Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 2: Implementation
Abstract
1. Introduction
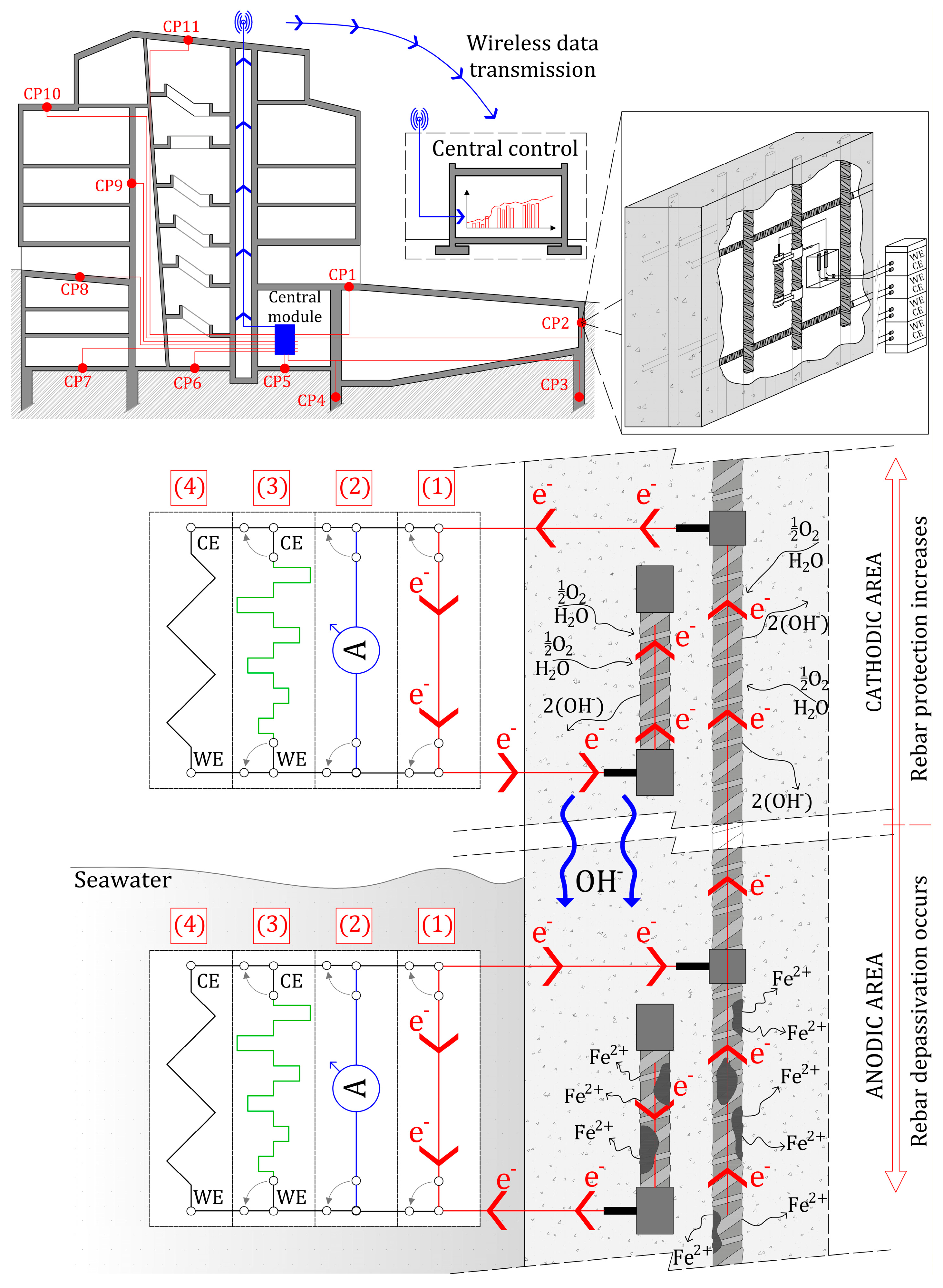
2. Background: The INESSCOM Monitoring System
3. Experimental Plan
3.1. Test Specimens
3.2. Materials
3.3. Exposure Conditions
- Column A, where the part’s LZ was free of the aggressive agents that could generate rebar depassivation;
- Column B, where the part’s LZ was affected by the presence of the chlorides introduced during mixing (LZ);
- Column C, where the LZ underwent an accelerated carbonation process.
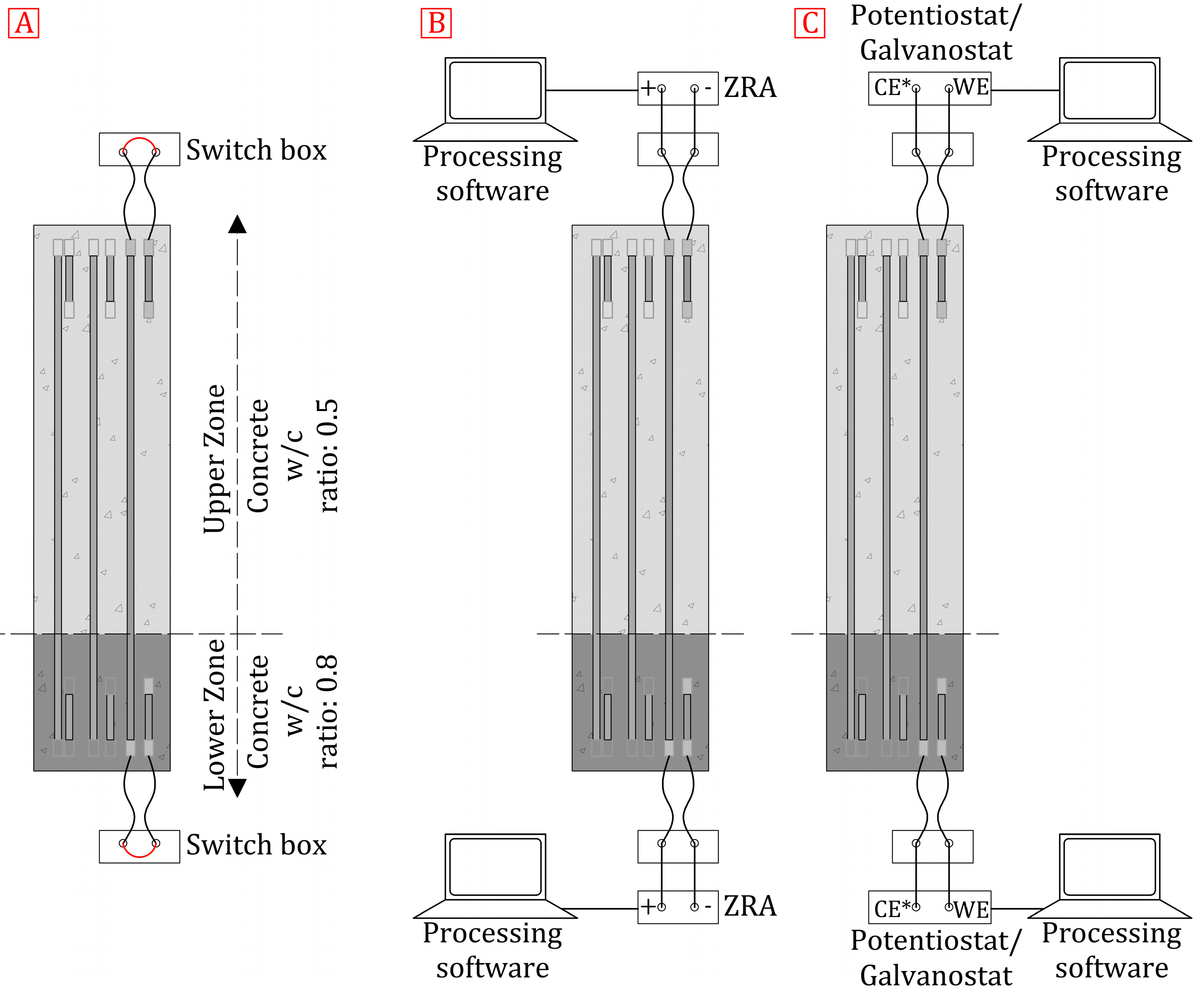
3.4. Testing Procedure
- First, the macrocell current () between the sensor and column reinforcement was measured by a Zero Resistance Ammeter (ZRA; Keithley 2000 Tektronix model). The value obtained