, J.; Jacobs, P.A.; De Vos, D.E. Recent progress in the immobilization of catalysts for selective oxidation in the liquid phase. Chem. Commun. 2008, 15, 1727–1737. [Google Scholar] [CrossRef] [PubMed]
- Bantz, C.; Koshkina, O.; Lang, T.; Galla, H.-J.; Kirkpatrick, C.J.; Stauber, R.H.; Maskos, M. The surface properties of nanoparticles determine the agglomeration state and the size of the particles under physiological conditions. Beilstein J. Nanotechnol. 2014, 5, 1774–1786. [Google Scholar] [CrossRef]
- Bu, F.X.; Hu, M.; Xu, L.; Meng, Q.; Mao, G.Y.; Jiang, D.M.; Jiang, J.S. Coordination polymers for catalysis: Enhancement of catalytic activity through hierarchical structuring. Chem. Commun. 2014, 62, 8543–8546. [Google Scholar] [CrossRef]
- Wang, X.; Du, Y.; Ding, S.; Wang, Q.; Xiong, G.; Xie, M.; Shen, X.; Pang, D. Preparation and Third-Order Optical Nonlinearity of Self-Assembled Chitosan/CdSe−ZnS Core−Shell Quantum Dots Multilayer Films. J. Phys. Chem. B 2006, 110, 1566–1570. [Google Scholar] [CrossRef]
- Li, H.; Wang, N.; Li, H.; Ren, Z.; Ma, W.; Li, J.; Du, Y.; Xu, Q. Polyvinylpyrrolidone-induced size-dependent catalytic behavior of Fe sites on N-doped carbon substrate and mechanism conversion in Fenton-like oxidation reaction. Appl. Catal. B 2024, 341, 123323. [Google Scholar] [CrossRef]
- Miguel-Garcha, I.; Berenguer-Murcia, B.; Garcha, T.; Cazorla-Amorуsa, D. Effect of the aging time of PVP coated palladium nanoparticles colloidal suspensions on their catalytic activity in the preferential oxidation of CO. Catal. Today 2012, 187, 2–9. [Google Scholar] [CrossRef]
- Chremos, A.; Glynos, E.; Koutsos, V.; Camp, P.J. Adsorption and self-assembly of linear polymers on surfaces: A computer simulation study. Soft Matter 2009, 5, 637–645. [Google Scholar] [CrossRef]
- Aiken, J.D.; Finke, R.G. A review of modern transition-metal nanoclusters: Their synthesis, characterization, and applications in catalysis. J. Mol. Catal. A Chem. 1999, 145, 1–44. [Google Scholar] [CrossRef]
- Ruas, C.P.; Fischer, D.K.; Gelesky, M.A. PVP-Stabilized Palladium Nanoparticles in Silica as Effective Catalysts for Hydrogenation Reactions. J. Nanotechnol. 2013, 2013, 906740. [Google Scholar] [CrossRef]
- Mohammadi-Samani, S.; Miri, R.; Salmanpour, M.; Khalighian, N.; Sotoudeh, S.; Erfani, N. Preparation and assessment of chitosan-coated superparamagnetic Fe3O4 nanoparticles for controlled delivery of methotrexate. Res. Pharm. Sci. 2013, 8, 25–33. [Google Scholar]
- Singh, A.N.; Singh, S.; Suthar, N.; Dubey, V.K. Glutaraldehyde-Activated Chitosan Matrix for Immobilization of a Novel Cysteine Protease, Procerain B. J. Agric. Food Chem. 2011, 59, 6256–6262. [Google Scholar] [CrossRef]
- Inbaraj, S.B.; Tsai, T.Y.; Chen, B.H. Synthesis, characterization and antibacterial activity of superparamagnetic nanoparticles modified with glycol chitosan. Sci. Technol. Adv. Mater. 2012, 13, 015002. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Mei, Y.; Ballauff, M.; Drechsler, M. Thermosensitive Core−Shell Particles as Carrier Systems for Metallic Nanoparticles. J. Phys. Chem. B 2006, 110, 3930–3937. [Google Scholar] [CrossRef]
- Vollath, D.; Fischer, F.D.; Holec, D. Surface energy of nanoparticles—Influence of particle size and structure. Beilstein J. Nanotechnol. 2018, 9, 2265–2276. [Google Scholar] [CrossRef] [PubMed]
- Paukshtis, E.A.; Yurchenko, E.N. Study of the Acid–Base Properties of Heterogeneous Catalysts by Infrared Spectroscopy. Russ. Chem. Rev. 1983, 52, 242–258. [Google Scholar] [CrossRef]
- Tsyganenko, A.A.; Smirnov, K.S.; Rzhevskij, A.M.; Mardilovich, P.P. Infrared spectroscopic evidence for the structural OH groups of spinel alumina modifications. Mater. Chem. Phys. 1990, 26, 35–46. [Google Scholar] [CrossRef]
- Moharram, M.A.; Khafagi, M.G. Application of FTIR spectroscopy for structural characterization of ternary poly(acrylic acid)–metal–poly(vinyl pyrrolidone) complexes. J. Appl. Polym. Sci. 2007, 105, 1888–1893. [Google Scholar] [CrossRef]
- Proskurnin, M.A.; Loginova, E.; Volkov, D.S. Analysis of Water-Soluble Polymers—Polyvinylpyrrolidone and Polyethyleneimine—By FTIR-Spectroscopy. Available online: https://www.researchgate.net/publication/312116573_Analysis_of_water-soluble_polymers-polyvinylpyrrolidone_and_polyethyleneimine-by_FTIR-spectroscopy (accessed on 29 July 2024).
