Anti-Hyperlipidemic Effect of Ruta chalepensis Ethanolic Extract in Triton WR-1339-Induced Hyperlipidemia in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Plant Material and Preparation of Extract
2.2. LC-MS Analysis
2.3. Animal Experiment
2.4. Induction of Hyperlipidemia Using the Triton WR-1339 Model
2.5. Pharmacological Study Design
2.6. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Hyperlipidemia Induction Using Triton WR-1339
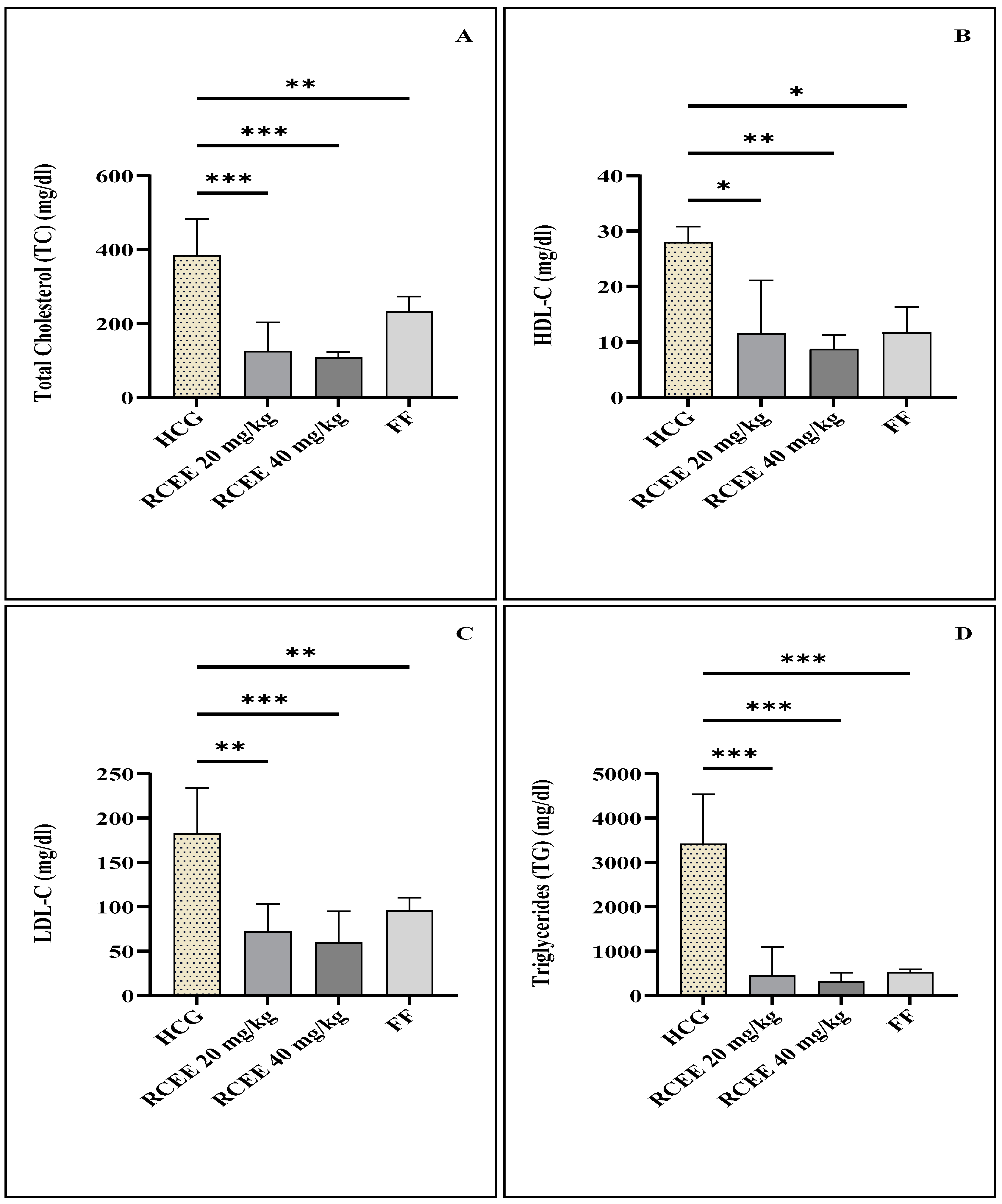
3.3. Effect of R. chalepensis Ethanolic Extract and Fenofibrate on Plasma Lipid Profile
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khalaf, R.A.; NasrAllah, A.; Jarrar, W.; Sabbah, D.A. Cholesteryl ester transfer protein inhibitory oxoacetam-ido-benzamide derivatives: Glide docking, pharmacophore mapping, and synthesis. Braz. J. Pharm. Sci. 2022, 58, e20028. [Google Scholar] [CrossRef]
- Mahemuti, N.; Jing, X.; Zhang, N.; Liu, C.; Li, C.; Cui, Z.; Liu, Y.; Chen, J. Association between Systemic Immunity-Inflammation Index and Hyperlipidemia: A Population-Based Study from the NHANES (2015–2020). Nutrients 2023, 15, 1177. [Google Scholar] [CrossRef] [PubMed]
- Ruscica, M.; Ferri, N.; Banach, M.; Sirtori, C.R.; Corsini, A. Side effects of statins: From pathophysiology and epidemiology to diagnostic and therapeutic implications. Cardiovasc. Res. 2022, 118, 3288–3304. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Grande, F.; Occhiuzzi, M.A.; Sicari, V.; Loizzo, M.R.; Cappello, A.R. Lavandula angustifolia mill. (Lamiaceae) ethanol extract and its main constituents as promising agents for the treatment of metabolic disorders: Chemical profile, in vitro biological studies, and molecular docking. J. Enzym. Inhib. Med. Chem. 2023, 38, 2269481. [Google Scholar] [CrossRef] [PubMed]
- El-Tantawy, W.H.; Temraz, A. Natural products for controlling hyperlipidemia. Arch. Physiol. Biochem. 2019, 125, 128–135. [Google Scholar] [CrossRef]
- Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from citrus Tacle® extract endowed with HMGCR inhibitory activity: An antihypercholesterolemia natural remedy. Molecules 2021, 26, 5718. [Google Scholar] [CrossRef]
