The Influence of Helium Addition on the Combustion Process in a Hydrogen-Fueled Turbulent Jet Ignition Engine
Abstract
:1. Introduction
2. Combustion of Hydrogen in an Internal Combustion Engine
2.1. Combustion of Pure Hydrogen in an Internal Combustion Engine
2.2. Combustion of Hydrogen with Additives in an Internal Combustion Engine
2.3. Purpose of the Work
3. Materials and Methods
3.1. Test Bench
3.2. Measurement Equipment
3.3. Research Methods
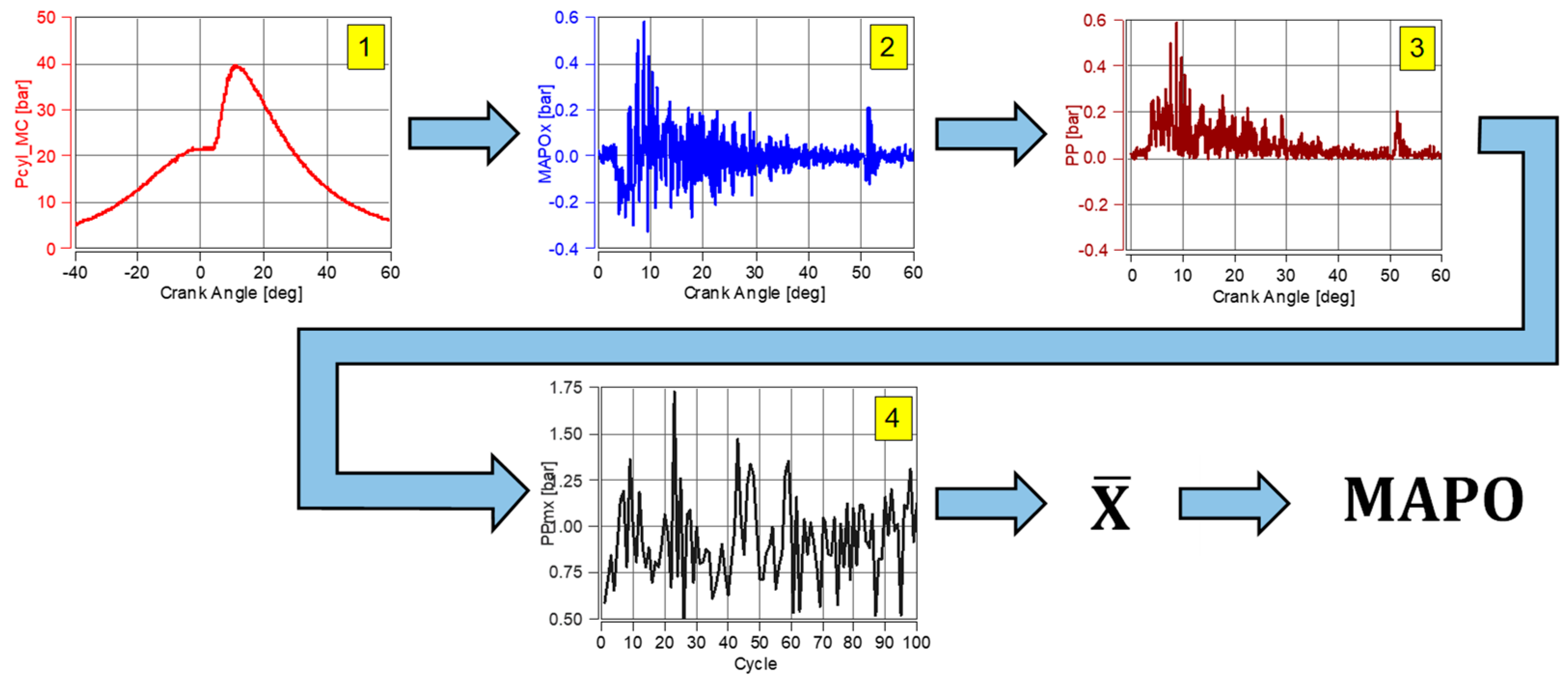
3.4. Data Processing
4. Evaluation of Hydrogen Combustion with the Addition of Helium
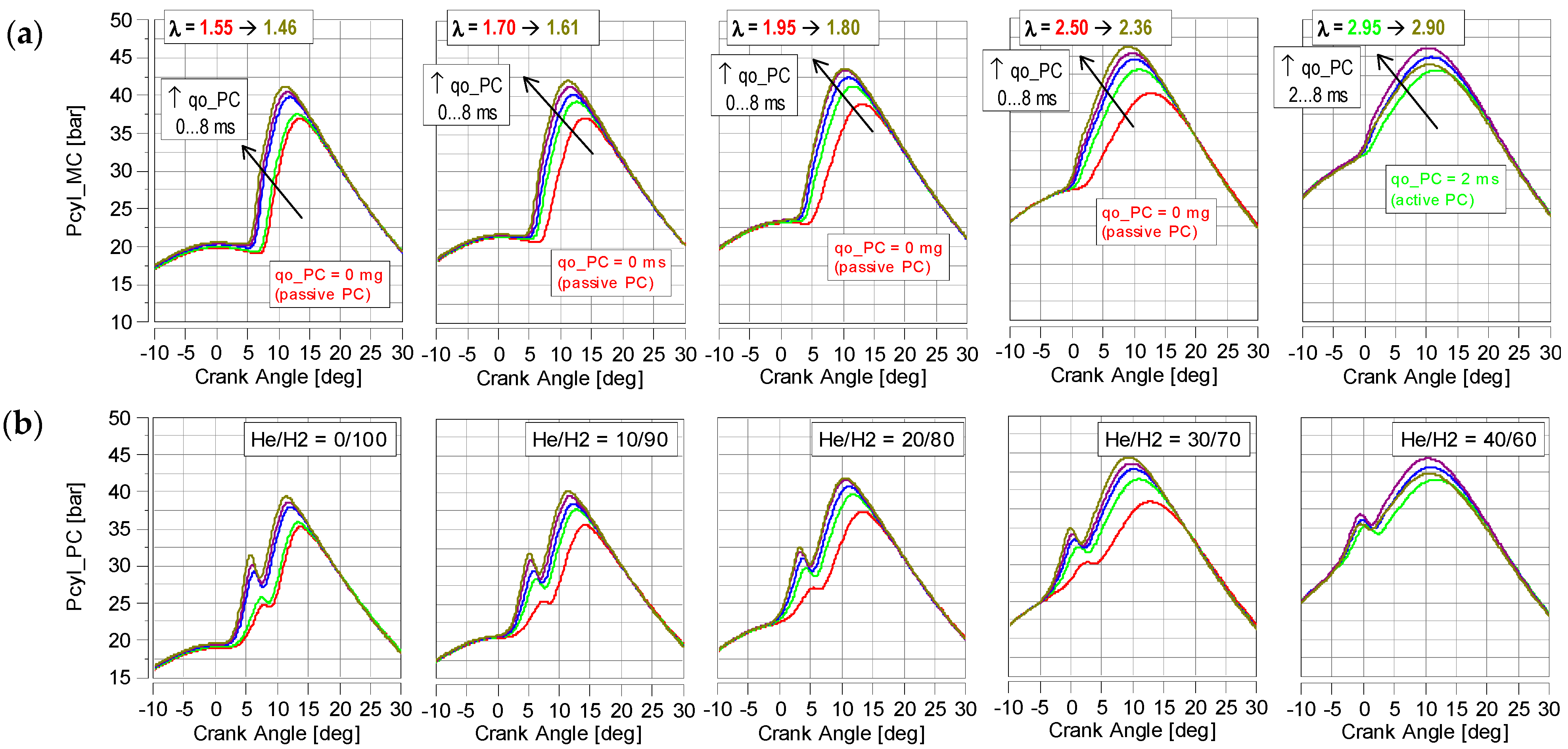
4.1. Cylinder Pressure
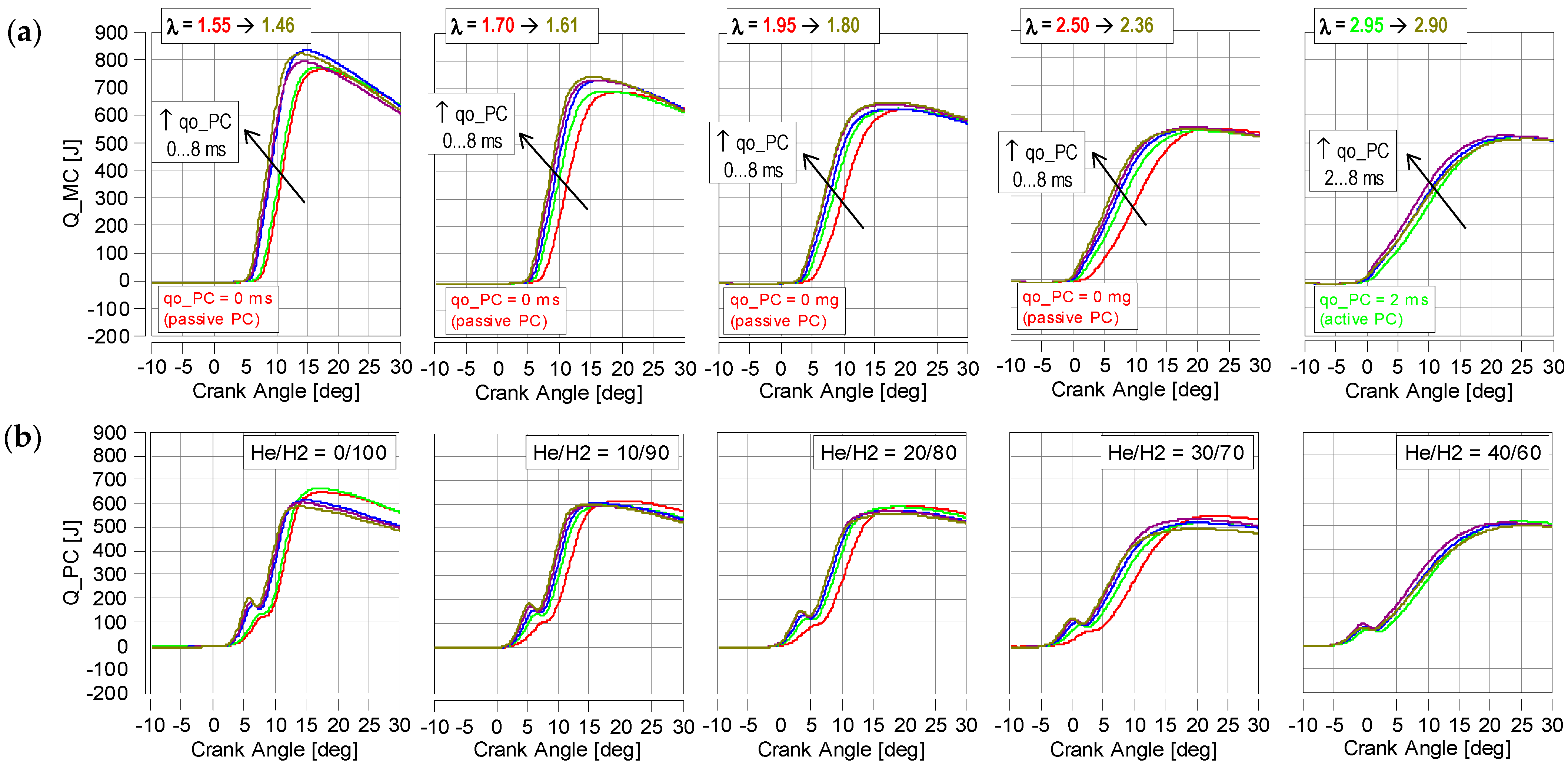
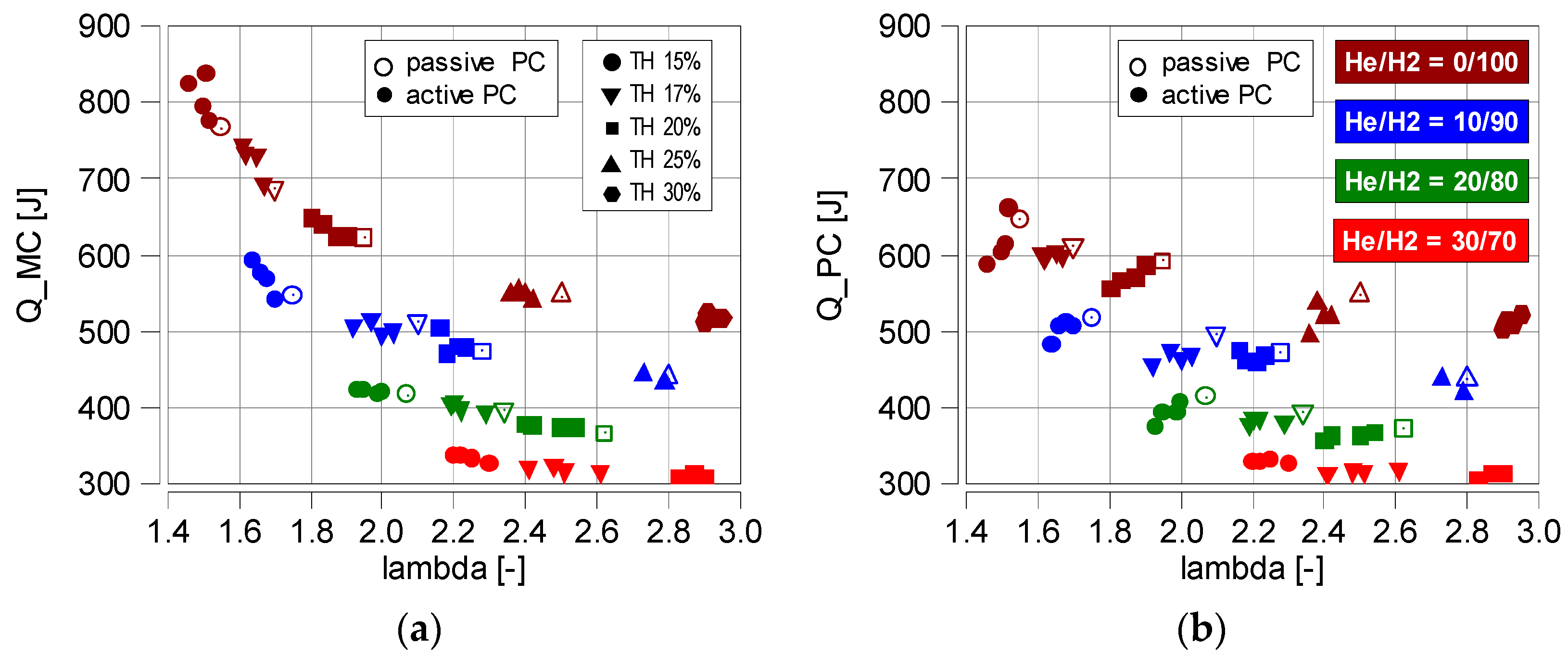
4.2. Thermodynamic Parameters
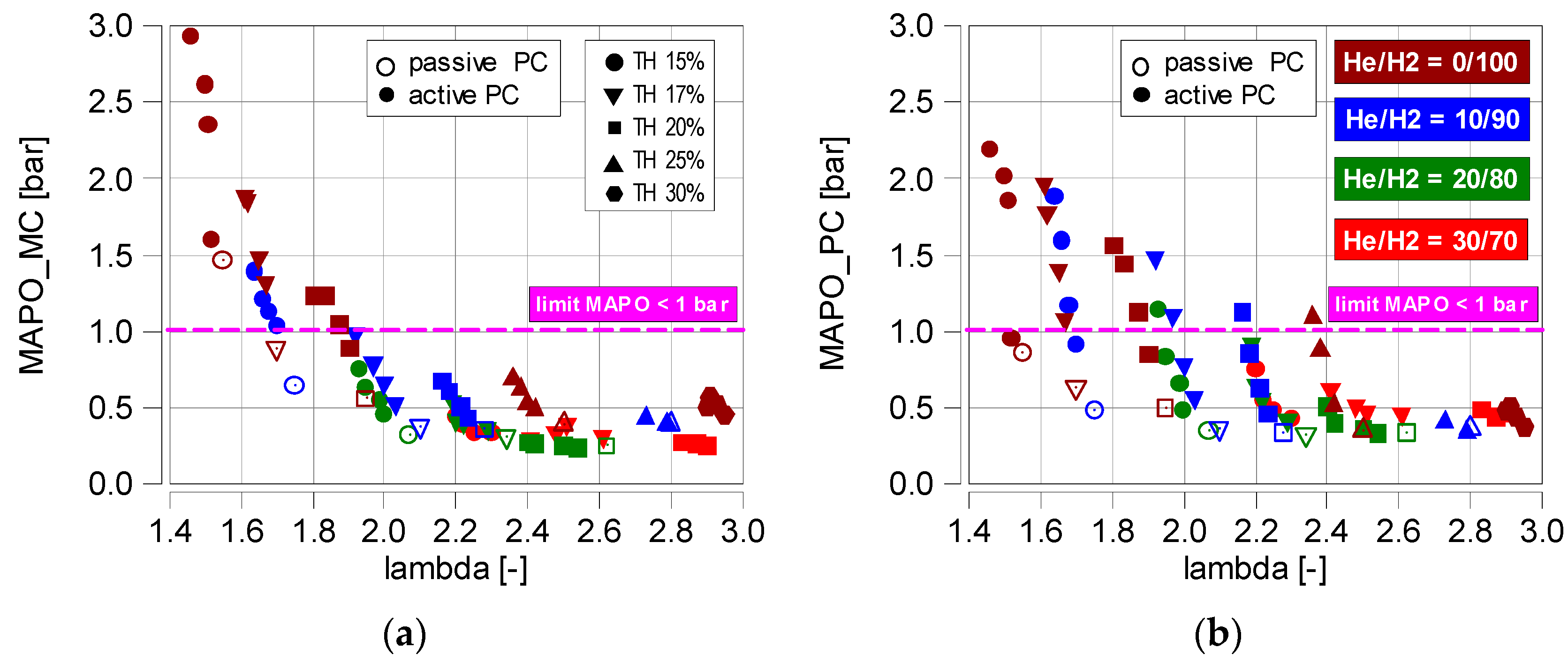
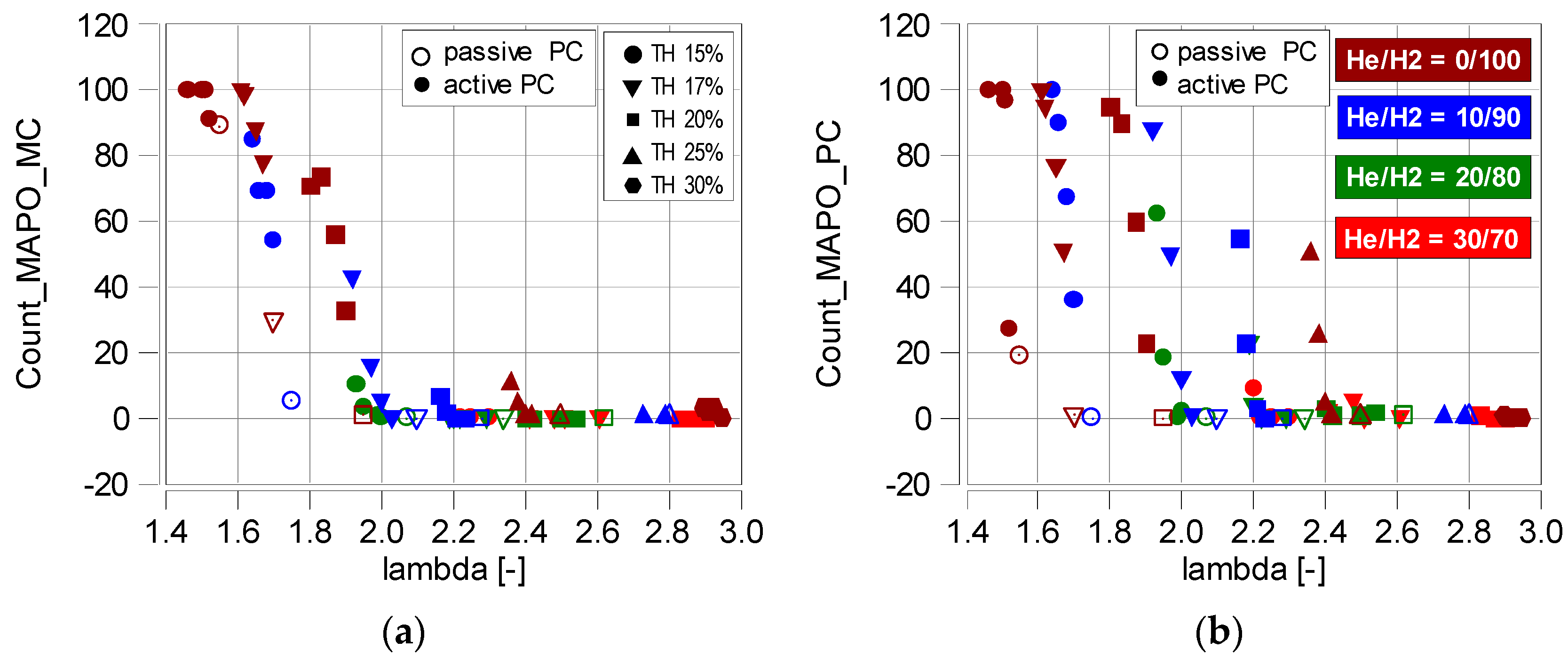
4.3. Knock Combustion
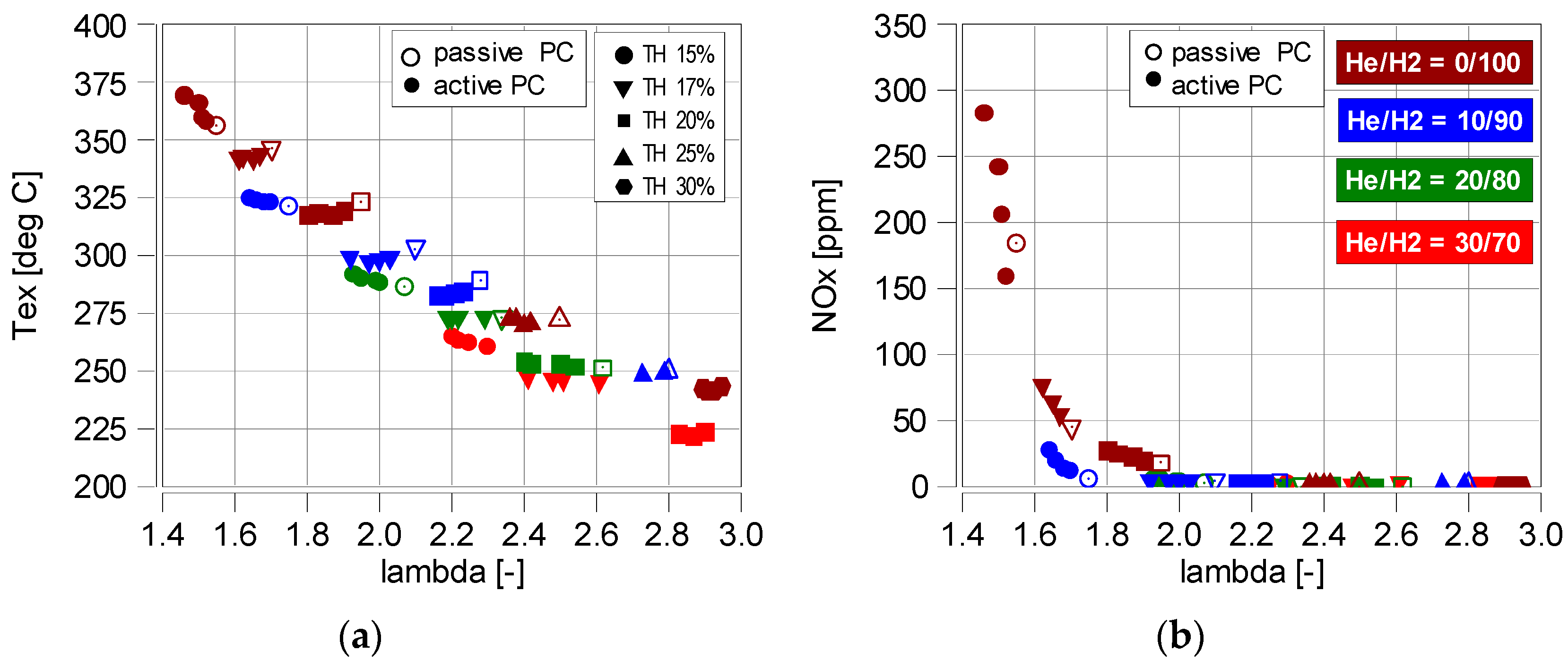
4.4. Exhaust Gas Emission
5. Conclusions
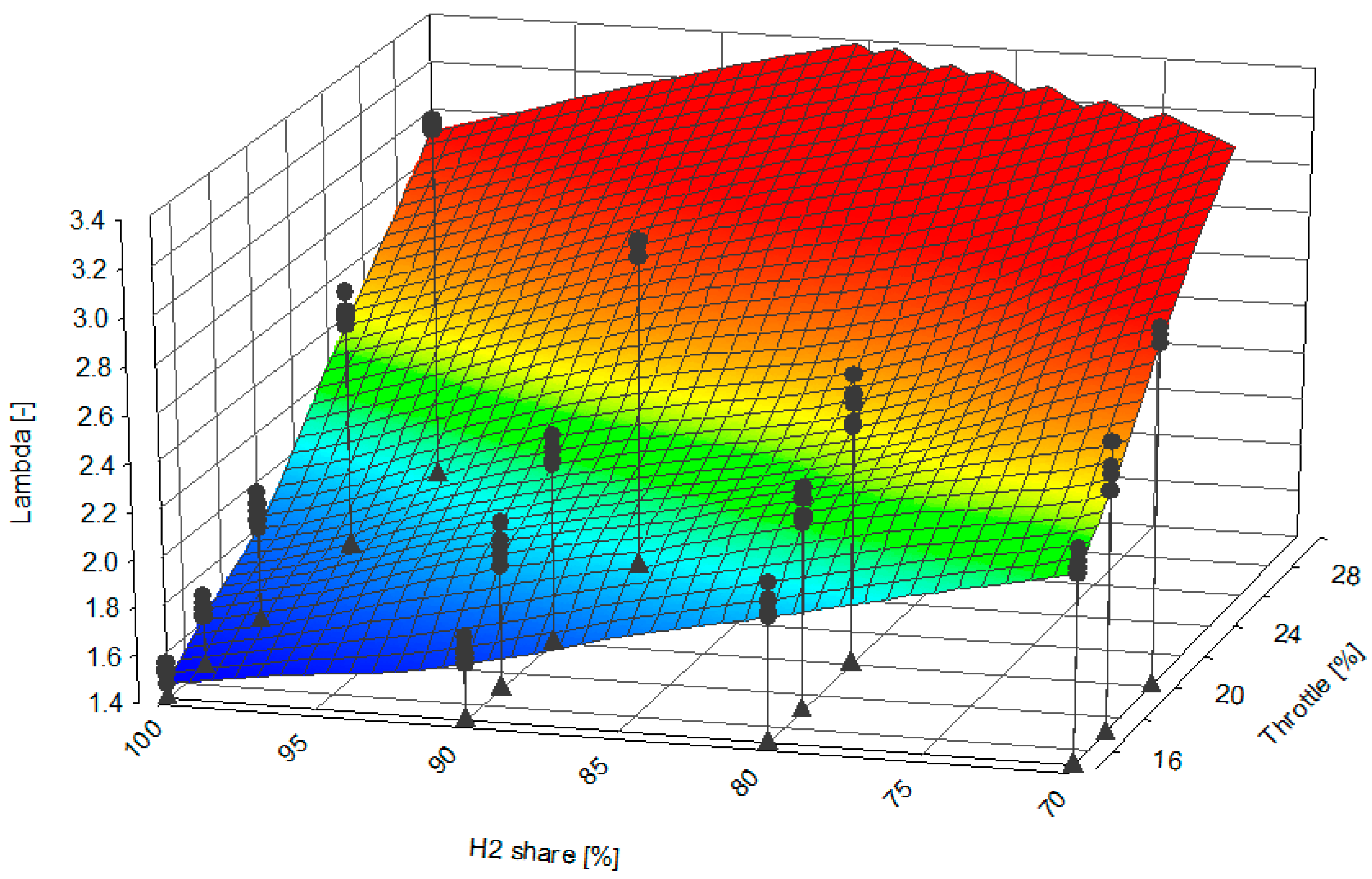
- The increase in the proportion of helium in the hydrogen fuel (at the same throttle opening) resulted in a higher excess air ratio. Therefore, the maximum combustion pressure Pmx decreased (due to a reduction in the energy content of the fuel)—Figure 10;
- The average decrease in IMEP is about 10% for every 10% increase in He. It is worth mentioning that λ does not affect the changes in this value—Figure 6a;
- Increasing the proportion of helium in the hydrogen fuel reduces the unevenness of the engine’s CoV(IMEP) from 2% to less than 1%;