for each sensor was normalised by its surface (≈1571 mm2). The value was recorded 3 min after measurements commenced to ensure that the recorded signal was stable enough. Macrocell currents were measured according to the criterion set out in Figure 4B.
- Second, the local corrosion () of each sensor was determined by the Potential Step Voltammetry method. This technique, used by the INESSCOM system, has been introduced in former works and previously validated [45,46,48,49,50]. This measurement was taken with an Autolab PGSTAT 100 Potentiostat/Galvanostat, and the Nova 1.11 software was employed for signal processing. The measurement cell configuration was a 2-electrode one, as described in Section 2. The working electrode was each sensor. The three continuous rebars of each column were used as the pseudoreference (Figure 4C).
- Third, the sensors’ double layer capacity () was determined from the voltammogram () obtained after applying CSV, ± 50 mV × 2 cycles at a sweep speed of 1 mV/s. This measurement was also taken with an Autolab PGSTAT 100 Potentiostat/Galvanostat, and the Nova 1.11 software was used for signal processing. This procedure, previously applied by other authors in the corrosion field [55,56,57], has been validated in a former work [33].
4. Results and Discussion
4.1. Monitoring Results
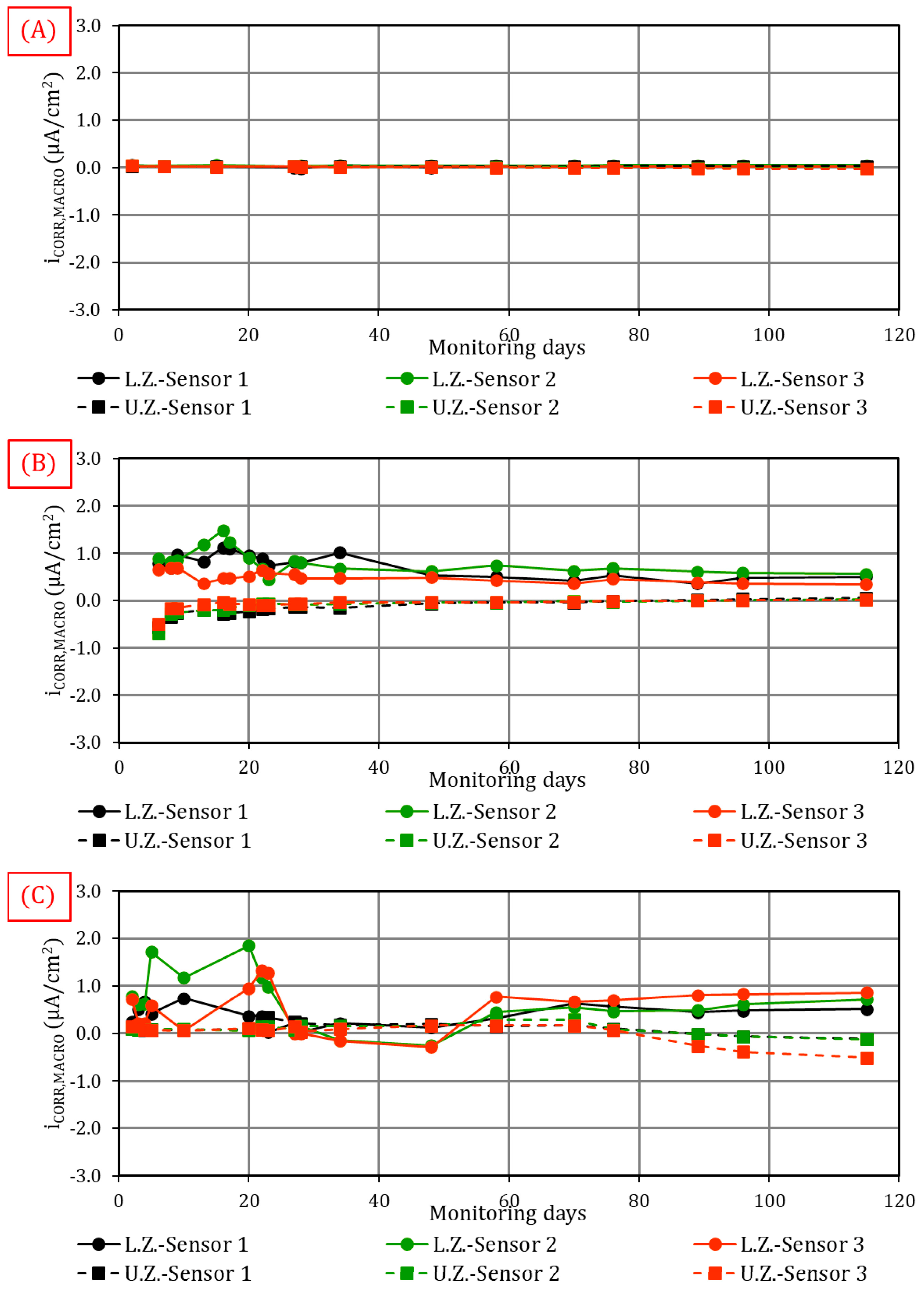
4.1.1. Macrocell Corrosion Current (iCORR,MACRO)
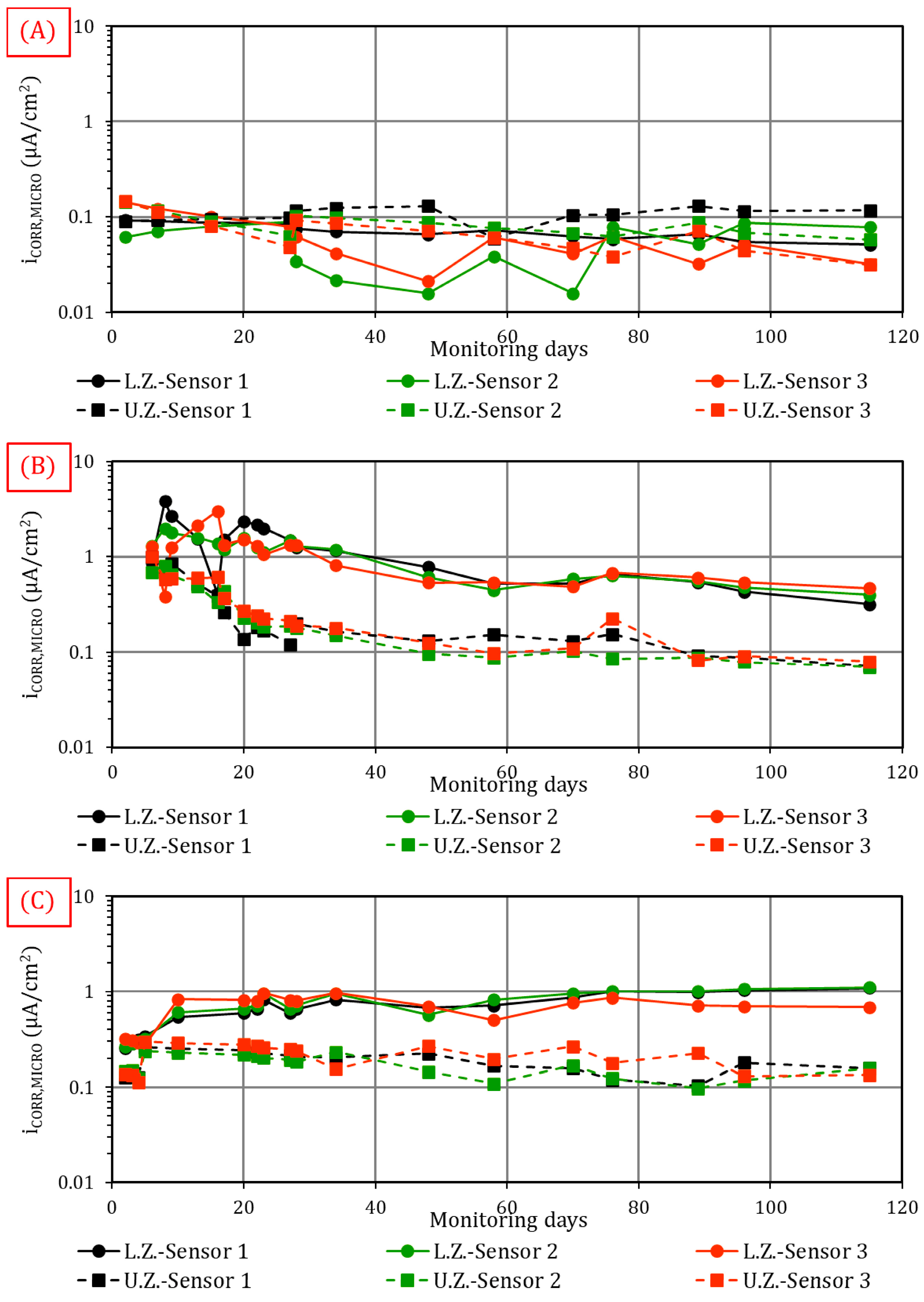
4.1.2. Microcell Corrosion Current (iCORR,MICRO)
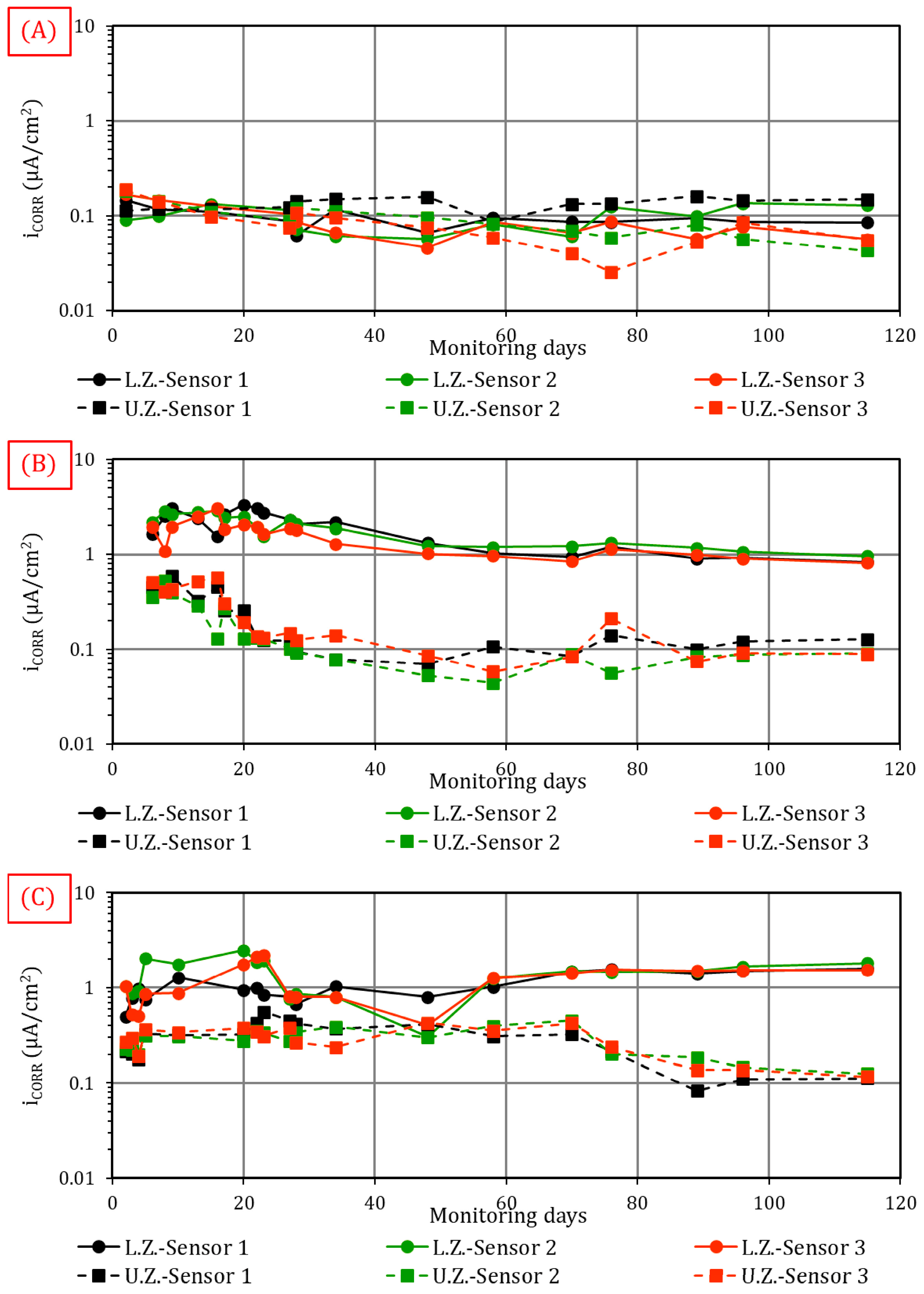
4.1.3. Corrosion (iCORR)