- Zhang, Y.; Lin, Z.; Wang, L.; Guo, X.; Hao, Z.; Li, Z.; Johnston, L.J.; Dong, B. Cooperative interaction of phenolic acids and flavonoids contained in activated charcoal with herb extracts, involving cholesterol, bile acid, and Fxr/Pxr activation in broilers fed with mycotoxin-containing diets. Antioxidants 2022, 11, 2200. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Tian, M.; Dong, Z.; Wang, C.; Guo, G.; Huang, Q.; Wang, J. In Vivo and Network Pharmacological Studies of Natural Bear Bile Powder against Hyperlipidemia. ChemistrySelect 2023, 8, e202300435. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A natural source of high value products with biological and pharmacological properties. J. Ethnopharmacol. 2020, 260, 113076. [Google Scholar] [CrossRef]
- Szewczyk, A.; Marino, A.; Molinari, J.; Ekiert, H.; Miceli, N. Phytochemical characterization, and antioxidant and antimicrobial properties of agitated cultures of three rue species: Ruta chalepensis, Ruta corsica, and Ruta graveolens. Antioxidants 2022, 11, 592. [Google Scholar] [CrossRef]
- Althaher, A.R.; Oran, S.A.; Awadallah, M.W.; Ameen, H.H.; Shehabi, R.F.; Bourghli, L.M.; Mastinu, A. Chemical Composition, Antioxidant, and Antibacterial Activity of Ruta chalepensis L. Ethanolic Extract. Chem. Biodivers. 2024, 21, e202400026. [Google Scholar] [CrossRef] [PubMed]
- Elizondo-Luévano, J.H.; Rodríguez-Garza, N.E.; Bazaldúa-Rodríguez, A.F.; Romo-Sáenz, C.I.; Tamez-Guerra, P.; Verde-Star, M.J.; Gomez-Flores, R.; Quintanilla-Licea, R. Cytotoxic, Anti-Hemolytic, and Antioxidant Activities of Ruta chalepensis L. (Rutaceae) Extract, Fractions, and Isolated Compounds. Plants 2023, 12, 2203. [Google Scholar] [CrossRef] [PubMed]
- Al-Qirim, T.; Al Bawab, A.Q.; Al-Hiari, Y.; Ahmad, N.H.; Alwahsh, M.; Al-Kouz, S.; Shattat, G. Synthesis and lipid-lowering properties of novel N-(4-benzoylphenyl) pyrrole-2-carboxamide derivatives in Triton WR-1339-induced hyperlipidemic rats. Indonesia. J. Appl. Pharm. Sci. 2024, 14, 64–70. [Google Scholar] [CrossRef]
