Improvement of Mechanical Properties of 3D Bioprinted Structures through Cellular Overgrowth
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. NIH/3T3 Cell Cultivation
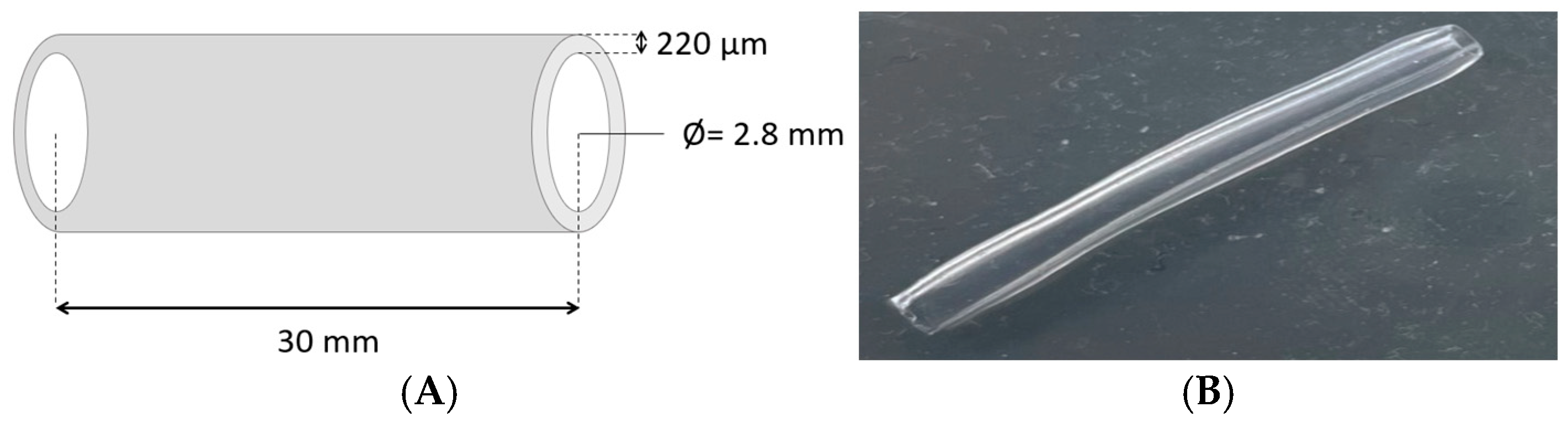
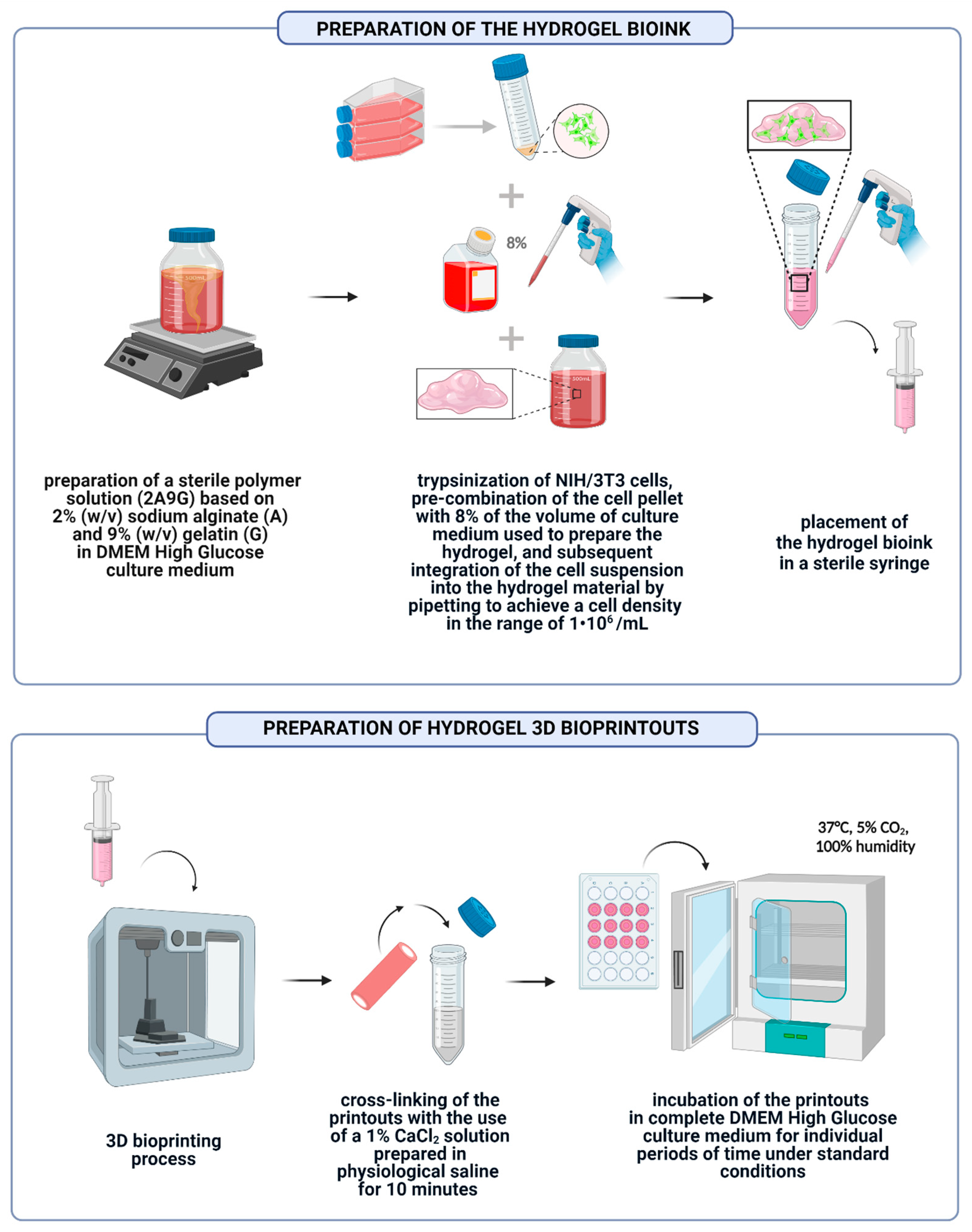
2.3. Preparation of Hydrogel Bioink and 3D Bioprinted Structures
2.4. Evaluation of Rheological Properties of Polymer Solutions
2.5. Assessment of the Viability of Fibroblast Cells of the NIH/3T3 Line Contained in Hydrogel Printouts after Individual Periods of Cultivation of Structures Using the Fluorescent Live/Dead Assay
2.6. Evaluation of the Proliferation of Fibroblast Cells of the NIH/3T3 Line Embedded inside the Printouts after Individual Periods of Cultivation of the Structures Using a Scanning Electron Microscope (SEM)
2.7. Evaluation of the Mechanical Properties of Bioprinted Hydrogel Structures Subjected to Individual Cultivation Periods Using a Static Tensile Test
3. Results and Discussion
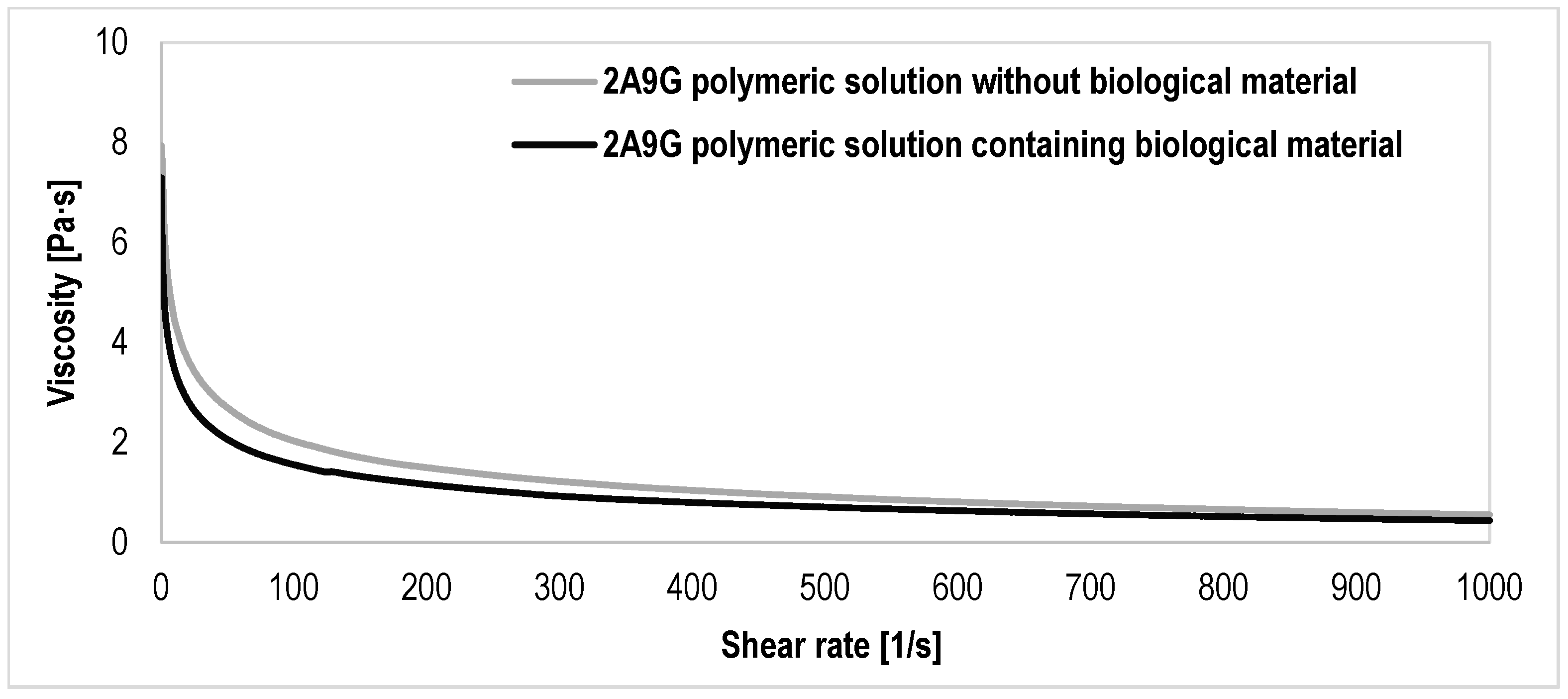
3.1. Results of the Evaluation of the Rheological Properties of the Polymer Solutions
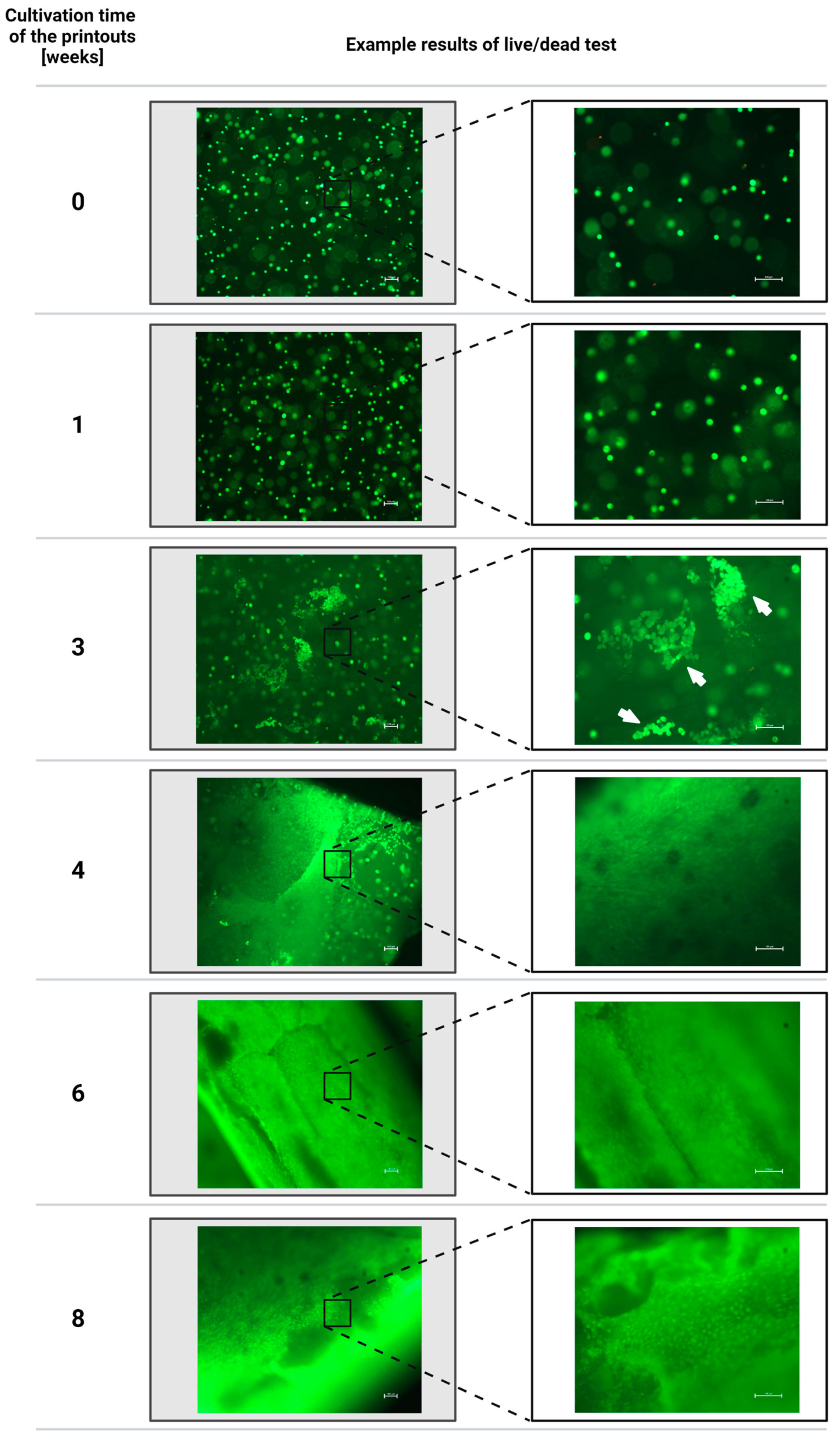
3.2. Results of the Assessment of the Viability of Fibroblast Cells of the NIH/3T3 Line Contained in Hydrogel Printouts after Individual Periods of Cultivation of Structures Using the Fluorescent Live/Dead Assay
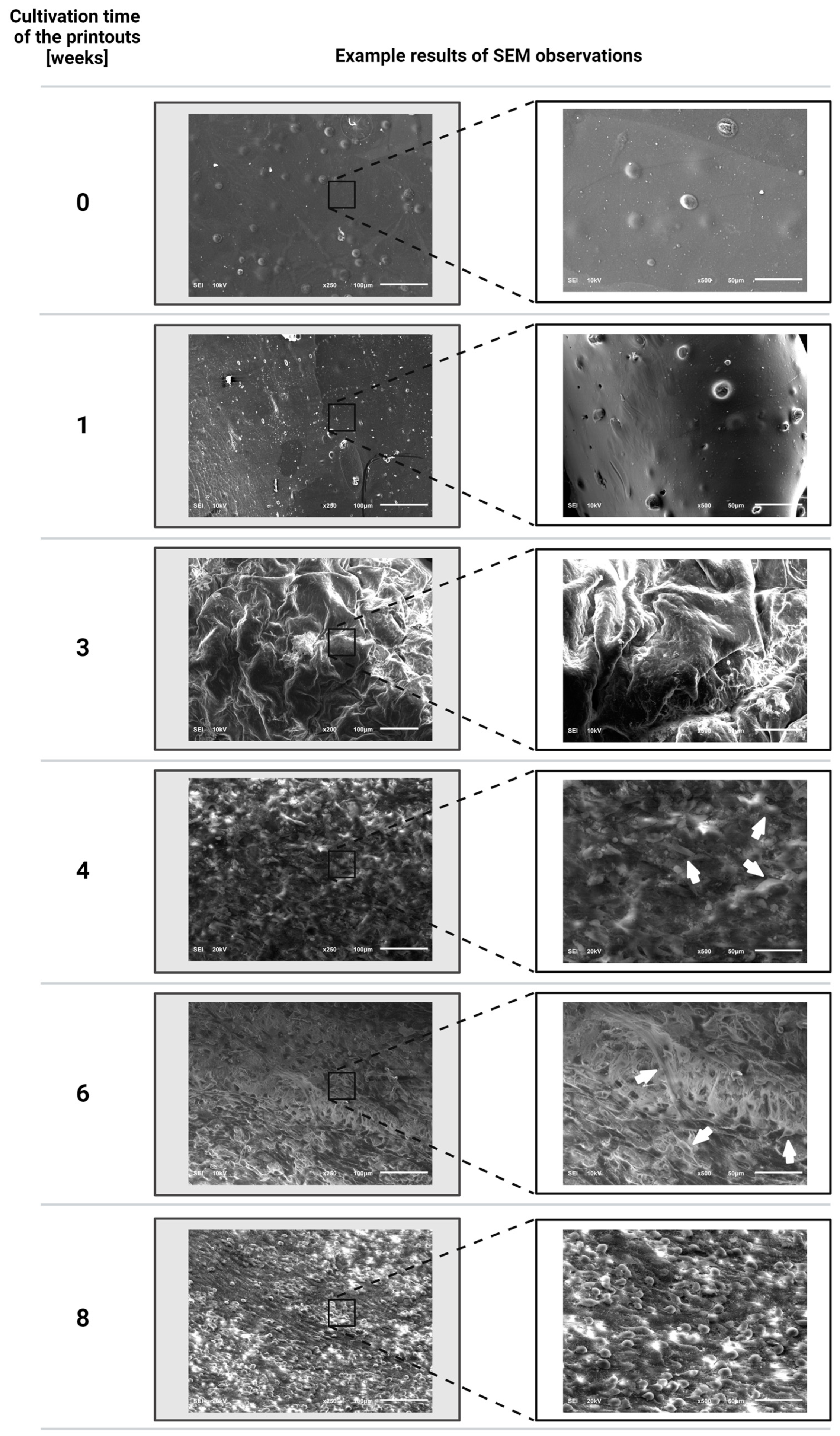
3.3. Evaluation Results of the Proliferation of Fibroblast Cells of the NIH/3T3 Line Incorporated into the Printouts after Individual Periods of Cultivation of the Structures Using a Scanning Electron Microscope
