Nitrite: From Application to Detection and Development
Abstract
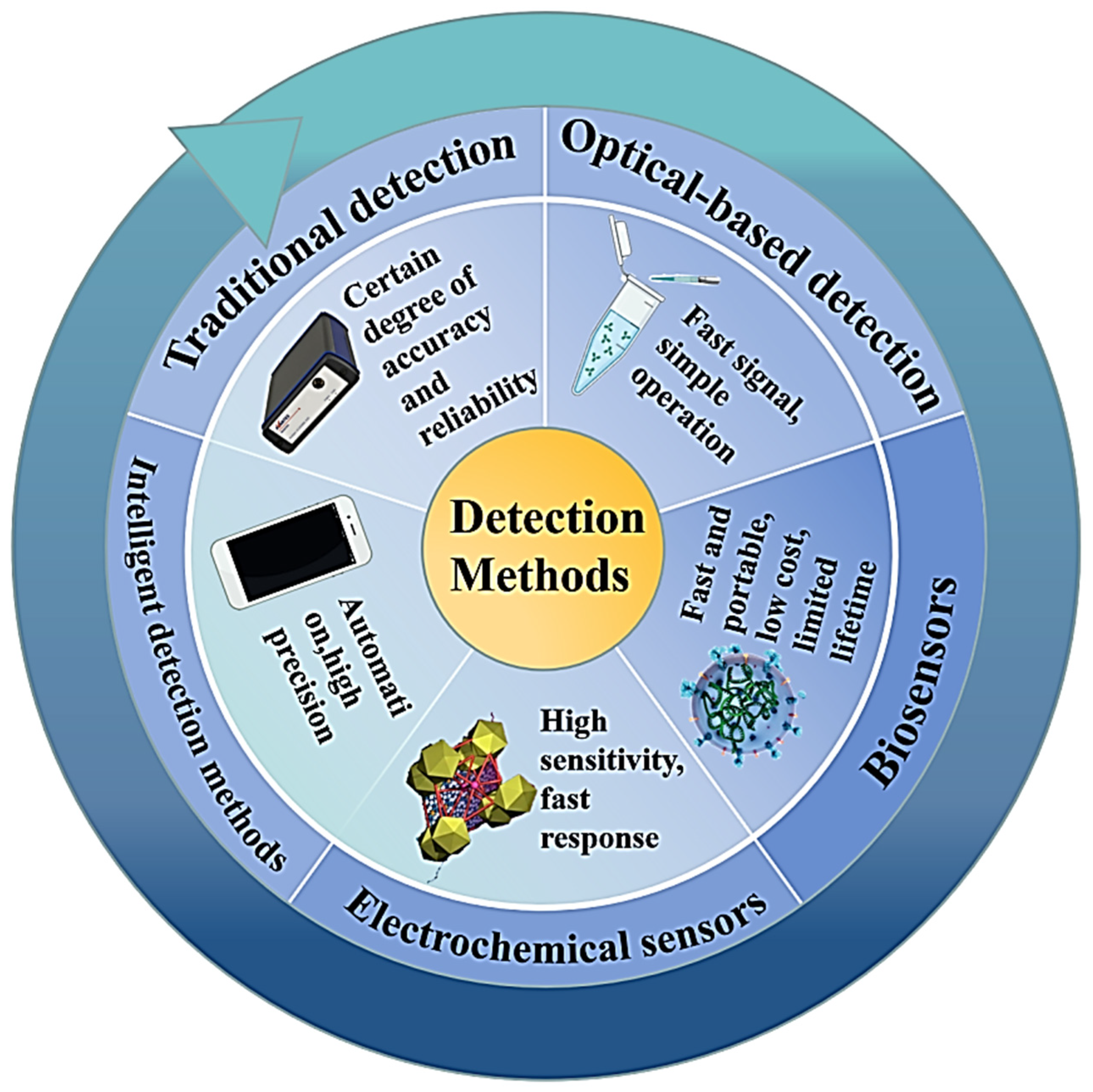
:1. Introduction
2. Traditional Detection Methods
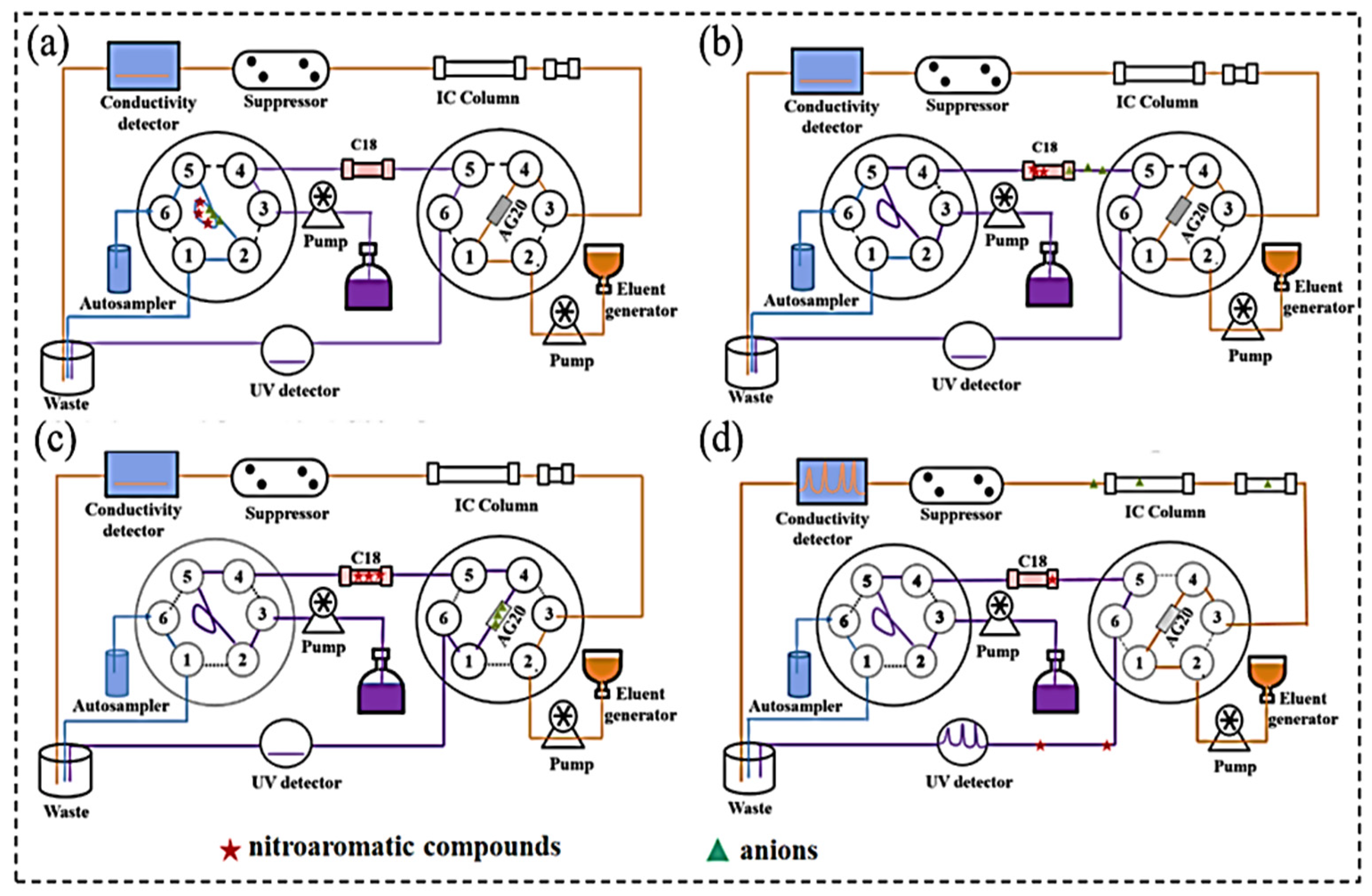
Ion Chromatography
3. Optical-Based Detection Methods
3.1. Spectrophotometry
3.2. Detection Methods Based on Colorimetric Methods
3.3. Methods Based on Fluorescence Detection
4. Biosensors
5. Electrochemical Sensors
| Electrodes | Detection Range | Detection Limit | References |
|---|---|---|---|
| PdAg/C/TP/GCE | 5.0–1000 μM | 1.24 μM | [95] |
| Nafion/Hb/AuNRs-WS2/CILE | 1.0–22.0 mM | 0.33 mM | [96] |
| AgNPs@PANI/rGO/GCE | 1.0–28.2 μM | 56 nM | [97] |
| FeTMPyP/Sr2Nb3O10 | 0.02–1.35 mM | 1.2 μM | [98] |
| Bi2MoO6@PDA-Au | 0.3 μM–4.4 mM | 0.08 μM | [99] |
| CuO NPs/CC | 0.5–3000 μM | 0.043 μM | [101] |
| Ag-coated Nanofiber SERS Platform | 10−1–10−4 M | 2.216 × 10−12 μM | [102] |
| AuNPs-PTH/SGN/GCE | 0.0002–0.1277 0.1277–2.8 mM | 0.06 μM | [103] |
| AuNP/MnOx-VOx/ERGO/GCE | 30–1000 μM | 10.0 μM | [104] |
| CLS/GCE | 0.5–4000 nM | 0.09 μM | [105] |
| Bi/HCNFs-SPE | 0.1–800 μM | 19 nM | [106] |
| Au/Ni-60s NTs | 0.4–40,000 μM 40–130 mM | 0.13 μM | [109] |
| Fe3O4@SiO2 | 0.01–1.0 mM | 3.33 μM | [110] |
| PA-TaCoPc@ZnO | 1–10 μM | 21 nM | [112] |
| AGO | 0.5–85 μM | 250 nM | [116] |
| Nomenclature | Definitions and Explanations |
|---|---|
| Nafion/Hb/AuNRs-WS2/CILE | Nafion/Hb/AuNRs-WS2/CILE is a composite system containing a combination of Nafion, haemoglobin (Hb), gold nanorods—tungsten disulphide (AuNRs-WS2), and carbon fiber ionic liquid electrode (CILE) |
| AgNPs@PANI/rGO/GCE | A glassy carbon electrode (GCE) modified by silver nanoparticles modified polyaniline/reduced graphene oxide complexes |
| FeTMPyP/Sr2Nb3O10 | A complex consisting of tetramethylpyrrolidine cationic iron complex (FeTMPyP) with strontium niobate columbium (Sr2Nb3O10) in a layered chalcogenide structure |
| PDA | Polydopamine (PDA), which can form a uniform coating on a wide range of surfaces and enhance material stability and functionality |
| CuO NPs/CC | Carbon fiber complexes modified by copper oxide nanoparticles |
| Ag-coated Nanofiber SERS Platform | Platform with superb surface-enhanced Raman scattering effect from silver-coated nanofibers |
| AuNPs-PTH/SGN/GCE | Composite combining gold nanoparticles, polythiophene and graphite nanosheets with glassy carbon electrodes |
| AuNP/MnOx-VOx/ERGO/GCE | An innovative composite system combining the advantages of gold nanoparticles, manganese oxides, vanadium oxides and graphene oxide with glassy carbon electrodes |
| PdAg/C/TP/GCE | Composite structure combining palladium-silver alloy, carbon material and triphenylamine on a glassy carbon electrode |
| CLS/GCE | Cell lysate conjugate to glassy carbon electrode |
| AGO | amine-functionalized graphene oxide |
| Bi/HCNFs-SPE | A composite material formed by combining bismuth with hyperbranched carbon nanofibers and applying it in a solid phase extraction technique |
| Au/Ni-60s NTs | Composites composed of gold, nickel and nanotubes |
| PA-TaCoPc@ZnO | A polyaniline-linked tetra amino cobalt phthalocyanine surface functionalized ZnO hybrid nanomaterial |
6. Intelligent Detection Methods
7. Nitrite Alternatives and Degradation
8. Conclusions and Prospects
