Antimicrobial Action of Lactobacillus spp. Isolated from Yoghurt against Escherichia coli, Salmonella Enteritidis and Listeria monocytogenes: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yoghurt Samples
- Group I (n = 10)—products with a long shelf life (from 15 to 10 days until the end of the expiration date) (bio natural (n = 2), bio with forest fruits (n = 2), bio drinkable with strawberries (n = 2), probiotic (n = 2), natural Greek type (n = 2)), evaluation conducted immediately after purchase;
- Group II (n = 10)—products directly before the end of shelf life (1 day before the expiration date) (bio natural (n = 2), bio with forest fruits (n = 2), bio drinkable with strawberries (n = 2), probiotic (n = 2), natural Greek type (n = 2)).
2.2. Qualitative and Quantitative Evaluation of Lactic Acid Bacteria (LAB)
2.3. Species Identification
2.4. Evaluation of Antimicrobial Effects of Selected Lactobacillus spp. Strains Isolated from Yoghurts
- CN—changes in the number of bacteria [log CFU × cm−3];
- logA—logarithm of the initial number of bacteria in suspension;
- logB—logarithm of the number of bacteria after 72 h of incubation.
2.5. Statistical Analysis
3. Results
3.1. Identification and Quantitative Changes of Bacteria Present in Yoghurts
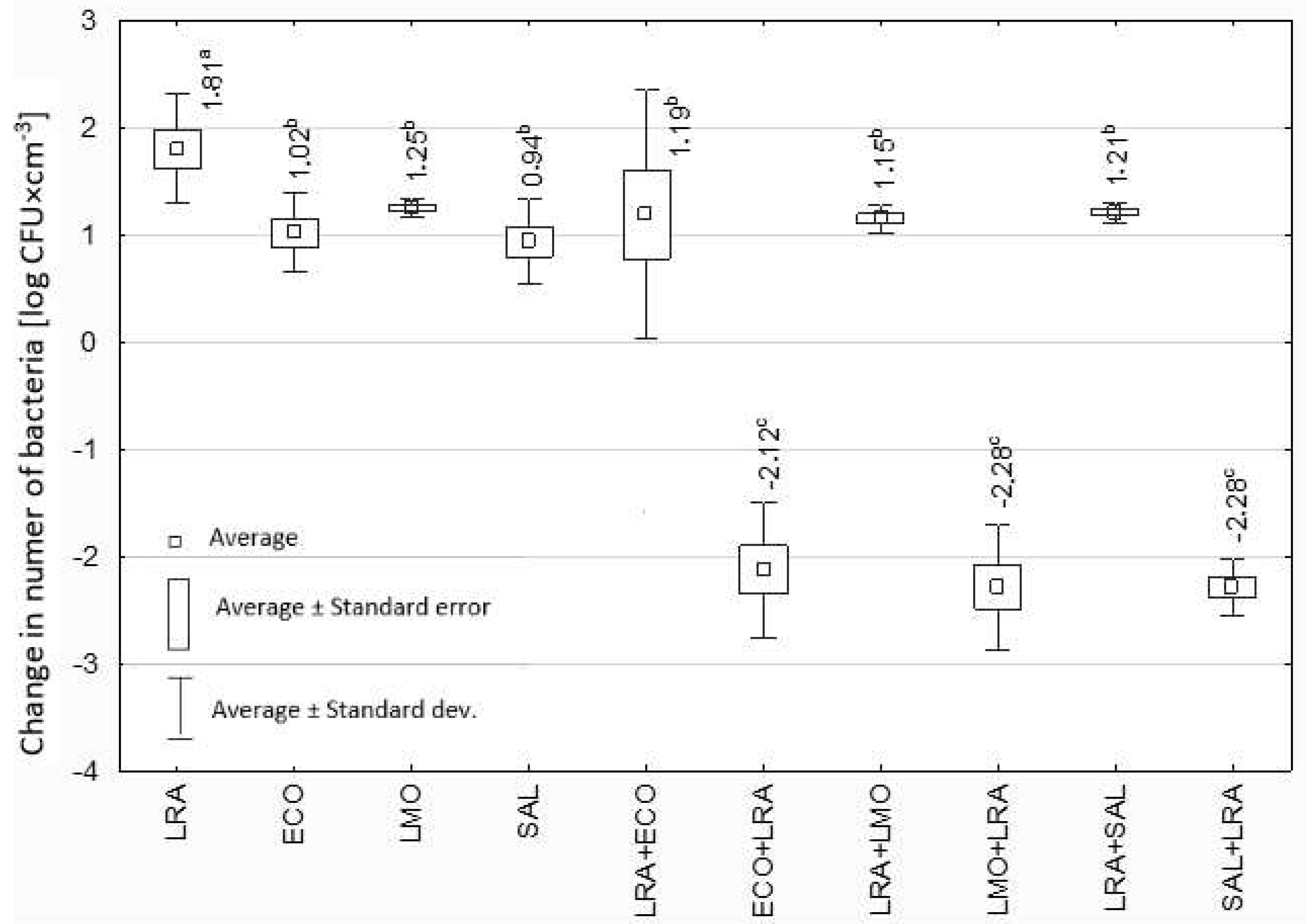