- Increasing the proportion of He in the fuel significantly reduces the rate of heat release. Raising the percentage of He by 10% reduces dQmx by an average of 20%—Figure 8. This is significantly more than during the IMEP analysis;
- The analysis shows that with a fixed throttle position and high He/H2 ratio, the combustion process has to be accelerated (ignition time) to reach CoC = 8–10 deg aTDC;
- Knock combustion only occurs at low helium values in the fuel. In the main chamber, knock combustion does not occur when λ > 1.9 (MAPO < 1 bar). In the prechamber (into which pure hydrogen was injected), the absence of knock combustion was only noted at λ > 2.4;
- A temperature of 325 °C is the limit below which the concentration of NOx in the exhaust gas decreases rapidly. This temperature reduces the NOx concentration by more than 80% to a level of 30 ppm. At lower exhaust gas temperatures (with higher λ), the proportion of NOx in the exhaust gas was below 10 ppm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| aTDC | after TDC |
| BMEP | Break Mean Effective Pressure |
| CH4 | Methane |
| CoC | Center of combustion |
| CoV(IMEP) | Coefficient of Variation of IMEP |
| CVC | Constant Volume Chamber |
| dQ | Rate of Heat Release |
| H2 | Hydrogen |
| He | Helium |
| IMEP | Indicating Mean Effective Pressure |
| MAPO | Maximum Amplitude Pressure Oscillation |
| MC | Main Chamber |
| MFM | Mass Flow Meter |
| n | Engine Speed |
| NOx | Nitrogen Oxides |
| PC | Prechamber |
| PFI | Port Fuel Injection |
| Pmx | Maximum Cylinder Pressure |
| SI | Spark Ignition |
| TDC | Top Dead Center |
| TH | Throttle |
| TJI | Turbulent Jet Ignition |
| Q | Heat Release |