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, T.T.; Zhang, M.; Law, C.L.; Mujumdar, A.S. Novel strategies for controlling nitrite content in prepared dishes: Current status, potential benefits, limitations and future challenges. Food Res. Int. 2023, 170, 112984. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zeng, X.; Kong, L.; Sun, X.; Shi, J.; Wu, Z.; Guo, Y.; Pan, D. Research Progress of Nitrite Metabolism in Fermented Meat Products. Foods 2023, 12, 1485. [Google Scholar] [CrossRef]
- Honikel, K.O. The use and control of nitrate and nitrite for the processing of meat products. Meat Sci. 2008, 78, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Sriboonyong, T.; Kawamatawong, T.; Sriwantana, T.; Srihirun, S.; Titapiwatanakun, V.; Vivithanaporn, P.; Pornsuriyasak, P.; Sibmooh, N.; Kamalaporn, H. Efficacy and safety of inhaled nebulized sodium nitrite in asthmatic patients. Pulm. Pharmacol. Ther. 2021, 66, 101984. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Truver, M.T.; Hoyer, J.L.; Chronister, C.W.; Goldberger, B.A. Presumptive identification of nitrite by Griess reagent test strips-Case reports of fatal poisoning with sodium nitrite. J. Anal. Toxicol. 2023, 47, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Chazelas, E.; Pierre, F.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaesse, C.; De Sa, A.; Lutchia, R.; Gigandet, S.; Srour, B.; et al. Nitrites and nitrates from food additives and natural sources and cancer risk: Results from the NutriNet-Sante cohort. Int. J. Epidemiol. 2022, 51, 1106–1119. [Google Scholar] [CrossRef] [PubMed]
- El-Nabarawy, N.A.; Gouda, A.S.; Khattab, M.A.; Rashed, L.A. Effects of nitrite graded doses on hepatotoxicity and nephrotoxicity, histopathological alterations, and activation of apoptosis in adult rats. Environ. Sci. Pollut. Res. 2020, 27, 14019–14032. [Google Scholar] [CrossRef] [PubMed]
- National Primary Drinking Water Regulations. Technical Factsheet on Nitrate/Nitrite. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P10154E3.txt (accessed on 25 June 2024).
- Kotopoulou, S.; Zampelas, A.; Magriplis, E. Dietary nitrate and nitrite and human health: A narrative review by intake source. Nutr. Rev. 2022, 80, 762–773. [Google Scholar] [CrossRef]
- GB 5009.33-2016; National Food Safety Standard-Determination of Nitrite and Nitrate in Foods. National Health and Family Planning Commission of the People’s Republic of China; State Food and Drug Administration: Beijing, China, 2016.
- Charles, J.P.; Jennifer, R.K. Colorimetric Determination of Nitrate Plus Nitrite in Water by Enzymatic Reduction, Automated Discrete Analyzer Methods; USGS Numbered Series; US Geological Survey (USGS): Reston, VA, USA, 2011; Volume 34. [CrossRef]
- Ding, H.; Zhang, J.Y.; Zhang, J.B. Discussion on standard management of food additives nitrate and nitrite in meat products. Chin. J. Food Hyg. 2021, 33, 364–368. [Google Scholar]
- Hu, J.; Christison, T.; Rohrer, J. Determination of dimethylamine and nitrite in pharmaceuticals by ion chromatography to assess the likelihood of nitrosamine formation. Heliyon 2021, 7, e06179. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Feng, Y.; Tao, X.; Yao, S.; Chong, X.; Yin, L. Determination of nitrite ion in rifampicin and rifapentine capsules by solid phase extraction-ion chromatography. Chin. J. New Drugs 2023, 32, 2218–2224. [Google Scholar]