3.4. Evaluation Results of the Mechanical Properties of Bioprinted Hydrogel Structures Subjected to Individual Cultivation Periods Using a Static Tensile Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Z.; Fu, J.; Lin, H.; He, Y. Development of 3D bioprinting: From printing methods to biomedical applications. Asian J. Pharm. Sci. 2020, 15, 529–557. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef] [PubMed]
- Ke, D.; Niu, C.; Yang, X. Evolution of 3D bioprinting-from the perspectives of bioprinting companies. Bioprinting 2022, 25, e00193. [Google Scholar] [CrossRef]
- Ma, Y.; Deng, B.; He, R.; Huang, P. Advancements of 3D bioprinting in regenerative medicine: Exploring cell sources for organ fabrication. Heliyon 2024, 10, e24593. [Google Scholar] [CrossRef]
- Gillispie, G.; Prim, P.; Copus, J.; Fisher, J.; Mikos, A.G.; Yoo, J.J.; Atala, A.; Lee, S.J. Assessment methodologies for extrusion-based bioink printability. Biofabrication 2020, 12, 022003. [Google Scholar] [CrossRef]
- Moroni, L.; Boland, T.; Burdick, J.A.; De Maria, C.; Derby, B.; Forgacs, G.; Groll, J.; Li, Q.; Malda, J.; Mironov, V.A.; et al. Biofabrication: A Guide to Technology and Terminology. Trends Biotechnol. 2018, 36, 384–402. [Google Scholar] [CrossRef]
- Panwar, A.; Tan, L.P. Current status of bioinks for micro-extrusion-based 3D bioprinting. Molecules 2016, 21, 685. [Google Scholar] [CrossRef]