| Qmx | Maximum of Heat Release |
| λ | Air Access Ratio |
| γ | Ratio of the Specific Heats (cp/cv) |
References
- Kweku, D.W.; Bismark, O.; Maxwell, A.; Desmond, K.A.; Danso, K.B.; Oti-Mensah, E.A.; Quachie, A.T.; Adormaa, B.B. Greenhouse effect: Greenhouse gases and their impact on global warming. J. Sci. Res. Rep. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Ruddiman, W.; Vavrus, S.; Kutzbach, J.; He, F. Does pre-industrial warming double the anthropogenic total? Anthr. Rev. 2014, 1, 147–153. [Google Scholar] [CrossRef]
- Holland, G.; Bruyère, C.L. Recent intense hurricane response to global climate change. Clim. Dynam. 2014, 42, 617–627. [Google Scholar] [CrossRef]
- Onorati, A.; Payri, R.; Vaglieco, B.M.; Agarwal, A.K.; Bae, C.; Bruneaux, G.; Canakci, M.; Gavaises, M.; Günthner, M.; Hasse, C.; et al. The role of hydrogen for future internal combustion engines. Int. J. Engine Res. 2022, 23, 529–540. [Google Scholar] [CrossRef]
- Fayaz, H.; Saidur, R.; Razali, N.; Anuar, F.S.; Saleman, A.R.; Islam, M.R. An overview of hydrogen as a vehicle fuel. Renew. Sust. Energy Rev. 2012, 16, 5511–5528. [Google Scholar] [CrossRef]
- Verhelst, S.; Wallner, T. Hydrogen-fueled internal combustion engines. Prog. Energy Combust. 2009, 35, 490–527. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Ps, V.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sust. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Qiang, Y.; Cai, X.; Xu, S.; Wang, F.; Zhang, L.; Wang, S.; Ji, C. Effect of injection strategy on the hydrogen mixture distribution and combustion of the hydrogen-fueled engine with passive prechamber ignition under lean burn condition. Fuel 2024, 375, 132610. [Google Scholar] [CrossRef]