- Leyva, E.; Moctezuma, E.; Baines, K.M.; Noriega, S.; Zarazua, E. A Review on Chemical Advanced Oxidation Processes for Pharmaceuticals with Paracetamol as a Model Compound. Reaction Conditions, Intermediates and Total Mechanism. Curr. Org. Chem. 2017, 22, 2–17. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of Catalysis on the Stability of Metallic Nanoparticles: Suzuki Reaction Catalyzed by PVP-Palladium Nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Lagunas, A.; Jimeno, C.; Font, D.; Solà, L.; Pericàs, M.A. Mechanistic Studies on the Conversion of Dicobalt Octacarbonyl into Colloidal Cobalt Nanoparticles. Langmuir 2006, 22, 3823–3829. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.S.; Diaz de Tuesta, J.L.; Gomes, H.T. Мulti-core shell nanoparticles for efficient removal of nitrophenols from contaminated streams. Mech. Technol. Sci. J. 2023, 2, 133–141. [Google Scholar] [CrossRef]
- Li, R.Z.; Hu, A.; Bridges, D.; Zhang, T.; Oakes, K.D.; Peng, R.; Tumuluri, U.; Wu, Z.; Zhili Feng, Z. Robust Ag nanoplate ink for flexible electronics packaging. Nanoscale 2015, 7, 7368–7377. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]

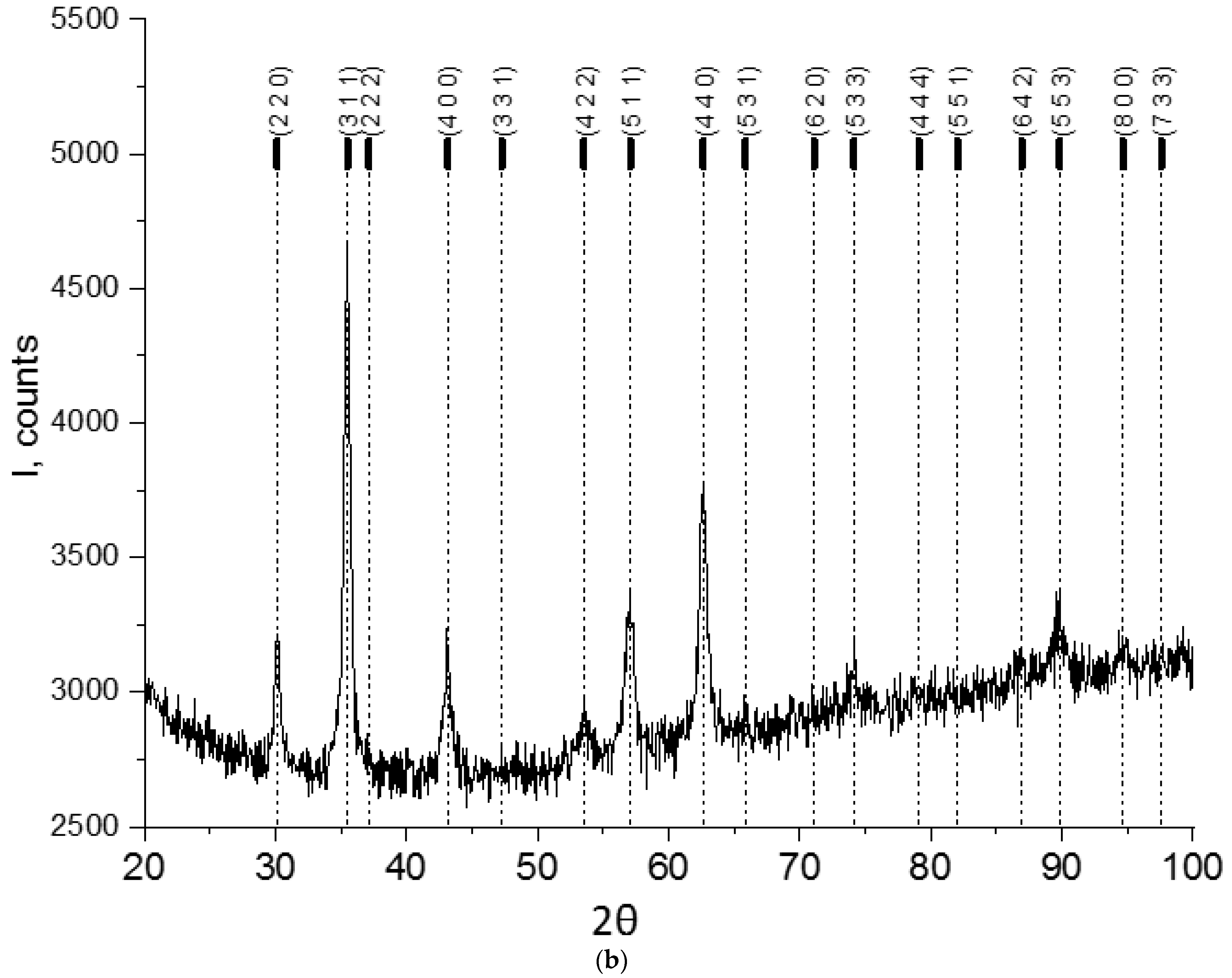

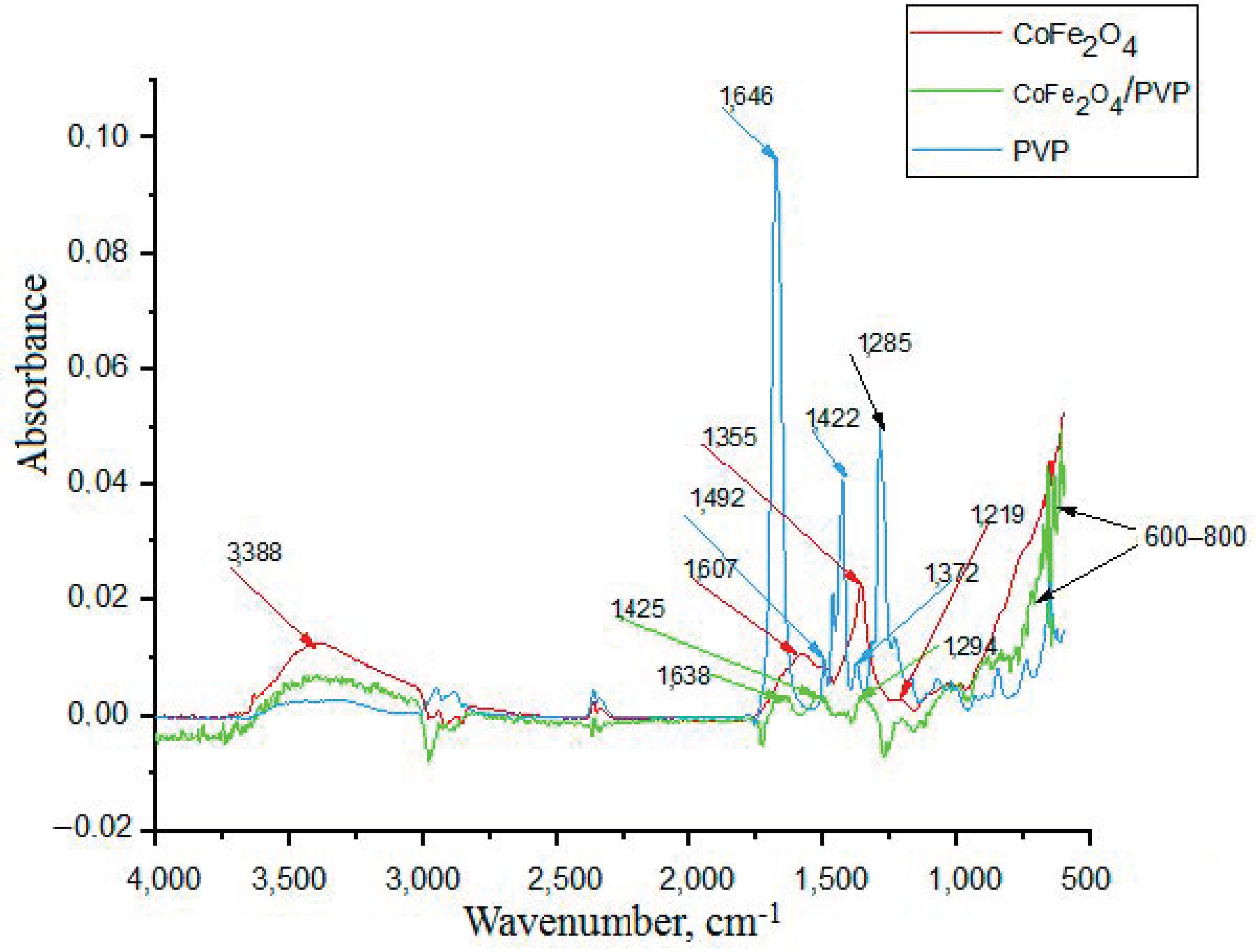
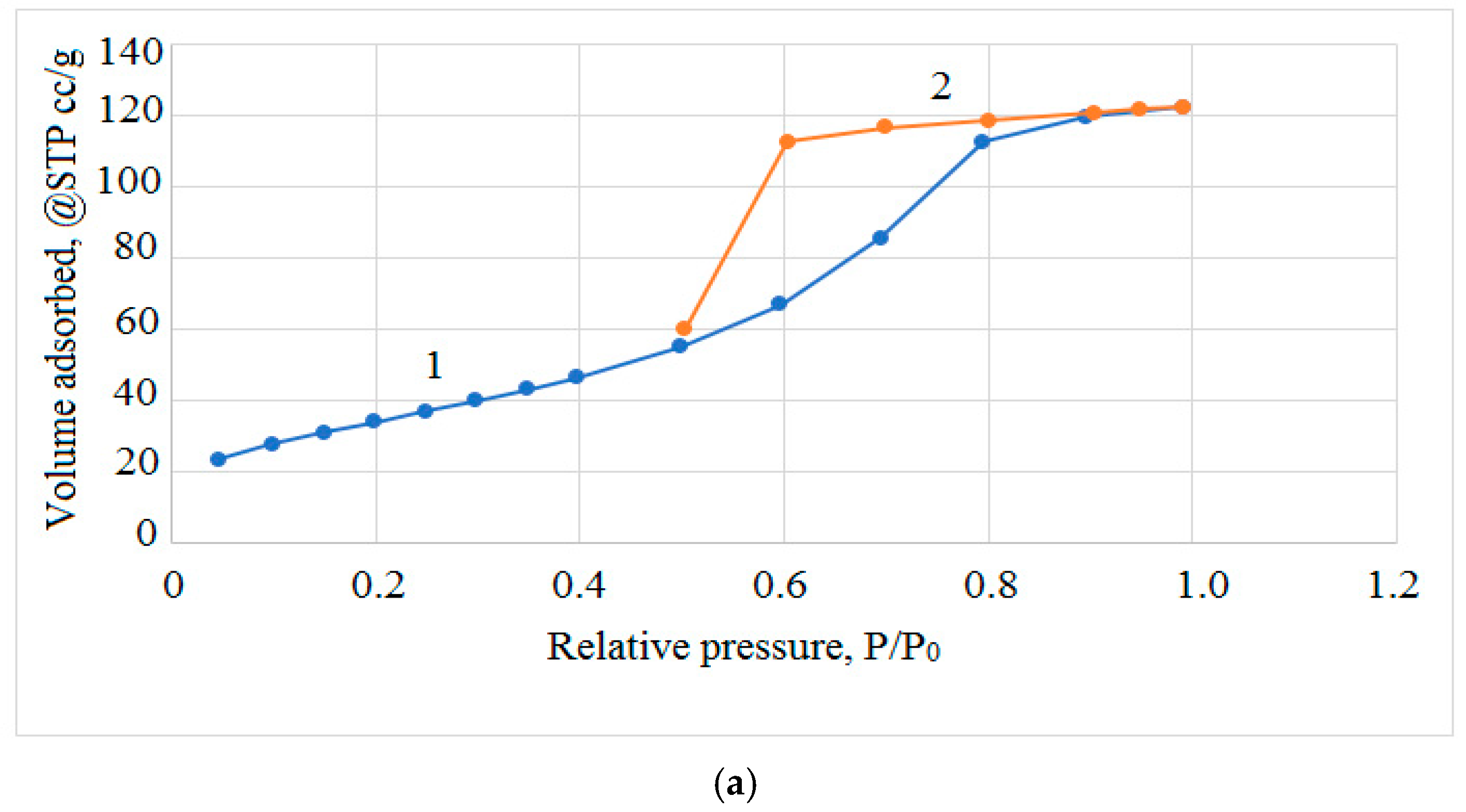


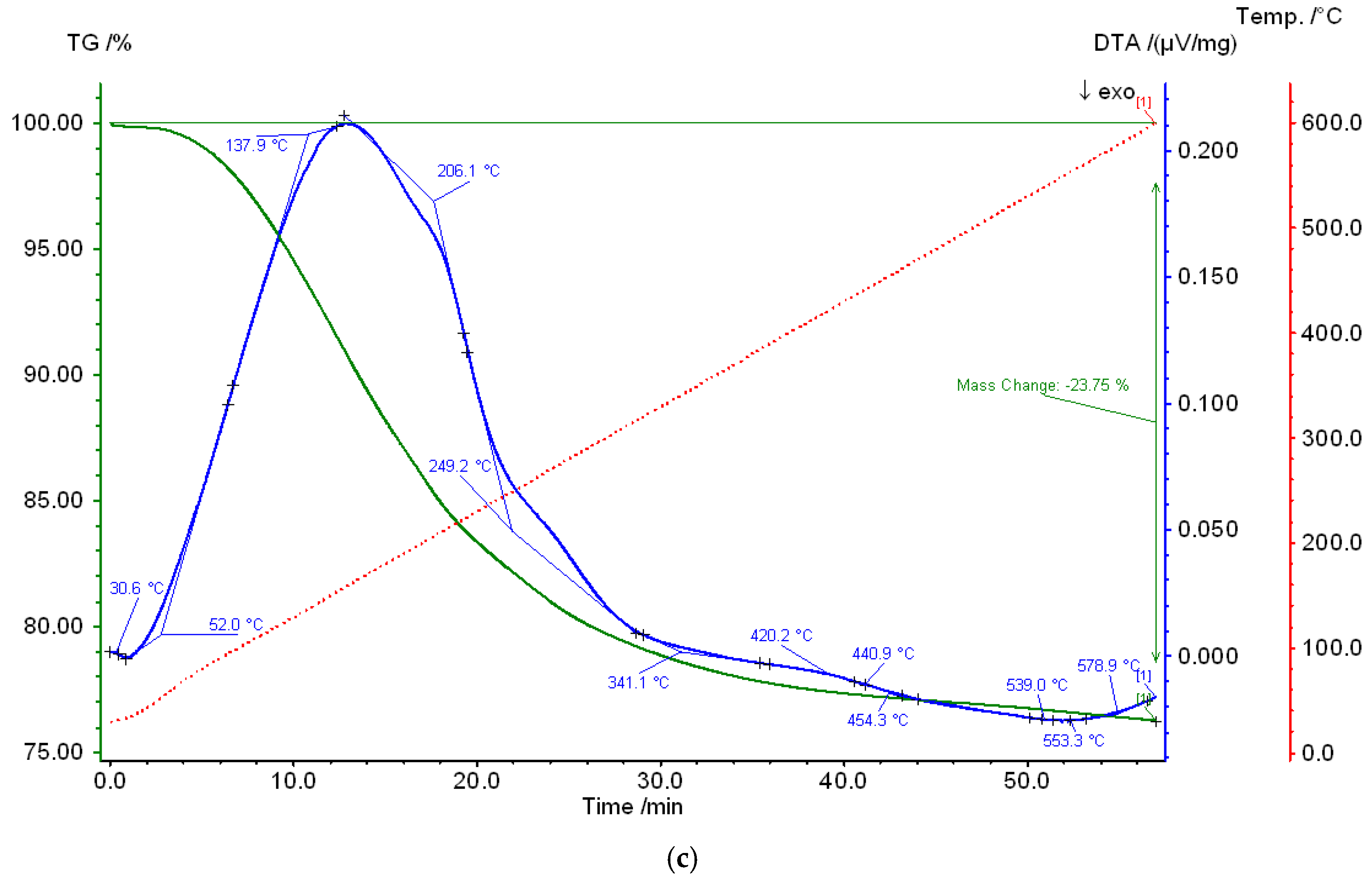
