Physical–Chemical and Thermal Properties of Clays from Porto Santo Island, Portugal
Abstract
:1. Introduction
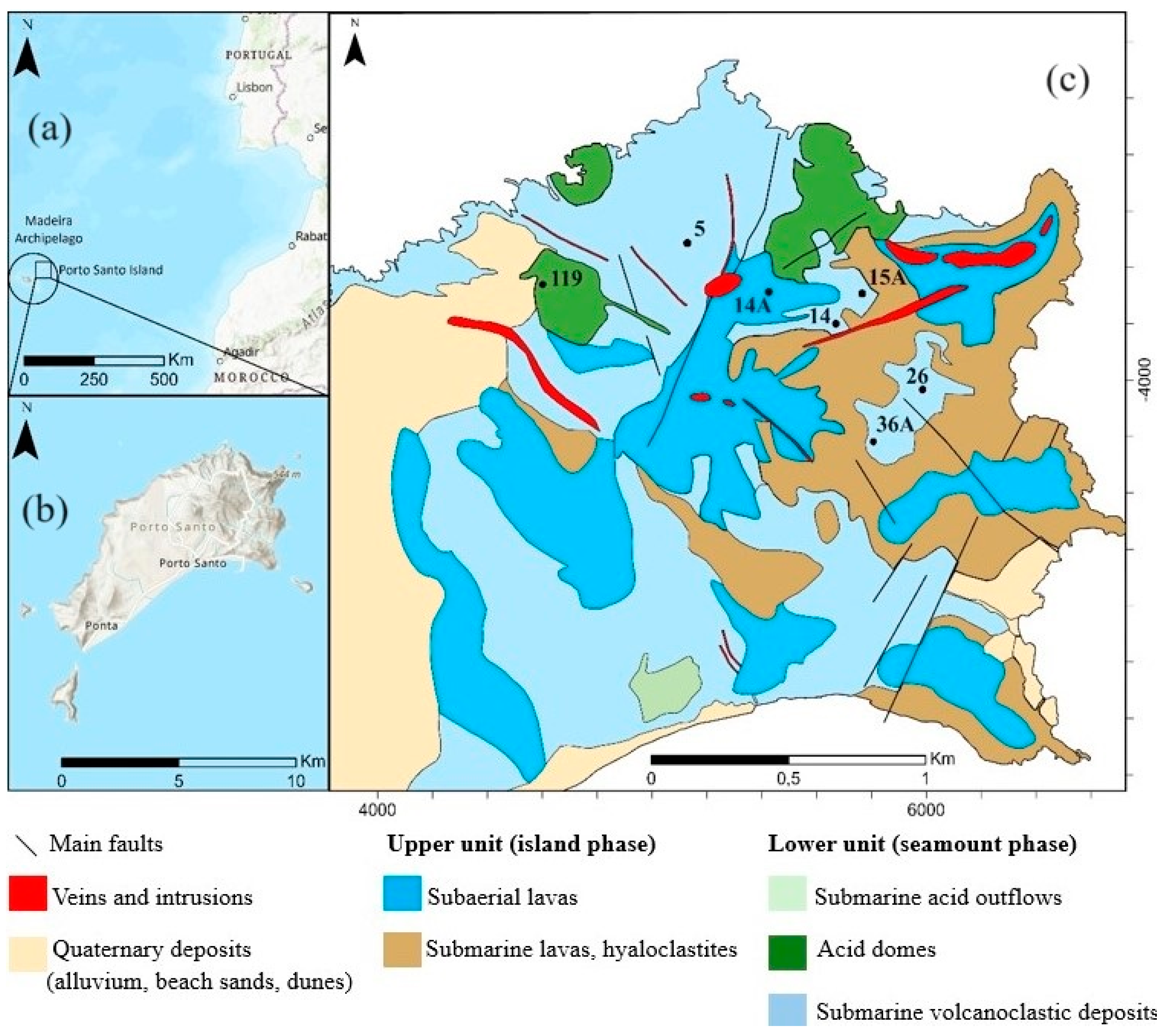
2. Geological Settings
3. Materials and Methods
3.1. Materials Description
3.2. Methods
3.2.1. Textural Analysis
3.2.2. Chemical Analysis
3.2.3. Mineralogical Analysis
3.2.4. Physical–Chemical Analysis
3.2.5. Thermal Analysis
4. Results and Discussion
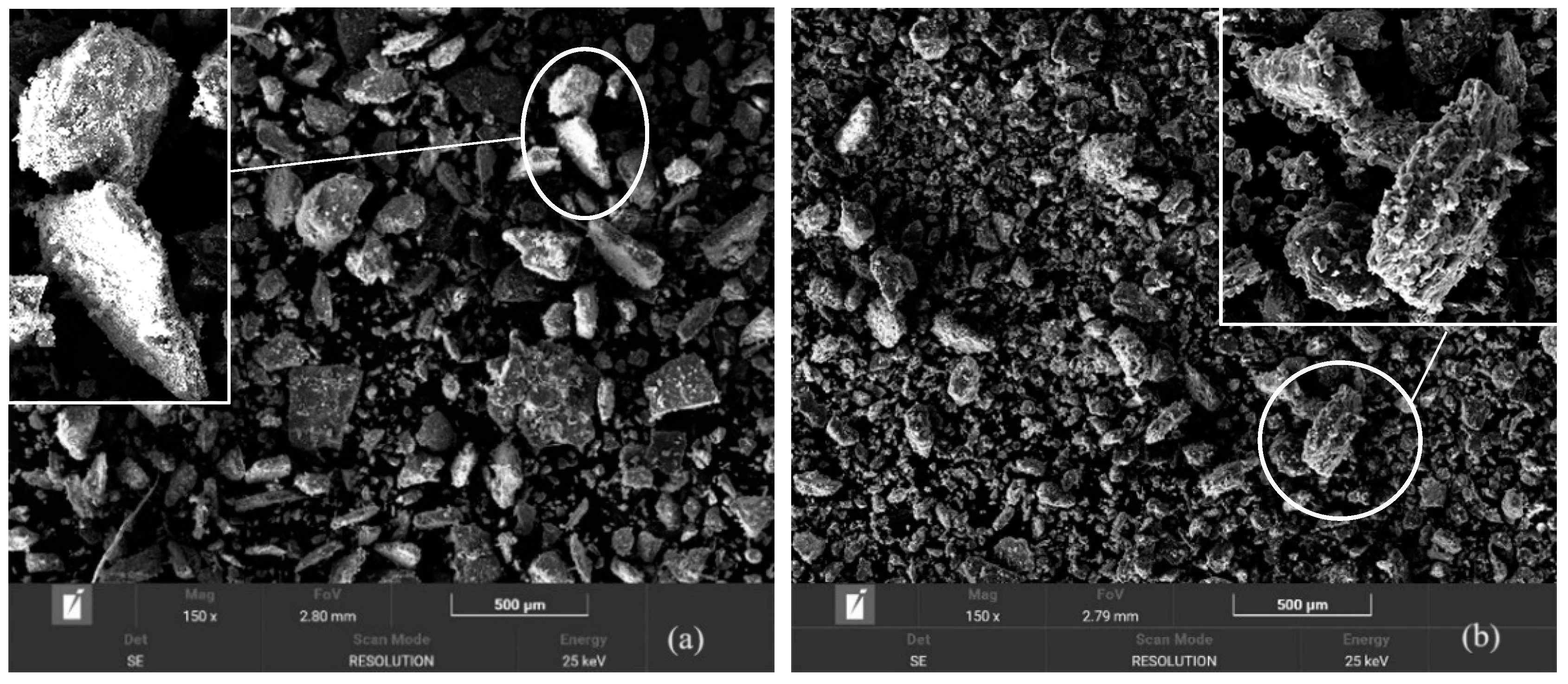
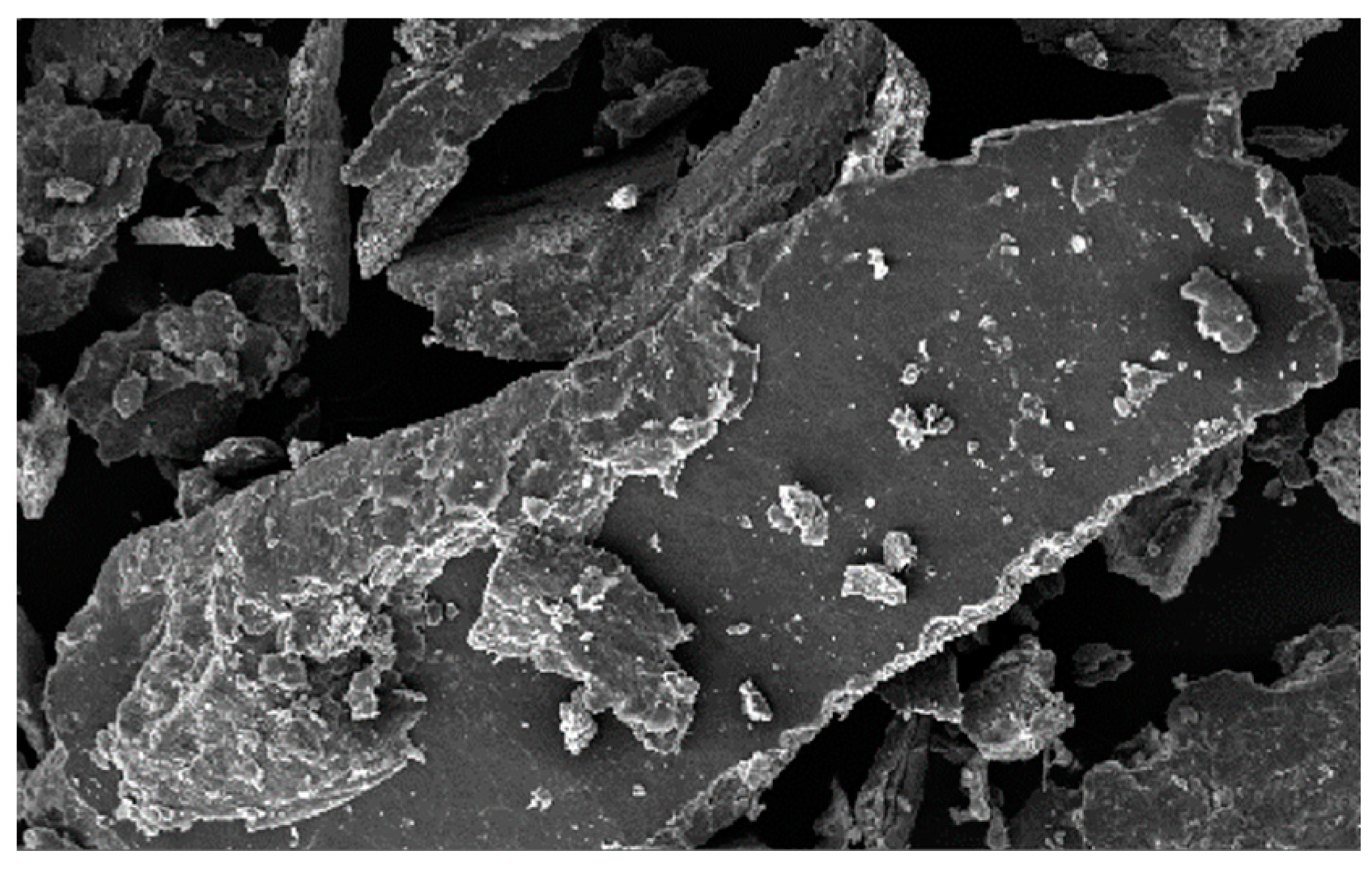
4.1. Morphology and Grain Size Analysis
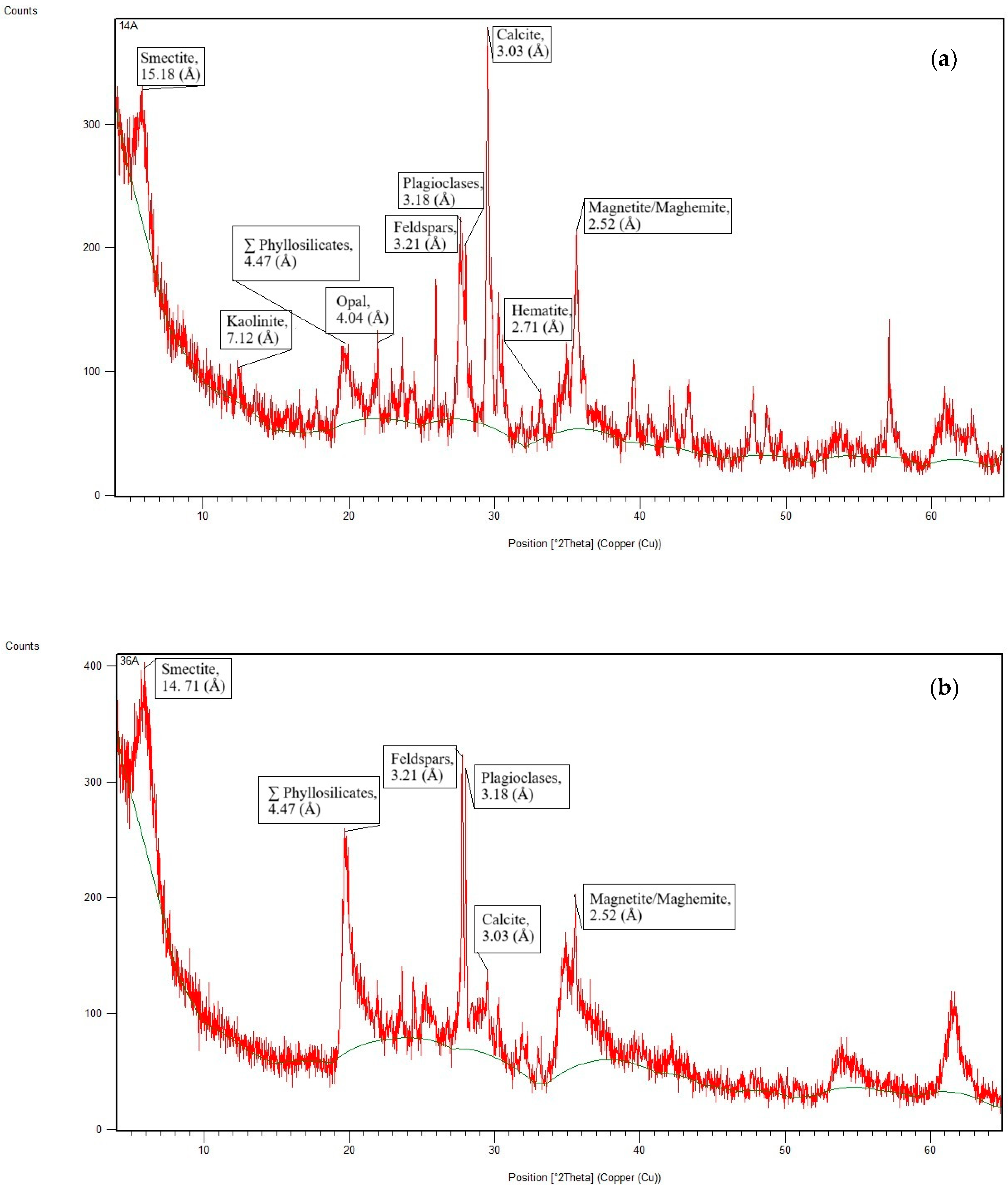
4.2. Chemical and Mineralogical Analysis
4.3. Physicochemical Properties
4.4. Thermal Characterization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carretero, M.I.; Pozo, M. Clay and non-clay minerals in the pharmaceutical and cosmetic industries Part II. Active ingredients. Appl. Clay Sci. 2010, 47, 171–181. [Google Scholar] [CrossRef]
- Gomes, C.S.F. Healing and edible clays: A review of basic concepts, benefits and risks. Environ. Geochem. Health 2018, 40, 1739–1765. [Google Scholar] [CrossRef]
- Meunier, A. Clays, 1st ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005; pp. 366–376. [Google Scholar] [CrossRef]
- Bastos, C.M.; Rocha, F. Assessment of Some Clay-Based Products Available on Market and Designed for Topical Use. Geosciences 2022, 12, 453. [Google Scholar] [CrossRef]
- Veniale, F.; Bettero, A.; Jobstraibizer, P.G.; Setti, M. Thermal muds: Perspectives of innovations. Appl. Clay Sci. 2007, 36, 141–147. [Google Scholar] [CrossRef]
- Armijo, F.; Maraver, F.; Pozo, M.; Carretero, M.I.; Armijo, O.; Fernández-Torán, M.Á.; Corvillo, I. Thermal behaviour of clays and clay-water mixtures for pelotherapy. Appl. Clay Sci. 2016, 126, 50–56. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Silva, J.B.P. Minerals and Clay Minerals in Medical Geology. Appl. Clay Sci. 2007, 36, 4–21. [Google Scholar] [CrossRef]
- Williams, L.B.; Haydel, S.E. Evaluation of the medicinal use of clay minerals as antibacterial agents. Int. Geol. Rev. 2010, 52, 745–770. [Google Scholar] [CrossRef]
