Nanostructured Drug Delivery Systems in Immunotherapy: An Updated Overview of Nanotechnology-Based Therapeutic Innovations
Abstract
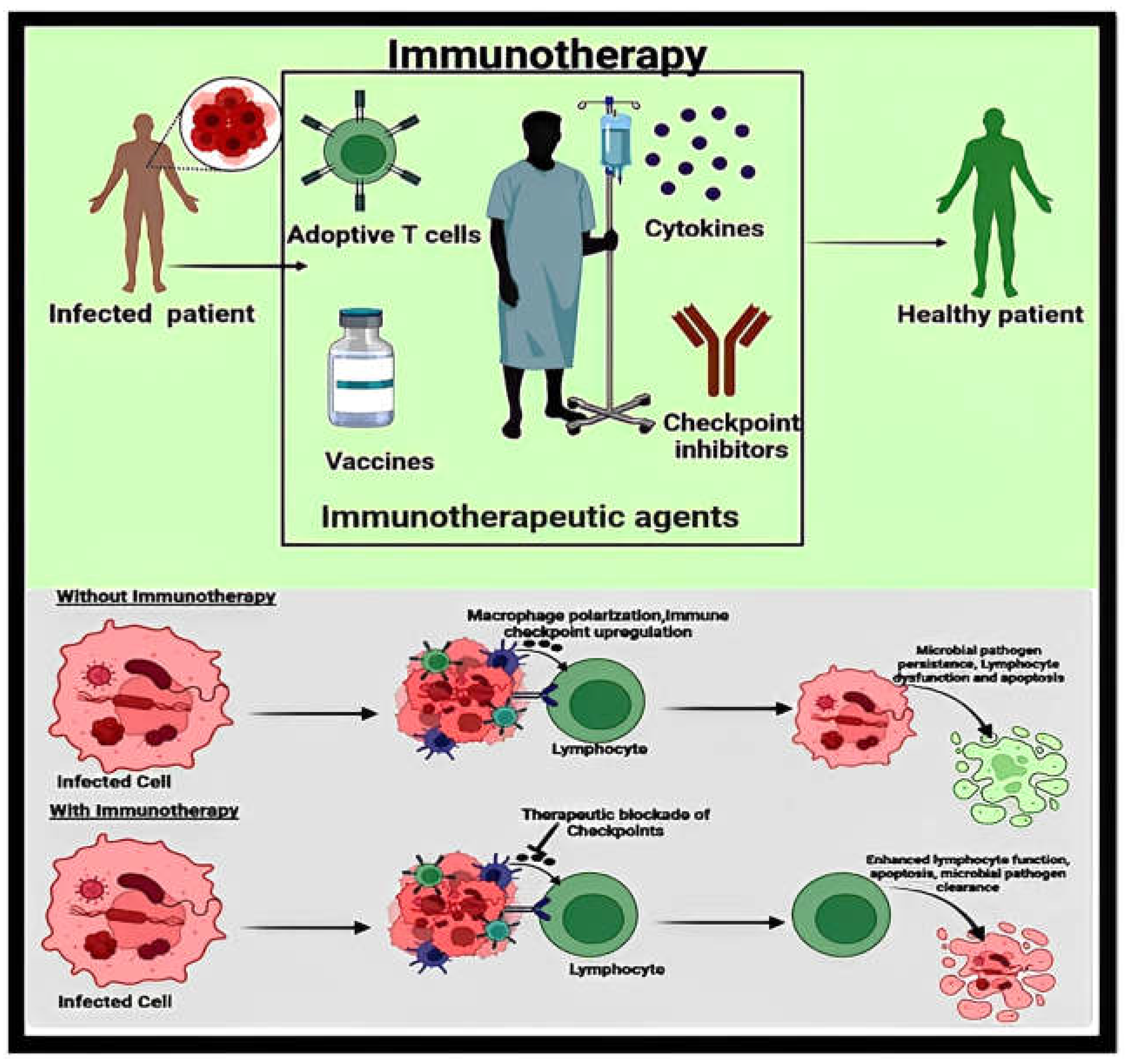
1. Introduction
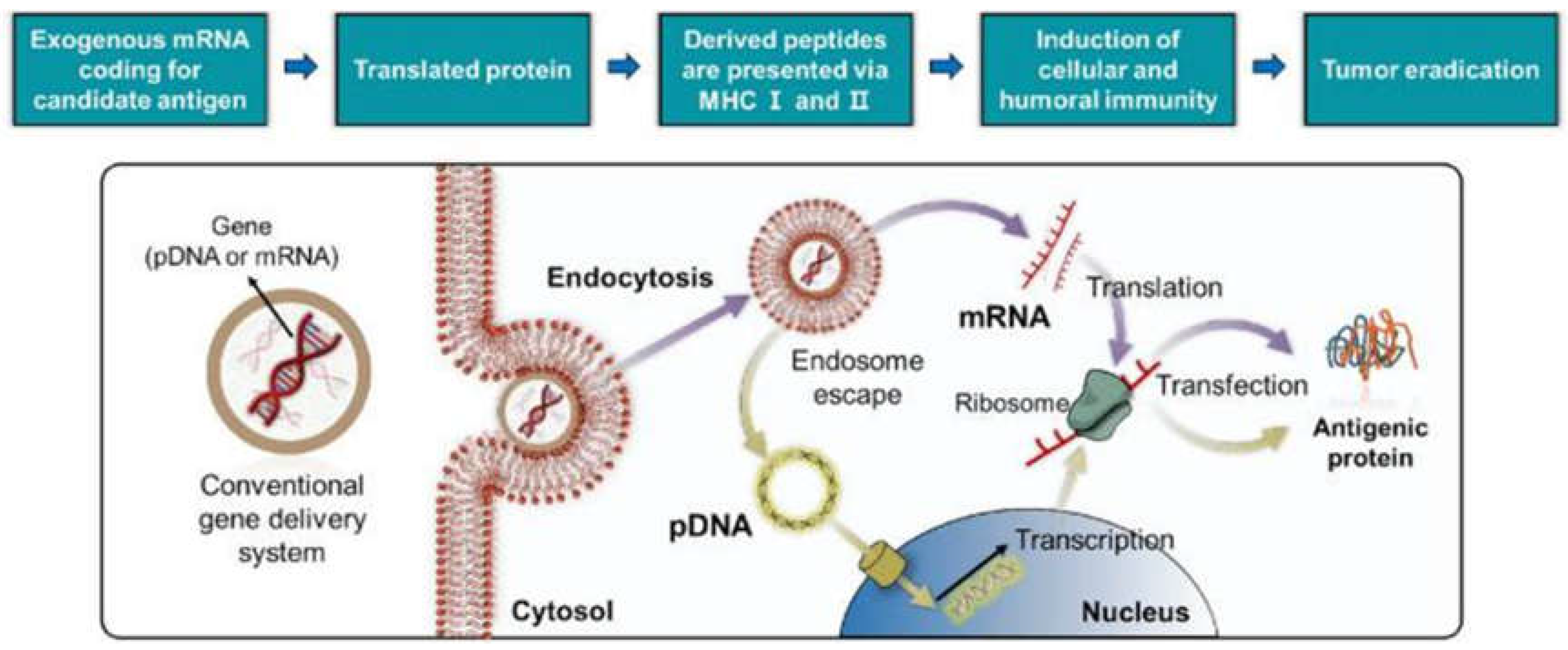
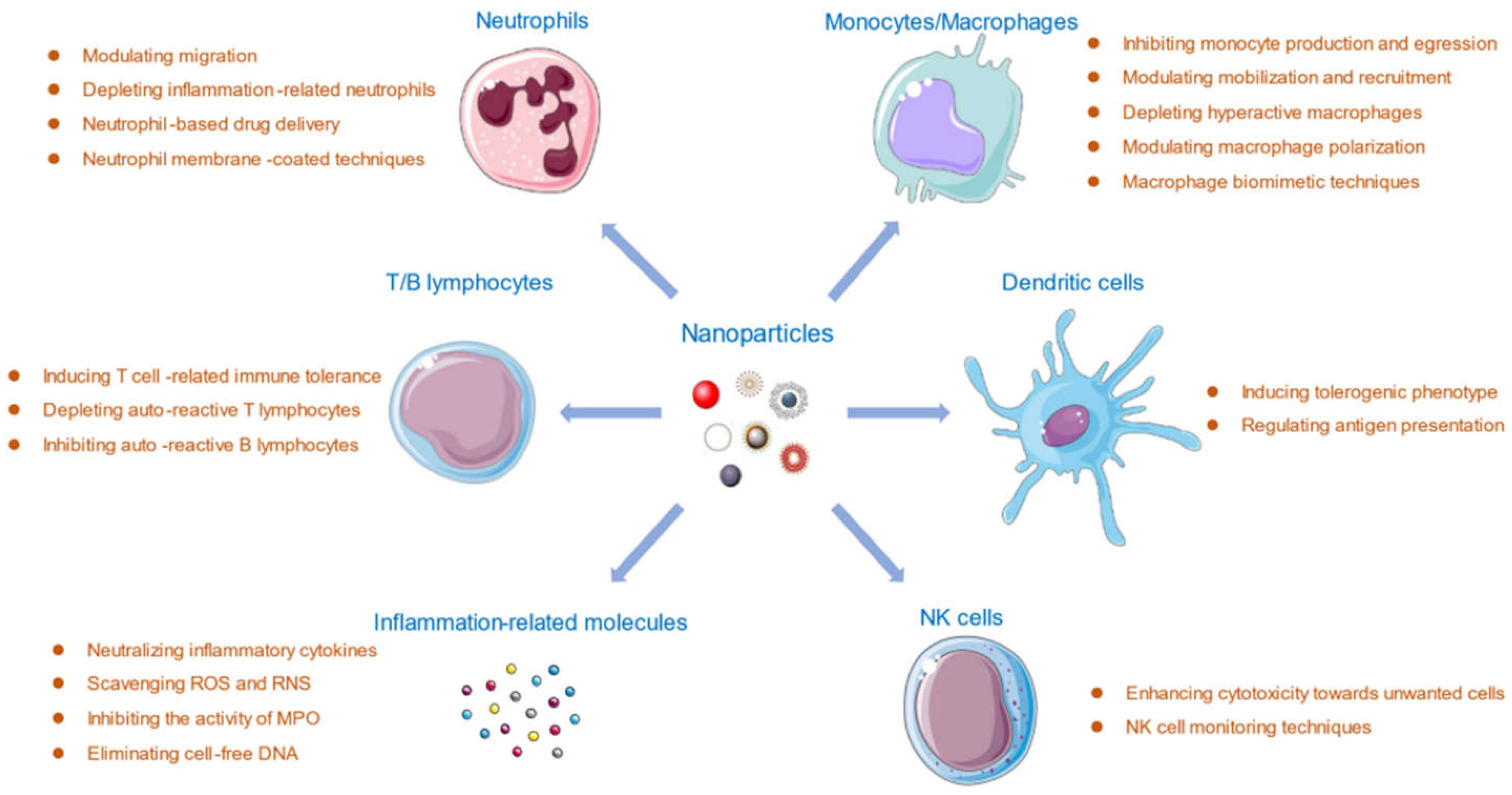
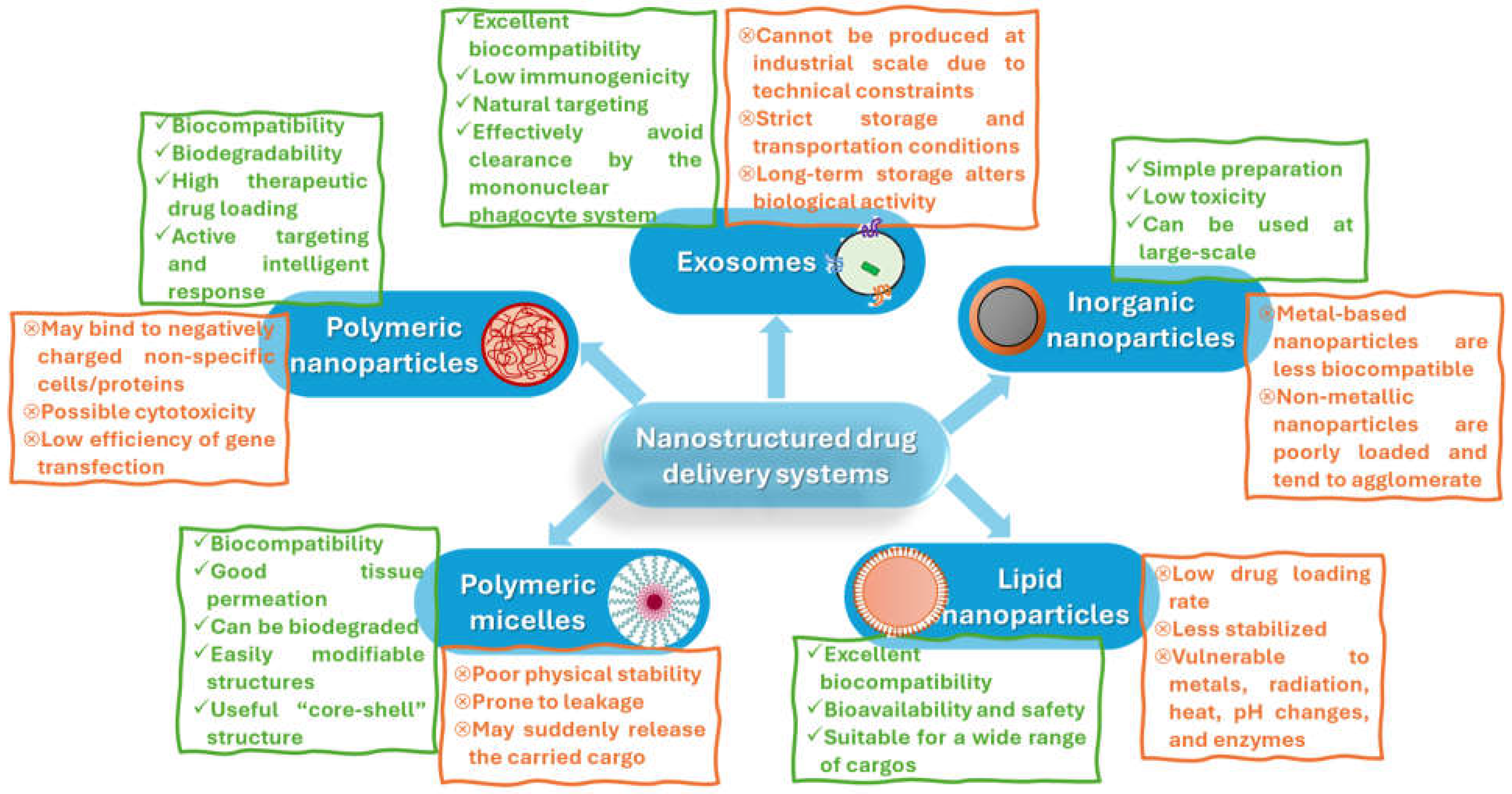
2. Design and Engineering of Nanostructured Drug Delivery Systems
3. Nanostructured Systems for Cancer Immunotherapy
4. Nanostructured Systems for Immunotherapeutic Delivery in Non-Malignant Applications
4.1. Autoimmune Diseases
4.2. Infectious Diseases
4.3. Allergic and Inflammatory Disorders
5. Clinically Approved Nanostructured Drug Delivery Systems
6. Remaining Challenges, Emerging Trends, and Future Perspectives
7. Conclusions
Funding
Conflicts of Interest
References
- Sorci, G.; Cornet, S.; Faivre, B. Immune Evasion, Immunopathology and the Regulation of the Immune System. Pathogens 2013, 2, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Mitarotonda, R.; Giorgi, E.; Eufrasio-da-Silva, T.; Dolatshahi-Pirouz, A.; Mishra, Y.K.; Khademhosseini, A.; Desimone, M.F.; De Marzi, M.; Orive, G. Immunotherapeutic nanoparticles: From autoimmune disease control to the development of vaccines. Biomater. Adv. 2022, 135, 212726. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa-Almeida, R.; Santos, S.G.; Magalhães, F.D.; Pinto, A.M. Advances in carbon nanomaterials for immunotherapy. Appl. Mater. Today 2022, 27, 101397. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, W.; Wei, H.; Dean, M.N.; Standaert, D.G.; Cutter, G.R.; Benveniste, E.N.; Qin, H. Dysregulation of the adaptive immune system in patients with early-stage Parkinson disease. Neurol. Neuroimmunol. Neuroinflammation 2021, 8, e1036. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Kleiner, A.; Looney, R.J. Immune dysregulation. J. Allergy Clin. Immunol. 2023, 151, 70–80. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Vaillant, A.A.J.; Qurie, A. Immunodeficiency. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Anfray, C.; Mainini, F.; Andón, F.T. Nanoparticles for Immunotherapy. Front. Nanosci. 2020, 16, 265–306. [Google Scholar]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef]
- Varshney, D.; Qiu, S.Y.; Graf, T.P.; McHugh, K.J. Employing drug delivery strategies to overcome challenges using TLR7/8 agonists for cancer immunotherapy. AAPS J. 2021, 23, 90. [Google Scholar] [CrossRef]
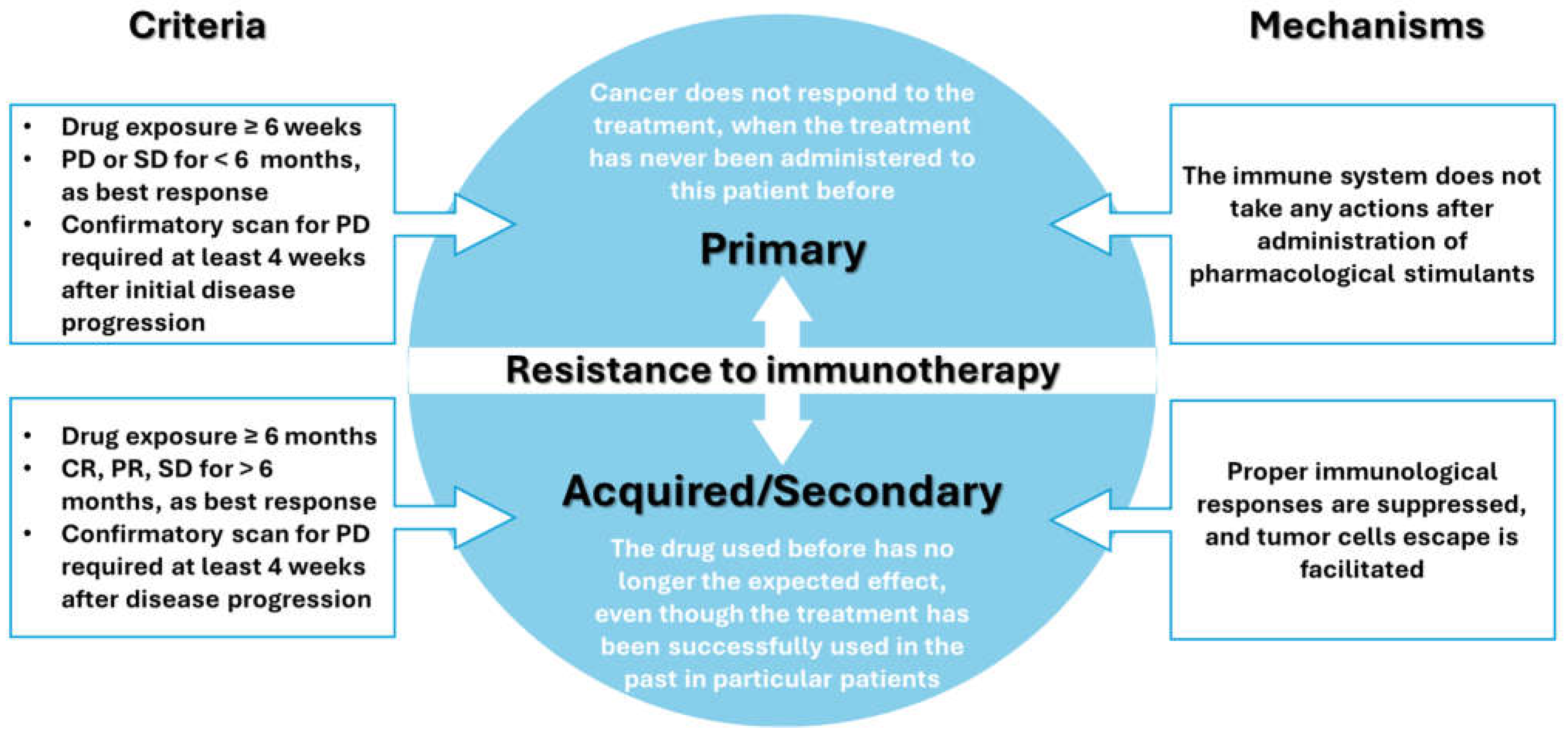
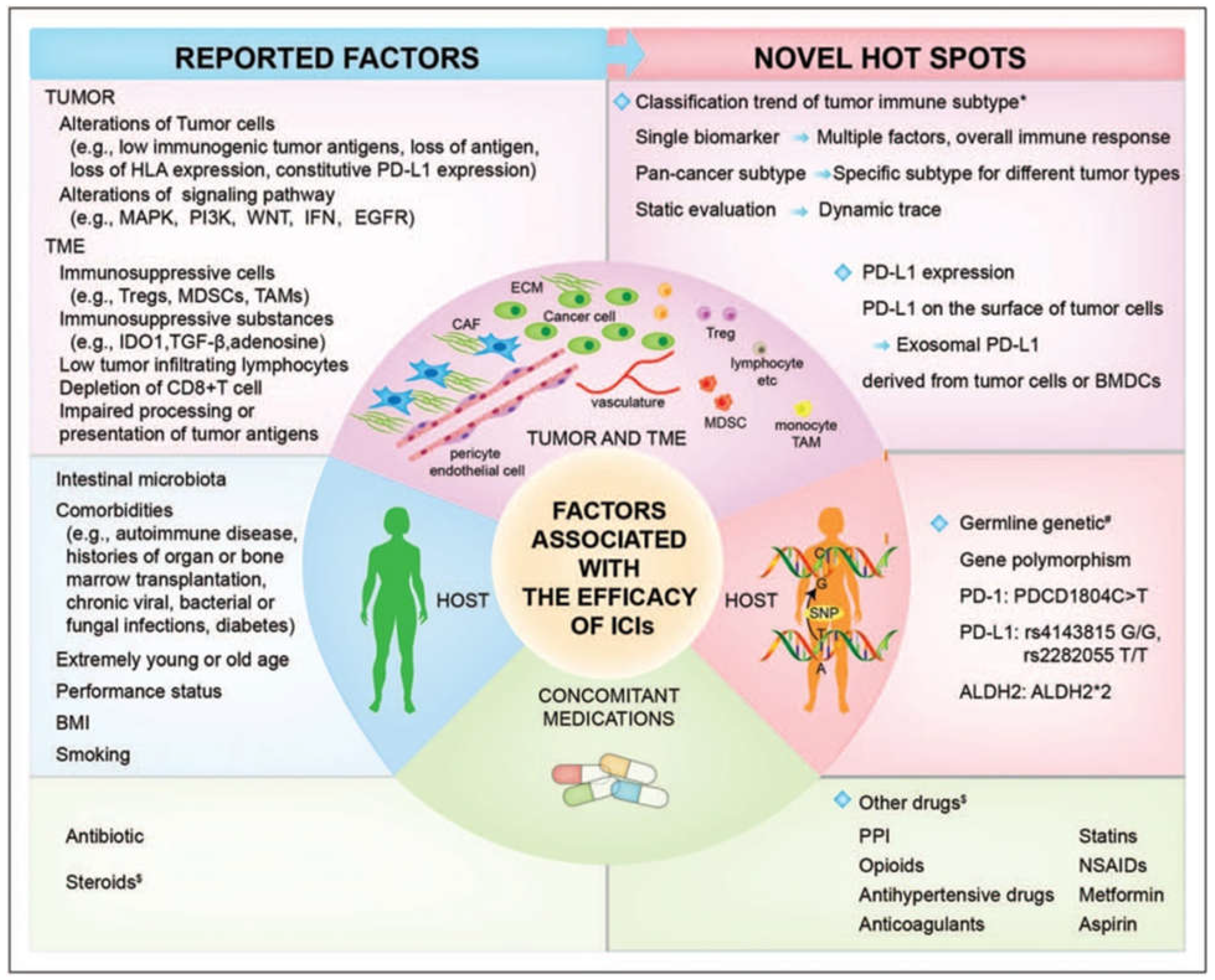
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Dobosz, P.; Stępień, M.; Golke, A.; Dzieciątkowski, T. Challenges of the Immunotherapy: Perspectives and Limitations of the Immune Checkpoint Inhibitor Treatment. Int. J. Mol. Sci. 2022, 23, 2847. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Z.; Zhang, H.-M. Recent advances in primary resistance mechanisms against immune checkpoint inhibitors. Curr. Opin. Oncol. 2022, 34, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Kluger, H.M.; Tawbi, H.A.; Ascierto, M.L.; Bowden, M.; Callahan, M.K.; Cha, E.; Chen, H.X.; Drake, C.G.; Feltquate, D.M.; Ferris, R.L.; et al. Defining tumor resistance to PD-1 pathway blockade: Recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce. J. Immunother. Cancer 2020, 8, e000398. [Google Scholar] [CrossRef] [PubMed]
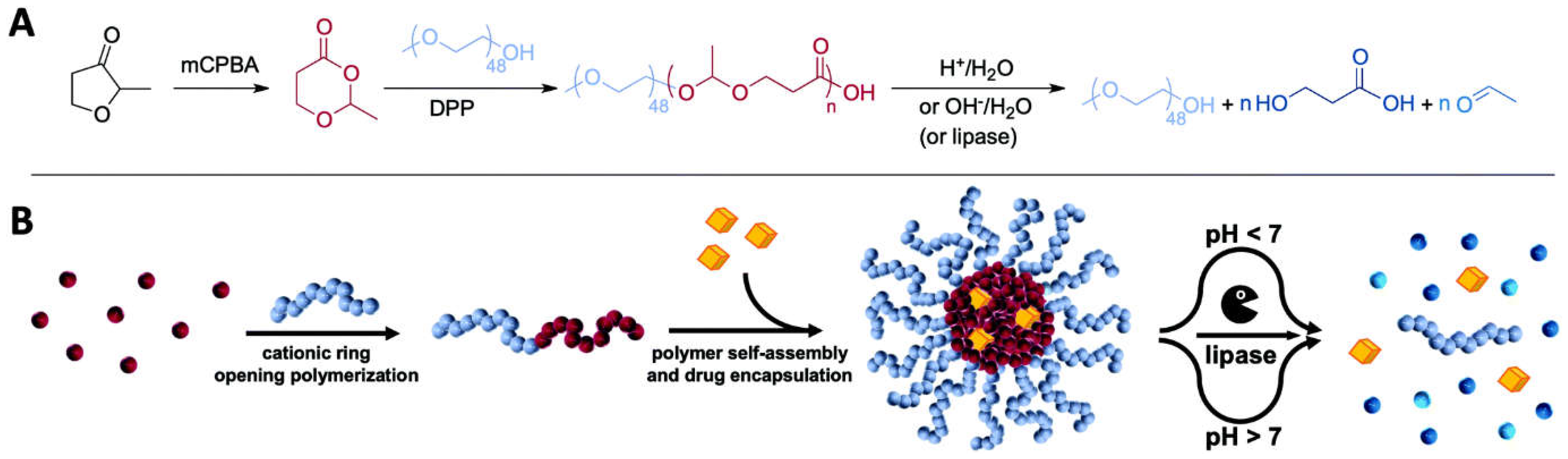
- Bixenmann, L.; Stickdorn, J.; Nuhn, L. Amphiphilic poly (esteracetal) s as dual pH-and enzyme-responsive micellar immunodrug delivery systems. Polym. Chem. 2020, 11, 2441–2456. [Google Scholar] [CrossRef]
- Croitoru, G.-A.; Pîrvulescu, D.-C.; Niculescu, A.-G.; Epistatu, D.; Rădulescu, M.; Grumezescu, A.M.; Nicolae, C.-L. Nanomaterials in Immunology: Bridging Innovative Approaches in Immune Modulation, Diagnostics, and Therapy. J. Funct. Biomater. 2024, 15, 225. [Google Scholar] [CrossRef]
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A comprehensive review of nanomaterials: Types, synthesis, characterization, and applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar]
- Wang, X.; Podila, R.; Shannahan, J.H.; Rao, A.M.; Brown, J.M. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int. J. Nanomed. 2013, 8, 1733–1748. [Google Scholar] [CrossRef]
- Sharma, N.; Singh, S.; Behl, T.; Gupta, N.; Gulia, R.; Kanojia, N. Explicating the applications of quality by design tools in optimization of microparticles and nanotechnology based drug delivery systems. Biointerface Res. Appl. Chem. 2022, 12, 4317–4336. [Google Scholar]
- Panda, B.S. A review on synthesis of silver nanoparticles and their biomedical applications. Lett. Appl. NanoBioScience 2022, 11, 3218–3231. [Google Scholar]
- Horwitz, D.A.; Bickerton, S.; La Cava, A. Strategies to use nanoparticles to generate CD4 and CD8 regulatory T cells for the treatment of SLE and other autoimmune diseases. Front. Immunol. 2021, 12, 681062. [Google Scholar] [CrossRef]