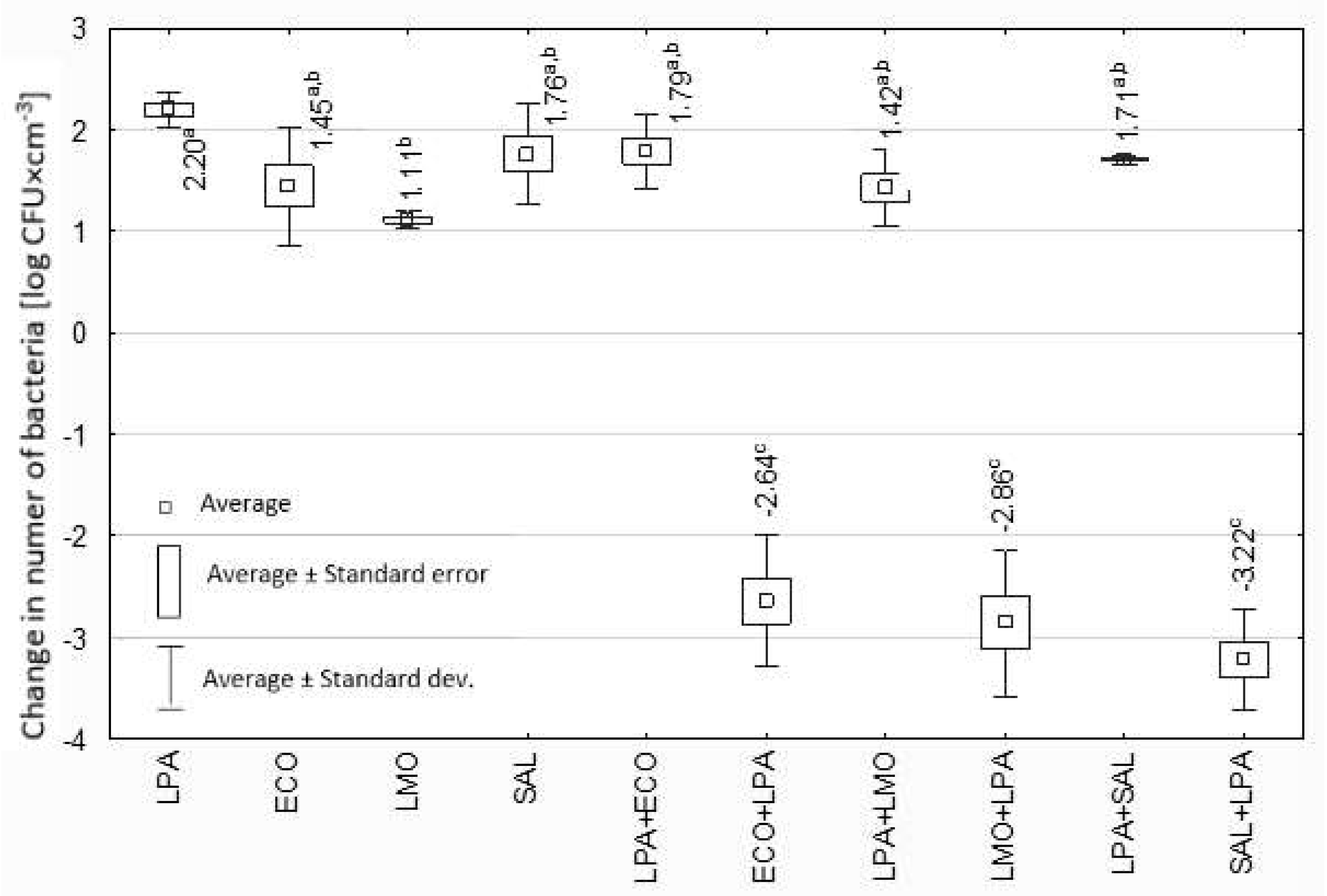
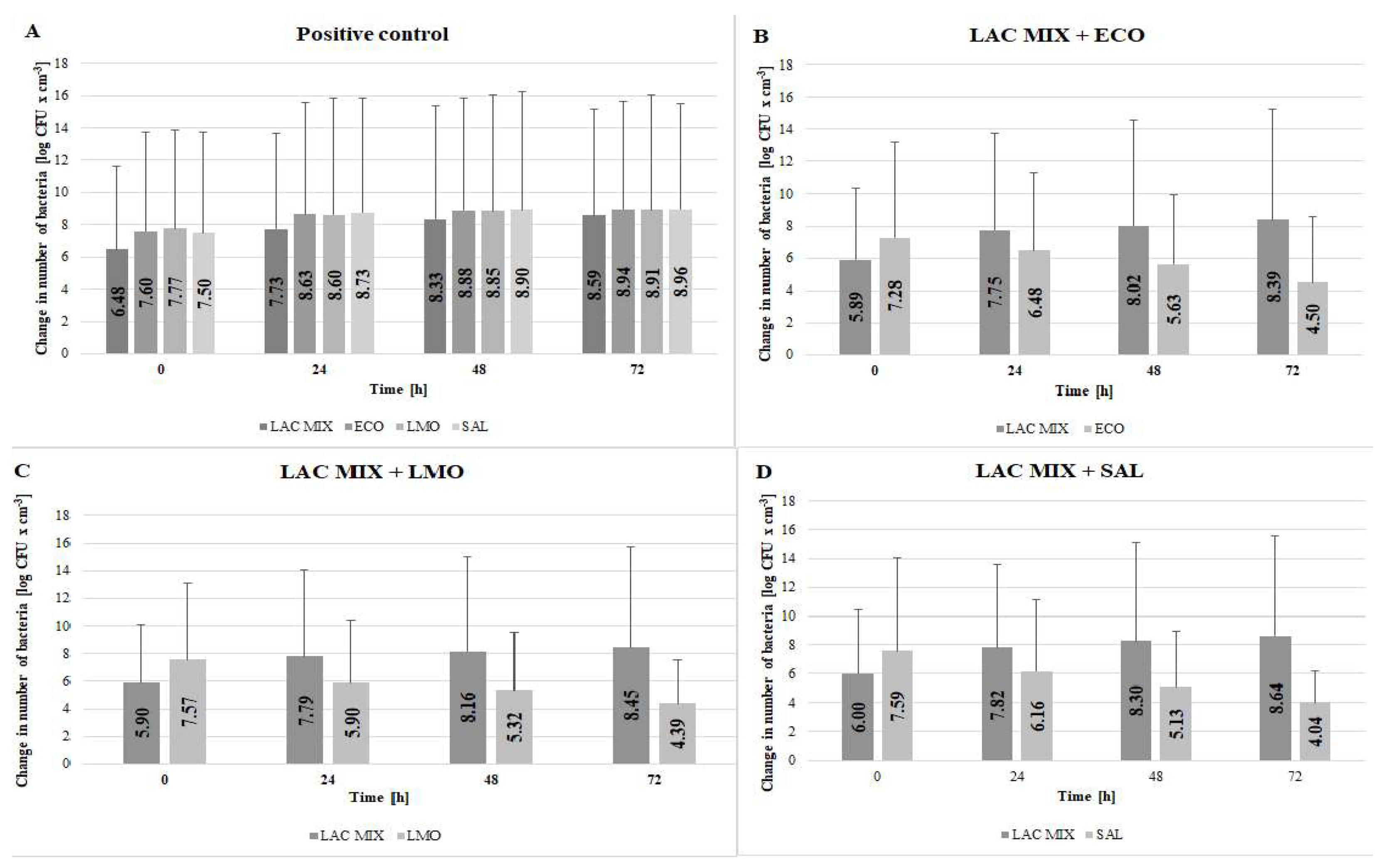
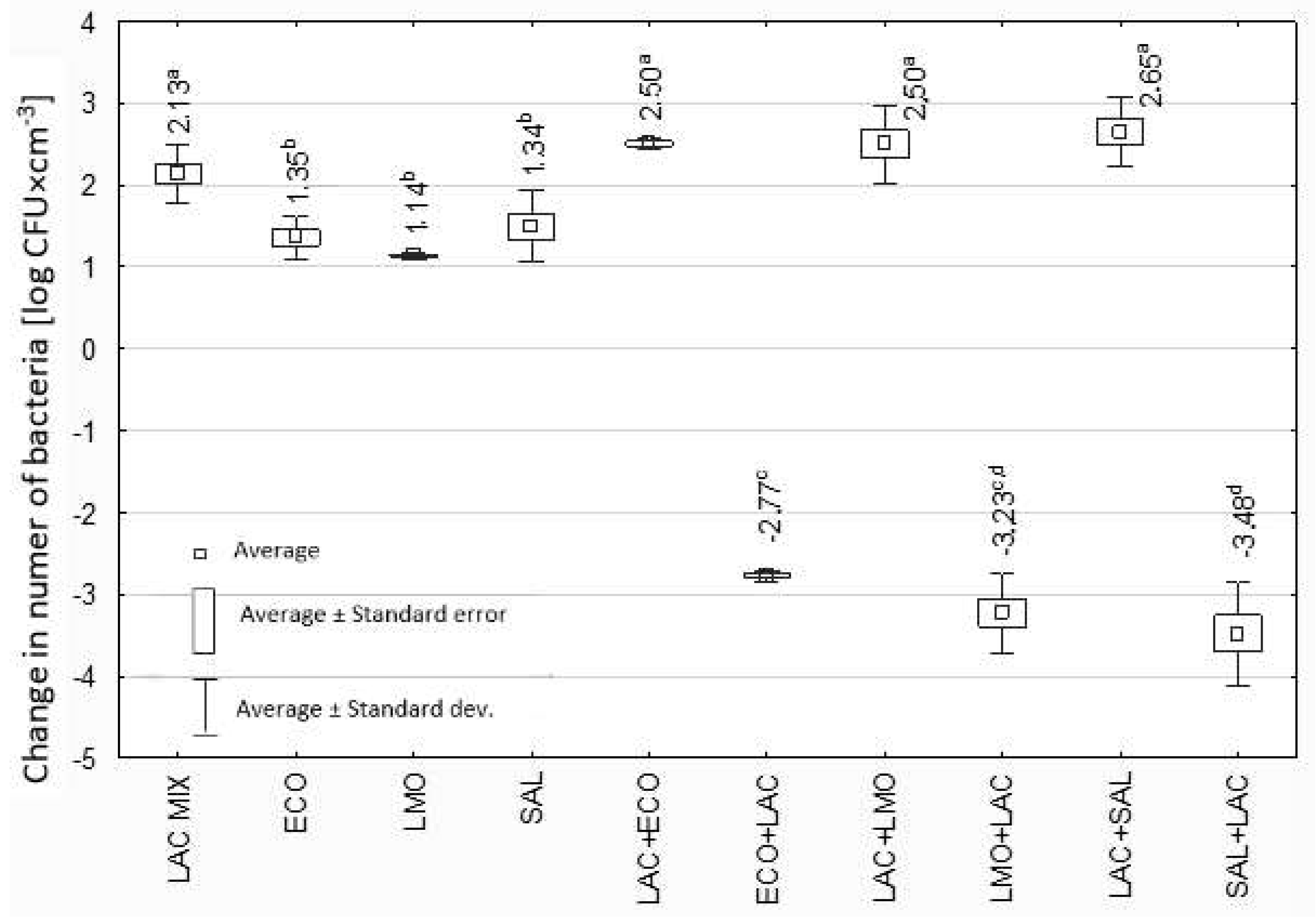
3.2. Effect of Lactobacillus spp. Isolated from Yoghurt on Selected Potentially Pathogenic Bacteria
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed; December 2020. Available online: http://www.dietaryguidelines.gov/resources/2020-2025-dietary-guidelines-online-materials (accessed on 10 July 2024).