- Ferraz, E.; Silva, J.; Gomes, C. Properties and applications of bentonites from Porto Santo Island (Madeira Archipelago). Acta Univ. Carol.-Geológica 1994, 38, 175–182. [Google Scholar]
- Gomes, C.; Silva, J. Beach Sand and Bentonite of Porto Santo Island: Potentialities for Applications in Geomedicine. O Liberal. Camara Lobos Madeira 2001, 60. [Google Scholar]
- Silva, J.; Gomes, C.; Rocha, F. Portuguese clays used in geomedicine: A study of their relevant properties. In 2001—A Clay Odyssey; Dominguez, E., Mas, G., Cravero, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 315–322. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Silva, J.B.P. Psammotherapy in Porto Santo Island (Madeira archipelago). An. Hidrol. Medica 2012, 4, 11–32. [Google Scholar] [CrossRef]
- Gomes, C.S.F.; Silva, J.B.P.; Viegas Fernandes, J.; Viegas Fernandes, F.M. Thalassotherapy in Porto Santo Island of the Madeira Archipelago: Facts and prospects. Bol. Soc. Esp. Hidrol. Méd. 2019, 34, 9–33. [Google Scholar] [CrossRef]
- Legido, J.L.; Medina, C.; Mourelle, M.L.; Carretero, M.I.; Pozo, M. Comparative study of the cooling rates of bentonite, sepiolite and common clays for their use in pelotherapy. Appl. Clay Sci. 2007, 36, 148–160. [Google Scholar] [CrossRef]
- Karakaya, M.; Karakaya, N.; Vural, H. Thermal Properties of Some Turkish Peloids and Clay Minerals for Their Use in Pelotherapy. Geomaterials 2016, 6, 79–90. [Google Scholar] [CrossRef]
- Innocentini, S.; Quartau, R.; Casalbore, D.; Roque, C.; Vinhas, A.; Santos, R.; Rodrigues, A. Morpho-stratigraphic characterization of the southern shelf of Porto Santo Island (Madeira Archipelago): Insights for small-scale instability processes and post-LGM sedimentary architecture. Mar. Geol. 2022, 444, 106729. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Neiva, J.C. Carta Geológica de Portugal, Folha de Porto Santo à escala 1:25,000; Universidade de Coimbra/Instituto Geológico e Mineiro: Coimbra, Portugal, 1996. [Google Scholar]