- Placone, J.K.; Engler, A.J. Recent Advances in Extrusion-Based 3D Printing for Biomedical Applications. Adv. Healthc. Mater. 2018, 7, e1701161. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Dell, A.C.; Wagner, G.; Own, J.; Geibel, J.P. 3D Bioprinting Using Hydrogels: Cell Inks and Tissue Engineering Applications. Pharmaceutics 2022, 14, 2596. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Hussain, C.M. 3D-Printed Hydrogel for Diverse Applications: A Review. Gels 2023, 9, 960. [Google Scholar] [CrossRef] [PubMed]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomater. Res. 2018, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Bom, S.; Ribeiro, R.; Ribeiro, H.M.; Santos, C.; Marto, J. On the progress of hydrogel-based 3D printing: Correlating rheological properties with printing behaviour. Int. J. Pharm. 2022, 615, 121506. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef]
- Leonardo, M.; Prajatelistia, E.; Judawisastra, H. Alginate-based bioink for organoid 3D bioprinting: A review. Bioprinting 2022, 28, e00246. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D Bioplotting of Gelatin/Alginate Scaffolds for Tissue Engineering: Influence of Crosslinking Degree and Pore Architecture on Physicochemical Properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Jia, J.; Richards, D.J.; Pollard, S.; Tan, Y.; Rodriguez, J.; Visconti, R.P.; Trusk, T.C.; Yost, M.J.; Yao, H.; Markwald, R.R.; et al. Engineering alginate as bioink for bioprinting. Acta Biomater. 2014, 10, 4323–4331. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Q.; Liu, C.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Wang, X. Natural polymers for organ 3D bioprinting. Polymers 2018, 10, 1278. [Google Scholar] [CrossRef]