- Coviello, D.; Pascale, R.; Ciriello, R.; Salvi, A.M.; Guerrieri, A.; Contursi, M.; Scrano, L.; Bufo, S.A.; Cataldi, T.R.I.; Bianco, G. Validation of an Analytical Method for Nitrite and Nitrate Determination in Meat Foods for Infants by Ion Chromatography with Conductivity Detection. Foods 2020, 9, 1238. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lee, S.J.; Choi, E.; Lee, S.B.; Nam, H.S.; Lee, J.K. Development and validation of an ionic chromatography method for nitrite determination in processed foods and estimation of daily nitrite intake in Korea. Food Chem. 2022, 382, 132280. [Google Scholar] [CrossRef]
- Fitzhenry, C.; Jowett, L.; Roche, P.; Harrington, K.; Moore, B.; Paull, B.; Murray, E. Portable analyser using two-dimensional ion chromatography with ultra-violet light-emitting diode-based absorbance detection for nitrate monitoring within both saline and freshwaters. J. Chromatogr. A 2021, 1652, 462368. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Y.; Qian, C.; Li, J.; Jiang, X.; Shao, P.; Zu, W. Determination of Nitrite in Hams by Gas Phase Molecular Absorption Spectrometry with Ultrasonic Assisted Extraction. J. Anal. Sci. 2022, 38, 89–93. [Google Scholar]
- Liu, X.; Hu, T. Simultaneous Determination of Nitrite and Azide Ions in Valsartan. J. Chromatogr. Sci. 2021, 59, 758–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Kim, S.; Yang, W.; Sim, J. Determination of nitrite and nitrate in postmortem whole blood samples of 10 sodium nitrite poisoning cases: The importance of nitrate in determining nitrite poisoning. Forensic Sci. Int. 2022, 335, 111279. [Google Scholar] [CrossRef]
- Zhu, K.; Kerry, M.; Serr, B.; Mintert, M. Parts per billion of nitrite in microcrystalline cellulose by ion chromatography mass spectrometry with isotope labeled internal standard. J. Pharm. Biomed. Anal. 2023, 235, 115648. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Zheng, H.; Liu, L.; Li, W. Simultaneous determination of six nitroaromatic compounds and three anions in environmental matrices using a liquid chromatography-ion chromatography coupled system. Se Pu=Chin. J. Chromatogr. 2024, 42, 92–98. [Google Scholar] [CrossRef]
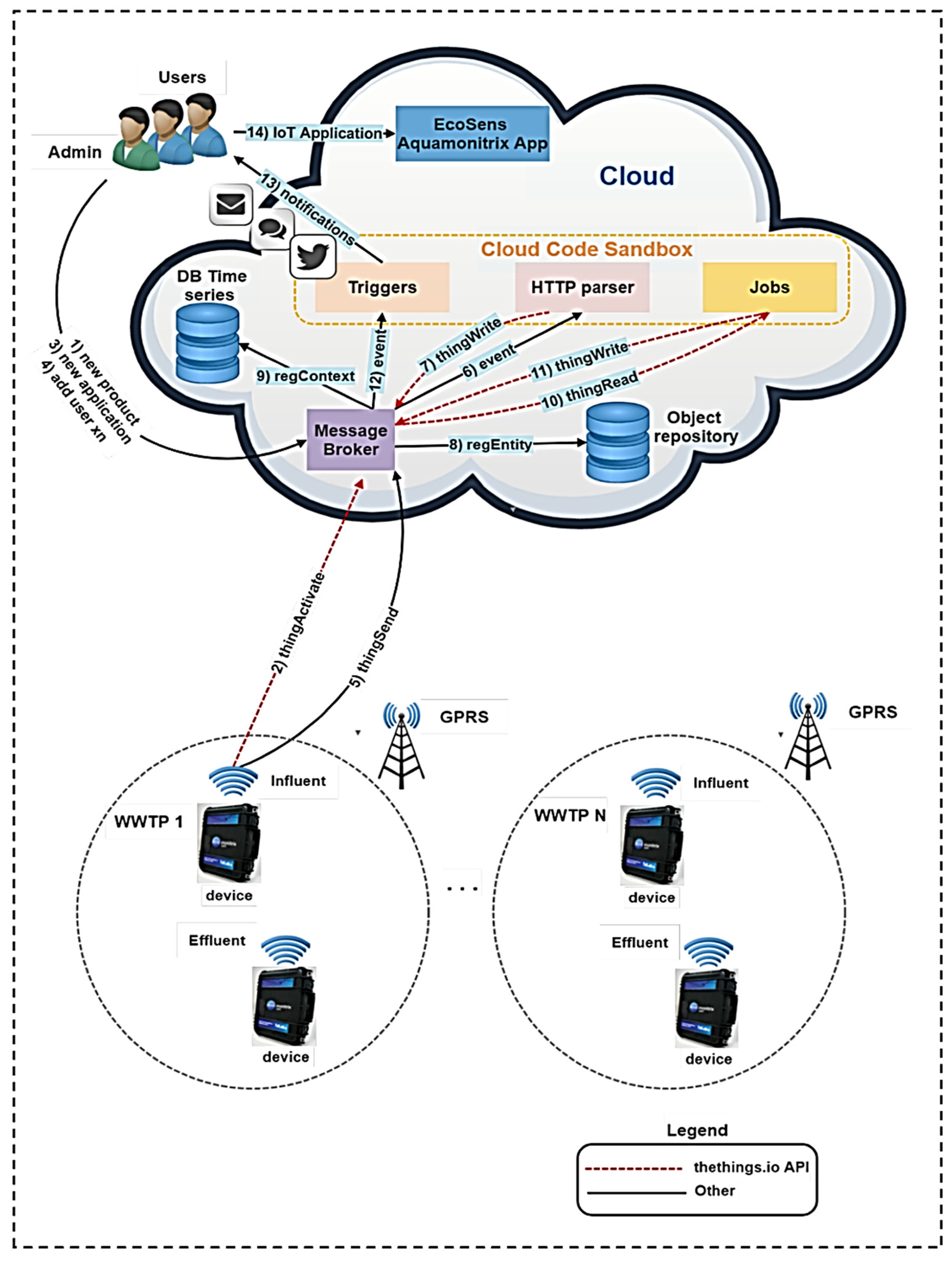
- Martinez, R.; Vela, N.; El Aatik, A.; Murray, E.; Roche, P.; Navarro, J.M. On the Use of an IoT Integrated System for Water Quality Monitoring and Management in Wastewater Treatment Plants. Water 2020, 12, 1096. [Google Scholar] [CrossRef]
- Yuan, L.; Zhang, J.Y.; Wu, X. The Determination of Nitrite Content in Market Sausages. In Proceedings of the 3rd International Conference on Air Pollution and Environmental Engineering, ELECTR NETWORK, Xi’an, China, 28–29 September 2020. [Google Scholar]
- Wang, X.; Hou, J.; Shen, X.; He, Q.; Hou, C.; Huo, D. Fluorescence-based measurements for the determination of nitrite using a coumarin derivative sensor based on inner filter effect. Anal. Methods 2020, 12, 1107–1114. [Google Scholar] [CrossRef]
- Salimi, M.; Nouroozi, S. Inexpensive spectrophotometric determination of nitrite with a laboratory-constructed flow cell. Instrum. Sci. Technol. 2023, 51, 132–143. [Google Scholar] [CrossRef]
- Pai, S.-C.; Su, Y.-T.; Lu, M.-C.; Chou, Y.; Ho, T.-Y. Determination of Nitrate in Natural Waters by Vanadium Reduction and the Griess Assay: Reassessment and Optimization. ACS EST Water 2021, 1, 1524–1532. [Google Scholar] [CrossRef]
- Lim, H.S.; Choi, E.; Lee, S.J.; Nam, H.S.; Lee, J.K. Improved spectrophotometric method for nitrite determination in processed foods and dietary exposure assessment for Korean children and adolescents. Food Chem. 2022, 367, 130628. [Google Scholar] [CrossRef]