- Schmidt, R.; Schmincke, H.U. From seamount to oceanic island, Porto Santo, central East-Atlantic. Int. J. Earth Sci. 2002, 91, 594–614. [Google Scholar] [CrossRef]
- Ribeiro, M.L.; Ramalho, M. A Geological Tour of the Archipelago of Madeira; Main Geo-Touristic Sites; Direção Regional do Comércio, Indústria e Energia, Laboratório Nacional de Energia e Geologia, I.P.: Lisboa, Portugal, 2009; Volume 92, ISBN 978-989-675-008-4. [Google Scholar]
- Czajkowski, M. A geological tour of the islands of Madeira and Porto Santo. Geol. Today 2002, 18, 26–34. [Google Scholar] [CrossRef]
- López-Galindo, A.; Viseras, C.; Cerezo, P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl. Clay Sci. 2007, 36, 51–63. [Google Scholar] [CrossRef]
- Rebelo, M.; Rocha, F.; Silva, E.F. Mineralogical and physicochemical characterization of selected Portuguese Mesozoic-Cenozoic muddy/clayey raw materials to be potentially used as healing clays. Clay Miner. 2010, 45, 229–240. [Google Scholar] [CrossRef]
- Rebelo, M.; Viseras, C.; López-Galindo, A.; Rocha, F.; Ferreira, E. Characterization of Portuguese geological materials to be used in medical hydrology. Appl. Clay Sci. 2011, 51, 258–266. [Google Scholar] [CrossRef]
- Quintela, A.; Terroso, D.; Silva, E.F.; Rocha, F. Certification and quality criteria of peloids used for therapeutic purposes. Clay Miner. 2012, 47, 441–451. [Google Scholar] [CrossRef]
- USDA. Soil survey manual. In Soil Survey Division Staff, Soil Conservation Service; Handbook No. 18; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; U.S. Department of Agriculture: Washington, DC, USA, 2017; Volume 3, pp. 80–233. [Google Scholar]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-ray Identification, 5th ed.; Mineralogical Society Monograph. Mineralogical Society: London, UK, 1980. [Google Scholar] [CrossRef]