- Milewska, S.; Niemirowicz-Laskowska, K.; Siemiaszko, G.; Nowicki, P.; Wilczewska, A.Z.; Car, H. Current Trends and Challenges in Pharmacoeconomic Aspects of Nanocarriers as Drug Delivery Systems for Cancer Treatment. Int. J. Nanomed. 2021, 16, 6593–6644. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Aftab, S.; Nisar, J.; Ashiq, M.N.; Iftikhar, F.J. Nanocarriers for targeted drug delivery. J. Drug Deliv. Sci. Technol. 2021, 62, 102426. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-based drug delivery systems: Conventional drug delivery routes, recent developments and future prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Radzi, M.R.M.; Lim, C.K.; Sulaiman, N.; Jemon, K. Nanoparticles-based Chemo-Phototherapy Synergistic Effects for Breast Cancer Treatment: A Systematic Review. Biointerface Res. Appl. Chem. 2023, 13, 14764. [Google Scholar]
- Llop, J.; Lammers, T. Nanoparticles for Cancer Diagnosis, Radionuclide Therapy and Theranostics. ACS Nano 2021, 15, 16974–16981. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Z.; Pang, Y.; Zhou, H. The interaction between nanoparticles and immune system: Application in the treatment of inflammatory diseases. J. Nanobiotechnol. 2022, 20, 127. [Google Scholar] [CrossRef]
- Eljack, S.; David, S.; Faggad, A.; Chourpa, I.; Allard-Vannier, E. Nanoparticles design considerations to co-deliver nucleic acids and anti-cancer drugs for chemoresistance reversal. Int. J. Pharm. X 2022, 4, 100126. [Google Scholar] [CrossRef]
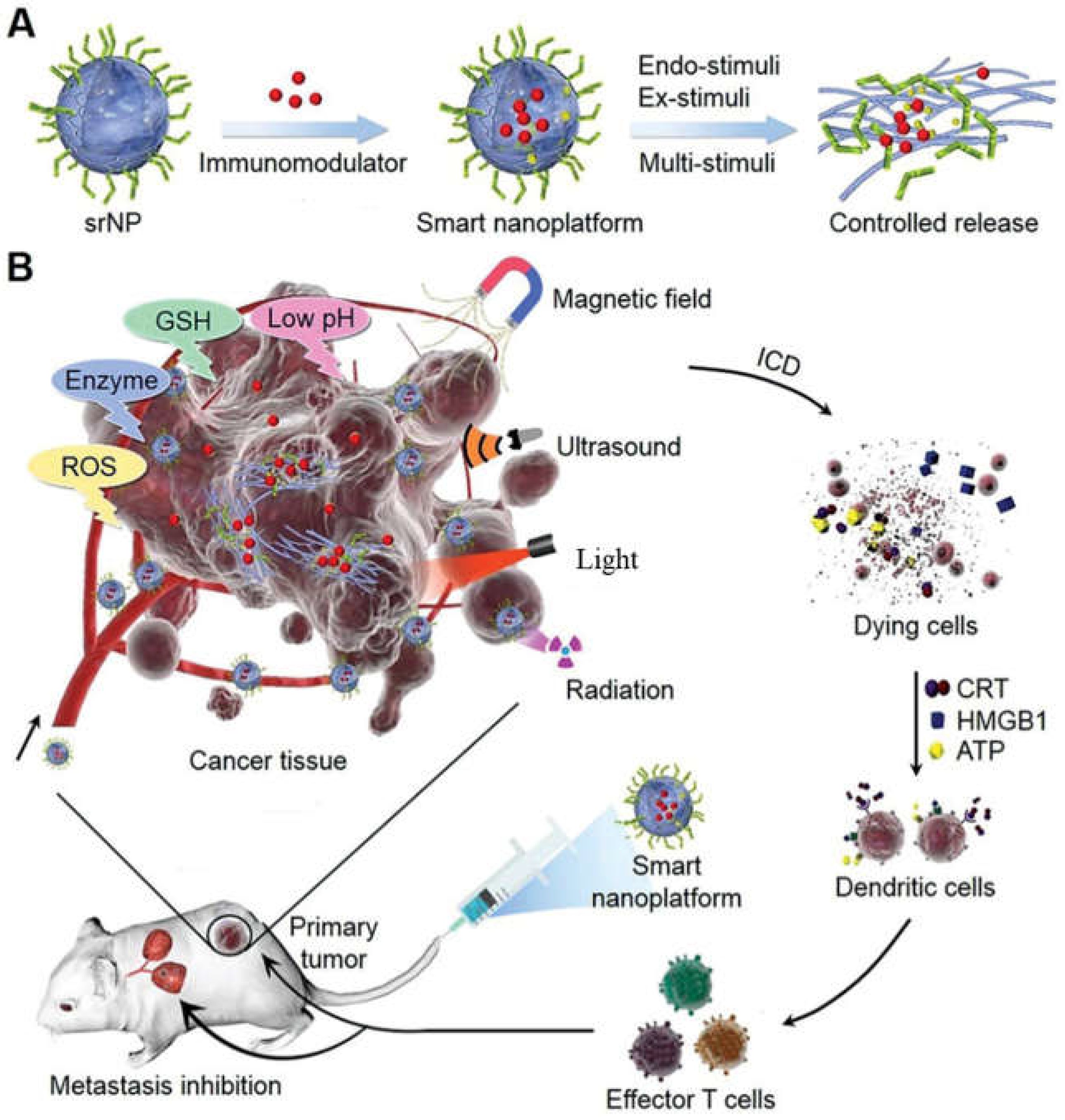
- Zhang, J.; Lin, Y.; Lin, Z.; Wei, Q.; Qian, J.; Ruan, R.; Jiang, X.; Hou, L.; Song, J.; Ding, J.; et al. Stimuli-Responsive Nanoparticles for Controlled Drug Delivery in Synergistic Cancer Immunotherapy. Adv. Sci. 2022, 9, 2103444. [Google Scholar] [CrossRef]
- Mohammapdour, R.; Ghandehari, H. Mechanisms of immune response to inorganic nanoparticles and their degradation products. Adv. Drug Deliv. Rev. 2022, 180, 114022. [Google Scholar] [CrossRef]
- Bi, J.; Mo, C.; Li, S.; Huang, M.; Lin, Y.; Yuan, P.; Liu, Z.; Jia, B.; Xu, S. Immunotoxicity of metal and metal oxide nanoparticles: From toxic mechanisms to metabolism and outcomes. Biomater. Sci. 2023, 11, 4151–4183. [Google Scholar] [CrossRef]
- Ahamad, N.; Kar, A.; Mehta, S.; Dewani, M.; Ravichandran, V.; Bhardwaj, P.; Sharma, S.; Banerjee, R. Immunomodulatory nanosystems for treating inflammatory diseases. Biomaterials 2021, 274, 120875. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Shen, X.; Yu, H.; Tu, H.; Chittasupho, C.; Zhao, Y. Smart Polymeric Nanoparticles in Cancer Immunotherapy. Pharmaceutics 2023, 15, 775. [Google Scholar] [CrossRef] [PubMed]
- Craparo, E.F.; Bondì, M.L. Application of polymeric nanoparticles in immunotherapy. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 658–664. [Google Scholar] [CrossRef]
- Samaridou, E.; Heyes, J.; Lutwyche, P. Lipid nanoparticles for nucleic acid delivery: Current perspectives. Adv. Drug Deliv. Rev. 2020, 154, 37–63. [Google Scholar] [CrossRef]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid nanoparticles as delivery systems for RNA-based vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Bîrcă, A.C.; Grumezescu, A.M. New Applications of Lipid and Polymer-Based Nanoparticles for Nucleic Acids Delivery. Pharmaceutics 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Lim, J.; Wang, C.J.; Han, J.H.; Shin, H.E.; Kim, S.N.; Jeong, D.; Lee, S.H.; Chun, B.H.; Park, C.G.; et al. Lipid nanoparticle-based mRNA delivery systems for cancer immunotherapy. Nano Converg. 2023, 10, 36. [Google Scholar] [CrossRef]
- Wang, H.-L.; Wang, Z.-G.; Liu, S.-L. Lipid Nanoparticles for mRNA Delivery to Enhance Cancer Immunotherapy. Molecules 2022, 27, 5607. [Google Scholar] [CrossRef]
- Wu, L.; Li, X.; Qian, X.; Wang, S.; Liu, J.; Yan, J. Lipid Nanoparticle (LNP) Delivery Carrier-Assisted Targeted Controlled Release mRNA Vaccines in Tumor Immunity. Vaccines 2024, 12, 186. [Google Scholar] [CrossRef]
- Papi, M.; De Spirito, M.; Palmieri, V. Nanotechnology in the COVID-19 era: Carbon-based nanomaterials as a promising solution. Carbon 2023, 210, 118058. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; He, L.; Cao, W.; Huang, X.; Jia, K.; Dai, J. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant. Acta Biomater. 2020, 112, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Mohammadnejad, J.; Najafi-Taher, R.; Zadeh, Z.B.; Tanhaei, M.; Ramakrishna, S. Multifunctional Carbon-Based Nanoparticles: Theranostic Applications in Cancer Therapy and Diagnosis. ACS Appl. Bio Mater. 2023, 6, 1323–1338. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Vaswani, P.; Bhatia, D. Revolutionizing cancer therapy using tetrahedral DNA nanostructures as intelligent drug delivery systems. Nanoscale Adv. 2024, 6, 3714–3732. [Google Scholar] [CrossRef] [PubMed]