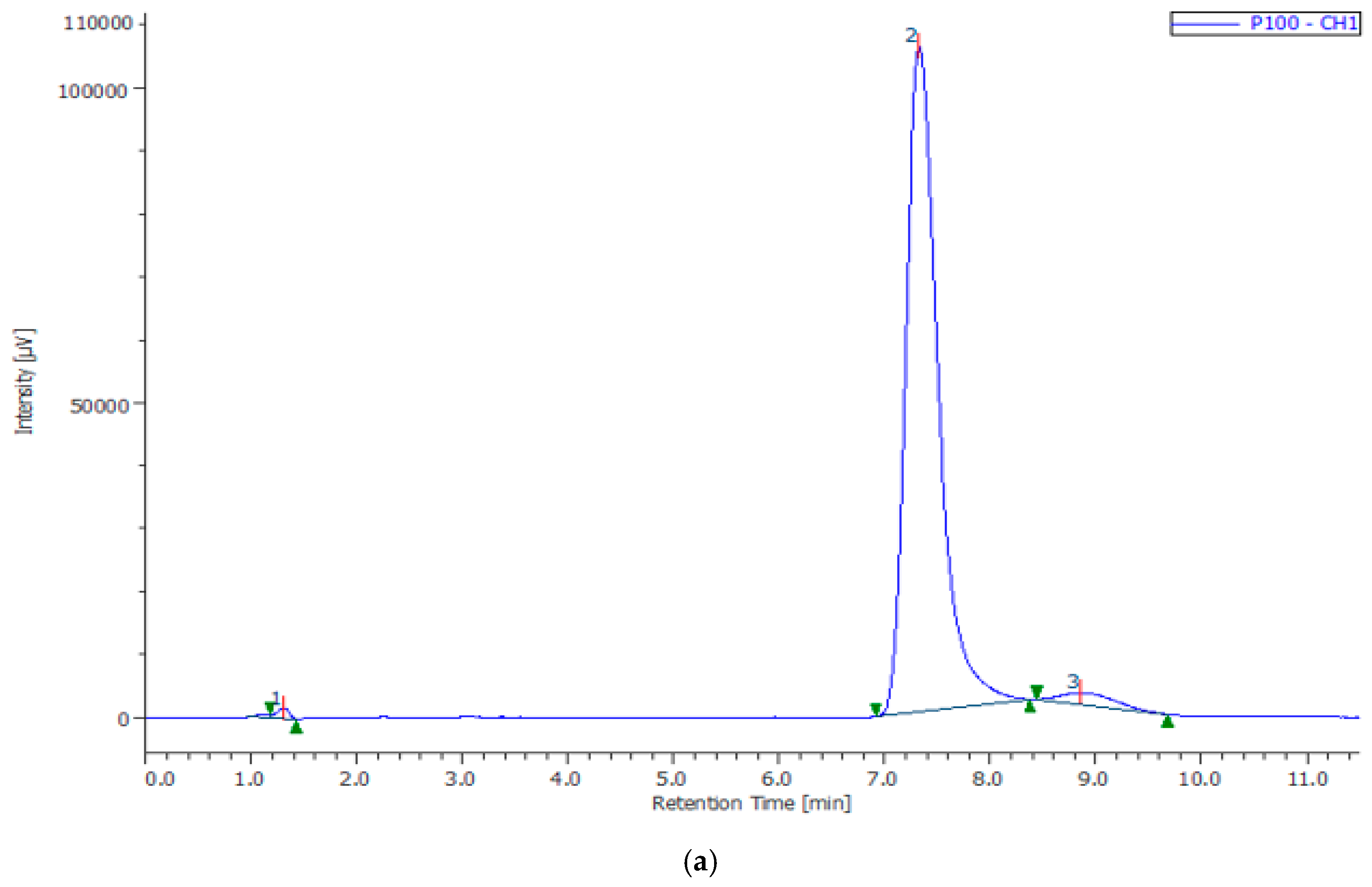
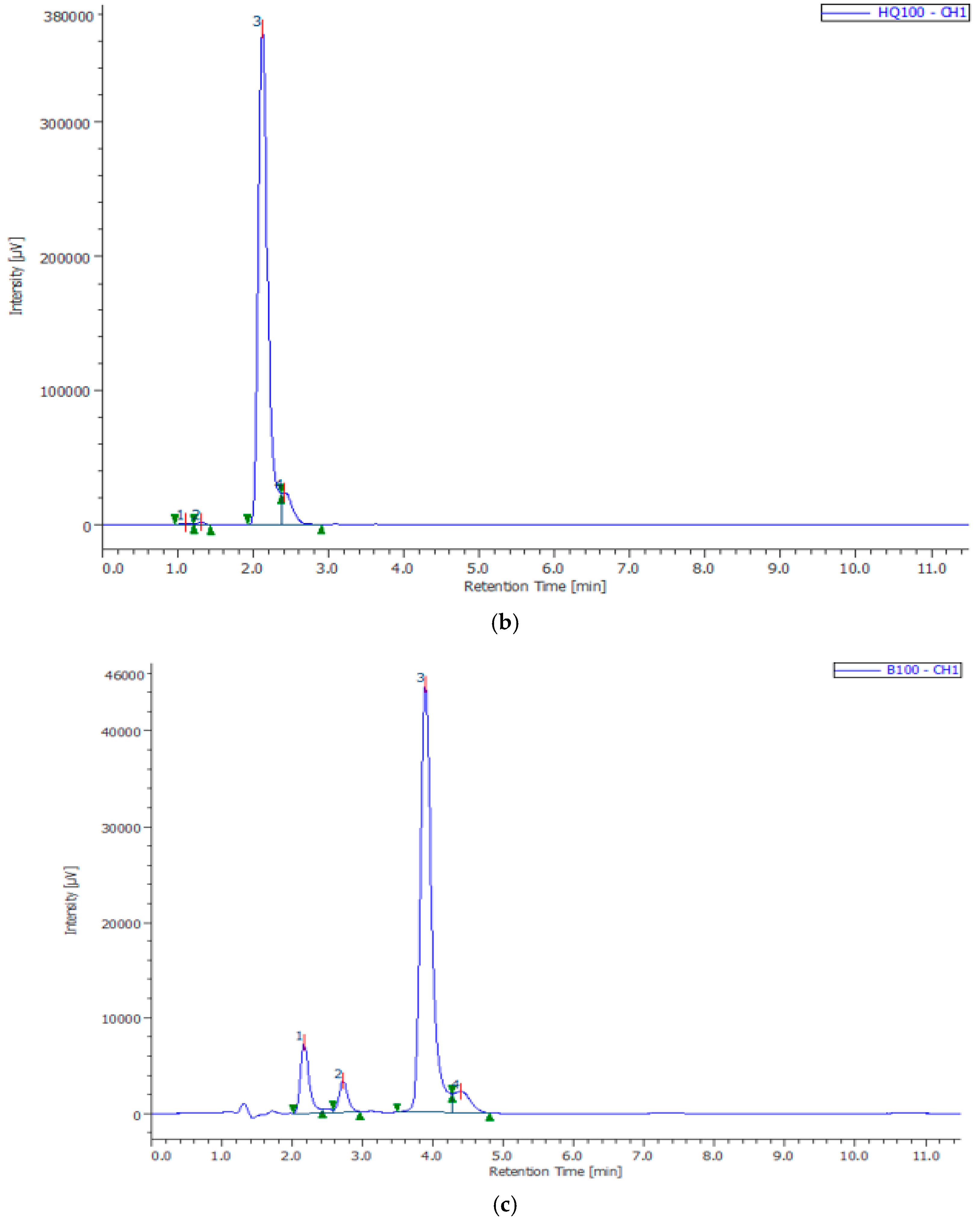


| Composites | Phase | a, nm | c, nm | Concentration, % | X-ray Density, g/cm3 | Space Group |
|---|---|---|---|---|---|---|
| CoFe2O4 | Fe2O3 (hematite) | 0.50367 | 1.37514 | 36.80 | 5.266 | R-3 c |
| CoFe2O4 (spinel) | 0.83880 | - | 63.20 | 5.281 | Fd-3m | |
| CoFe2O4/PVP | CoFe2O4 (spinel) | 0.83939 | - | 100 | 5.270 | Fd-3m |
| No | Name | Is, mm/с | Qs, mm/s | H, keV | Srelative, % | G, mm/s |
|---|---|---|---|---|---|---|
| 1-C | Fe3+_octahedron | 0.3680 | −0.2091 | 514.38 | 50.88 | 0.3216 |
| 2-C | Fe3+_octahedron | 0.4369 | 0.3681 | 510.92 | 8.12 | 0.2215 |