- Galhano, C.; Rocha, F.; Gomes, C. Geostatistical analysis of the influence of textural, mineralogical and geochemical parameters on the geotechnical behaviour of the ‘Argilas de Aveiro’ Formation (Portugal). Clay Miner. 1999, 34, 109–116. [Google Scholar] [CrossRef]
- Oliveira, A.; Rocha, F.; Rodrigues, A.; Jouanneau, J.; Dias, A.; Weber, O.; Gomes, C. Clay minerals from the sedimentary cover from the Northwest Iberian shelf. Prog. Oceanogr. 2002, 52, 233–247. [Google Scholar] [CrossRef]
- Cerqueira, Â.; Costa, C.; Terroso, D.; Sequeira, C.; Rocha, F. Assessment of clayey materials from Santa Maria (Azores, Portugal) for preparation of peloids. Clay Miner. 2019, 54, 299–307. [Google Scholar] [CrossRef]
- Quintela, A.; Terroso, D.; Costa, C.; Sá, H.; Nunes, J.C.; Rocha, F. Characterization and evaluation of hydrothermally influenced clayey sediments from Caldeiras da Ribeira Grande fumarolic field (Azores Archipelago, Portugal) used for aesthetic and pelotherapy purposes. Environ. Earth Sci. 2015, 73, 2833–2842. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge—Determination of pH. International Organization of Standardization (ISO): Geneva, Switzerland, 2021.
- ISO 11265:1994; Soil Quality—Determination of the Specific Electrical Conductivity. International Organization of Standardization (ISO): Geneva, Switzerland, 1994.
- Goldberg, K.; Humayun, M. The applicability of the Chemical Index of Alteration as a paleoclimatic indicator: An example from the Permian of the Paraná Basin, Brazil. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 293, 175–183. [Google Scholar] [CrossRef]
- Armijo, F.; Maraver, F. Granulometría y textura de los peloides españoles. An. Hidrol. Médica 2006, 1, 79–96. [Google Scholar]
- Rebelo, M.; Gonçalves, P.; Silva, E.; Rocha, F. Some portuguese clay sediments used as raw materials for curative clay pastes: A study of physical and technological properties. Acta Geodyn. Geomater. 2005, 2, 151–155. [Google Scholar]