4.1.4. Double Layer Capacitance (CDL)
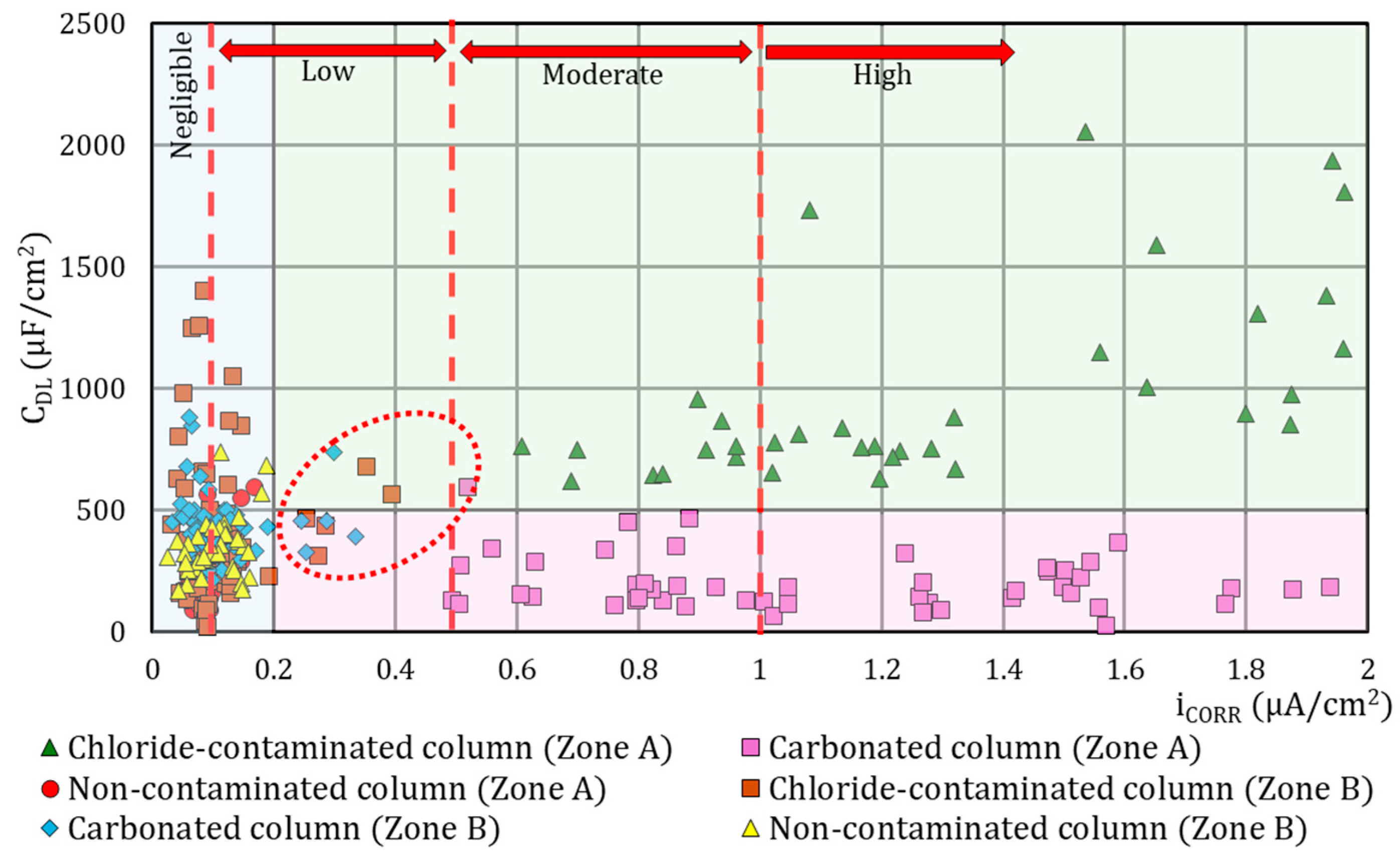
4.2. Classification Model: Degree of Corrosion vs. Attack Type
4.3. Validation
4.3.1. Validating the Capacity to Identify the Environment
4.3.2. The Probability of Classification Model Success
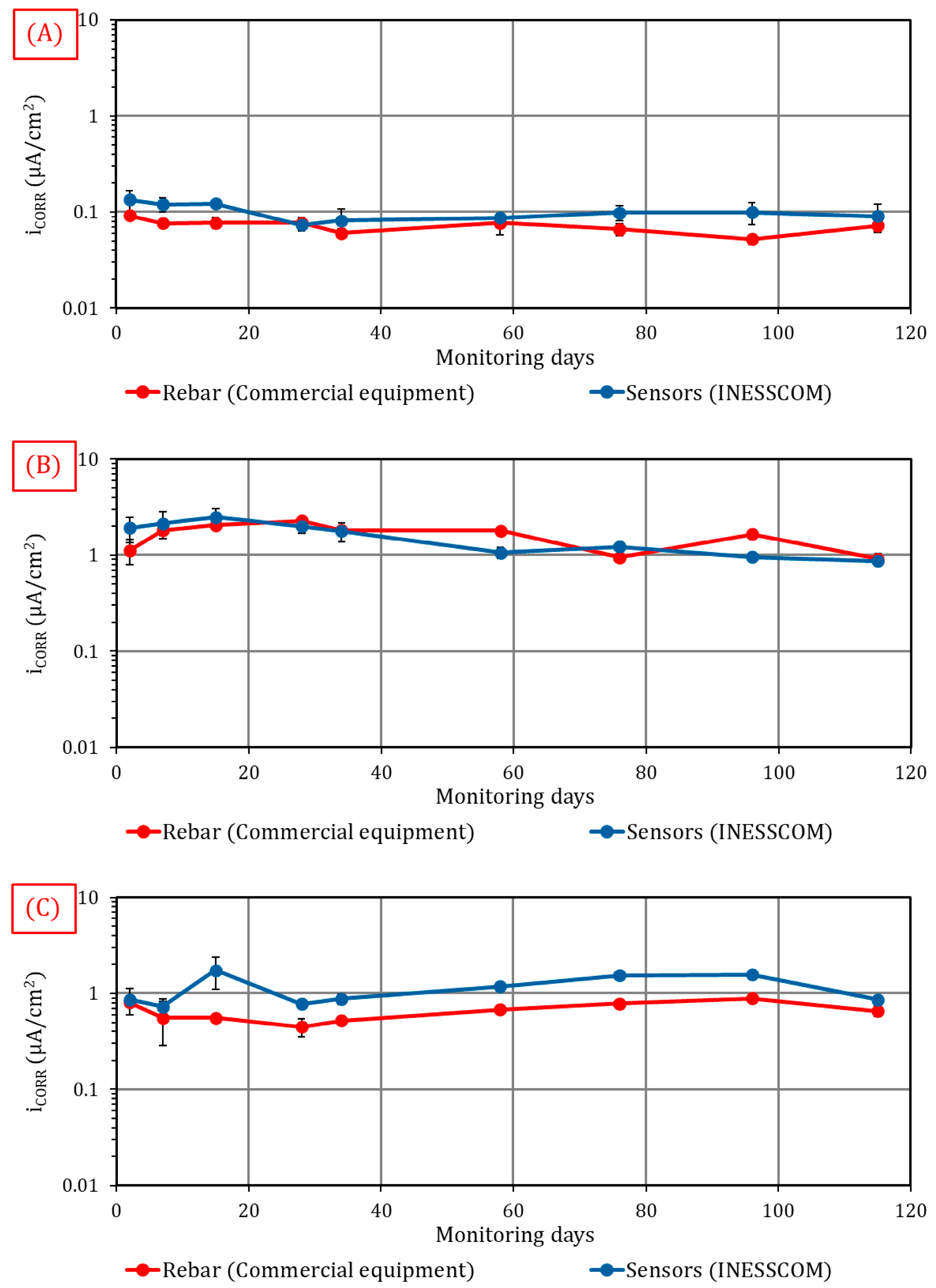
4.3.3. Validating the Capacity to Estimate Degree of Corrosion
5. Conclusions
- The protocol designed to be implemented in the INESSCOM monitoring system allows different parameters to be followed up, of which corrosion intensity and (iCORR) double layer capacity (CDL) are highlighted.
- The proposed multivariate classification model (iCORR and CDL) quickly and simply allows the precursor agent of corrosion to be identified, even when corrosion kinetics are similar. The developed model simplifies the techniques used for defining classification and estimation models, commonly employed in voltammetric sensor systems. Some of these techniques, such as PCA and PLS, require advanced computational software, whereas the proposed model can be processed with simple tools like spreadsheets. This makes it easier for non-expert users to apply it and allows its more widespread use.
- Implementing the proposed measurement protocol is simple and does not require modifying changes in technology, but merely a change in the data management of the control software. So, it can be implemented in already existing remote-testing systems.
- The study data were validated with in situ measurement tools, with deviation close to 10% when the remote-sensing system INESSCOM was used.
6. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Power, G.J.; Tandja M, C.D.; Bastien, J.; Grégoire, P. Measuring infrastructure investment option value. J. Risk Financ. 2015, 16, 49–72. [Google Scholar] [CrossRef]
- Ellingwood, B.R. Risk-informed condition assessment of civil infrastructure: State of practice and research issues. Struct. Infrastruct. Eng. 2005, 1, 7–18. [Google Scholar] [CrossRef]
- McCarter, W.J.; Vennesland, Ø. Sensor systems for use in reinforced concrete structures. Constr. Build. Mater. 2004, 18, 351–358. [Google Scholar] [CrossRef]
- Stewart, M.G.; Wang, X.; Nguyen, M.N. Climate change adaptation for corrosion control of concrete infrastructure. Struct. Saf. 2012, 35, 29–39. [Google Scholar] [CrossRef]
- Saad, L.; Aissani, A.; Chateauneuf, A.; Raphael, W. Reliability-based optimization of direct and indirect LCC of RC bridge elements under coupled fatigue-corrosion deterioration processes. Eng. Fail. Anal. 2016, 59, 570–587. [Google Scholar] [CrossRef]
- Ožbolt, J.; Oršanić, F.; Balabanić, G.; Kušter, M. Modeling damage in concrete caused by corrosion of reinforcement: Coupled 3D FE model. Int. J. Fract. 2012, 178, 233–244. [Google Scholar] [CrossRef]
- Ball, B.J.C.; Whitmore, D.W. Corrosion Mitigation Systems for Concrete Structures. Concr. Repair Bull. 2003, 16, 6–11. [Google Scholar]
- Cavaleri, L.; Barkhordari, M.S.; Repapis, C.C.; Armaghani, D.J.; Ulrikh, D.V.; Asteris, P.G. Convolution-based ensemble learning algorithms to estimate the bond strength of the corroded reinforced concrete. Constr. Build. Mater. 2022, 359, 129504. [Google Scholar] [CrossRef]
- Phulara, N.R.; Bhattarai, J. Assessment on Corrosion Damage of Steel Reinforced Concrete Structures of Kathmandu Valley Using Corrosion Potential Mapping Method. J. Inst. Eng. 2019, 15, 45–54. [Google Scholar] [CrossRef]
- Cavaco, E.; Pimenta, R.; Valença, J. A new method for corrosion assessment of reinforcing bars based on close-range photogrammetry: Experimental validation. Struct. Concr. 2019, 20, 996–1009. [Google Scholar] [CrossRef]
- François, R.; Laurens, S.; Deby, F. Corrosion and its Consequences for Reinforced Concrete Structures; ISTE Press Ltd.: London, UK; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; ISBN 978-1-78548-234-2. [Google Scholar]
- Angst, U.M. Challenges and opportunities in corrosion of steel in concrete. Mater. Struct. Constr. 2018, 51, 4. [Google Scholar] [CrossRef]
- Polder, R.B.; Peelen, W.H.A.; Courage, W.M.G. Non-traditional assessment and maintenance methods for aging concrete structures—Technical and non-technical issues. Mater. Corros. 2012, 63, 1147–1153. [Google Scholar] [CrossRef]
- Helal, J.; Sofi, M.; Mendis, P. Non-destructive testing of concrete: A review of methods. Electron. J. Struct. Eng. 2015, 14, 97–105. [Google Scholar] [CrossRef]
- Li, C.; Jiang, Z.; Myers, R.J.; Chen, Q.; Wu, M.; Li, J.; Monteiro, P.J.M. Understanding the sulfate attack of Portland cement–based materials exposed to applied electric fields: Mineralogical alteration and migration behavior of ionic species. Cem. Concr. Compos. 2020, 111, 103630. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Li, W. Preparation and characterization of autolytic mineral microsphere for self-healing cementitious materials. Cem. Concr. Compos. 2019, 103, 112–120. [Google Scholar] [CrossRef]
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction—A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Brenna, A.; Bolzoni, F.; Beretta, S.; Ormellese, M. Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings. Constr. Build. Mater. 2013, 48, 734–744. [Google Scholar] [CrossRef]
- Tuutti, K. Corrosion of Steel in Concrete; Swedish Cement and Concrete Research Instittute: Stockholm, Sweden, 1982. [Google Scholar]
- Solla, M.; Lagüela, S.; Fernández, N.; Garrido, I. Assessing rebar corrosion through the combination of nondestructive GPR and IRT methodologies. Remote Sens. 2019, 11, 1705. [Google Scholar] [CrossRef]
- Medvedev, V.; Pustovgar, A. A Review of Concrete Carbonation and Approaches to Its Research under Irradiation. Buildings 2023, 13, 1998. [Google Scholar] [CrossRef]
- Chang, C.F.; Chen, J.W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- Stefanoni, M.; Angst, U.; Elsener, B. Corrosion rate of carbon steel in carbonated concrete—A critical review. Cem. Concr. Res. 2018, 103, 35–48. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahmad, S.; Al-Gahtani, H.J. Chloride-Induced Corrosion of Steel in Concrete: An Overview on Chloride Diffusion and Prediction of Corrosion Initiation Time. Int. J. Corros. 2017, 2017, 5819202. [Google Scholar] [CrossRef]
- Glass, G.K.; Buenfeld, N.R. Chloride-induced corrosion of steel. Prog. Struct. Eng. Mater. 2000, 2, 448–458. [Google Scholar] [CrossRef]
- Feng, W.; Tarakbay, A.; Ali Memon, S.; Tang, W.; Cui, H. Methods of accelerating chloride-induced corrosion in steel-reinforced concrete: A comparative review. Constr. Build. Mater. 2021, 289, 123165. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. Constr. 2019, 52, 88. [Google Scholar] [CrossRef]
- Vera, R.; Villarroel, M.; Carvajal, A.M.; Vera, E.; Ortiz, C. Corrosion products of reinforcement in concrete in marine and industrial environments. Mater. Chem. Phys. 2009, 114, 467–474. [Google Scholar] [CrossRef]
- Hwang, W.; Yong Ann, K. Determination of rust formation to cracking at the steel–concrete interface by corrosion of steel in concrete. Constr. Build. Mater. 2023, 367, 130215. [Google Scholar] [CrossRef]
- Bertolini, L.; Carsana, M.; Gastaldi, M.; Lollini, F.; Redaelli, E. Corrosion assessment and restoration strategies of reinforced concrete buildings of the cultural heritage. Mater. Corros. 2011, 62, 146–154. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Jamali, A.; Adey, B. Concrete cover cracking owing to reinforcement corrosion—Theoretical considerations and practical experience. Mater. Corros. 2012, 63, 1069–1077. [Google Scholar] [CrossRef]
- Reichling, K.; Raupach, M.; Broomfield, J.; Gulikers, J.; L’Hostis, V.; Kessler, S.; Osterminski, K.; Pepenar, I.; Schneck, U.; Sergi, G. Full surface inspection methods regarding reinforcement corrosion of concrete structures. Mater. Corros. 2013, 64, 116–127. [Google Scholar] [CrossRef]