- Durbin, J.K.; Miller, D.K.; Niekamp, J.; Khisamutdinov, E.F. Modulating Immune Response with Nucleic Acid Nanoparticles. Molecules 2019, 24, 3740. [Google Scholar] [CrossRef]
- Khisamutdinov, E.F.; Bui, M.N.H.; Jasinski, D.; Zhao, Z.; Cui, Z.; Guo, P. Simple Method for Constructing RNA Triangle, Square, Pentagon by Tuning Interior RNA 3WJ Angle from 60° to 90° or 108°. In RNA Scaffolds: Methods and Protocols; Ponchon, L., Ed.; Springer New York: New York, NY, USA, 2015; pp. 181–193. [Google Scholar]
- Khisamutdinov, E.F.; Li, H.; Jasinski, D.L.; Chen, J.; Fu, J.; Guo, P. Enhancing immunomodulation on innate immunity by shape transition among RNA triangle, square and pentagon nanovehicles. Nucleic Acids Res. 2014, 42, 9996–10004. [Google Scholar] [CrossRef]
- Afonin, K.A.; Bindewald, E.; Yaghoubian, A.J.; Voss, N.; Jacovetty, E.; Shapiro, B.A.; Jaeger, L. In vitro assembly of cubic RNA-based scaffolds designed in silico. Nat. Nanotechnol. 2010, 5, 676–682. [Google Scholar] [CrossRef]
- Afonin, K.A.; Kasprzak, W.; Bindewald, E.; Puppala, P.S.; Diehl, A.R.; Hall, K.T.; Kim, T.J.; Zimmermann, M.T.; Jernigan, R.L.; Jaeger, L. Computational and Experimental Characterization of RNA Cubic Nanoscaffolds. In Therapeutic RNA Nanotechnology; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; pp. 121–149. [Google Scholar]
- Iinuma, R.; Ke, Y.; Jungmann, R.; Schlichthaerle, T.; Woehrstein, J.B.; Yin, P. Polyhedra Self-Assembled from DNA Tripods and Characterized with 3D DNA-PAINT. Science 2014, 344, 65–69. [Google Scholar] [CrossRef]
- Khisamutdinov, E.F.; Jasinski, D.L.; Li, H.; Zhang, K.; Chiu, W.; Guo, P. Fabrication of RNA 3D nanoprism for loading and protection of small RNAs and model drugs. Adv. Mater. 2016, 28, 10079. [Google Scholar] [CrossRef]
- Grabow, W.W.; Zakrevsky, P.; Afonin, K.A.; Chworos, A.; Shapiro, B.A.; Jaeger, L. Self-Assembling RNA Nanorings Based on RNAI/II Inverse Kissing Complexes. In Therapeutic RNA Nanotechnology; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2021; pp. 67–93. [Google Scholar]
- Illig, M.; Jahnke, K.; Weise, L.P.; Scheffold, M.; Mersdorf, U.; Drechsler, H.; Zhang, Y.; Diez, S.; Kierfeld, J.; Göpfrich, K. Triggered contraction of self-assembled micron-scale DNA nanotube rings. Nat. Commun. 2024, 15, 2307. [Google Scholar] [CrossRef]
- Glynn, A.T.; Davidson, S.R.; Qian, L. Developmental Self-Assembly of a DNA Ring with Stimulus-Responsive Size and Growth Direction. J. Am. Chem. Soc. 2022, 144, 10075–10079. [Google Scholar] [CrossRef] [PubMed]
- Roller, E.-M.; Khorashad, L.K.; Fedoruk, M.; Schreiber, R.; Govorov, A.O.; Liedl, T. DNA-Assembled Nanoparticle Rings Exhibit Electric and Magnetic Resonances at Visible Frequencies. Nano Lett. 2015, 15, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Piao, X.; Li, H.; Guo, P. Methods for construction and characterization of simple or special multifunctional RNA nanoparticles based on the 3WJ of phi29 DNA packaging motor. Methods 2018, 143, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Sawada, T.; Mori, Y.; Morita, K.; Ishimatsu, R. Covalent Hyperbranched Polymer Self-Assemblies of Three-Way Junction DNA for Single-Molecule Devices. Langmuir 2020, 36, 10166–10174. [Google Scholar] [CrossRef] [PubMed]
- Vittala, S.K.; Saraswathi, S.K.; Ramesan, A.B.; Joseph, J. Nanosheets and 2D-nanonetworks by mutually assisted self-assembly of fullerene clusters and DNA three-way junctions. Nanoscale Adv. 2019, 1, 4158–4165. [Google Scholar] [CrossRef]
- Takezawa, Y.; Shionoya, M. Supramolecular DNA three-way junction motifs with a bridging metal center. Front. Chem. 2020, 7, 925. [Google Scholar] [CrossRef]
- Haque, F.; Shu, D.; Shu, Y.; Shlyakhtenko, L.S.; Rychahou, P.G.; Evers, B.M.; Guo, P. Ultrastable synergistic tetravalent RNA nanoparticles for targeting to cancers. Nano Today 2012, 7, 245–257. [Google Scholar] [CrossRef]
- Bi, S.; Xiu, B.; Ye, J.; Dong, Y. Target-Catalyzed DNA Four-Way Junctions for CRET Imaging of MicroRNA, Concatenated Logic Operations, and Self-Assembly of DNA Nanohydrogels for Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 23310–23319. [Google Scholar] [CrossRef]
- Li, J.; Lu, W.; Yang, Y.; Xiang, R.; Ling, Y.; Yu, C.; Zhou, Y. Hybrid Nanomaterials for Cancer Immunotherapy. Adv. Sci. 2023, 10, 2204932. [Google Scholar] [CrossRef]
- Gautam, A.; Beiss, V.; Wang, C.; Wang, L.; Steinmetz, N.F. Plant Viral Nanoparticle Conjugated with Anti-PD-1 Peptide for Ovarian Cancer Immunotherapy. Int. J. Mol. Sci. 2021, 22, 9733. [Google Scholar] [CrossRef]
- Pruteanu, L.-L.; Braicu, C.; Módos, D.; Jurj, M.-A.; Raduly, L.-Z.; Zănoagă, O.; Magdo, L.; Cojocneanu, R.; Paşca, S.; Moldovan, C.; et al. Targeting Cell Death Mechanism Specifically in Triple Negative Breast Cancer Cell Lines. Int. J. Mol. Sci. 2022, 23, 4784. [Google Scholar] [CrossRef] [PubMed]