- Freeman, F.E.; Kelly, D.J. Tuning alginate bioink stiffness and composition for controlled growth factor delivery and to spatially direct MSC Fate within bioprinted tissues. Sci. Rep. 2017, 7, 17042. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, S.; Feng, Q.; Dai, Q.; Yao, L.; Zhang, Y.; Gao, H.; Dong, H.; Chen, D.; Cao, X. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact. Mater. 2021, 6, 3396–3410. [Google Scholar] [CrossRef]
- Sawyer, S.W.; Takeda, K.; Alayoubi, A.; Mirdamadi, E.; Zidan, A.; Bauer, S.R.; Degheidy, H. 3D bioprinting optimization of human mesenchymal stromal cell laden gelatin-alginate-collagen bioink. Biomed. Mater. 2023, 18, 015016. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.P.; Shah, H.B.; Maniar, K.K.; Özel, T. Extrusion-based 3D bioprinting of alginate-based tissue constructs. Proc. CIRP 2020, 95, 143–148. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Time dependent gelling properties of cuboid alginate gels made by external gelation method: Effects of alginate-CaCl2 solution ratios and pH. Food Hydrocoll. 2019, 90, 232–240. [Google Scholar] [CrossRef]
- Savić Gajić, I.M.; Savić, I.M.; Svirčev, Z. Preparation and Characterization of Alginate Hydrogels with High Water-Retaining Capacity. Polymers 2023, 15, 2592. [Google Scholar] [CrossRef]