- Fisberg, M.; Machado, R. History of yogurt and current patterns of consumption. Nutr. Rev. 2015, 73 (Suppl. S1), 4–7. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.C.; Gandhi, A.; Shah, N.P. Yogurt: Historical background, health benefits, and global trade. In Yogurt in Health and Disease Prevention; Academic Press: London, UK, 2017; pp. 3–29. [Google Scholar] [CrossRef]
- Ouweh, G.J.; Vest, A.C. Anti-microbial components from lactic acid bacteria. In Lactic Acid Bacteria: Microbiology and Functional Aspects, 2nd ed.; Salminen, S., Von, A., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; pp. 139–160. [Google Scholar]
- Molaee Parvarei, M.; Fazeli, M.R.; Mortazavian, A.M.; Sarem Nezhad, S.; Mortazavi, S.A.; Golabchifar, A.A.; Khorshidian, N. Comparative effects of probiotic and paraprobiotic addition on microbiological, biochemical and physical properties of yogurt. Food Res. Int. 2021, 140, 110030. [Google Scholar] [CrossRef]
- Kapka-Skrzypczak, L.; Niedźwiecka, J.; Wojtyła, A.; Kruszewski, M. Probiotyki i prebiotyki jako aktywny składnik żywności funkcjonalnej. Pediatr. Endocrinol. Diabetes Metab. 2012, 18, 79–83. [Google Scholar] [PubMed]
- Presti, I.; D’Orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. App. Microbiol. Biotech. 2015, 99, 613–5626. [Google Scholar] [CrossRef]
- Abdi-Moghadam, Z.; Darroudi, M.; Mahmoudzadeh, M.; Mohtashami, M.; Jamal, A.M.; Shamloo, E.; Rezaei, Z. Functional yogurt, enriched and probiotic: A focus on human health. Clin. Nutr. ESPEN 2023, 57, 575–586. [Google Scholar] [CrossRef]
- Cifelli, C.J.; Agarwal, S.; Fulgoni, V.L. Association of Yogurt Consumption with Nutrient Intakes, Nutrient Adequacy, and Diet Quality in American Children and Adults. Nutrients 2020, 12, 3435. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jain, N.; Holschuh, N.; Smith, J. Associations between frequency of yogurt consumption and nutrient intake and diet quality in the United Kingdom. J. Nutr. Sci. 2021, 10, e85. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, Y.; Bolling, B.W. Yogurt consumption for improving immune health. Curr. Opin. Food Sci. 2023, 51, 101017. [Google Scholar] [CrossRef]
- Wyka, J.; Tajner-Czopek, A.; Rytel, E.; Habanova, M.; Malczyk, E.; Misiarz, M. Żywność fermentowana—Znaczenie dla zdrowia człowieka. Acta Sci. Pol. Biotechnol. 2017, 16, 101–106. [Google Scholar]
- Świderski, F.; Waszkiewicz-Robak, B. Towaroznawstwo Żywności Przetworzonej z Elementami Technologii; Wydawnictwo SGGW: Warszawa, Poland, 2010. [Google Scholar]
- Nami, Y.; Tavallaei, O.; Kiani, A.; Moazami, N.; Samari, M.; Derakhshankhah, H.; Jaymand, M.; Haghshenas, B. Anti-oral cancer properties of potential probiotic lactobacilli isolated from traditional milk, cheese, and yogurt. Sci. Rep. 2024, 14, 6398. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 15 September 2024).
- Yang, H.; Hewes, D.; Salaheen, S.; Federman, C.; Biswas, D. Effects of blackberry juice on growth inhibition of foodborne pathogens and growth promotion of Lactobacillus. Food Control 2014, 37, 15–20. [Google Scholar] [CrossRef]
- Alvarez-Sieiro, P.; Montalbán-López, M.; Mu, D.; Kuipers, O.P. Bacteriocins of lactic acid bacteria: Extending the family. Appl. Microbiol. Biotechnol. 2016, 100, 2939–2951. [Google Scholar] [CrossRef]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control): The European Union One Health 2021 Zoonoses Report. EFSA J. 2022, 20, 7666. [CrossRef]
- World Health Organization (WHO). Listeriosis. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/listeriosis (accessed on 15 September 2024).