- Yahya, S.; Abdelmenym Mohamed, S.I.; Yahya, S. Gene Editing: A Powerful Tool for Cancer Immunotherapy. Biointerface Res. Appl. Chem. 2023, 13, 98. [Google Scholar]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Tumor microenvironment-mediated immune evasion in hepatocellular carcinoma. Front. Immunol. 2023, 14, 1133308. [Google Scholar] [CrossRef]
- Zhang, Y.; He, X.; Zhang, Y.; Zhao, Y.; Lu, S.; Peng, Y.; Lu, L.; Hu, X.; Zhan, M. Native Mitochondria-Targeting polymeric nanoparticles for mild photothermal therapy rationally potentiated with immune checkpoints blockade to inhibit tumor recurrence and metastasis. Chem. Eng. J. 2021, 424, 130171. [Google Scholar] [CrossRef]
- Lee, C.K.; Atibalentja, D.F.; Yao, L.E.; Park, J.; Kuruvilla, S.; Felsher, D.W. Anti-PD-L1 F(ab) Conjugated PEG-PLGA Nanoparticle Enhances Immune Checkpoint Therapy. Nanotheranostics 2022, 6, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.O.D. Overview of nanomaterials and cellular interactions. Biointerface Res. Appl. Chem. 2023, 13, 367. [Google Scholar]
- Fávaro, W.J.; dos Santos, M.M.; Pereira, M.M.; Garcia, P.V.; Durán, N. Effects of P-MAPA immunotherapy associated with gemcitabine on chemically-induced pancreatic cancer in animal model: New therapeutic perspectives. Biointerface Res. Appl. Chem. 2022, 12, 7540–7555. [Google Scholar]
- Zhang, H.; Tang, W.-L.; Kheirolomoom, A.; Fite, B.Z.; Wu, B.; Lau, K.; Baikoghli, M.; Raie, M.N.; Tumbale, S.K.; Foiret, J.; et al. Development of thermosensitive resiquimod-loaded liposomes for enhanced cancer immunotherapy. J. Control. Release 2021, 330, 1080–1094. [Google Scholar] [CrossRef]
- Park, W.; Heo, Y.-J.; Han, D.K. New opportunities for nanoparticles in cancer immunotherapy. Biomater. Res. 2018, 22, 24. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, M.; Vishwakarma, R.; Behera, K.; Trivedi, S. Green solvents tailored nanostructures of block copolymers and their potential applications in drug delivery. J. Mol. Liq. 2024, 410, 125642. [Google Scholar] [CrossRef]
- Dias, M.F.; de Figueiredo, B.C.P.; Teixeira-Neto, J.; Guerra, M.C.A.; Fialho, S.L.; Silva Cunha, A. In vivo evaluation of antitumoral and antiangiogenic effect of imiquimod-loaded polymeric nanoparticles. Biomed. Pharmacother. 2018, 103, 1107–1114. [Google Scholar] [CrossRef] [PubMed]
- Gazzi, R.P.; Frank, L.A.; Onzi, G.; Pohlmann, A.R.; Guterres, S.S. New pectin-based hydrogel containing imiquimod-loaded polymeric nanocapsules for melanoma treatment. Drug Deliv. Transl. Res. 2020, 10, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Gazzi, R.P.; Mello, P.A.; Chaves, P.; Peña, F.; Beck, R.C.R.; Buffon, A.; Pohlmann, A.R.; Guterres, S.S. Anti-HPV Nanoemulsified-Imiquimod: A New and Potent Formulation to Treat Cervical Cancer. AAPS PharmSciTech 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S.; Kohler, R.H.; Pittet, M.J.; Weissleder, R. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Widmer, J.; Thauvin, C.; Mottas, I.; Nguyen, V.N.; Delie, F.; Allémann, E.; Bourquin, C. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int. J. Pharm. 2018, 535, 444–451. [Google Scholar] [CrossRef]
- Komura, F.; Okuzumi, K.; Takahashi, Y.; Takakura, Y.; Nishikawa, M. Development of RNA/DNA Hydrogel Targeting Toll-Like Receptor 7/8 for Sustained RNA Release and Potent Immune Activation. Molecules 2020, 25, 728. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Grumezescu, A.M. Novel Tumor-Targeting Nanoparticles for Cancer Treatment—A Review. Int. J. Mol. Sci. 2022, 23, 5253. [Google Scholar] [CrossRef]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef]
- Kim, H.; Khanna, V.; Kucaba, T.A.; Zhang, W.; Sehgal, D.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. TLR7/8 Agonist-Loaded Nanoparticles Augment NK Cell-Mediated Antibody-Based Cancer Immunotherapy. Mol. Pharm. 2020, 17, 2109–2124. [Google Scholar] [CrossRef]
- Lu, Q.; Qi, S.; Li, P.; Yang, L.; Yang, S.; Wang, Y.; Cheng, Y.; Song, Y.; Wang, S.; Tan, F.; et al. Photothermally activatable PDA immune nanomedicine combined with PD-L1 checkpoint blockade for antimetastatic cancer photoimmunotherapy. J. Mater. Chem. B 2019, 7, 2499–2511. [Google Scholar] [CrossRef]
- Jia, Y.P.; Shi, K.; Yang, F.; Liao, J.F.; Han, R.X.; Yuan, L.P.; Hao, Y.; Pan, M.; Xiao, Y.; Qian, Z.Y.; et al. Multifunctional Nanoparticle Loaded Injectable Thermoresponsive Hydrogel as NIR Controlled Release Platform for Local Photothermal Immunotherapy to Prevent Breast Cancer Postoperative Recurrence and Metastases. Adv. Funct. Mater. 2020, 30, 2001059. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; You, S.; Zhang, K.; Li, M.; Cao, Y.; Wang, C.; Dong, H.; Zhang, X. Bacterial Vesicle-Cancer Cell Hybrid Membrane-Coated Nanoparticles for Tumor Specific Immune Activation and Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 41138–41147. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Wu, M.; Chen, J.; Liu, Y.; Chen, Y.; Fan, G.; Liu, Y.; Cheng, J.; Wang, Z.; Wang, S.; et al. Cancer-Erythrocyte Hybrid Membrane-Camouflaged Magnetic Nanoparticles with Enhanced Photothermal-Immunotherapy for Ovarian Cancer. ACS Nano 2021, 15, 19756–19770. [Google Scholar] [CrossRef]