- Pozo, M.; Carretero, M.; Maraver, F.; Pozo, E.; Gómez, I.; Armijo, F.; Rubí, J. Composition and physico-chemical properties of peloids used in Spanish spas: A comparative study. Appl. Clay Sci. 2013, 83–84, 270–279. [Google Scholar] [CrossRef]
- Carretero, M.; Pozo, M.; Legido Soto, J.L.; Fernández-González, M.; Delgado, R.; Gómez, I.; Armijo, F.; Maraver, F. Assessment of three Spanish clays for their use in pelotherapy. Appl. Clay Sci. 2014, 99, 131–143. [Google Scholar] [CrossRef]
- Karakaya, M.Ç.; Karakaya, N.; Aydin, S. The physical and physicochemical properties of some Turkish thermal muds and pure clay minerals and their uses in therapy. Turk. J. Earth Sci. 2017, 26, 1. [Google Scholar] [CrossRef]
- Antunes, J.; Martins, N.; Naudin, J.; Silva, J.; Almeida, F.; Rocha, F.; Gomes, C. Bentonites do Porto Santo (Arquipélago da Madeira): Prospecção Geoeléctrica e Propriedades. Publicações Comun. Geológicas 1988, 84, 62–65. [Google Scholar]
- Kumari, N.; Mohan, C. Basics of Clay Minerals and Their Characteristic Properties. In Clay and Clay Minerals; Do Nascimento, G.M., Ed.; IntechOpen: London, UK, 2021; pp. 1–29. [Google Scholar] [CrossRef]
- Ferreira, M.P.; Serrano, L. O Problema das Esmectites na Ilha de Porto Santo. In Proceedings of the I Congresso Hispano-Luso-Americano de Geologia Económica, Lisboa, Portugal, 24–25 September 1971. [Google Scholar]
- Reimann, C.; De Caritat, P. Chemical Elements in the Environment. In Facts Heets for the Geochemist and Environmental Scientist; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- USP. United States Pharmacopoeia 40-NF 35. In US Pharmacopoeial Convention; EUA: Rockville, MD, USA, 2017. [Google Scholar]
- Carretero, M. Clays in pelotherapy. A review. Part I: Mineralogy, chemistry, physical and physicochemical properties. Appl. Clay Sci. 2020, 189, 105526. [Google Scholar] [CrossRef]
- Health, Canada. Guidance on Heavy Metals Impurities in Cosmetics. 2012. Available online: https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guidance-heavy-metal-impurities-cosmetics.html (accessed on 18 September 2023).