| 3-C | Fe3+_tetrahedron | 0.2844 | −0.0071 | 482.75 | 22.60 | 0.5139 |
| 4-C | Fe3+_octahedron | 0.3052 | −0.1597 | 495.34 | 18.41 | 0.2637 |
| No | Name | Is, mm/с | Qs, mm/s | H, keV | Srelative, % | G, mm/s |
|---|---|---|---|---|---|---|
| 1-C | Fe3+_octahedron | 0.4299 | −0.0044 | 408.93 | 11.98 | 0.7759 |
| 2-C | Fe3+_octahedron | 0.3909 | 0.0293 | 446.46 | 23.32 | 0.5719 |
| 3-C | Fe3+_tetrahedron | 0.2870 | 0.0280 | 486.82 | 21.45 | 0.5049 |
| 4-D | Fe3+_octahedron | 0.3555 | −0.0396 | 475.15 | 38.75 | 0.6014 |
| 5-D | Doublet_1 | 0.0905 | 0.5371 | - | 4.50 | 0.7759 |
| Sample | Parameters | |||
|---|---|---|---|---|
| Sspecific, m2/g | Vpore, cm3/g | Average Crystallite Size According to Scherrer, nm (311) | Lattice Parameter, nm | |
| CoFe2O4 | 126.00 | 0.23952 | 32.43965 | 0.83880 |
| CoFe2O4/PVP | 156.00 | 0.211097 | 15.50987 | 0.83939 |
| No | Indicators | Research Results | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | Amount of H2O2, µL | 60 | 120 | 240 | 480 | ||||
| 2 | Reaction duration, h. | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| 3 | Hydroquinone yield, % | 0.6 | 0.3 | 19.8 | 28.2 | 20.5 | 21 | 18.8 | 17.0 |
| 4 | Benzoquinone yield, % | 0.3 | 0.2 | 39.2 | 49.6 | 38.73 | 40 | 17.8 | 17.7 |