- Chen, C.; Song, M.; Du, Y.; Yu, Y.; Li, C.; Han, Y.; Yan, F.; Shi, Z.; Feng, S. Tumor-Associated-Macrophage-Membrane-Coated Nanoparticles for Improved Photodynamic Immunotherapy. Nano Lett. 2021, 21, 5522–5531. [Google Scholar] [CrossRef]
- Neek, M.; Tucker, J.A.; Butkovich, N.; Nelson, E.L.; Wang, S.-W. An Antigen-Delivery Protein Nanoparticle Combined with Anti-PD-1 Checkpoint Inhibitor Has Curative Efficacy in an Aggressive Melanoma Model. Adv. Ther. 2020, 3, 2000122. [Google Scholar] [CrossRef]
- Crispen, P.L.; Kusmartsev, S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol. Immunother. 2020, 69, 3–14. [Google Scholar] [CrossRef]
- Ni, Q.; Zhang, F.; Liu, Y.; Wang, Z.; Yu, G.; Liang, B.; Niu, G.; Su, T.; Zhu, G.; Lu, G.; et al. A bi-adjuvant nanovaccine that potentiates immunogenicity of neoantigen for combination immunotherapy of colorectal cancer. Sci. Adv. 2020, 6, eaaw6071. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Zhang, C.; Yan, J.; Hou, X.; Du, S.; Zeng, C.; Zhao, W.; Deng, B.; McComb, D.W.; et al. Biomimetic nanoparticles deliver mRNAs encoding costimulatory receptors and enhance T cell mediated cancer immunotherapy. Nat. Commun. 2021, 12, 7264. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Yang, Y.; Wang, X.; Tian, X.; Qin, W.; Wang, X.; Liang, J.; Zhang, H.; Leng, X. Polydopamine as the Antigen Delivery Nanocarrier for Enhanced Immune Response in Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2330–2342. [Google Scholar] [CrossRef]
- Sordo-Bahamonde, C.; Lorenzo-Herrero, S.; Gonzalez-Rodriguez, A.P.; Martínez-Pérez, A.; Rodrigo, J.P.; García-Pedrero, J.M.; Gonzalez, S. Chemo-Immunotherapy: A New Trend in Cancer Treatment. Cancers 2023, 15, 2912. [Google Scholar] [CrossRef]
- Jiang, M.; Zeng, J.; Zhao, L.; Zhang, M.; Ma, J.; Guan, X.; Zhang, W. Chemotherapeutic drug-induced immunogenic cell death for nanomedicine-based cancer chemo–immunotherapy. Nanoscale 2021, 13, 17218–17235. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Chu, Q.; Liu, Y.; Zhang, N. A Review on Nano-Based Drug Delivery System for Cancer Chemoimmunotherapy. Nano-Micro Lett. 2020, 12, 142. [Google Scholar] [CrossRef]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/Redox Dual-Responsive Polyplex with Effective Endosomal Escape for Codelivery of siRNA and Doxorubicin against Drug-Resistant Cancer Cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef] [PubMed]
- Akkın, S.; Varan, G.; Aksüt, D.; Malanga, M.; Ercan, A.; Şen, M.; Bilensoy, E. A different approach to immunochemotherapy for colon Cancer: Development of nanoplexes of cyclodextrins and Interleukin-2 loaded with 5-FU. Int. J. Pharm. 2022, 623, 121940. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ren, X.; Guo, T.; Sun, X.; Chen, X.; Patterson, L.H.; Li, H.; Zhang, J. NLG919/cyclodextrin complexation and anti-cancer therapeutic benefit as a potential immunotherapy in combination with paclitaxel. Eur. J. Pharm. Sci. 2019, 138, 105034. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, Y.; Chen, X.; Ning, T.; Chen, H.; Guo, Q.; Zhang, Y.; Liu, P.; Zhang, Y.; Li, C.; et al. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials 2021, 268, 120546. [Google Scholar] [CrossRef]
- Shen, F.; Sun, L.; Wang, L.; Peng, R.; Fan, C.; Liu, Z. Framework Nucleic Acid Immune Adjuvant for Transdermal Delivery Based Chemo-immunotherapy for Malignant Melanoma Treatment. Nano Lett. 2022, 22, 4509–4518. [Google Scholar] [CrossRef]
- Liu, M.; Hao, L.; Zhao, D.; Li, J.; Lin, Y. Self-Assembled Immunostimulatory Tetrahedral Framework Nucleic Acid Vehicles for Tumor Chemo-immunotherapy. ACS Appl. Mater. Interfaces 2022, 14, 38506–38514. [Google Scholar] [CrossRef]
- Shabbir, R.; Mingarelli, M.; Cabello, G.; van Herk, M.; Choudhury, A.; Smith, T.A.D. EGFR targeting of [177Lu] gold nanoparticles to colorectal and breast tumour cells: Affinity, duration of binding and growth inhibition of Cetuximab-resistant cells. J. King Saud Univ. Sci. 2021, 33, 101573. [Google Scholar] [CrossRef]
- Mireștean, C.C.; Iancu, R.I.; Iancu, D.T. Radiotherapy and Immunotherapy—A Future Partnership towards a New Standard. Appl. Sci. 2023, 13, 5643. [Google Scholar] [CrossRef]
- Rebegea, L.; Firescu, D.; Stoleriu, G.; Arbune, M.; Anghel, R.; Dumitru, M.; Mihailov, R.; Neagu, A.I.; Bacinschi, X. Radiotherapy and Immunotherapy, Combined Treatment for Unresectable Mucosal Melanoma with Vaginal Origin. Appl. Sci. 2022, 12, 7734. [Google Scholar] [CrossRef]
- Shun, L. NGS-Based Large-Panel in Targeted Drug Delivery and Immunotherapy of Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT04159337 (accessed on 15 August 2024).