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223 (accessed on 18 September 2023).
- European Medicines Agency. Guideline on the Specification Limits for Residual Metal Catalysts for Metal Reagents. 2008. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-specification-limits-residues-metal-catalysts_en.pdf (accessed on 18 September 2023).
- Bernardo, B.; Candeias, C.; Rocha, F. Geophagic Materials Characterization and Potential Impact on Human Health: The Case Study of Maputo City (Mozambique). Appl. Sci. 2022, 12, 4832. [Google Scholar] [CrossRef]
- Gomes, C.F.; Silva, J.B.P. Minerals and Human Health. Benefits and Risks; Centro de Investigação Minerais Industriais e Argilas: Aveiro, Portugal, 2006; p. 142. ISBN 972-99004-2-6. [Google Scholar]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of lead and cadmium on the immune system and cancer progression. J. Environ. Health Sci. Eng. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.T.; Nelson, P.N.; Li, M.H.; Cai, J.P.; Zhang, Y.Y.; Zhang, Y.G.; Yang, S.; Wang, R.Z.; Wang, Z.W.; Wu, Y.N.; et al. Contrasting pH buffering patterns in neutral-alkaline soils along a 3600 km transect in northern China. Biogeosciences 2015, 12, 7047–7056. [Google Scholar] [CrossRef]
- Klee, S.; Farwick, M.; Lersch, P. Triggered release of sensitive active ingredients upon response to the skin’s natural pH. Colloids Surf. A-Physicochem. Eng. Asp. 2009, 338, 162–166. [Google Scholar] [CrossRef]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef]
- Selinus, O.; Finkelman, R.B.; Centeno, J.A. Medical Geology: A Regional Synthesis; Springer: Dordrecht, Switzerland, 2010; p. 409. [Google Scholar] [CrossRef]
- Carretero, M.I.; Pozo, M.; Sánchez, C.; García, F.J.; Medina, J.A.; Bernabé, J.M. Comparison of saponite and montmorillonite behaviour during static and stirring maturation with seawater for pelotherapy. Appl. Clay Sci. 2007, 36, 161–173. [Google Scholar] [CrossRef]
| Soil Samples | % Clay (<2 μm) | % Silt (<63 μm) | % Sand (>63 μm) | Soil Texture (USDA, 2017) | Specific Surface Area (m2/g) |
|---|---|---|---|---|---|
| 5 | 14 | 67 | 19 | Silt loam | 83 |
| 14 | 6 | 30 | 64 | Sandy loam | 90 |
| 14A | 2 | 20 | 78 | Loamy sand | 39 |
| 15A | 8 | 30 | 62 | Sandy loam | 90 |
| 26 | 15 | 61 | 24 | Silt loam | 90 |
| 36A | 11 | 56 | 33 | Silt loam | 89 |
| 119 | 5 | 45 | 50 | Sandy loam | 76 |
| Smectite * | - | - | - | - | 150–800 |
| Pelotherapy Applications ** | - | - | - | - | >10 |
| 5 | 14 | 14A | 15A | 26 | 36A | 119 | |
|---|---|---|---|---|---|---|---|
| SiO2 | 54.02 | 48.04 | 41.36 | 46.56 | 46.92 | 47.96 | 51.21 |
| TiO2 | 1.93 | 2.88 | 2.04 | 2.88 | 2.40 | 2.60 | 2.10 |
| Al2O3 | 17.65 | 16.13 | 12.52 | 16.22 | 16.40 | 15.65 | 16.31 |
| Fe2O3 | 8.20 | 12.50 | 12.28 | 12.49 | 11.22 | 11.64 | 9.86 |
| MgO | 2.81 | 3.88 | 6.01 | 4.23 | 3.19 | 4.25 | 3.29 |
| CaO | 4.64 | 4.33 | 9.94 | 4.64 | 6.12 | 4.20 | 4.93 |
| Na2O | 2.62 | 1.57 | 1.69 | 1.78 | 1.97 | 1.59 | 2.62 |