- Aljabri, H.; Silva, M.; Houidi, M.B.; Liu, X.; Allehaibi, M.; Almatrafi, F.; AlRamadan, A.S.; Mohan, B.; Cenker, E.; Im, H.G. Comparative study of spark-ignited and prechamber hydrogen-fueled engine: A computational approach. Energies 2022, 15, 8951. [Google Scholar] [CrossRef]
- Özyalcin, C.; Sterlepper, S.; Roiser, S.; Eichlseder, H.; Pischinger, S. Exhaust gas aftertreatment to minimize NOx emissions from hydrogen-fueled internal combustion engines. Appl. Energy 2024, 353, 122045. [Google Scholar] [CrossRef]
- Taib, N.M.; Mansor, M.R.A.; Wan Mahmood, W.M.F. Combustion characteristics of hydrogen in a noble gas compression ignition engine. Energy Rep. 2021, 7, 200–218. [Google Scholar] [CrossRef]
- Taib, N.M.; Mahmood, W.M.F.; Mansor, M.R.A. Combustion characteristics of hydrogen direct injection in a helium–oxygen compression ignition engine. Energy Rep. 2022, 8, 494–511. [Google Scholar] [CrossRef]
- Taib, N.M.; Mahmood, W.M.F.; Ghopa, W.A.W.; Köten, H.; Mansor, M.R.A. Numerical study of heat transfer of hydrogen combustion in noble gases atmosphere in compression ignition engine. Int. J. Hydrogen Energy 2023, 48, 28970–28979. [Google Scholar] [CrossRef]
- Dimitriou, P.; Tsujimura, T. A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrogen Energy 2017, 42, 24470–24486. [Google Scholar] [CrossRef]
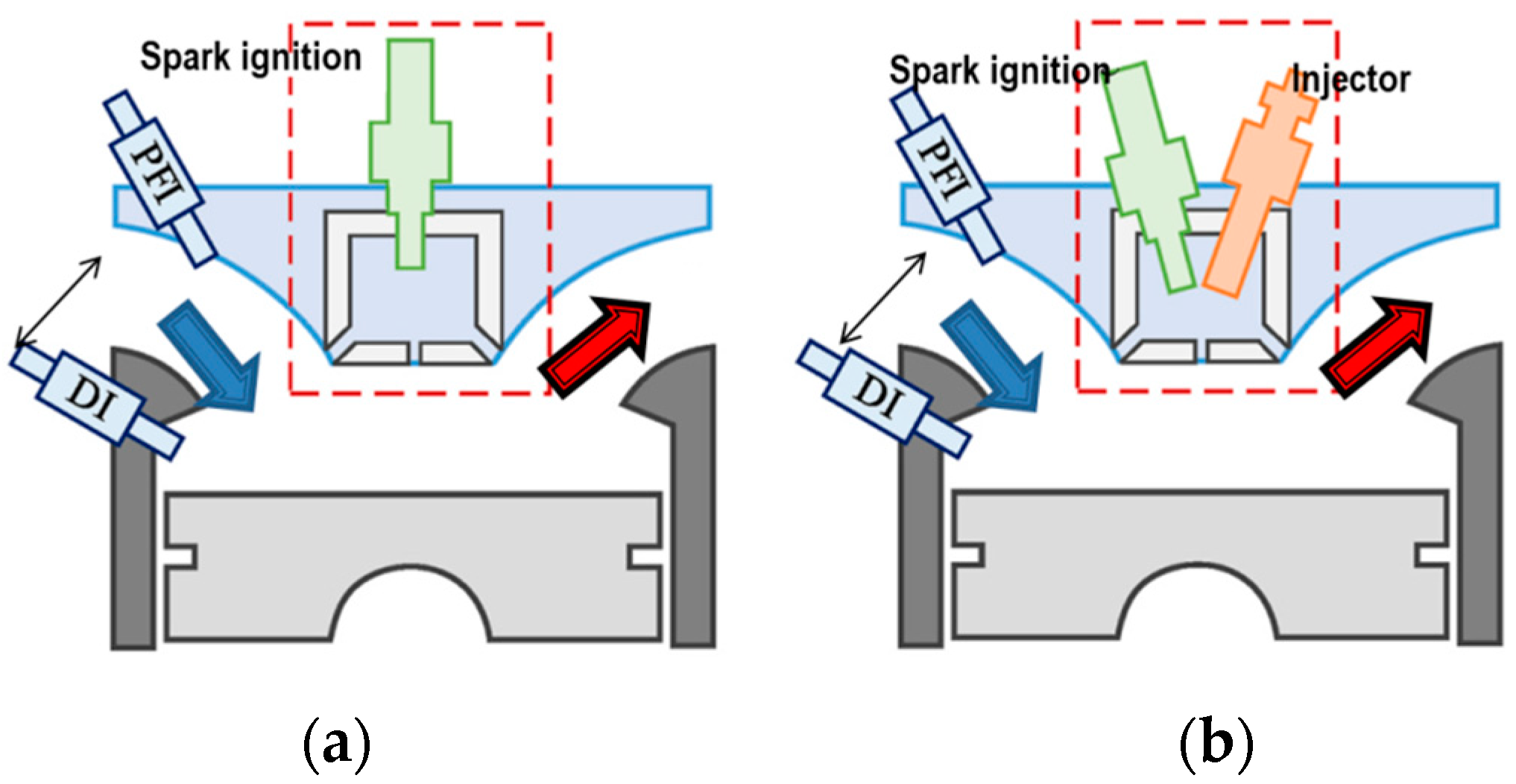
- Zhu, S.; Akehurst, S.; Lewis, A.; Yuan, H. A review of the prechamber ignition system applied on future low-carbon spark ignition engines. Renew. Sust. Energy Rev. 2022, 154, 111872. [Google Scholar] [CrossRef]