- Ramón-Zamora, J.E.; Lliso-Ferrando, J.R.; Martínez-Ibernón, A.; Gandiá-Romero, J.M. Corrosion Assessment in Reinforced Concrete Structures by means of Embedded Sensors and Multivariate Analysis. Part 1: Laboratory Validation. Sensors 2023, 23, 8869. [Google Scholar] [CrossRef] [PubMed]
- Broomfield, J.P.; Davies, K.; Hladky, K. The use of permanent corrosion monitoring in new and existing reinforced concrete structures. Cem. Concr. Compos. 2002, 24, 27–34. [Google Scholar] [CrossRef]
- Kersey, A.D.; Davis, M.A.; Patrick, H.J.; LeBlanc, M.; Koo, K.P.; Askins, C.G.; Putnam, M.A.; Friebele, E.J. Fiber grating sensors. J. Light. Technol. 1997, 15, 1442–1462. [Google Scholar] [CrossRef]
- Fan, L.; Bao, Y. Review of fiber optic sensors for corrosion monitoring in reinforced concrete. Cem. Concr. Compos. 2021, 120, 104029. [Google Scholar] [CrossRef]
- Bao, Y.; Valipour, M.; Meng, W.; Khayat, K.H.; Chen, G. Distributed fi ber optic sensor-enhanced detection and prediction of shrinkage- induced delamination of ultra-high- performance concrete overlay. Smart Mater. Struct. 2017, 26, 085009. [Google Scholar] [CrossRef]
- Kadkhodazadeh, S.; Ihamouten, A.; Souriou, D.; Dérobert, X.; Guilbert, D. Parametric Study to Evaluate the Geometry and Coupling Effect on the Efficiency of a Novel FMM Tool Embedded in Cover Concrete for Corrosion Monitoring. Remote Sens. 2022, 14, 5593. [Google Scholar] [CrossRef]
- Martínez, I.; Andrade, C. Examples of reinforcement corrosion monitoring by embedded sensors in concrete structures. Cem. Concr. Compos. 2009, 31, 545–554. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, C.; Gulikers, J.; Polder, R.; Cigna, R.; Vennesland, Ø.; Salta, M.; Raharinaivo, A.; Elsener, B. Recommendations of RILEM TC-154-EMC: “Electrochemical techniques for measuring metallic corrosion” Test methods for on-site corrosion rate measurement of steel reinforcement in concrete by means of the polarization resistance method. Mater. Struct. 2004, 37, 623–643. [Google Scholar] [CrossRef]
- Lliso-Ferrando, J.R. Monitorizacion de la Durabilidad de Estructuras Existentes de Hormigon Armado Mediante la Inserción de una red de Sensores. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2022. [Google Scholar]
- Pallarés, F.J.; Betti, M.; Bartoli, G.; Pallarés, L. Structural health monitoring (SHM) and Nondestructive testing (NDT) of slender masonry structures: A practical review. Constr. Build. Mater. 2021, 297, 123768. [Google Scholar] [CrossRef]
- Sakiyama, F.I.H.; Lehmann, F.; Garrecht, H. Structural health monitoring of concrete structures using fibre-optic-based sensors: A review. Mag. Concr. Res. 2021, 73, 174–194. [Google Scholar] [CrossRef]
- Ramón, J.E.; Gandía-Romero, J.M.; Bataller, R.; López, J.A.; Valcuende, M.; Soto, J. Real-time corrosion monitoring of an ultra-high performance fibre-reinforced concrete offshore raft by using an autonomous sensor system. Struct. Control Heal. Monit. 2022, 29, e3102. [Google Scholar] [CrossRef]
- Ramon Zamora, J.E. Sistema de Sensores Embebidos para Monitorizar la Corrosión en Estructuras de Hormigón Armado. Fundamentos, Metodología y Aplicaciones. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2018. [Google Scholar]
- Ramón, J.E.; Gandía-Romero, J.M.; Valcuende, M.; Bataller, R. Integrated sensor network for monitoring steel corrosion in concrete structures. Vitr. Int. J. Archit. Technol. Sustain. 2016, 1, 65. [Google Scholar] [CrossRef]
- Lliso-Ferrando, J.R.; Gasch, I.; Martínez-Ibernón, A.; Valcuende, M. Effect of macrocell currents on rebar corrosion in reinforced concrete structures exposed to a marine environment. Ocean Eng. 2022, 257, 111680. [Google Scholar] [CrossRef]
- Martínez-Ibernón, A.; Ramón, J.E.; Gandía-Romero, J.M.; Gasch, I.; Valcuende, M.; Alcañiz, M.; Soto, J. Characterization of electrochemical systems using potential step voltammetry. Part II: Modeling of reversible systems. Electrochim. Acta 2019, 328, 135111. [Google Scholar] [CrossRef]
- Ramón, J.E.; Gandía-Romero, J.M.; Bataller, R.; Alcañiz, M.; Valcuende, M.; Soto, J. Potential step voltammetry: An approach to corrosion rate measurement of reinforcements in concrete. Cem. Concr. Compos. 2020, 110, 103590. [Google Scholar] [CrossRef]
- Ramón, J.E.; Martínez-Ibernón, A.; Gandía-Romero, J.M.; Fraile, R.; Bataller, R.; Alcañiz, M.; García-Breijo, E.; Soto, J. Characterization of electrochemical systems using potential step voltammetry. Part I: Modeling by means of equivalent circuits. Electrochim. Acta 2019, 323, 134702. [Google Scholar] [CrossRef]
- Duffó, G.S.; Farina, S.B.; Giordano, C.M. Characterization of solid embeddable reference electrodes for corrosion monitoring in reinforced concrete structures. Electrochim. Acta 2009, 54, 1010–1020. [Google Scholar] [CrossRef]
- Andrade, C. Propagation of reinforcement corrosion: Principles, testing and modelling. Mater. Struct. 2019, 52, 2. [Google Scholar] [CrossRef]
- Andrade, C.; Garcés, P.; Martínez, I. Galvanic currents and corrosion rates of reinforcements measured in cells simulating different pitting areas caused by chloride attack in sodium hydroxide. Corros. Sci. 2008, 50, 2959–2964. [Google Scholar] [CrossRef]
- Qian, S.; Zhang, J.; Qu, D. Theoretical and experimental study of microcell and macrocell corrosion in patch repairs of concrete structures. Cem. Concr. Compos. 2006, 28, 685–695. [Google Scholar] [CrossRef]
- Rodriguez, P.; Ramirez, E.; Gonzalez, J.A. Methods for studying corrosion in reinforced concrete. Mag. Concr. Res. 1994, 46, 81–90. [Google Scholar] [CrossRef]
- González, J.A.; Feliú, S.; Rodríguez, P.; Ramírez, E.; Alonso, C.; Andrade, C. Some questions on the corrosion of steel in concrete—Part I: When, how and how much steel corrodes. Mater. Struct. Constr. 1996, 29, 40–46. [Google Scholar] [CrossRef]
- González, J.A.; Feliú, S.; Rodríguez, P.; López, W.; Ramírez, E.; Alonso, C.; Andrade, C. Some questions on the corrosion of steel in concrete. Part II: Corrosion mechanism and monitoring, service life prediction and protection methods. Mater. Struct. Constr. 1996, 29, 97–104. [Google Scholar] [CrossRef]
- UNE-EN 12390-3:2020; Ensayos de Hormigón Endurecido. Parte 3: Determinación de la Resistencia a Compresión de Probetas. UNE: Madrid, Spain, 2020.