- Thipwimonmas, Y.; Jaidam, J.; Samoson, K.; Khunseeraksa, V.; Phonchai, A.; Thiangchanya, A.; Chang, K.H.; Abdullah, A.F.L.; Limbut, W. A Simple and Rapid Spectrophotometric Method for Nitrite Detection in Small Sample Volumes. Chemosensors 2021, 9, 161. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Zhang, J.; Fu, Z.; Shan, M.e. Spectrophotometric Determination of Nitrite Nitrogen in Environmental Water with Sulfanilamide and L-Histidine System. Environ. Monit. China 2021, 37, 186–192. [Google Scholar]
- El Hani, O.; Karrat, A.; Digua, K.; Amine, A. Development of a simplified spectrophotometric method for nitrite determination in water samples. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2022, 267, 120574. [Google Scholar] [CrossRef] [PubMed]
- Shichijo, M.; Okamoto, K.; Takahashi, T.; Nomura, M.; Ohira, S.-I.; Mizuguchi, H.; Tanaka, H.; Takeuchi, M. Feedback standard addition method coupled flow injection analysis-Validation by spectrophotometric determination of nitrite in seawater. Microchem. J. 2023, 190, 108721. [Google Scholar] [CrossRef]
- Hatta, M.; Ruzicka, J.; Measures, C.I. The performance of a new linear light path flow cell is compared with a liquid core waveguide and the linear cell is used for spectrophotometric determination of nitrite in sea water at nanomolar concentrations. Talanta 2020, 219, 121240. [Google Scholar] [CrossRef]
- Xu, L.; Wu, H.; Wang, X.; Chen, Q.; Ostrikov, K. A simple derivative spectrophotometric method for simultaneously detecting nitrate and nitrite in plasma treated water. Plasma Sci. Technol. 2022, 24, 085502. [Google Scholar] [CrossRef]
- Yardimci, B. Spectrophotometric and smartphone-based facile green chemistry approach to determine nitrite ions using green tea extract as a natural source. Sustain. Chem. Pharm. 2023, 34, 101175. [Google Scholar] [CrossRef]
- Nishan, U.; Rehman, S.; Ullah, R.; Bari, A.; Afridi, S.; Shah, M.; Iqbal, J.; Asad, M.; Badshah, A.; Khan, N.; et al. Fabrication of a colorimetric sensor using acetic acid-capped drug-mediated copper oxide nanoparticles for nitrite biosensing in processed food. Front. Mater. 2023, 10, 1169945. [Google Scholar] [CrossRef]
- Tai, Y.T.; Cheng, C.-Y.; Chen, Y.-S.; Ko, F.-H. A hydrogel-based chemosensor applied in conjunction with a Griess assay for real-time colorimetric detection of nitrite in the environment. Sens. Actuators B-Chem. 2022, 369, 132298. [Google Scholar] [CrossRef]
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Rapid and highly selective colorimetric detection of nitrite based on the catalytic-enhanced reaction of mimetic Au nanoparticle-CeO2 nanoparticle-graphene oxide hybrid nanozyme. Talanta 2021, 224, 121875. [Google Scholar] [CrossRef]
- Taweekarn, T.; Wongniramaikul, W.; Limsakul, W.; Sriprom, W.; Phawachalotorn, C.; Choodum, A. A novel colorimetric sensor based on modified mesoporous silica nanoparticles for rapid on-site detection of nitrite. Microchim. Acta 2020, 187, 643. [Google Scholar] [CrossRef]
- Nishan, U.; Khan, H.U.; Rahim, A.; Asad, M.; Qayum, M.; Khan, N.; Shah, M.; Muhammad, N. Non-enzymatic colorimetric sensing of nitrite in fortified meat using functionalized drug mediated manganese dioxide. Mater. Chem. Phys. 2022, 278, 125729. [Google Scholar] [CrossRef]