| 5 | Phenol conversion, % | 57.0 | 56.0 | 78.0 | 95.0 | 89.0 | 89.0 | 97.4 | 99.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakiyeva, T.V.; Dossumova, B.T.; Sassykova, L.R.; Ilmuratova, M.S.; Dzhatkambayeva, U.N.; Abildin, T.S. Study of the Oxidation of Phenol in the Presence of a Magnetic Composite Catalyst CoFe2O4/Polyvinylpyrrolidone. Appl. Sci. 2024, 14, 8907. https://doi.org/10.3390/app14198907
Shakiyeva TV, Dossumova BT, Sassykova LR, Ilmuratova MS, Dzhatkambayeva UN, Abildin TS. Study of the Oxidation of Phenol in the Presence of a Magnetic Composite Catalyst CoFe2O4/Polyvinylpyrrolidone. Applied Sciences. 2024; 14(19):8907. https://doi.org/10.3390/app14198907
Chicago/Turabian StyleShakiyeva, Tatyana V., Binara T. Dossumova, Larissa R. Sassykova, Madina S. Ilmuratova, Ulzhan N. Dzhatkambayeva, and Tleutai S. Abildin. 2024. "Study of the Oxidation of Phenol in the Presence of a Magnetic Composite Catalyst CoFe2O4/Polyvinylpyrrolidone" Applied Sciences 14, no. 19: 8907. https://doi.org/10.3390/app14198907
APA StyleShakiyeva, T. V., Dossumova, B. T., Sassykova, L. R., Ilmuratova, M. S., Dzhatkambayeva, U. N., & Abildin, T. S. (2024). Study of the Oxidation of Phenol in the Presence of a Magnetic Composite Catalyst CoFe2O4/Polyvinylpyrrolidone. Applied Sciences, 14(19), 8907. https://doi.org/10.3390/app14198907