- Center, S.Z.M. Phase 1b Study of Pegylated Liposomal Doxorubicin and Pembrolizumab in Endocrine-Resistant Breast Cancer (KEYDOX). Available online: https://clinicaltrials.gov/study/NCT03591276 (accessed on 15 August 2024).
- Serra, P.; Santamaria, P. Nanoparticle-based approaches to immune tolerance for the treatment of autoimmune diseases. Eur. J. Immunol. 2018, 48, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Khademi, Z.; Falsafi, M.; Taghdisi, S.M.; Abnous, K. Cell Membrane Surface-Engineered Nanoparticles for Autoimmune Diseases and Immunotherapy. In Cell Membrane Surface-Engineered Nanoparticles: Biomimetic Nanomaterials for Biomedical Applications; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2024; Volume 1464, pp. 217–247. [Google Scholar]
- Moraes, A.S.; Paula, R.F.O.; Pradella, F.; Santos, M.P.A.; Oliveira, E.C.; von Glehn, F.; Camilo, D.S.; Ceragioli, H.; Peterlevitz, A.; Baranauskas, V.; et al. The Suppressive Effect of IL-27 on Encephalitogenic Th17 Cells Induced by Multiwalled Carbon Nanotubes Reduces the Severity of Experimental Autoimmune Encephalomyelitis. CNS Neurosci. Ther. 2013, 19, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Latha, T.S.; Lomada, D.; Dharani, P.K.; Muthukonda, S.V.; Reddy, M.C. Ti–O based nanomaterials ameliorate experimental autoimmune encephalomyelitis and collagen-induced arthritis. RSC Adv. 2016, 6, 8870–8880. [Google Scholar] [CrossRef]
- Tosic, J.; Stanojevic, Z.; Vidicevic, S.; Isakovic, A.; Ciric, D.; Martinovic, T.; Kravic-Stevovic, T.; Bumbasirevic, V.; Paunovic, V.; Jovanovic, S.; et al. Graphene quantum dots inhibit T cell-mediated neuroinflammation in rats. Neuropharmacology 2019, 146, 95–108. [Google Scholar] [CrossRef]
- Xiao, Q.; Li, X.; Li, Y.; Wu, Z.; Xu, C.; Chen, Z.; He, W. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm. Sin. B 2021, 11, 941–960. [Google Scholar] [CrossRef]
- Lee, Y.; Sugihara, K.; Gillilland Iii, M.G.; Jon, S.; Kamada, N.; Moon, J.J. Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 2020, 19, 118–126. [Google Scholar] [CrossRef]
- Lee, B.-C.; Lee, J.Y.; Kim, J.; Yoo, J.M.; Kang, I.; Kim, J.-J.; Shin, N.; Kim, D.J.; Choi, S.W.; Kim, D.; et al. Graphene quantum dots as anti-inflammatory therapy for colitis. Sci. Adv. 2020, 6, eaaz2630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, C.; Fu, H.; Yu, J.; Sun, Y.; Huang, H.; Tang, Y.; Shen, N.; Duan, Y. MicroRNA-125a-Loaded Polymeric Nanoparticles Alleviate Systemic Lupus Erythematosus by Restoring Effector/Regulatory T Cells Balance. ACS Nano 2020, 14, 4414–4429. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; He, M.; Lin, M.; Tu, C.; Zhang, B. Polymeric nanoparticles containing rapamycin and autoantigen induce antigen-specific immunological tolerance for preventing vitiligo in mice. Hum. Vaccines Immunother. 2021, 17, 1923–1929. [Google Scholar] [CrossRef]
- Mercan, D.-A.; Niculescu, A.-G.; Grumezescu, A.M. Nanoparticles for Antimicrobial Agents Delivery—An Up-to-Date Review. Int. J. Mol. Sci. 2022, 23, 13862. [Google Scholar] [CrossRef] [PubMed]
- Qadri, H.; Shah, A.H.; Alkhanani, M.; Almilaibary, A.; Mir, M.A. Immunotherapies against human bacterial and fungal infectious diseases: A review. Front. Med. 2023, 10, 1135541. [Google Scholar] [CrossRef] [PubMed]
- Kheirollahpour, M.; Mehrabi, M.; Dounighi, N.M.; Mohammadi, M.; Masoudi, A. Nanoparticles and vaccine development. Pharm. Nanotechnol. 2020, 8, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Abusalah, M.A.H.; Chopra, H.; Sharma, A.; Mustafa, S.A.; Choudhary, O.P.; Sharma, M.; Dhawan, M.; Khosla, R.; Loshali, A.; Sundriyal, A.; et al. Nanovaccines: A game changing approach in the fight against infectious diseases. Biomed. Pharmacother. 2023, 167, 115597. [Google Scholar] [CrossRef]
- Curley, S.M.; Putnam, D. Biological nanoparticles in vaccine development. Front. Bioeng. Biotechnol. 2022, 10, 867119. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef] [PubMed]
- ModernaTX, Inc. Safety, Tolerability, and Immunogenicity of VAL-506440 in Healthy Adult Subjects; ModernaTX, Inc.: Cambridge, MA, USA, 2015. [Google Scholar]
- ModernaTX, Inc. Safety, Tolerability, and Immunogenicity of VAL-339851 in Healthy Adult Subjects; ModernaTX, Inc.: Cambridge, MA, USA, 2016. [Google Scholar]
- Scarpini, S.; Morigi, F.; Betti, L.; Dondi, A.; Biagi, C.; Lanari, M. Development of a Vaccine against Human Cytomegalovirus: Advances, Barriers, and Implications for the Clinical Practice. Vaccines 2021, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; Porter, E.; Zhang, Y.; Silva, M.; Li, N.; Dobosh, B.; Liguori, A.; Skog, P.; Landais, E.; Menis, S.; et al. Immunogenicity of RNA Replicons Encoding HIV Env Immunogens Designed for Self-Assembly into Nanoparticles. Mol. Ther. 2019, 27, 2080–2090. [Google Scholar] [CrossRef]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. NPJ Vaccines 2021, 6, 50. [Google Scholar] [CrossRef]
- Flisiak, R.; Jaroszewicz, J.; Łucejko, M. siRNA drug development against hepatitis B virus infection. Expert Opin. Biol. Ther. 2018, 18, 609–617. [Google Scholar] [CrossRef]
- Thi, E.P.; Dhillon, A.P.; Ardzinski, A.; Bidirici-Ertekin, L.; Cobarrubias, K.D.; Cuconati, A.; Kondratowicz, A.S.; Kwak, K.; Li, A.H.L.; Miller, A.; et al. ARB-1740, a RNA Interference Therapeutic for Chronic Hepatitis B Infection. ACS Infect. Dis. 2019, 5, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tateno, C.; Thi, E.P.; Kakuni, M.; Snead, N.M.; Ishida, Y.; Barnard, T.R.; Sofia, M.J.; Shimada, T.; Lee, A.C.H. Hepatitis B Virus Therapeutic Agent ARB-1740 Has Inhibitory Effect on Hepatitis Delta Virus in a New Dually-Infected Humanized Mouse Model. ACS Infect. Dis. 2019, 5, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Dallas, A.; Ilves, H.; Shorenstein, J.; Judge, A.; Spitler, R.; Contag, C.; Wong, S.P.; Harbottle, R.P.; MacLachlan, I.; Johnston, B.H. Minimal-length synthetic shRNAs formulated with lipid nanoparticles are potent inhibitors of hepatitis C virus IRES-linked gene expression in mice. Mol. Ther. Nucleic Acids 2013, 2, e123. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Kundu, A.K.; Hazari, S.; Chandra, S.; Bao, L.; Ooms, T.; Morris, G.F.; Wu, T.; Mandal, T.K.; Dash, S. Inhibition of hepatitis C virus replication by intracellular delivery of multiple siRNAs by nanosomes. Mol. Ther. 2012, 20, 1724–1736. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Lee, S.-H.; Kim, E.-J.; Cho, H.; Lee, W.; Kim, G.-W.; Park, H.-J.; Cho, S.-W.; Lee, C.; Oh, J.-W. Inhibition of Hepatitis C Virus in Mice by a Small Interfering RNA Targeting a Highly Conserved Sequence in Viral IRES Pseudoknot. PLoS ONE 2016, 11, e0146710. [Google Scholar] [CrossRef]
- Egan, K.P.; Hook, L.M.; Naughton, A.; Pardi, N.; Awasthi, S.; Cohen, G.H.; Weissman, D.; Friedman, H.M. An HSV-2 nucleoside-modified mRNA genital herpes vaccine containing glycoproteins gC, gD, and gE protects mice against HSV-1 genital lesions and latent infection. PLoS Pathog. 2020, 16, e1008795. [Google Scholar] [CrossRef]
- Xu, L.; Xiang, J.; Liu, Y.; Xu, J.; Luo, Y.; Feng, L.; Liu, Z.; Peng, R. Functionalized graphene oxide serves as a novel vaccine nano-adjuvant for robust stimulation of cellular immunity. Nanoscale 2016, 8, 3785–3795. [Google Scholar] [CrossRef]
- Vicente, S.; Peleteiro, M.; Díaz-Freitas, B.; Sanchez, A.; González-Fernández, Á.; Alonso, M.J. Co-delivery of viral proteins and a TLR7 agonist from polysaccharide nanocapsules: A needle-free vaccination strategy. J. Control. Release 2013, 172, 773–781. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Chen, X.; Zhou, Z.; Pang, J.; Luo, X.; Kong, M. A surface charge dependent enhanced Th1 antigen-specific immune response in lymph nodes by transfersome-based nanovaccine-loaded dissolving microneedle-assisted transdermal immunization. J. Mater. Chem. B 2019, 7, 4854–4866. [Google Scholar] [CrossRef]
- Umeyor, C.E.; Okonkwo, A.U.; Ejielo, O.D.; Umeyor, I.C.; Uronnachi, E.M.; Nwakile, C.D.; Okeke, I.J.; Attama, A.A. Formulation design and preclinical evaluations of surface modified lipid nanoparticles-coupled gel encapsulating dihydroartemisinin for treatment of localized inflammation. Lett. Appl. NanoBioSci 2021, 11, 3745–3769. [Google Scholar]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Morcuende, A.; Navarrete, F.; Nieto, E.; Manzanares, J.; Femenía, T. Inflammatory Biomarkers in Addictive Disorders. Biomolecules 2021, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Widhiati, S.; Purnomosari, D.; Wibawa, T.; Soebono, H. The role of gut microbiome in inflammatory skin disorders: A systematic review. Dermatol. Rep. 2022, 14, 9188. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.S.; Farhana, S.A.; Tp, A.; Hussain, S.M.; Viswanad, V.; Nasr, M.H.; Sahu, R.K.; Khan, J. Modulation of immune response by nanoparticle-based immunotherapy against food allergens. Front. Immunol. 2023, 14, 1229667. [Google Scholar]