- Huang, Z.-J.; Luo, J.-Y.; Zheng, F.-Y.; Li, S.-X.; Liu, F.-J.; Lin, L.-X.; Huang, Y.-J.; Man, S.; Cao, G.-X.; Huang, X.-G. Long-term stable, high accuracy, and visual detection platform for In-field analysis of nitrite in food based on colorimetric test paper and deep convolutional neural networks. Food Chem. 2022, 373, 131593. [Google Scholar] [CrossRef]
- Ratnarathorn, N.; Dungchai, W. Paper-based Analytical Device (PAD) for the Determination of Borax, Salicylic Acid, Nitrite, and Nitrate by Colorimetric Methods. J. Anal. Chem. 2020, 75, 487–494. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Fu, L.-M.; Ju, W.-J.; Wu, P.-Y. Microfluidic colorimetric system for nitrite detection in foods. Chem. Eng. J. 2020, 398, 125573. [Google Scholar] [CrossRef]
- Hou, J.; Wu, H.; Shen, X.; Zhang, C.; Hou, C.; He, Q.; Huo, D. Phenosafranin-Based Colorimetric-Sensing Platform for Nitrite Detection Enabled by Griess Assay. Sensors 2020, 20, 1501. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Chen, J.; Zhu, Y.; Hu, K.; Ma, Q.; Zuo, Y. A novel propylene glycol alginate gel based colorimetric tube for rapid detection of nitrite in pickled vegetables. Food Chem. 2022, 373, 131678. [Google Scholar] [CrossRef]
- Jantra, J.; Arsawiset, S.; Teepoo, S.; Keeratirawee, K. Rapid colorimetric assay based on the oxidation of 2,2-azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid-diammonium salt for nitrite detection in meat products. J. Environ. Sci. Health Part B-Pestic. Food Contam. Agric. Wastes 2024, 59, 72–80. [Google Scholar] [CrossRef]
- Nam, J.; Jung, I.-B.; Kim, B.; Lee, S.-M.; Kim, S.-E.; Lee, K.-N.; Shin, D.-S. A colorimetric hydrogel biosensor for rapid detection of nitrite ions. Sens. Actuators B-Chem. 2018, 270, 112–118. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, H.; Liu, Y.; Zhang, Y.; Shang, M.; Wang, L.; Zhuang, Y.; Lv, X. A colorimetric and fluorescent dual-readout probe based on red emission carbon dots for nitrite detection in meat products. Food Chem. 2022, 374, 131768. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, L.; Zhang, Y.; Lu, L.; Fu, H.; She, Y. A naphthalimide-based fluorescent probe for rapid detection of nitrite and its application in food quality monitoring. Anal. Chim. Acta 2023, 1268, 341403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, Y.; Li, J.; Ren, W.; Dou, X. High-performance fluorescent and colorimetric dual-mode nitrite sensor boosted by a versatile coumarin probe equipped with diazotization-coupling reaction-sites. Sens. Actuators B-Chem. 2023, 379, 133261. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, H.; Liu, B.; Hu, P.; Pan, J.; Niu, X. Bifunctional Mn-Doped N-Rich Carbon Dots with Tunable Photoluminescence and Oxidase-Mimetic Activity Enabling Bimodal Ratiometric Colorimetric/Fluorometric Detection of Nitrite. Acs Appl. Mater. Interfaces 2022, 14, 44762–44771. [Google Scholar] [CrossRef] [PubMed]
- Murfin, L.C.; Lopez-Alled, C.M.; Sedgwick, A.C.; Wenk, J.; James, T.D.; Lewis, S.E. A simple, azulene-based colorimetric probe for the detection of nitrite in water. Front. Chem. Sci. Eng. 2020, 14, 90–96. [Google Scholar] [CrossRef]
- Xu, J.; Shi, Y.; Yang, S.; Yang, J.; Zhang, X.; Xu, L.; Bian, Z.; Xu, Z.; Zhu, B. Highly selective colorimetric fluorescent probe for detecting nitrite in aqueous solution. Microchem. J. 2021, 169, 106342. [Google Scholar] [CrossRef]