- Pielecha, I.; Szwajca, F.; Skobiej, K. Experimental investigation on knock characteristics from prechamber gas engine fueled by hydrogen. Energies 2024, 17, 937. [Google Scholar] [CrossRef]
- Lou, D.; Rao, X.; Zhang, Y.; Fang, L.; Tan, P.; Hu, Z. Investigation of impacts of hydrogen injection and spark strategy on knock in hydrogen engine. Int. J. Hydrogen Energy 2024, 82, 1252–1262. [Google Scholar] [CrossRef]
- Lai, F.; Sun, B.; Wang, X.; Zhang, D.; Luo, Q.; Bao, L. Research on the inducing factors and characteristics of knock combustion in a DI hydrogen internal combustion engine in the process of improving performance and thermal efficiency. Int. J. Hydrogen Energy 2023, 48, 7488–7498. [Google Scholar] [CrossRef]
- Soltic, P.; Hilfiker, T. Efficiency and raw emission benefits from hydrogen addition to methane in a prechamber–equipped engine. Int. J. Hydrogen Energy 2020, 45, 23638–23652. [Google Scholar] [CrossRef]
- Liu, P.; Zhong, L.; Zhou, L.; Wei, H. The ignition characteristics of the prechamber turbulent jet ignition of the hydrogen and methane based on different orifices. Int. J. Hydrogen Energy 2021, 46, 37083–37097. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988; ISBN 0-07-028637-X. [Google Scholar]
- Min, K.; Chung, J.; Sunwoo, M. Torque balance control for light-duty diesel engines using an individual cylinder IMEP estimation model with a single cylinder pressure sensor. Appl. Therm. Eng. 2016, 109, 440–448. [Google Scholar] [CrossRef]