- Broos, S.; Lundberg, K.; Akagi, T.; Kadowaki, K.; Akashi, M.; Greiff, L.; Borrebaeck, C.A.K.; Lindstedt, M. Immunomodulatory nanoparticles as adjuvants and allergen-delivery system to human dendritic cells: Implications for specific immunotherapy. Vaccine 2010, 28, 5075–5085. [Google Scholar] [CrossRef]
- Ryan, J.J.; Bateman, H.R.; Stover, A.; Gomez, G.; Norton, S.K.; Zhao, W.; Schwartz, L.B.; Lenk, R.; Kepley, C.L. Fullerene Nanomaterials Inhibit the Allergic Response. J. Immunol. 2007, 179, 665–672. [Google Scholar] [CrossRef]
- Pali-Schöll, I.; Szöllösi, H.; Starkl, P.; Scheicher, B.; Stremnitzer, C.; Hofmeister, A.; Roth-Walter, F.; Lukschal, A.; Diesner, S.C.; Zimmer, A.; et al. Protamine nanoparticles with CpG-oligodeoxynucleotide prevent an allergen-induced Th2-response in BALB/c mice. Eur. J. Pharm. Biopharm. 2013, 85, 656–664. [Google Scholar] [CrossRef]
- Pereira, M.A.; de Souza Rebouças, J.; de Siqueira Ferraz-Carvalho, R.; de Redín, I.L.; Guerra, P.V.; Gamazo, C.; Brodskyn, C.I.; Irache, J.M.; Santos-Magalhães, N.S. Poly(anhydride) nanoparticles containing cashew nut proteins can induce a strong Th1 and Treg immune response after oral administration. Eur. J. Pharm. Biopharm. 2018, 127, 51–60. [Google Scholar] [CrossRef]
- Garaczi, E.; Szabó, K.; Francziszti, L.; Csiszovszki, Z.; Lőrincz, O.; Tőke, E.R.; Molnár, L.; Bitai, T.; Jánossy, T.; Bata-Csörgő, Z.; et al. DermAll nanomedicine for allergen-specific immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 1245–1254. [Google Scholar] [CrossRef][Green Version]
- Tasaniyananda, N.; Chaisri, U.; Tungtrongchitr, A.; Chaicumpa, W.; Sookrung, N. Mouse Model of Cat Allergic Rhinitis and Intranasal Liposome-Adjuvanted Refined Fel d 1 Vaccine. PLoS ONE 2016, 11, e0150463. [Google Scholar] [CrossRef]
- Aliu, H.; Rask, C.; Brimnes, J.; Andresen, T.L. Enhanced efficacy of sublingual immunotherapy by liposome-mediated delivery of allergen. Int. J. Nanomed. 2017, 12, 8377–8388. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ji, Z.; Zhou, H.; Wu, D.; Gu, Z.; Wang, D.; ten Dijke, P. Lipid-based nanoparticles as drug delivery systems for cancer immunotherapy. MedComm 2023, 4, e339. [Google Scholar] [CrossRef]
- Gao, Y.; Joshi, M.; Zhao, Z.; Mitragotri, S. PEGylated therapeutics in the clinic. Bioeng. Transl. Med. 2024, 9, e10600. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, Y.; Liu, Y.; Hu, X.; Wen, T. Diverse drug delivery systems for the enhancement of cancer immunotherapy: An overview. Front. Immunol. 2024, 15, 1328145. [Google Scholar] [CrossRef]
- Namiot, E.D.; Sokolov, A.V.; Chubarev, V.N.; Tarasov, V.V.; Schiöth, H.B. Nanoparticles in Clinical Trials: Analysis of Clinical Trials, FDA Approvals and Use for COVID-19 Vaccines. Int. J. Mol. Sci. 2023, 24, 787. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, Z.; Ding, Z.; Lv, W.; Li, J.; Li, X.; Liu, H.; Yu, P.; Yang, X.; Gan, L. Boosting doxil-based chemoimmunotherapy via reprogramming tumor-associated macrophages. Chem. Eng. J. 2023, 451, 138971. [Google Scholar] [CrossRef]
- Rodríguez, F.; Caruana, P.; De la Fuente, N.; Español, P.; Gámez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Gajera, K.; Patel, A. An overview of FDA approved liposome formulations for cancer therapy. J. Adv. Med. Pharm. Sci. 2022, 24, 1–7. [Google Scholar] [CrossRef]
- Leung, A.W.Y.; Amador, C.; Wang, L.C.; Mody, U.V.; Bally, M.B. What Drives Innovation: The Canadian Touch on Liposomal Therapeutics. Pharmaceutics 2019, 11, 124. [Google Scholar] [CrossRef]
- Dawidczyk, C.M.; Kim, C.; Park, J.H.; Russell, L.M.; Lee, K.H.; Pomper, M.G.; Searson, P.C. State-of-the-art in design rules for drug delivery platforms: Lessons learned from FDA-approved nanomedicines. J. Control. Release 2014, 187, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, K.; Liu, Y.; Noe, D.; Mertz, J.; Bargfrede, M.; Marbury, T.; Farbakhsh, K.; Oliva, C.; Milton, A. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br. J. Clin. Pharmacol. 2014, 77, 998–1010. [Google Scholar] [CrossRef] [PubMed]
- Múdry, P.; Kýr, M.; Rohleder, O.; Mahdal, M.; Staniczková Zambo, I.; Ježová, M.; Tomáš, T.; Štěrba, J. Improved osteosarcoma survival with addition of mifamurtide to conventional chemotherapy—Observational prospective single institution analysis. J. Bone Oncol. 2021, 28, 100362. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Kumar, V.; Tiwari, R.K.; Ravidas, V.; Pandey, K.; Kumar, A. Amphotericin B: A drug of choice for Visceral Leishmaniasis. Acta Trop. 2022, 235, 106661. [Google Scholar] [CrossRef] [PubMed]
- Urits, I.; Swanson, D.; Swett, M.C.; Patel, A.; Berardino, K.; Amgalan, A.; Berger, A.A.; Kassem, H.; Kaye, A.D.; Viswanath, O. A Review of Patisiran (ONPATTRO®) for the Treatment of Polyneuropathy in People with Hereditary Transthyretin Amyloidosis. Neurol. Ther. 2020, 9, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Fernández, M.; Melguizo-Rodríguez, L.; Costela-Ruiz, V.J.; González-Acedo, A.; Ramos-Torrecillas, J.; Illescas-Montes, R. The current status of COVID-19 vaccines. A scoping review. Drug Discov. Today 2022, 27, 103336. [Google Scholar] [CrossRef]
- Timmins, P. Industry Update: The Latest Developments in the Field of Therapeutic delivery, November 2021. Ther. Deliv. 2022, 13, 141–156. [Google Scholar] [CrossRef]
- Wang, F.; Porter, M.; Konstantopoulos, A.; Zhang, P.; Cui, H. Preclinical development of drug delivery systems for paclitaxel-based cancer chemotherapy. J. Control. Release 2017, 267, 100–118. [Google Scholar] [CrossRef]
- Crintea, A.; Dutu, A.G.; Samasca, G.; Florian, I.A.; Lupan, I.; Craciun, A.M. The Nanosystems Involved in Treating Lung Cancer. Life 2021, 11, 682. [Google Scholar] [CrossRef]
- Wileński, S.; Koper, A.; Śledzińska, P.; Bebyn, M.; Koper, K. Innovative strategies for effective paclitaxel delivery: Recent developments and prospects. J. Oncol. Pharm. Pract. 2024, 30, 367–384. [Google Scholar] [CrossRef]
- Wang, X.; Dormont, F.; Lorenzato, C.; Latouche, A.; Hernandez, R.; Rouzier, R. Current perspectives for external control arms in oncology clinical trials: Analysis of EMA approvals 2016–2021. J. Cancer Policy 2023, 35, 100403. [Google Scholar] [CrossRef] [PubMed]
- Binkhathlan, Z.; Yusuf, O.; Ali, R.; Alomrani, A.H.; Alshamsan, A.; Alshememry, A.K.; Almomen, A.; Alkholief, M.; Aljuffali, I.A.; Alqahtani, F. Polycaprolactone–Vitamin E TPGS micelles for delivery of paclitaxel: In vitro and in vivo evaluation. Int. J. Pharm. X 2024, 7, 100253. [Google Scholar] [CrossRef] [PubMed]
- Serras, A.; Faustino, C.; Pinheiro, L. Functionalized Polymeric Micelles for Targeted Cancer Therapy: Steps from Conceptualization to Clinical Trials. Pharmaceutics 2024, 16, 1047. [Google Scholar] [CrossRef]
- Patel, P.; Vedarethinam, V.; Korsah, M.A.; Danquah, M.K.; Jeevanandam, J. Exploring the Potential of Nanoparticles in the Treatment of Breast Cancer: Current Applications and Future Directions. Appl. Sci. 2024, 14, 1809. [Google Scholar] [CrossRef]
- Yousefpour, P.; Ni, K.; Irvine, D.J. Targeted modulation of immune cells and tissues using engineered biomaterials. Nat. Rev. Bioeng. 2023, 1, 107–124. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Scheetz, L.; Park, K.S.; Li, Q.; Lowenstein, P.R.; Castro, M.G.; Schwendeman, A.; Moon, J.J. Engineering patient-specific cancer immunotherapies. Nat. Biomed. Eng. 2019, 3, 768–782. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Fallica, A.N.; Virzì, N.; Kesharwani, P.; Pittalà, V.; Greish, K. The promise of nanotechnology in personalized medicine. J. Pers. Med. 2022, 12, 673. [Google Scholar] [CrossRef]
- Lu, Q.; Kou, D.; Lou, S.; Ashrafizadeh, M.; Aref, A.R.; Canadas, I.; Tian, Y.; Niu, X.; Wang, Y.; Torabian, P.; et al. Nanoparticles in tumor microenvironment remodeling and cancer immunotherapy. J. Hematol. Oncol. 2024, 17, 16. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Malachowski, T.; Hassel, A. Engineering nanoparticles to overcome immunological barriers for enhanced drug delivery. Eng. Regen. 2020, 1, 35–50. [Google Scholar] [CrossRef]