- Kelle, B.P.; Ćesić, A.K.; Čustović, S.; Ćosović, E.; Lagumdžija, D.; Jordamović, N.; Kusturica, J. Improvement of a diet-induced model of hyperlipidemia in Wistar rats: Assessment of biochemical parameters, the thickness of the abdominal aorta and liver histology. J. King Saud Univ. Sci. 2024, 36, 103068. [Google Scholar] [CrossRef]
- Hussein, B.; Alwahsh, M.; Al-Hiari, Y.; Bourghli, L.; Al-Jammal, B.; Al-Qirim, T.; Al Bujuq, N.; Abu-zaid, R.; Saqallah, F.G.; Hamadneh, L. Substituted furan-carboxamide and Schiff base derivatives as potential hypolipidemic compounds: Evaluation in Triton WR-1339 hyperlipidemic rat model. Med. Chem. Res. 2024, 33, 1643–1656. [Google Scholar] [CrossRef]
- Bouhlali ED, T.; Hmidani, A.; Bourkhis, B.; Moussafir, Z.; Filali-Zegzouti, Y.; Alem, C. Date (Phoenix dactylifera L.) Fruits as a Potential Lipid-Lowering Therapy: Effect on High-Fat Diet and Triton-WR-1339-Induced Hyperlipidemic Rats. Drugs Drug Candidates 2023, 2, 422–432. [Google Scholar] [CrossRef]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Najem, M.; Bammou, M.; Bachiri, L.; Bouiamrine, E.H.; Ibijbijen, J.; Nassiri, L. Ruta chalepensis L. Essential Oil Has a Biological Potential for a Natural Fight against the Pest of Stored Foodstuffs: Tribolium castaneum Herbst. Evid.-Based Complement. Altern. Med. eCAM 2020, 2020, 5739786. [Google Scholar] [CrossRef]
- Al-Majmaie, S.; Nahar, L.; Rahman, M.M.; Nath, S.; Saha, P.; Talukdar, A.D.; Sharples, G.P.; Sarker, S.D. Anti-MRSA constituents from Ruta chalepensis (rutaceae) grown in Iraq, and in silico studies on two of most active compounds, chalepensin and 6-hydroxy-rutin 3′,7-dimethyl ether. Molecules 2021, 26, 1114. [Google Scholar] [CrossRef]
- Kacem, M.; Kacem, I.; Simon, G.; Mansour, A.B.; Chaabouni, S.; Elfeki, A.; Bouaziz, M. Phytochemicals and biological activities of Ruta chalepensis L. growing in Tunisia. Food Biosci. 2015, 12, 73–83. [Google Scholar] [CrossRef]
- Zouidi, F. Ruta Chalepensis Leaf Extract’s Protects Metabolic Indices Abnormalities, Type 2 Diabetes, and Various Organs Toxicities in Obese Rat. Chelonian Res. Found. 2024, 19, 675–685. [Google Scholar]