- Wiktorczyk-Kapischke, N.; Skowron, K.; Wałecka-Zacharska, E. Genomic and pathogenicity islands of Listeria monocytogenes-overview of selected aspects. Front. Mol. Biosci. 2023, 10, 1161486. [Google Scholar] [CrossRef]
- Khaneghah, A.M.; Abhari, K.; Eş, I.; Soares, M.B.; Oliveira, R.B.A.; Hasseini, H.; Rezai, M.; Balthazar, C.F.; Silva, R.; Cruz, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Schulthess, B.; Brodner, K.; Bloemberg, G.V.; Zbinden, R.; Böttger, E.C.; Hombach, M. Identification of Gram-positive cocci by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry: Comparison of different preparation methods and implementation of a practical algorithm for routine diagnostics. J. Clin. Microbiol. 2013, 51, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Sady, M.; Domagała, J.; Grega, T.; Kalicka, D. Wpływ czasu przechowywania na mikroflorę jogurtów z dodatkiem nasion amarantusa i ziaren owsa. Żywność Nuka Technol. Jakość 2007, 6, 242–250. [Google Scholar]
- Nikmaram, P.; Mousavi, S.M.; Kiani, H.; Emamdjomeh, Z.; Razavi, S.H.; Mousavi, Z. Modeling the effect of inulin, pH and storage time on the viability of selected Lactobacillus in a probiotic fruity yogurt drink using the Monte Carlo simulation. J. Food Qual. 2016, 39, 362–369. [Google Scholar] [CrossRef]
- Ng, E.W.; Yeung, M.; Tong, P.S. Effects of starter cultures on the survival of Lactobacillus acidophilus. Int. J. Food Microbiol. 2011, 45, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Nikmaram, P.; Mousavi, S.M.; Emam-Djomeh, Z.; Kiani, H.; Razavi, S.H. Evaluation and prediction of metabolite production, antioxidant activities, and survival of Lactobacillus casei 431 in a pomegranate juice supplemented yogurt drink using support vector regression. Food Sci. Biotechnol. 2015, 24, 2105–2112. [Google Scholar] [CrossRef]
- Xu, X.; Cui, H.; Xu, J.; Yuan, Z.; Li, J.; Yang, L.; Wang, S.; Liu, H.; Zhu, D. Effects of cold storage time on the quality and active probiotics of yogurt fermented by Bifidobacterium lactis and commercial bacteria Danisco. J. Food Sci. 2023, 88, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic viability and storage stability of yogurts and fermented milks prepared with several mixtures of lactic acid bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Beal, C.; Skokanova, J.; Latrille, E.; Martin, N.; Corrieu, G. Combined effects of culture conditions and storage time on acidification and viscosity of stirred yogurt. J. Dairy Sci. 1999, 82, 673–681. [Google Scholar] [CrossRef]
- Skryplonek, K.; Jasińska, M. Jakość fermentowanych napojów probiotycznych otrzymanych z mrożonej serwatki kwasowej i mleka w czasie chłodniczego przechowywania. Żywność Nauka Technol. Jakość 2016, 1, 32–44. [Google Scholar]
- Li, C.; Song, J.; Kwok, L.; Wang, J.; Dong, Y.; Yu, H.; Hou, Q.; Zhang, H.; Chen, Y. Influence of Lactobacillus plantarum on yogurt fermentation properties and subsequent changes during postfermentation storage. Am. Dairy Sci. Assoc. 2017, 1, 2512–2525. [Google Scholar] [CrossRef]
- Kaptan, B.; Kayisoglu, S. Influence of forest fruit purees (strawberry or blackberry) on the biochemical, microbiological, sensory properties, and consumer preferences of standard and probiotic cultured yogurts. J. Food Saf. Food Qual. 2024, 75, 77–85. [Google Scholar] [CrossRef]
- Turgut, T.; Cakmakci, S. Probiotic Strawberry Yogurts: Microbiological, Chemical and Sensory Properties. Probiotics Antimicrob. Prot. 2018, 10, 64–70. [Google Scholar] [CrossRef]