- Szwajca, F.; Gawrysiak, C.; Pielecha, I. Effects of passive prechamber jet ignition on knock combustion at hydrogen engine. Combust. Engines 2024, 198, 110–122. [Google Scholar] [CrossRef]
- Bucherer, S.; Rothe, P.; Sobek, F.; Gottwald, T.; Kraljevic, I.; Vacca, A.; Gal, T.; Chiodi, M.; Kulzer, A. Experimental and numerical investigation of spark plug and passive prechamber ignition on a single-cylinder engine with hydrogen port fuel injection for lean operations. In SAE Technical; SAE International: Warrendale, PA, USA, 2023. [Google Scholar] [CrossRef]
- Hountalas, D.T.; Kouremenos, A.D. Development and application of a fully automatic troubleshooting method for large marine diesel engines. Appl. Therm. Eng. 1999, 19, 299–324. [Google Scholar] [CrossRef]
- Papagiannakis, R.G.; Hountalas, D.T. Combustion and exhaust emission characteristics of a dual fuel compression ignition engine operated with pilot diesel fuel and natural gas. Energy Convers. Manag. 2004, 45, 2971–2987. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Sun, B.G.; Zhang, S.W.; Lai, F.Y.; Ma, N.; Ju, X.; Luo, Q.H.; Bao, L.Z. Experimental investigation of the working boundary limited by abnormal combustion and the combustion characteristics of a turbocharged direct injection hydrogen engine. Energy Convers. Manag. 2024, 299, 117861. [Google Scholar] [CrossRef]
- Hong, C.; Xu, S.; Zhao, S.; Zhang, H.; Su, F.; Wang, S.; Ji, C. Analysis of ammonia as a combustion inhibitor for combustion knock and power expansion in a DI hydrogen engine. Fuel 2024, 375, 132481. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, L.; Hua, J.; Liu, C.; Wei, H. Effects of prechamber jet ignition on knock and combustion characteristics in a spark ignition engine fueled with kerosene. Fuel 2021, 293, 120278. [Google Scholar] [CrossRef]