| K2O | 0.92 | 1.03 | 1.25 | 0.82 | 1.53 | 0.81 | 0.83 |
| P2O5 | 0.57 | 1.18 | 0.58 | 1.78 | 0.91 | 1.52 | 1.56 |
| SO3 | 0.02 | 0.04 | 0.03 | 0.05 | 0.09 | 0.03 | 0.09 |
| LOI | 7.70 | 7.90 | 13.85 | 7.90 | 13.85 | 9.42 | 6.13 |
| CIA | 68.33 | 69.95 | 49.29 | 69.12 | 63.03 | 70.33 | 66.05 |
| 5 | 14 | 14A | 15A | 26 | 36A | 119 | |
|---|---|---|---|---|---|---|---|
| Smectite | 61 | 68 | 52 | 73 | 56 | 82 | 68 |
| Kaolinite | - | - | 3 | - | - | - | - |
| Plagioclases | 19 | 13 | 13 | 7 | 14 | 5 | 12 |
| K-Feldspars | 6 | 3 | 3 | 1 | 9 | 2 | 4 |
| Calcite | 3 | 3 | 17 | 2 | 2 | 2 | 3 |
| Hematite | - | - | 2 | - | - | - | - |
| Magnetite/maghemite | 5 | 10 | 4 | 6 | 11 | 9 | 6 |
| Opal | 6 | 3 | 6 | 6 | 6 | - | 7 |
| Anatase | - | - | - | 5 | - | - | - |
| Quartz | - | - | - | - | 2 | - | - |
| Smectite crystallinity index | 0.24 | 0.22 | 0.36 | 0.39 | 0.54 | 0.37 | 0.23 |
| d060 | 1.50 | 1.50 | 1.52 | 1.50 | 1.49 | 1.50 | 1.49 |
| Al | Ca | Cl | Fe | Mg | O | K | Si | Na | Ti | |
|---|---|---|---|---|---|---|---|---|---|---|
| EDS1 | 4.7 | 0.4 | 0.0 | 0.4 | 1.8 | 79.0 | 0.1 | 11.5 | 2.1 | 0.1 |
| (Si3.7Al0.3)(Al1.3Fe0.1Mg0.6)(Ca0.1Na0.7)O10(OH)2(H2O) | ||||||||||
| EDS2 | 7.0 | 1.9 | 0.2 | 10.4 | 1.3 | 54.5 | 1.0 | 19.8 | 1.6 | 2.3 |
| (Si2.9Ti0.3Al0.8)(Al0.3Fe1.5Mg0.2)(Ca0.3Na0.2K0.1)O10(OH)2(H2O) | ||||||||||
| 5 | 14 | 14A | 15A | 26 | 36A | 119 | HC (2012) | EC (2009) | EMEA (2008) | |
|---|---|---|---|---|---|---|---|---|---|---|
| As | <0.1 | 0.1 | <0.1 | 0.1 | <0.1 | 0.2 | 0.7 | 3 | 0 | - |
| B | 42 | 51 | 19 | 50 | 31 | 51 | 34 | - | - | - |
| Ba | 46.1 | 68.1 | 75.8 | 59.1 | 95.6 | 74.4 | 177 | 1300 | - | - |
| Be | 1.9 | 1.2 | 1.2 | 1.5 | 1.4 | 1.5 | 3.4 | |||
| Cd | 0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.2 | 3 | 0 | - |
| Co | 7.4 | 25.2 | 37.6 | 17.6 | 22.7 | 13.0 | 16.3 | 5 | - | - |
| Cr | 5.0 | 5.0 | 57.0 | 6.0 | 20.0 | 4.0 | 7.0 | - | - | 25 |
| Cs | 0.2 | 0.2 | 0.2 | 0.3 | 0.4 | 0.3 | 0.5 | |||
| Cu | 14.4 | 22.1 | 70.1 | 10.7 | 19.7 | 8.4 | 11.9 | - | - | 250 |
| Ga | 10.4 | 13 | 13.1 | 13.1 | 9.63 | 13.5 | 11 | |||
| Hg | 0.04 | 0.04 | 0.05 | 0.05 | 0.03 | 0.07 | 0.03 | 3 | 0 | - |
| In | 0.09 | 0.07 | 0.05 | 0.06 | 0.08 | 0.08 | 0.08 | |||
| Li | 4.8 | 6.1 | 11.2 | 7 | 9 | 8 | 13.5 | |||
| Mn | 572 | 1070 | 1430 | 881 | 1300 | 940 | 3900 | - | - | 250 |
| Mo | 0.29 | 0.56 | 0.67 | 0.36 | 0.76 | 0.25 | 0.97 | - | - | 25 |
| Nb | <0.1 | <0.1 | 0.9 | <0.1 | 0.7 | <0.1 | 0.3 | |||
| Ni | 5.5 | 20.4 | 114 | 8.2 | 24.4 | 4.2 | 44.4 | - | - | 25 |
| Pb | 3.1 | 2.1 | 2.8 | 2.8 | 4.6 | 3.3 | 4.8 | 10 | 0 | - |
| Rb | 15.3 | 9.7 | 8.9 | 9.1 | 12.7 | 11.5 | 10.1 | |||
| Sb | 0.05 | 0.05 | 0.07 | 0.04 | 0.08 | 0.02 | 0.09 | 5 | 0 | - |
| Sc | 5.7 | 11.4 | 10.7 | 9.8 | 8.7 | 8.2 | 6.1 | |||
| Se | <0.1 | <0.1 | 0.4 | 0.1 | 0.2 | <0.1 | <0.1 | 17 | - | - |
| Sn | 1.97 | 1.38 | 1.13 | 1.39 | 1.37 | 1.48 | 1.87 | |||
| Sr | 75.7 | 177 | 169 | 183 | 207 | 126 | 125 | |||
| Th | 8.7 | 5.1 | 3.1 | 5.4 | 3.6 | 6.8 | 8.3 | |||