- Solberg, A.; Draget, K.I.; Schatz, C.; Christensen, B.E. Alginate Blocks and Block Polysaccharides: A Review. Macromol. Symp. 2023, 408, 2200072. [Google Scholar] [CrossRef]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Gorroñogoitia, I.; Urtaza, U.; Zubiarrain-Laserna, A.; Alonso-Varona, A.; Zaldua, A.M. A Study of the Printability of Alginate-Based Bioinks by 3D Bioprinting for Articular Cartilage Tissue Engineering. Polymers 2022, 14, 354. [Google Scholar] [CrossRef]
- Williams, P.A.; Campbell, K.T.; Silva, E.A. Alginate hydrogels of varied molecular weight distribution enable sustained release of sphingosine-1-phosphate and promote angiogenesis. J. Biomed. Mater. Res. A 2018, 106, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Kong, H.-J.; Mooney, D.J. Controlling alginate gel degradation utilizing partial oxidation and bimodal molecular weight distribution. Biomaterials 2005, 26, 2455–2465. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2020, 573, 118803. [Google Scholar] [CrossRef]
- Heid, S.; Boccaccini, A.R. Advancing bioinks for 3D bioprinting using reactive fillers: A review. Acta Biomater. 2020, 113, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yang, H.; Chen, D.; Xie, Y.; Tai, C.; Wang, L.; Wang, P.; Wang, B. Three-dimensional bioprinting sodium alginate/gelatin scaffold combined with neural stem cells and oligodendrocytes markedly promoting nerve regeneration after spinal cord injury. Regen. Biomater. 2022, 9, rbac038. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef]
- Kaliampakou, C.; Lagopati, N.; Pavlatou, E.A.; Charitidis, C.A. Alginate–Gelatin Hydrogel Scaffolds; An Optimization of Post-Printing Treatment for Enhanced Degradation and Swelling Behavior. Gels 2023, 9, 857. [Google Scholar] [CrossRef]