- Sementa, P.; De Vargas Antolini, J.B.; Tornatore, C.; Catapano, F.; Vaglieco, B.M.; López Sánchez, J.J. Exploring the potentials of lean-burn hydrogen SI engine compared to methane operation. Int. J. Hydrogen Energy 2022, 47, 25044–25056. [Google Scholar] [CrossRef]
- Molina, S.; Novella, R.; Gomez-Soriano, J.; Olcina-Girona, M. Experimental activities on a hydrogen-fueled spark-ignition engine for light-duty applications. Appl. Sci. 2023, 13, 12055. [Google Scholar] [CrossRef]
- Pan, S.; Wang, J.; Liang, B.; Duan, H.; Huang, Z. Experimental study on the effects of hydrogen injection strategy on the combustion and emissions of a hydrogen/gasoline dual fuel SI engine under lean burn condition. Appl. Sci. 2022, 12, 10549. [Google Scholar] [CrossRef]
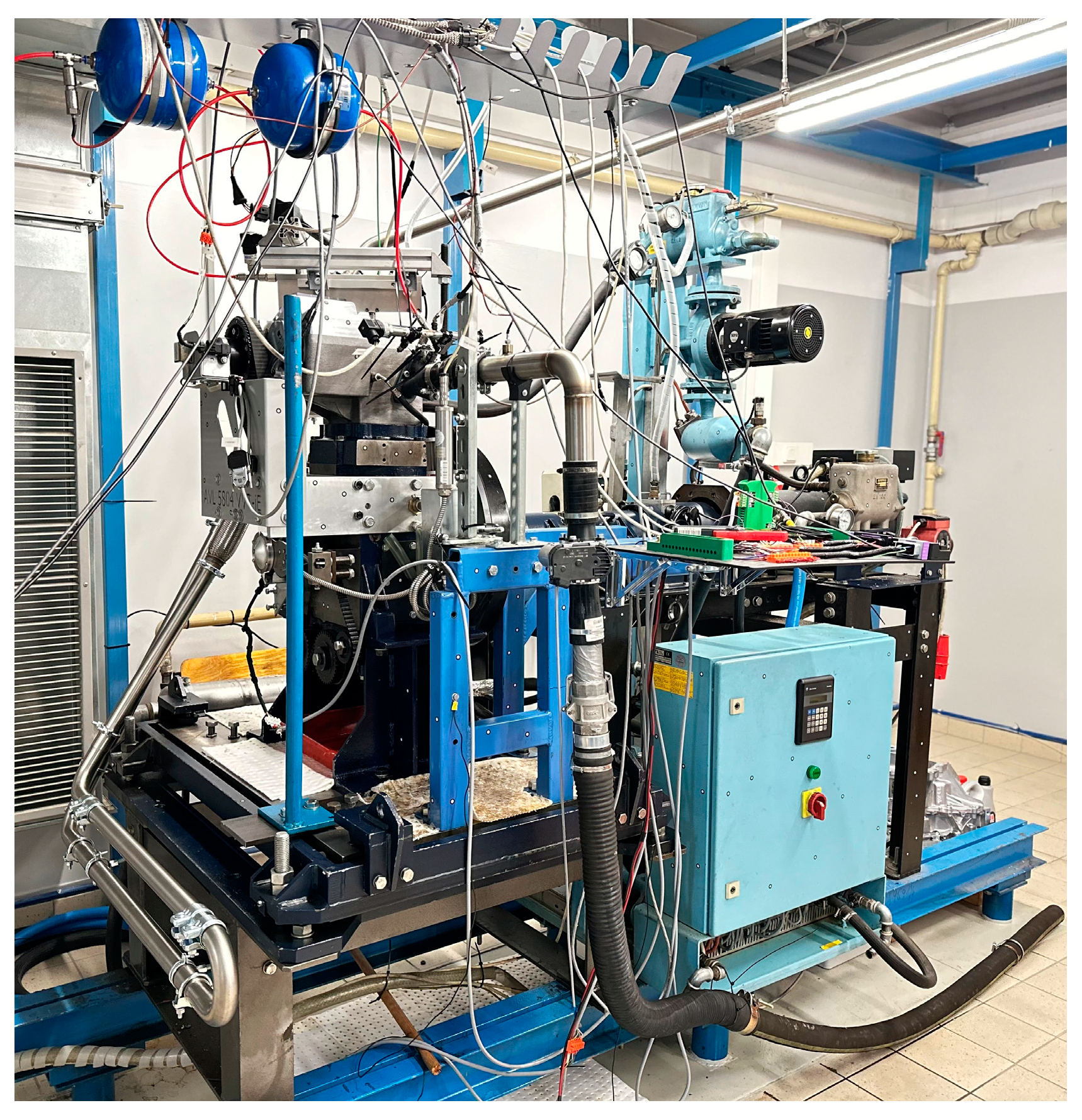
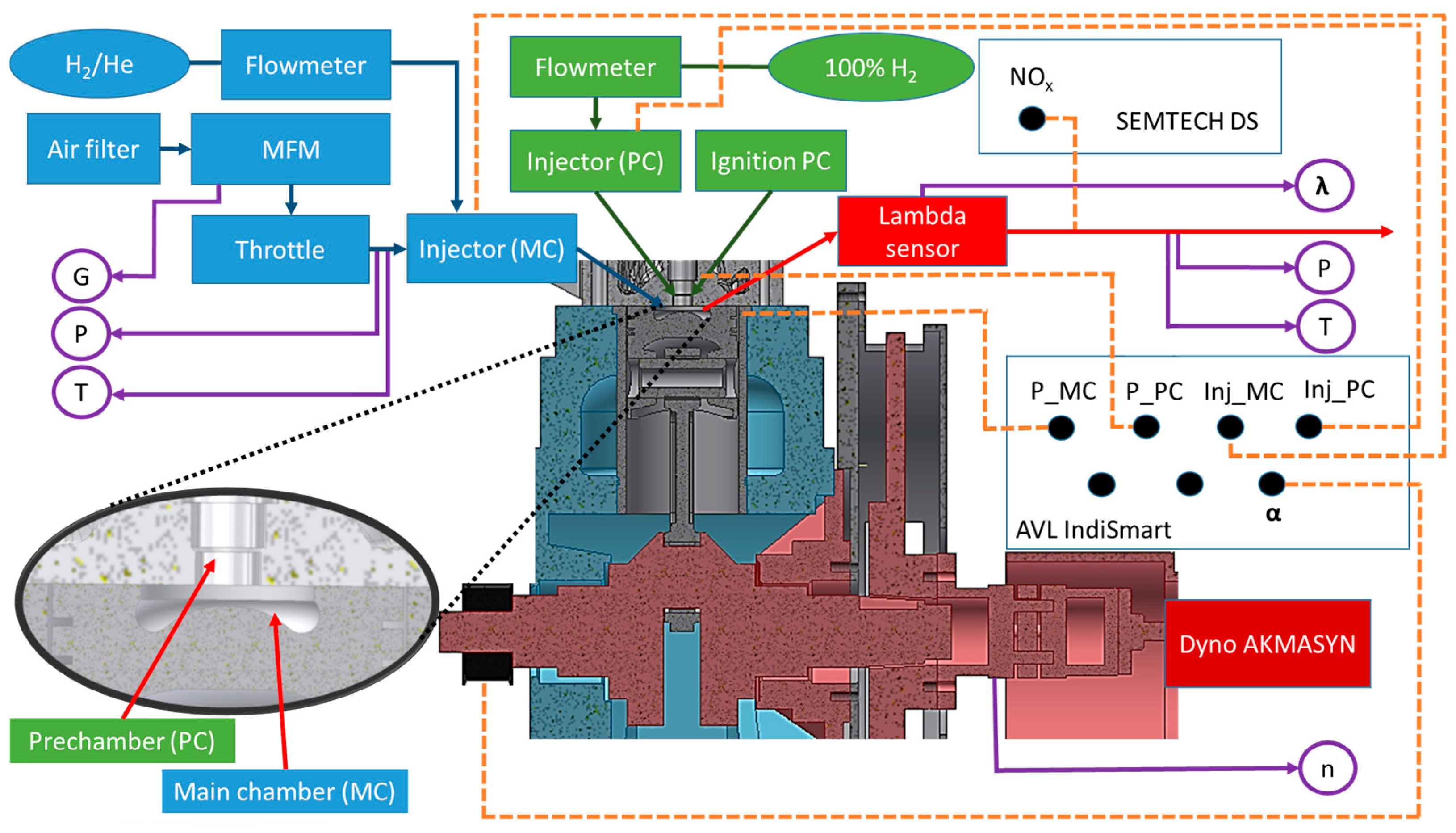




| Parameter | Name | Value |
|---|---|---|
| Engine | AVL 5804 | 1-cyl.; 4 valves; TJI |
| Injectors | PFI (EM) | – |
| Brake | AMKASYN AVL AMK DW13-170 | – |
| Parameter recorder | AVL IndiSmart | 8-chanell, 0.5 CA |
| Pressure sensor (MC) | AVL GH14D | 0–25 MPa |
| Pressure sensor (PC) | Kistler M3.5 6081 AQ22 | 0–25 MPa |
| Airflow meter | ABB SensyFlow | 0–720 kg/h, error < ±0.8% |
| Injection controller | Mechatronika | 0–20 ms, ±0.1 ms |
| Fuel flowmeter (MC) | Micro Motion ELITE CMFS010M Coriolis Meter | 0.1–2 kg/h, accuracy ±0.25% |