- Schillinger, U. Isolation and identification of lactobacilli from novel-type probiotic and mild yoghurts and their stability during refrigerated storage. Int. J. Food Microbiol. 1999, 47, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Pyar, H.; Peh, K.K. Characterization and identification of Lactobacillus acidophilus using biolog rapid identification system. Int. J. Pharm. Pharm. Sci. 2014, 6, 189–193. [Google Scholar]
- Yolmeh, M.; Khomeiri, M.; Ahmadi, Z. Application of mixture design to introduce an optimum cell-free supernatant of multi-strain mixture (MSM) for Lactobacillus against food-borne pathogens. LWT 2017, 83, 298–304. [Google Scholar] [CrossRef]
- Kamal, R.M.; Alnakap, M.E.; Abal El Aal, S.F.; Bayoumi, M.A. Bio-controlling capability of probiotic strain Lactobacillus rhamnosus against some common foodborne pathogens in yoghurt. Int. Dairy J. 2018, 85, 1–7. [Google Scholar] [CrossRef]
- Ołdak, A.; Zielińska, D.; Rzepkowska, A.; Kołożyn-Krajewska, D. Comparison of Antibacterial Activity of Lactobacillus plantarum Strains Isolated from Two Different Kinds of Regional Cheeses from Poland: Oscypek and Korycinski Cheese. BioMed Res. Int. 2017, 2017, 6820369. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D. Characterization of Lactobacillus isolated from dairy samples for probiotic properties. Clin. Microbiol. 2015, 33, 117–123. [Google Scholar] [CrossRef]
- Iglesias, M.N.; Viñas, I.; Colás-Medà, P.; Collazo, C.; Serrano, J.C.E.; Abadias, M. Adhesion and invasion of Listeria monocytogenes and interaction with Lactobacillus rhamnosus GG after habituation on fresh-cut pear. J. Func. Foods 2017, 34, 453–460. [Google Scholar] [CrossRef]
- Prezzi, L.E.; Lee, S.H.I.; Nunes, V.M.R.; Corassin, C.H.; Pimentel, T.C.; Rocha, R.S.; Ramos, G.L.P.A.; Guimarães, J.T.; Balthazar, C.F.; Durate, M.C.K.H.; et al. Effect of Lactobacillus rhamnosus on growth of Listeria monocytogenes and Staphylococcus aureus in a probiotic Minas Frescal cheese. Food Microbiol. 2020, 92, 103557. [Google Scholar] [CrossRef]
- Echresh, S.; Behbahani, B.A.; Falah, F.; Noshad, M.; Ibrahim, S.A. Assessment of the probiotic, anti-bacterial, and anti-biofilm characteristics of Lacticaseibacillus rhamnosus CWKu-12, along with its potential impact on the expression of virulence genes in Listeria monocytogenes ATCC 19115. LWT. 2024, 203, 116391. [Google Scholar] [CrossRef]
- Gutiérrez, S.; Martínez-Blanco, H.; Rodríguez-Aparicio, L.B.; Ferrero, M.A. Effect of fermented broth from lactic acid bacteria on pathogenic bacteria proliferation. J. Dairy Sci. 2016, 9, 2654–2665. [Google Scholar] [CrossRef] [PubMed]
- Aryantini, N.P.; Yamasaki, E.; Kurozono, H.; Sujaya, N.; Urshima, T.; Fukuda, K. In vitro safety assessments and antimicrobial activities of Lactobacillus rhamnosus strains isolated from a fermented mare’s milk. Anim. Sci. J. 2017, 88, 517–525. [Google Scholar] [CrossRef]
- Shi, S.; Gong, L.; Yu, H.; He, G.; Zhang, J.; Han, Y.; Liu, Y.; Hu, J.; Dong, J.; Liu, J.; et al. Antagonistic activity and mechanism of Lactobacillus rhamnosus SQ511 against Salmonella enteritidis. 3 Biotech 2022, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Muyyarikkandy, M.S.; Amalaradjou, M.A. Lactobacillus bulgaricus, Lactobacillus rhamnosus and Lactobacillus paracasei Attenuate Salmonella Enteritidis, Salmonella Heidelberg and Salmonella Typhimurium Colonization and Virulence Gene Expression In Vitro. Int. J. Mol. Sci. 2017, 18, 2381. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Du, M.; Yi, H.; Guo, C.; Tuo, Y.; Han, X.; Li, J.; Zhang, L.; Yang, L. Antimicrobial activity against Shigella sonnei and probiotic properties of wild Lactobacillus from fermented food. Microbiol. Res. 2011, 167, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Haghshenas, B.; Kiani, A.; Mansoori, S.; Mohammadi-Noori, E.; Nami, Y. Probiotic properties and antimicrobial evaluation of silymarin-enriched Lactobacillus bacteria isolated from traditional curd. Sci. Rep. 2023, 13, 10916. [Google Scholar] [CrossRef] [PubMed]