- Pan, Y.; Jiang, J.; Kan, X. Diazo-reaction based dual-mode colorimetric-electrochemical sensing of nitrite in pickled food. Analyst 2023, 148, 4869–4876. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Javed, R.; Li, R.; Zhao, H.; Liu, X.; Zhang, C.; Cao, H.; Ye, D. Nonmetal catalyst boosting amplification of both colorimetric and electrochemical signal for multi-mode nitrite sensing. Food Chem. 2024, 441, 138315. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Huang, Z.; Lu, X.; Wang, T.; Cheng, W.; Yang, H.; Huang, T.; Li, T.; Li, Z. Plasmon-Mediated Oxidase-like Activity on Ag@ZnS Heterostructured Hollow Nanowires for Rapid Visual Detection of Nitrite. Inorg. Chem. 2023, 62, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ruan, G.; Sun, Y.; Zhao, D.; Yu, H.; Zhang, C.-W.; Li, L.; Liu, J. A full-wavelength coverage colorimetric sensor depending on polymer-carbon nanodots from blue to red for visual detection of nitrite via smartphone. Dye. Pigment. 2021, 191, 109383. [Google Scholar] [CrossRef]
- Choodum, A.; Tiengtum, J.; Taweekarn, T.; Wongniramaikul, W. Convenient environmentally friendly on-site quantitative analysis of nitrite and nitrate in seawater based on polymeric test kits and smartphone application. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2020, 243, 118812. [Google Scholar] [CrossRef] [PubMed]
- Polat, F. Development of a Simple and Accurate Analytical Method for the Determination of Nitrite in Processed Meat Products by Using an Optical Solid Chemosensor and Smartphone. Food Anal. Methods 2022, 15, 700–706. [Google Scholar] [CrossRef]
- Sargazi, M.; Kaykhaii, M. Application of a smartphone-based spectrophotometer for rapid in-field determination of nitrite and chlorine in environmental water samples. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2020, 227, 117672. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Nath, P. Smartphone-based Photometric Detection of Nitrite Level in Water. In Proceedings of the IEEE Workshop on Recent Advances in Photonics (WRAP), Mumbai, India, 4–6 March 2022. [Google Scholar]
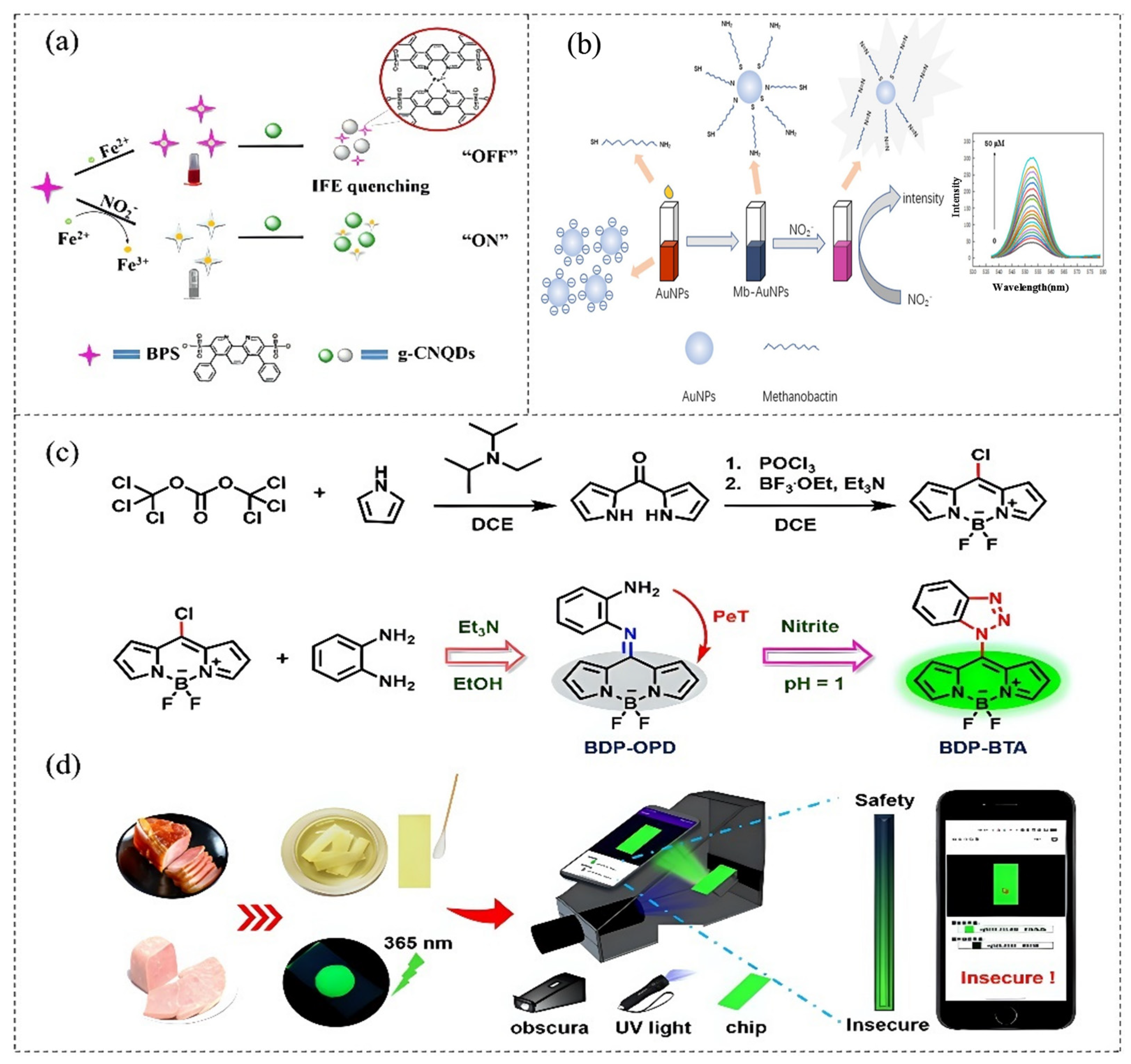
- Kong, Y.; Cheng, Q.; He, Y.; Ge, Y.; Zhou, J.; Song, G. A dual-modal fluorometric and colorimetric nanoprobe based on graphitic carbon nitrite quantum dots and Fe (II)-bathophenanthroline complex for detection of nitrite in sausage and water. Food Chem. 2020, 312, 126089. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, J.; Hu, X.; Cai, Z.; Dou, X. Ultrasensitive, Specific, and Rapid Fluorescence Turn-On Nitrite Sensor Enabled by Precisely Modulated Fluorophore Binding. Adv. Sci. 2020, 7, 2002991. [Google Scholar] [CrossRef] [PubMed]
- Omer, K.M.; Idrees, S.A.; Hassan, A.Q.; Jamil, L.A. Amphiphilic fluorescent carbon nanodots as a selective nanoprobe for nitrite and tetracycline both in aqueous and organic solutions. New J. Chem. 2020, 44, 5120–5126. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Y.; Shi, H.; Liu, E.; Hu, X.; Zhang, X.; Fan, J. A novel fluorescent sensors for sensitive detection of nitrite ions. Mater. Chem. Phys. 2020, 239, 122121. [Google Scholar] [CrossRef]
- Li, R.; Li, L.; Wang, B.; Yu, L. Preparation of Quantum Dot-Embedded Photonic Crystal Hydrogel and Its Application as Fluorescence Sensor for the Detection of Nitrite. Nanomaterials 2021, 11, 3126. [Google Scholar] [CrossRef]