| Fuel flowmeter (PC) | Bronkhorst 111B | 0.1–100 g/h; accuracy 0.1% FS |
| Oxygen sensor | Bosch LSU 4.9 | 0.7 do 12.5 |
| Throttle | Bosch ETB 32 mm | ±1° |
| Measurement Series | Helium Content of the Fuel | Hydrogen Content of the Fuel |
|---|---|---|
| 1 | 0% | 100% |
| 2 | 10% | 90% |
| 3 | 20% | 80% |
| 4 | 30% | 70% |
| 5 | 40% | 60% |
| He/H2 | Density [kg/m3] | Energy in a Unit of Mass [MJ/kg] | Energy in a Unit of Volume [MJ/m3] |
|---|---|---|---|
| 0/100 | 0.08985 | 120 | 10.782 |
| 10/90 | 0.09871 | 108 | 10.661 |
| 20/80 | 0.10758 | 96 | 10.327 |
| 30/70 | 0.11644 | 84 | 9.781 |
| 40/60 | 0.12531 | 72 | 9.022 |
| Parameter | Unit | Value |
|---|---|---|
| Engine speed, rpm | rpm | 1500 |
| Oil temperature | °C | 90 |
| Coolant temperature | °C | 90 |
| Injection time (MC) | ms | 9.5 |
| Injection time (PC) | ms | 0; 2; 4; 6; 8 |
| Throttle | % | 8, 14, 19, 27, 36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielcarzewicz, D.; Pielecha, I. The Influence of Helium Addition on the Combustion Process in a Hydrogen-Fueled Turbulent Jet Ignition Engine. Appl. Sci. 2024, 14, 8996. https://doi.org/10.3390/app14198996
Mielcarzewicz D, Pielecha I. The Influence of Helium Addition on the Combustion Process in a Hydrogen-Fueled Turbulent Jet Ignition Engine. Applied Sciences. 2024; 14(19):8996. https://doi.org/10.3390/app14198996
Chicago/Turabian StyleMielcarzewicz, Dawid, and Ireneusz Pielecha. 2024. "The Influence of Helium Addition on the Combustion Process in a Hydrogen-Fueled Turbulent Jet Ignition Engine" Applied Sciences 14, no. 19: 8996. https://doi.org/10.3390/app14198996