| Tl | 0.04 | 0.03 | 0.04 | 0.03 | 0.05 | 0.02 | 0.14 | 0.8 | - | - |
| U | 0.9 | 1.6 | 0.5 | 1.3 | 0.7 | 1.0 | 1.8 | |||
| V | 63 | 177 | 121 | 130 | 20 | 4 | 7 | - | - | 25 |
| W | <0.1 | 0.1 | 0.2 | 0.1 | 0.2 | 0.1 | 0.3 | |||
| Y | 28.6 | 25.7 | 18.3 | 26.9 | 23.4 | 30.5 | 51.5 | |||
| Zn | 93.8 | 90.9 | 87 | 85.8 | 87.9 | 96.4 | 108 | - | - | 1300 |
| Zr | 2.5 | 1.6 | 3.2 | 2.1 | 3.4 | 2.3 | 1.6 | |||
| Ce | 161 | 96.5 | 71.4 | 97.6 | 90.9 | 127 | 160 | |||
| Dy | 6.9 | 6.2 | 4.1 | 6.4 | 5.7 | 6.9 | 8.8 | |||
| Er | 3.1 | 2.9 | 2.0 | 3.1 | 2.7 | 3.4 | 5.1 | |||
| Eu | 3.1 | 2.8 | 1.7 | 2.7 | 2.2 | 3.1 | 3.4 | |||
| Gd | 9.7 | 8.2 | 5.6 | 8.3 | 7.3 | 9.7 | 11.3 | |||
| Ho | 1.2 | 1.1 | 0.7 | 1.1 | 1.0 | 1.2 | 1.7 | |||
| La | 74.8 | 46.6 | 34 | 47.4 | 43.1 | 62 | 80.1 | |||
| Lu | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 0.6 | |||
| Nd | 64.8 | 45.9 | 31.2 | 45.5 | 41.9 | 55.9 | 66.8 | |||
| Pr | 17.1 | 11.3 | 8 | 11.4 | 10.3 | 14.4 | 17.2 | |||
| Sm | 11.5 | 8.5 | 6.7 | 9.4 | 7.9 | 10.6 | 12.1 | |||
| Tb | 1.3 | 1.1 | 0.8 | 1.1 | 1.0 | 1.3 | 1.6 | |||
| Tm | 0.4 | 0.3 | 0.2 | 0.3 | 0.3 | 0.4 | 0.6 | |||
| Yb | 2.5 | 2.2 | 1.6 | 2.2 | 2.0 | 2.6 | 3.8 |
| Soil Samples | pH | Electrical Conductivity (µS/cm) | CEC (meq/100 g) | Exchange Ions (mg/L) | |||
|---|---|---|---|---|---|---|---|
| Na+ | K+ | Mg2+ | Ca2+ | ||||
| 5 | 9.3 | 710 | 50.4 | 93.5 | 21.6 | 57.3 | 131.2 |
| 14 | 9.6 | 730 | 74.0 | 155.6 | 20.5 | 78.5 | 6.5 |
| 14A | 9.3 | 646 | 60.2 | 2.9 | 3.9 | 7.6 | 4.3 |
| 15A | 9.5 | 972 | 74.6 | 168.5 | 24.1 | 7.6 | 6.4 |
| 26 | 8.8 | 242 | 43.8 | 10.9 | 25.9 | 65.6 | 7.4 |
| 36A | 9.6 | 772 | 86.8 | 185.4 | 18.3 | 58.6 | 8.0 |
| 119 | 8.7 | 940 | 59.8 | 139.7 | 29.7 | 80.9 | 75.8 |
| Smectite * | - | - | 60–150 | - | - | - | - |
| Pelotherapy applications ** | - | - | >10 | - | - | - | - |
| Specific Heat Capacity (J/g°C) | Cooling Kinetics (min) | |
|---|---|---|
| 5 | 0.6 | 14.5 |
| 14 | 0.7 | 15.5 |
| 14A | 0.8 | 18.0 |
| 15A | 0.8 | 16.5 |
| 26 | 0.9 | 15.5 |
| 36A | 0.9 | 19.0 |
| 119 | 0.8 | 17.0 |
| Pelotherapy * | 0.5 | - |
| Pelotherapy ** | - | ~20.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, A.; Carvalho, P.C.S.; Rocha, F. Physical–Chemical and Thermal Properties of Clays from Porto Santo Island, Portugal. Appl. Sci. 2024, 14, 8962. https://doi.org/10.3390/app14198962
Valente A, Carvalho PCS, Rocha F. Physical–Chemical and Thermal Properties of Clays from Porto Santo Island, Portugal. Applied Sciences. 2024; 14(19):8962. https://doi.org/10.3390/app14198962
Chicago/Turabian StyleValente, André, Paula C. S. Carvalho, and Fernando Rocha. 2024. "Physical–Chemical and Thermal Properties of Clays from Porto Santo Island, Portugal" Applied Sciences 14, no. 19: 8962. https://doi.org/10.3390/app14198962
APA StyleValente, A., Carvalho, P. C. S., & Rocha, F. (2024). Physical–Chemical and Thermal Properties of Clays from Porto Santo Island, Portugal. Applied Sciences, 14(19), 8962. https://doi.org/10.3390/app14198962