- Ray, P.; Haideri, N.; Haque, I.; Mohammed, O.; Chakraborty, S.; Banerjee, S.; Quadir, M.; Brinker, A.E.; Banerjee, S.K. The impact of nanoparticles on the immune system: A gray zone of nanomedicine. J. Immunol. Sci. 2021, 5, 19–33. [Google Scholar] [CrossRef]
- Zolnik, B.S.; González-Fernández, Á.; Sadrieh, N.; Dobrovolskaia, M.A. Minireview: Nanoparticles and the immune system. Endocrinology 2010, 151, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Caracciolo, G. Tuning the immune system by nanoparticle–biomolecular corona. Nanoscale Adv. 2022, 4, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Sushnitha, M.; Evangelopoulos, M.; Tasciotti, E.; Taraballi, F. Cell membrane-based biomimetic nanoparticles and the immune system: Immunomodulatory interactions to therapeutic applications. Front. Bioeng. Biotechnol. 2020, 8, 627. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Xu, W.; Li, Y.; Lai, R.; Qiu, X.; Chen, X.; Chen, Z.; Mi, B.; Wu, M. Translational challenges and prospective solutions in the implementation of biomimetic delivery systems. Pharmaceutics 2023, 15, 2623. [Google Scholar] [CrossRef]
- Sen, S.; Xavier, J.; Kumar, N.; Ahmad, M.Z.; Ranjan, O.P. Exosomes as natural nanocarrier-based drug delivery system: Recent insights and future perspectives. 3 Biotech 2023, 13, 101. [Google Scholar] [CrossRef]
- Đorđević, S.; Gonzalez, M.M.; Conejos-Sánchez, I.; Carreira, B.; Pozzi, S.; Acúrcio, R.C.; Satchi-Fainaro, R.; Florindo, H.F.; Vicent, M.J. Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Deliv. Transl. Res. 2022, 12, 1–26. [Google Scholar] [CrossRef]
- Desai, N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012, 14, 282–295. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Busnatu, Ș.; Niculescu, A.-G.; Bolocan, A.; Petrescu, G.E.D.; Păduraru, D.N.; Năstasă, I.; Lupușoru, M.; Geantă, M.; Andronic, O.; Grumezescu, A.M. Clinical applications of artificial intelligence—An updated overview. J. Clin. Med. 2022, 11, 2265. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Ma, Q.; Yin, W.; Ma, X.; He, Z. CRISPR/Cas9 gene-editing in cancer immunotherapy: Promoting the present revolution in cancer therapy and exploring more. Front. Cell Dev. Biol. 2021, 9, 674467. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, M.; Ren, Y.; Xu, H.; Weng, S.; Ning, W.; Ge, X.; Liu, L.; Guo, C.; Duo, M.; et al. Recent advances and applications of CRISPR-Cas9 in cancer immunotherapy. Mol. Cancer 2023, 22, 35. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials synthesis through microfluidic methods: An updated overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]

| ClinicalTrials.gov ID | Official Title | Study Type | Intervention | Enrollment (Estimated) | Study Completion (Estimated) | Ref. |
|---|---|---|---|---|---|---|
| NCT04159337 | A Prospective Study on Companion Diagnosis by NGS-based Large-panel in Targeted Drug Delivery and Immunotherapy of Lung Cancer | Observational | Other: The method of gene mutation detection | 2000 | 31 May 2022 | [106] |
| NCT03591276 | A Phase 1b Study of Combination Chemo-immunotherapy With Pegylated Liposomal Doxorubicin (Doxil/Caelyx) and Pembrolizumab (Keytruda) in Metastatic Endocrine-resistant Breast Cancer | Interventional | Drug: Chemotherapy drugs, cancer | 15 | 15 June 2021 | [107] |
| Commercial Name | Formulation | Benefits | Indication | Approval Status | Company/Approval Holder | Refs. |
|---|---|---|---|---|---|---|
| Doxil® | Doxorubicin hydrochloride encapsulated in PEGylated liposomes |
| Ovarian cancer, Multiple myeloma, AIDS-related Kaposi’s Sarcoma | First FDA-approved nano-drug (1995)Also approved by EMA (1996, marketed under the name of “Caelyx”), HC (1998), and PMDA (2009) | Schering-Plough Corporation (Kenilworth, NJ, USA) | [152,155,156,157] |
| Myocet® | Doxorubicin encapsulated in liposomes |
| Metastatic breast cancer | Approved by EMA (2000) and HC (2001) | Teva Pharmaceutical Industries (Tel Aviv, Israel) | [153,158,159] |
| DaunoXome® | Daunorubicin encapsulated in liposomes |
| AIDS-related Kaposi’s Sarcoma | Approved by FDA, EMA, and HC (1996) | Galen Pharma (Craigavon, Northern Ireland, UK) | [153,157,158,160] |
| Onivyde® | Irinotecan hydrochloride trihydrate encapsulated in PEGylated liposomes |
| Metastatic adenocarcinoma of the pancreas | Approved by FDA (2015), EMA (2016), HC (2017), and PMDA (2020) | Merrimack Pharmaceuticals (Cambridge, MA, USA) | [152,153,157] |
| Vyxeos® | Daunorubicin and cytarabine co-encapsulated in liposomes |
| Therapy-related AML or AML with myelodysplasia-related changes | Approved by FDA (2015), EMA (2016), and HC (2017) | Jazz Pharmaceuticals (Palo Alto, CA, USA) | [157,159] |
| Marqibo® | Vincristine encapsulated in liposomes |
| Acute lymphoblastic leukemia | Approved by FDA (2012) | Spectrum Pharmaceuticals (Henderson, NV, USA) | [157,158,159,160] |
| DepoCyt® | Aracytidine encapsulated in liposomes |
| Neoplastic meningitis | Approved by FDA, EMA, and HC (2007) | Pacira Pharmaceuticals (San Diego, CA, USA) | [158,159] |
| Mepact® | Mifamurtide encapsulated in liposomes |
| High-grade, resectable, non-metastatic osteosarcoma | Approved by EMA (2009) | Takeda Pharmaceuticals (Tokyo, Japan) | [153,158,161,162] |
| AmBisome® | Amphotercin B encapsulated in liposomes |
| Systemic fungal infection | Approved by FDA, HC (1997), EMA (1999), and PMDA (2001) | Astellas Pharma (Northbrook, Illinois, USA) and Gilead Sciences (San Dimas, CA, USA) | [159,163] |
| Onpattro® | siRNA encapsulated in LNPs |
| Polyneuropathy of hereditary transthyretin-mediated amyloidosis | Approved by FDA, EMA, HC (2018), and PMDA (2019) | Alnylam Pharmaceuticals (Cambridge, MA, USA) | [152,159,164] |
| Spikevax® | mRNA encapsulated in LNPs |
| COVID-19 | Approved by FDA (EUA in 2020, full approval in 2022), EMA, and PMDA (2021) | Moderna (Cambridge, MA, USA) | [152,165] |
| Comirnaty™ | mRNA encapsulated in LNPs |
| COVID-19 | Approved by FDA (EUA in 2020, full approval in 2021), EMA, and PMDA (2021) | BioNTech/Pfizer (Mainz, Germany) | [152,165] |
| Abraxane® | Nanoparticle albumin-bound Paclitaxel |
| Metastatic breast cancer, locally advanced or metastatic NSCLC, metastatic adenocarcinoma of the pancreas | Approved by FDA (2005), HC (2006), EMA (2008), and PMDA (2010) | Celgene Corporation (Summit, NJ, USA) | [153,160] |
| Fyarro® | Nanoparticle albumin-bound Sirolimus |
| Metastatic perivascular epithelioid cell tumor | Approved by FDA, EMA (2021), and HC (2022) | Aadi Bioscience (Pacific Palisades, CA, USA) | [153,166] |
| Genexol® | Paclitaxel encapsulated in polymer micelle |
| Metastatic breast cancer, NSCLC, ovarian cancer | Approved in Republic of Korea and China (2007) | Samyang Biopharm (Seoyoon Cheong, Republic of Korea) | [153,167,168,169] |
| Apealea® | Paclitaxel encapsulated in polymer micelle |
| Epithelial ovarian cancer, primary peritoneal cancer, fallopian tube cancer | Approved by EMA (2018) | Elevar Therapeutics (Fort Lee, NJ, USA) | [153,170,171] |
| Nanoxel® | Docetaxel encapsulated in polymer micelle |
| Metastatic esophageal squamous cell carcinoma | Approved in India (2012) | Samyang Biopharm (Seoyoon Cheong, Republic of Korea) | [153,172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Croitoru, G.-A.; Niculescu, A.-G.; Epistatu, D.; Mihaiescu, D.E.; Antohi, A.M.; Grumezescu, A.M.; Nicolae, C.-L. Nanostructured Drug Delivery Systems in Immunotherapy: An Updated Overview of Nanotechnology-Based Therapeutic Innovations. Appl. Sci. 2024, 14, 8948. https://doi.org/10.3390/app14198948
Croitoru G-A, Niculescu A-G, Epistatu D, Mihaiescu DE, Antohi AM, Grumezescu AM, Nicolae C-L. Nanostructured Drug Delivery Systems in Immunotherapy: An Updated Overview of Nanotechnology-Based Therapeutic Innovations. Applied Sciences. 2024; 14(19):8948. https://doi.org/10.3390/app14198948
Chicago/Turabian StyleCroitoru, George-Alexandru, Adelina-Gabriela Niculescu, Dragoș Epistatu, Dan Eduard Mihaiescu, Alexandru Mihai Antohi, Alexandru Mihai Grumezescu, and Carmen-Larisa Nicolae. 2024. "Nanostructured Drug Delivery Systems in Immunotherapy: An Updated Overview of Nanotechnology-Based Therapeutic Innovations" Applied Sciences 14, no. 19: 8948. https://doi.org/10.3390/app14198948
APA StyleCroitoru, G.-A., Niculescu, A.-G., Epistatu, D., Mihaiescu, D. E., Antohi, A. M., Grumezescu, A. M., & Nicolae, C.-L. (2024). Nanostructured Drug Delivery Systems in Immunotherapy: An Updated Overview of Nanotechnology-Based Therapeutic Innovations. Applied Sciences, 14(19), 8948. https://doi.org/10.3390/app14198948