- Yu, K.K.; Pan, S.L.; Li, K.; Shi, L.; Liu, Y.-H.; Chen, S.Y.; Yu, X.Q. A novel near-infrared fluorescent sensor for zero background nitrite detection via the “covalent-assembly” principle. Food Chem. 2021, 341, 128254. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Wei, M.X.; Huang, B.H.; Guo, X.F.; Wang, H. One-pot facile synthesis of green-emitting fluorescent silicon quantum dots for the highly selective and sensitive detection of nitrite in food samples. Dye. Pigment. 2021, 184, 108848. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Shi, Y.H.; Wu, W.; Zhao, Y.; Xu, Z.H. A fluorescent carbon dots synthesized at room temperature for automatic determination of nitrite in Sichuan pickles. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 286, 122025. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Liu, S.; Zhao, N.; Zhao, L. A new fluorescent probe based on metallic deep eutectic solvent for visual detection of nitrite and pH in food and water environment. Food Chem. 2023, 398, 133935. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Huang, S.; Wen, H.; Luo, Y.; Cheng, J.; Jia, Z.; Han, P.; Xue, W. Fluorescent recognition and selective detection of nitrite ions with carbon quantum dots. Anal. Bioanal. Chem. 2020, 412, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.D. A novel ratiometric and colorimetric probe for rapid and ultrasensitive detection of nitrite in water based on an Acenaphtho 1,2-d imidazole derivative. Anal. Chim. Acta 2021, 1166, 338597. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liu, H.; Zhang, C.; Yang, X.; Blecker, C. LMOF serve as food preservative nanosensor for sensitive detection of nitrite in meat products. LWT-Food Sci. Technol. 2022, 169, 114030. [Google Scholar] [CrossRef]
- Chen, L.; Fan, T.; Li, W.; Song, J.; Zhang, J.; Wang, L.; Han, K. A turn-on fluorescent nano-probe base on methanobactin-AuNPs for simple and efficient detection of nitrite. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 2023, 286, 121960. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Bai, Y.; Li, F.; Liu, F.; Yu, X. A Facile Synthesis of Tannic Acid-Protected Copper Nanoclusters and the Sensitive Fluorescence Detection of Nitrite Ion Under Mild Conditions. Spectroscopy 2021, 36, 22–27. [Google Scholar]
- Wang, M.; Liu, B.; Liu, J.; Zhu, H.; Bai, Q.; Hu, P.; Pan, J.; Liang, H.; Niu, X. Bifunctional manganese-doped silicon quantum dot-responsive smartphone-integrated paper sensor for visual multicolor/ multifluorescence dual-mode detection of nitrite. Sens. Actuators B-Chem. 2023, 392, 134143. [Google Scholar] [CrossRef]
- Yang, L.; Wang, F.; Zhao, J.; Kong, X.; Lu, K.; Yang, M.; Zhang, J.; Sun, Z.; You, J. A facile dual-function fluorescent probe for detection of phosgene and nitrite and its applications in portable chemosensor analysis and food analysis. Talanta 2021, 221, 121477. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, Y.; Zhang, L.; Cao, Y.; Guo, M.; Yu, Y.; Lin, B. Construction of integrated and portable fluorescence sensor and the application for visual detection in situ. Sens. Actuators B-Chem. 2022, 373, 132764. [Google Scholar] [CrossRef]
- Zeng, L.; Ke, Y.; Yang, X.; Lan, M.; Zhao, S.; Zhu, B. Intramolecular cascade reaction sensing platform for rapid, specific and ultrasensitive detection of nitrite. Food Chem. 2024, 438, 138044. [Google Scholar] [CrossRef]