- Saidi, A.; Hambaba, L.; Kucuk, B.; Cacan, E.; Erenler, R. Phenolic profile, acute toxicity, and hepatoprotective and antiproliferative activities of Algerian Ruta tuberculata Forssk. Curr. Bioact. Compd. 2022, 18, 72–83. [Google Scholar] [CrossRef]
- Hamdiken, M.; Bouhalit, S.; Kechrid, Z. Effect of Ruta chalepensis on zinc, lipid profile and antioxidant levels in the blood and tissue of streptozotocin-induced diabetes in rats fed zinc-deficient diets. Can. J. Diabetes 2018, 42, 356–364. [Google Scholar] [CrossRef]
- Tahri-Joutey, M.; Andreoletti, P.; Surapureddi, S.; Nasser, B.; Cherkaoui-Malki, M.; Latruffe, N. Mechanisms mediating the regulation of peroxisomal fatty acid beta-oxidation by PPARα. Int. J. Mol. Sci. 2021, 22, 8969. [Google Scholar] [CrossRef] [PubMed]
- Danielewski, M.; Rapak, A.; Kruszyńska, A.; Małodobra-Mazur, M.; Oleszkiewicz, P.; Dzimira, S.; Kucharska, A.Z.; Słupski, W.; Matuszewska, A.; Nowak, B.; et al. Cornelian cherry (Cornus mas L.) fruit extract lowers SREBP-1c and C/EBPα in liver and alters various PPAR-α, PPAR-γ, LXR-α target genes in cholesterol-rich diet rabbit model. Int. J. Mol. Sci. 2024, 25, 1199. [Google Scholar] [CrossRef]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
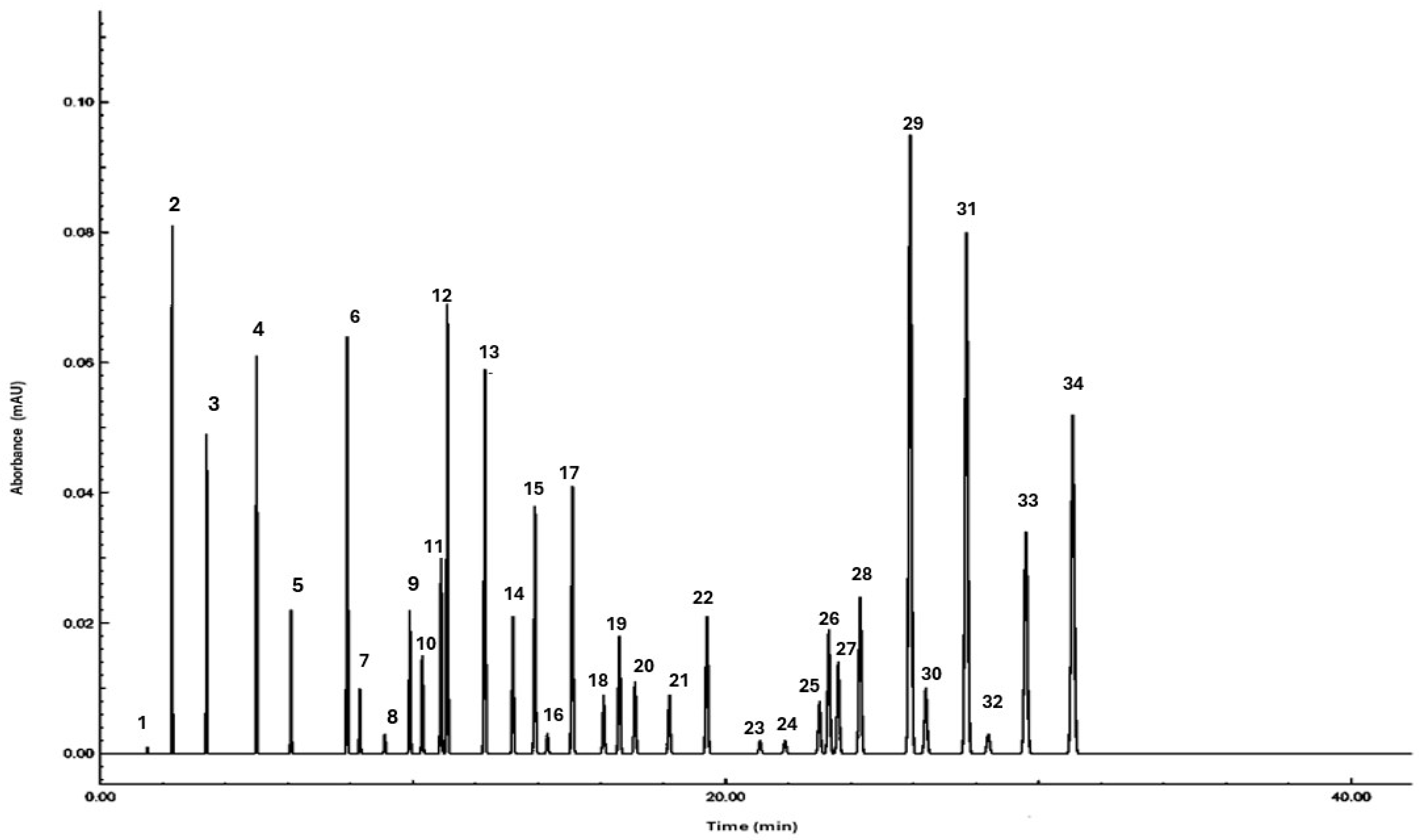
| No. | Compound | Retention Time (min) | Percentage (%) |
|---|---|---|---|
| 1 | Phellandrene | 1.5 | 0.1 |
| 2 | 2,2-Dimethyl-3-methylidenebicyclo [2.2.1] heptane | 2.3 | 8.1 |
| 3 | D-Limonene | 3.4 | 4.9 |
| 4 | Alpha-Pinene | 5 | 6.1 |
| 5 | Methyl heptyl ketone | 6.1 | 2.2 |
| 6 | 2-Bornanone | 7.9 | 6.4 |
| 7 | Piperitone | 8.3 | 1 |
| 8 | P-Menth-4(8)-en-3-one | 9.1 | 0.3 |
| 9 | Eucalyptol | 9.9 | 2.2 |
| 10 | 2-Bornanol | 10.3 | 1.5 |
| 11 | 2-Isoborneol | 10.9 | 3 |
| 12 | 3,7-Dimethylocta-1,6-dien-3-ol | 11.1 | 6.9 |
| 13 | γ-Terpinene | 12.3 | 5.9 |
| 14 | Methyl nonyl ketone | 13.2 | 2.1 |
| 15 | 4-Hydroxycinnamic acid | 13.9 | 3.8 |
| 16 | 4-Hydroxy-3-methoxybenzoic acid | 14.3 | 0.3 |
| 17 | 3,4,5-Trihydroxybenzoic acid | 15.1 | 4.1 |
| 18 | Methyl undecyl ketone | 16.1 | 0.9 |