- Mohd-Zubri, N.S.; Ramasamy, K.; Abdul-Rahman, N.Z. Characterization and potential oral probiotic properties of Lactobacillus plantarum FT 12 and Lactobacillus brevis FT 6 isolated from Malaysian fermented food. Arch. Oral Biol. 2022, 143, 105515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Singh, B.P.; Thakur, N.; Gulati, S.; Gupta, S.; Mishra, S.K.; Panwar, H. Antibacterial effects of Lactobacillus isolates of curd and human milk origin against food-borne and human pathogens. 3 Biotech 2017, 7, 31. [Google Scholar] [CrossRef]
- Çakmakoğlu, S.K.; Dere, S.; Bekiroğlu, H.; Bozkurt, F.; Karasu, S.; Dertli, E.; Türker, M.; Sagdic, O. Production of bioactive peptides during yogurt fermentation, their extraction and functional characterization. Food Biosci. 2024, 61, 104805. [Google Scholar] [CrossRef]



| Type of Yoghurt | Bio Natural (n = 2) | Bio with Forest Fruits (n = 2) | Bio Drinkable with Strawberries (n = 2) | Probiotic (n = 2) | Natural Greek Type (n = 2) | |
|---|---|---|---|---|---|---|
| Bacterial species declared by the manufacturer: “yoghurt bacteria cultures” and the listed species | L. acidophilus, B. lactis | L. acidophilus, B. lactis | L. acidophilus, B. lactis | L. casei, L. bulgaricus, L. acidophilus, S. thermophilus, Bifidobacterium spp. | S. thermophilus, L. delbrueckii, subsp. bulgaricus, L. acidophilus, B. lactis | |
| Long shelf life (n = 10) | Bacterial species isolated from the product | L. rhamnosus | L. rhamnosus | L. rhamnosus | L. paracasei | L. rhamnosus |
| Average number of bacteria [log CFU/g] | 8.33 (6.33) | 8.62 (6.71) | 8.66 (7.05) | 8.27 (6.87) | 8.46 (6.15) | |
| Directly before the end of shelf life (n = 10) | Bacterial species isolated from the product | L. rhamnosus | L. rhamnosus | L. rhamnosus | L. paracasei | L. rhamnosus |
| Average number of bacteria [log CFU/g] | 8.04 (7.10) | 9.06 (7.87) | 9.11 (6.55) | 8.09 (6.50) | 8.63 (7.00) | |
| Changes in the number of bacteria in stored yoghurt [log CFU/g] | −0.29 | 0.45 | 0.45 | −0.18 | 0.17 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiktorczyk-Kapischke, N.; Grudlewska-Buda, K.; Budzyńska, A.; Chomacka-Gollus, K.; Gospodarek-Komkowska, E.; Skowron, K. Antimicrobial Action of Lactobacillus spp. Isolated from Yoghurt against Escherichia coli, Salmonella Enteritidis and Listeria monocytogenes: A Pilot Study. Appl. Sci. 2024, 14, 9010. https://doi.org/10.3390/app14199010
Wiktorczyk-Kapischke N, Grudlewska-Buda K, Budzyńska A, Chomacka-Gollus K, Gospodarek-Komkowska E, Skowron K. Antimicrobial Action of Lactobacillus spp. Isolated from Yoghurt against Escherichia coli, Salmonella Enteritidis and Listeria monocytogenes: A Pilot Study. Applied Sciences. 2024; 14(19):9010. https://doi.org/10.3390/app14199010
Chicago/Turabian StyleWiktorczyk-Kapischke, Natalia, Katarzyna Grudlewska-Buda, Anna Budzyńska, Karolina Chomacka-Gollus, Eugenia Gospodarek-Komkowska, and Krzysztof Skowron. 2024. "Antimicrobial Action of Lactobacillus spp. Isolated from Yoghurt against Escherichia coli, Salmonella Enteritidis and Listeria monocytogenes: A Pilot Study" Applied Sciences 14, no. 19: 9010. https://doi.org/10.3390/app14199010