- Sonaye, S.Y.; Ertugral, E.G.; Kothapalli, C.R.; Sikder, P. Extrusion 3D (Bio)Printing of Alginate-Gelatin-Based Composite Scaffolds for Skeletal Muscle Tissue Engineering. Materials 2022, 15, 7945. [Google Scholar] [CrossRef]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Moghimi, N.; Vandvajdi, S.; Powathil, G.; Hamis, S.; Kohandel, M. Predicting and elucidating the post-printing behavior of 3D printed cancer cells in hydrogel structures by integrating in-vitro and in-silico experiments. Sci. Rep. 2023, 13, 1211. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, L.; Gu, L.; Yang, H.; Luo, Y.; Ma, L. High-resolution 3D Bioprinting System for Fabricating Cell-laden Hydrogel Scaffolds with High Cellular Activities. Proc. CIRP 2017, 65, 219–224. [Google Scholar] [CrossRef]
- Rosińska, K.; Bartniak, M.; Wierzbicka, A.; Sobczyk-Guzenda, A.; Bociaga, D. Solvent types used for the preparation of hydrogels determine their mechanical properties and influence cell viability through gelatine and calcium ions release. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 314–330. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, A.; Bartniak, M.; Rosińska, K.; Bociaga, D. Optimization of the preparation process stages of the bioink compositions based on sodium alginate and gelatin to improve the viability of biological material contained in hydrogel 3D printouts. Eng. Biomater. 2022, 25, 165. [Google Scholar] [CrossRef]
- Scientific Image and Illustration Software BioRender. Available online: https://www.biorender.com/ (accessed on 12 July 2024).