| 19 | 3,4-Dihydroxycinnamic acid | 16.6 | 1.8 |
| 20 | Methyl decyl ketone | 17.1 | 1.1 |
| 21 | 4-Hydroxy-3-methoxycinnamic acid | 18.2 | 0.9 |
| 22 | Linalool acetate, 3,7-Dimethylocta-1,6-dien-3-yl acetate | 19.4 | 2.1 |
| 23 | Alpha-Humulene | 21.1 | 0.2 |
| 24 | Unknown | 21.9 | 0.2 |
| 25 | (−)-β-Eudesmol | 23 | 0.8 |
| 26 | Elemane, Elemol alcohol | 23.3 | 1.9 |
| 27 | Viridifloral | 23.6 | 1.4 |
| 28 | Octadecanoic acid | 24.3 | 2.4 |
| 29 | Quercetin | 25.9 | 9.5 |
| 30 | 3‘-Methylquercetin | 26.4 | 1 |
| 31 | Myricetol | 27.7 | 8 |
| 32 | 5-Caffeoylquinic acid | 28.4 | 0.3 |
| 33 | Unknown | 29.6 | 3.4 |
| 34 | Rutin | 31.1 | 5.2 |
| Total identified compounds | |||
| 96.4% | |||
| Component | Percentage (%) |
|---|---|
| Fatty acid | 2.4% |
| Flavonoid | 23.7% |
| Phenol | 11.2% |
| Ketone | 6.3% |
| Monoterpene | 48.5% |
| Sesquiterpene | 4.3% |
| (Mean ± SD) | ||
|---|---|---|
| Lipid Parameter | NCG (Control) | HCG (Triton) |
| Total Cholesterol (TC) (mg/dL) | 87.6 ± 13.3 | 385.25 ± 96.8 ** |
| High-Density Lipoprotein (HDL-C) (mg/dL) | 37.2 ± 7.0 | 28 ± 2.8 * |
| Low-Density Lipoprotein (LDL-C) (mg/dL) | 9.0 ± 1.6 | 183 ± 51.3 ** |
| Triglycerides (TGs) (mg/dL) | 65.2 ± 9.4 | 3421 ± 1114.2 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althaher, A.R.; Alwahsh, M.; Hasan, A.; Al-Majali, D.; Awadallah, M.W.; Al-Qirim, T. Anti-Hyperlipidemic Effect of Ruta chalepensis Ethanolic Extract in Triton WR-1339-Induced Hyperlipidemia in Rats. Appl. Sci. 2024, 14, 9017. https://doi.org/10.3390/app14199017
Althaher AR, Alwahsh M, Hasan A, Al-Majali D, Awadallah MW, Al-Qirim T. Anti-Hyperlipidemic Effect of Ruta chalepensis Ethanolic Extract in Triton WR-1339-Induced Hyperlipidemia in Rats. Applied Sciences. 2024; 14(19):9017. https://doi.org/10.3390/app14199017
Chicago/Turabian StyleAlthaher, Arwa R., Mohammad Alwahsh, Aya Hasan, Dima Al-Majali, Mirna W. Awadallah, and Tariq Al-Qirim. 2024. "Anti-Hyperlipidemic Effect of Ruta chalepensis Ethanolic Extract in Triton WR-1339-Induced Hyperlipidemia in Rats" Applied Sciences 14, no. 19: 9017. https://doi.org/10.3390/app14199017