- UNE 83980; Durabilidad del Hormigón. Métodos de Ensayo. Determinación de la Absorción de Agua, la Densidad y la Porosidad Accesible al agua del Hormigón. UNE: Madrid, Spain, 2014.
- UNE-EN 12390-8; Ensayos de Hormigón Endurecido. Parte 8: Profundidad de Penetración de Agua bajo Presión. UNE: Madrid, Spain, 2020.
- UNE 83981:2008; Durabilidad del Hormigón. Métodos de Ensayo. Determinación de la Permeabilidad al Oxígeno del Hormigón Endurecido. UNE: Madrid, Spain, 2008.
- UNE-EN 12390-18:2021; Ensayos de Hormigón Endurecido. Parte 18: Determinación del Coeficiente de Migración de Cloruros. UNE: Madrid, Spain, 2021.
- UNE-EN 12390-19:2023; Ensayos de Hormigón Endurecido. Parte 19: Determinación de la Resistividad Eléctrica. UNE: Madrid, Spain, 2024.
- UNE 112072:2011; Determinación de la Velocidad de Corrosión de Armaduras en Laboratorio Mediante Medida de la Resistencia a la Polarización. UNE: Madrid, Spain, 2021.
- Lliso-Ferrando, J.R.; Soto, J.; Gasch, I.; Valcuende, M. Significance of macrocell currents in reinforced concrete columns partially immersed in seawater. Constr. Build. Mater. 2023, 389, 131739. [Google Scholar] [CrossRef]
- Sibatov, R.T.; Uchaikin, V.V. Fractional kinetics of charge carriers in supercapacitors. In Handbook of Fractional Calculus with Applications; De Gruyter: Berlin, Germany, 2019; ISBN 9783110571929. [Google Scholar]
- Conway, B.E.; Pell, W.G. Double-layer and pseudocapacitance types of electrochemical capacitors and their applications to the development of hybrid devices. J. Solid State Electrochem. 2003, 7, 637–644. [Google Scholar] [CrossRef]
- Martínez, M.; Marí, M. La Distribución Binomial; Universidad Politécnica de Valencia: Valencia, Spain, 2009; p. 8. [Google Scholar]
- Saturno, P.J. La distribución binomial y el muestreo para la aceptación de lotes (LQAS) como métodos de monitorización en servicios de salud. Rev. Calid. Asist. 2000, 15, 99–107. [Google Scholar]
- Jos, E.S.; Morales, R. Distribuciones de Probabilidad: Binomial y Normal Variables Aleatorias. pp. 1–13. Available online: https://www.google.com/url?sa=t&source=web&rct=j&opi=89978449&url=https://www.matematicasonline.es/BachilleratoCCSS/segundo/archivos/Inferencia_estadistica/binom.pdf&ved=2ahUKEwi7jsfH1OyIAxV72jgGHTiRMJIQFnoECBwQAQ&usg=AOvVaw3IDodTYE0p4SrWabvY4Cwr (accessed on 14 September 2024).
- Hodges, L. Common Univariate Distributions. In Methods in Experimental Physics; Elsevier Masson SAS: Amsterdam, The Netherlands, 1994; pp. 35–61. [Google Scholar]
- Tsokos, C.; Wooten, R. Normal Probability. In The Joy of Finite Mathematics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 231–261. [Google Scholar]





| Cement | Water | Sand (0/4) | Gravel (4/6) | w/c | |
|---|---|---|---|---|---|
| Lower zone | 300 | 240 | 1112.9 | 599.2 | 0.8 |
| Upper zone | 380 | 190 | 1152.3 | 620.5 | 0.5 |
| Test Specimens 1 | Geometry | Standard | Results 2 | |
|---|---|---|---|---|
| Compressive strength (fc) | 3 | Ø100 × 200 mm | UNE-EN 12390-3:2020 [58] | 0.8: 18.98 MPa |
| 0.5: 40.50 MPa | ||||
| Absorption coefficient | 3 | Ø100 × 50 mm | UNE 83980:2014 [59] | 0.8: 8.38 % |
| 0.5: 7.49 % | ||||
| Porosity accessible to water | 3 | Ø100 × 50 mm | 0.8: 18.24 % | |
| 0.5: 16.42 % | ||||
| Penetration of water under pressure (Z) | 4 | Ø150 × 150 mm | UNE-EN 12390-8: 2020 [60] | 0.8: 103 mm |
| 0.5: 18 mm | ||||
| Gas permeability (Kgas) | 3 | Ø150 × 50 mm | UNE 83981:2008 [61] | 0.8: 433.15 × 10−18 m2 |
| 0.5: 50.37 × 10−18 m2 | ||||
| Non-steady-state migration coefficient (Dnssm) | 3 | Ø100 × 50 mm | UNE-EN 12390-18:2021 [62] | 0.8: 50.04 × 10−12 m2/s |
| 0.5: 25.35 × 10−12 m2/s | ||||
| Concrete resistivity (Ω) | 3 | 40 × 40 × 160 mm | UNE-EN 12390-19:2023 [63] | 0.8: 170.36 Ωm |
| 0.5: 195.69 Ωm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lliso-Ferrando, J.R.; Martínez-Ibernón, A.; Ramón-Zamora, J.E.; Gandía-Romero, J.M. Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 2: Implementation. Appl. Sci. 2024, 14, 9002. https://doi.org/10.3390/app14199002
Lliso-Ferrando JR, Martínez-Ibernón A, Ramón-Zamora JE, Gandía-Romero JM. Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 2: Implementation. Applied Sciences. 2024; 14(19):9002. https://doi.org/10.3390/app14199002
Chicago/Turabian StyleLliso-Ferrando, Josep Ramon, Ana Martínez-Ibernón, José Enrique Ramón-Zamora, and José Manuel Gandía-Romero. 2024. "Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 2: Implementation" Applied Sciences 14, no. 19: 9002. https://doi.org/10.3390/app14199002
APA StyleLliso-Ferrando, J. R., Martínez-Ibernón, A., Ramón-Zamora, J. E., & Gandía-Romero, J. M. (2024). Corrosion Assessment in Reinforced Concrete Structures by Means of Embedded Sensors and Multivariate Analysis—Part 2: Implementation. Applied Sciences, 14(19), 9002. https://doi.org/10.3390/app14199002