- Majumder, N.; Mishra, A.; Ghosh, S. Effect of varying cell densities on the rheological properties of the bioink. Bioprinting 2022, 28, e00241. [Google Scholar] [CrossRef]
- Gregory, T.; Benhal, P.; Scutte, A.; Quashie, D.; Harrison, K., Jr.; Cargill, C.; Grandison, S.; Savitsky, M.J.; Ramakrishnan, S.; Ali, J. Rheological characterization of cell-laden alginate-gelatin hydrogels for 3D biofabrication. J. Mech. Behav. Biomed. Mater. 2022, 136, 105474. [Google Scholar] [CrossRef]
- Gao, T.; Gillispie, G.J.; Copus, J.S.; Pr, A.K.; Seol, Y.J.; Atala, A.; Yoo, J.J.; Lee, S.J. Optimization of gelatin-alginate composite bioink printability using rheological parameters: A systematic approach. Biofabrication 2018, 10, 034106. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, R.; Rodríguez-Rego, J.M.; Macías-García, A.; Mendoza-Cerezo, L.; Díaz-Parralejo, A. Relationship between shear-thinning rheological properties of bioinks and bioprinting parameters. Int. J. Bioprint 2023, 9, 422–431. [Google Scholar] [CrossRef]
- Gerdes, S.; Ramesh, S.; Mostafavi, A.; Tamayol, A.; Rivero, I.V.; Rao, P. Extrusion-based 3D (Bio)Printed Tissue Engineering Scaffolds: Process-Structure-Quality Relationships. ACS Biomater. Sci. Eng. 2021, 7, 4694–4717. [Google Scholar] [CrossRef]
- Hull, S.M.; Brunel, L.G.; Heilshorn, S.C. 3D Bioprinting of Cell-Laden Hydrogels for Improved Biological Functionality. Adv. Mater. 2022, 34, e2103691. [Google Scholar] [CrossRef] [PubMed]
- Ahearne, M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Vaughan, T.J.; Madl, C.M.; Raftery, R.M.; McNamara, L.M.; O’Brien, F.J.; Heilshorn, S.C. Investigating the interplay between substrate stiffness and ligand chemistry in directing mesenchymal stem cell differentiation within 3D macro-porous substrates. Biomaterials 2018, 171, 23–33. [Google Scholar] [CrossRef]
- Liu, J.; Zheng, H.; Poh, P.S.; Machens, H.G.; Schilling, A.F. Hydrogels for engineering of perfusable vascular networks. Int. J. Mol. Sci. 2015, 16, 15997–16016. [Google Scholar] [CrossRef]
- Choi, Y.J.; Jun, Y.J.; Kim, D.Y.; Yi, H.G.; Chae, S.H.; Kang, J.; Lee, J.; Gao, G.; Kong, J.S.; Jang, J.; et al. A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss. Biomaterials 2019, 206, 160–169. [Google Scholar] [CrossRef]
- Geevarghese, R.; Somasekharan, L.T.; Bhatt, A.; Kasoju, N.; Nair, R.P. Development and evaluation of a multicomponent bioink consisting of alginate, gelatin, diethylaminoethyl cellulose and collagen peptide for 3D bioprinting of tissue construct for drug screening application. Int. J. Biol. Macromol. 2022, 207, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Boucard, E.; Vidal, L.; Coulon, F.; Mota, C.; Hascoët, J.Y.; Halary, F. The degradation of gelatin/alginate/fibrin hydrogels is cell type dependent and can be modulated by targeting fibrinolysis. Front. Bioeng. Biotechnol. 2022, 10, 920929. [Google Scholar] [CrossRef] [PubMed]
- Andreazza, R.; Morales, A.; Pieniz, S.; Labidi, J. Gelatin-Based Hydrogels: Potential Biomaterials for Remediation. Polymers 2023, 15, 1026. [Google Scholar] [CrossRef]
- Giuseppe, M.D.; Law, N.; Webb, B.; A Macrae, R.; Liew, L.J.; Sercombe, T.B.; Dilley, R.J.; Doyle, B.J. Mechanical behaviour of alginate-gelatin hydrogels for 3D bioprinting. J. Mech. Behav. Biomed. Mater. 2018, 79, 150–157. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Sharma, S. Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym. 2004, 59, 129–140. [Google Scholar] [CrossRef]
- Khatun, M.R.; Bhattacharyya, A.; Gunbayar, M.; Jung, M.; Noh, I. Study on Bioresponsive Gelatin-Hyaluronic Acid-Genipin Hydrogel for High Cell-Density 3D Bioprinting. Gels 2023, 9, 601. [Google Scholar] [CrossRef]
- Martinez-Garcia, F.D.; Fischer, T.; Hayn, A.; Mierke, C.T.; Burgess, J.K.; Harmsen, M.C. A Beginner’s Guide to the Characterization of Hydrogel Microarchitecture for Cellular Applications. Gels 2022, 8, 535. [Google Scholar] [CrossRef]
- Wisdom, K.M.; Adebowale, K.; Chang, J.; Lee, J.Y.; Nam, S.; Desai, R.; Rossen, N.S.; Rafat, M.; West, R.B.; Hodgson, L.; et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018, 9, 4144. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef]
- Gong, C.; Kong, Z.; Wang, X. The effect of agarose on 3d bioprinting. Polymers 2021, 13, 4028. [Google Scholar] [CrossRef]
- Patiño Vargas, M.I.; Martinez-Garcia, F.D.; Offens, F.; Becerra, N.Y.; Restrepo, L.M.; van der Mei, H.C.; Harmsen, M.C.; van Kooten, T.G.; Sharma, P.K. Viscoelastic properties of plasma-agarose hydrogels dictate favorable fibroblast responses for skin tissue engineering applications. Biomater. Adv. 2022, 139, 212967. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.; Lippens, E.; Duda, G.N.; et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Koch, M.; Włodarczyk-Biegun, M.K. Faithful scanning electron microscopic (SEM) visualization of 3D printed alginate-based scaffolds. Bioprinting 2020, 20, e00098. [Google Scholar] [CrossRef]
- 3D Cell Culture vs. Traditional 2D Cell Culture Explained. Available online: https://www.mimetas.com/en/blogs/345/3d-cell-culture-vs--traditional-2d-cell-culture-explained.html (accessed on 12 July 2024).
- Marga, F.; Jakab, K.; Khatiwala, C.; Shepherd, B.; Dorfman, S.; Hubbard, B.; Colbert, S.; Gabor, F. Toward engineering functional organ modules by additive manufacturing. Biofabrication 2012, 4, 022001. [Google Scholar] [CrossRef]
- Du Plessis, L.H.; Gouws, C.; Nieto, D. The influence of viscosity of hydrogels on the spreading and migration of cells in 3D bioprinted skin cancer models. Front. Cell Dev. Biol. 2024, 12, 1391259. [Google Scholar] [CrossRef]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Morley, C.D.; Ellison, S.T.; Bhattacharjee, T.; O’Bryan, C.S.; Zhang, Y.; Smith, K.F.; Kabb, C.P.; Sebastian, M.; Moore, G.L.; Schulze, K.D.; et al. Quantitative characterization of 3D bioprinted structural elements under cell generated forces. Nat. Commun. 2019, 10, 3029. [Google Scholar] [CrossRef]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Cooper, D.; Dimri, M. Biochemistry, Calcium Channels. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Finan, J.D.; Guilak, F. The effects of osmotic stress on the structure and function of the cell nucleus. J. Cell Biochem. 2010, 109, 460–467. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzbicka, A.; Bartniak, M.; Grabarczyk, J.; Biernacka, N.; Aftyka, M.; Wójcik, T.; Bociaga, D. Improvement of Mechanical Properties of 3D Bioprinted Structures through Cellular Overgrowth. Appl. Sci. 2024, 14, 8977. https://doi.org/10.3390/app14198977
Wierzbicka A, Bartniak M, Grabarczyk J, Biernacka N, Aftyka M, Wójcik T, Bociaga D. Improvement of Mechanical Properties of 3D Bioprinted Structures through Cellular Overgrowth. Applied Sciences. 2024; 14(19):8977. https://doi.org/10.3390/app14198977
Chicago/Turabian StyleWierzbicka, Adrianna, Mateusz Bartniak, Jacek Grabarczyk, Nikola Biernacka, Mateusz Aftyka, Tomasz Wójcik, and Dorota Bociaga. 2024. "Improvement of Mechanical Properties of 3D Bioprinted Structures through Cellular Overgrowth" Applied Sciences 14, no. 19: 8977. https://doi.org/10.3390/app14198977
APA StyleWierzbicka, A., Bartniak, M., Grabarczyk, J., Biernacka, N., Aftyka, M., Wójcik, T., & Bociaga, D. (2024). Improvement of Mechanical Properties of 3D Bioprinted Structures through Cellular Overgrowth. Applied Sciences, 14(19), 8977. https://doi.org/10.3390/app14198977