- Duhan, J.; Obrai, S. Highly sensitive and selective fluorescence and smartphone-based sensor for detection of L-dopa using nitrogen sulphur graphene quantum dots. Microchem. J. 2023, 193, 109262. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, T.; Xiong, B.; Zheng, X.; Wang, R.; Zhu, P.; Chen, J.; Cong, T.; Li, Y.; Wang, X. Fluorescent probe/hydrogel-based portable platform for ultrasensitive on-site detection of explosive particles containing nitrite. Chem. Eng. J. 2023, 475, 146311. [Google Scholar] [CrossRef]
- Yilmaz, M.D. A simple yet effective colorimetric assay for nitrite based on nitration of a near-infrared (NIR) absorbing dye IR780. Microchem. J. 2024, 196, 109554. [Google Scholar] [CrossRef]
- Wulandari, A.; Sunarti, T.C.; Fahma, F.; Noor, E.; Enomae, T. Encapsulation of purple sweet potato’s anthocyanin in CMC-PVA matrix for development of paper strips as a colorimetric biosensor. Indian J. Biochem. Biophys. 2021, 58, 292–302. [Google Scholar]
- Chen, L.; Song, J.; Wang, L.; Hao, X.; Zhang, H.; Li, X.; Wu, J. A simple electrochemical biosensor based on HS-β-cyclodextrin coordination methanobactin/gold nanoparticles for highly sensitive detection of nitrite. J. Solid State Electrochem. 2024, 28, 305–316. [Google Scholar] [CrossRef]
- Revsbech, N.P.; Nielsen, M.; Fapyane, D. Ion Selective Amperometric Biosensors for Environmental Analysis of Nitrate, Nitrite and Sulfate. Sensors 2020, 20, 4326. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, P.; Kruse, T.; Bernhardt, P.V. A highly sensitive and stable electrochemical nitrate biosensor. Electrochim. Acta 2021, 386, 138480. [Google Scholar] [CrossRef]
- Xie, H.; Luo, G.; Niu, Y.; Weng, W.; Zhao, Y.; Ling, Z.; Ruan, C.; Li, G.; Sun, W. Synthesis and utilization of Co3O4 doped carbon nanofiber for fabrication of hemoglobin-based electrochemical sensor. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 107, 110209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, M.; Xiao, L.; Liu, H.; Hu, M.; Li, S.; Zhai, Q.; Chen, Y.; Jiang, Y. Enzymatic biosensor for nitrite detection based on direct electron transfer by CPO-ILEMB/Au@MoS2/GC. J. Appl. Electrochem. 2022, 52, 979–987. [Google Scholar] [CrossRef]
- Monteiro, T.; Coelho, A.R.; Moreira, M.; Viana, A.S.; Almeida, M.G. Interfacing the enzyme multiheme cytochrome c nitrite reductase with pencil lead electrodes: Towards a disposable biosensor for cyanide surveillance in the environment. Biosens. Bioelectron. 2021, 191, 113438. [Google Scholar] [CrossRef]
- Cardoso, R.M.; Silva, P.R.L.; Lima, A.P.; Rocha, D.P.; Oliveira, T.C.; do Prado, T.M.; Fava, E.L.; Fatibello-Filho, O.; Richter, E.M.; Munoz, R.A.A. 3D-Printed graphene/polylactic acid electrode for bioanalysis: Biosensing of glucose and simultaneous determination of uric acid and nitrite in biological fluids. Sens. Actuators B-Chem. 2020, 307, 127621. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Cheng, J. Bionic Enzyme-Assisted Ion-Selective Amperometric Biosensor Based on 3D Porous Conductive Matrix for Point-of-Care Nitrite Testing. Acs Nano 2022, 16, 14849–14859. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, S.; Li, H.; Jin, B.; He, X. Highly selective and sensitive nitrite biocathode biosensor prepared by polarity inversion method coupled with selective removal of interfering electroactive bacteria. Biosens. Bioelectron. 2022, 214, 114507. [Google Scholar] [CrossRef]
- Wang, J.; Zhan, G.; Yang, X.; Zheng, D.; Li, X.; Zhang, L.; Huang, T.; Wang, X. Rapid detection of nitrite based on nitrite-oxidizing bacteria biosensor and its application in surface water monitoring. Biosens. Bioelectron. 2022, 215, 114573. [Google Scholar] [CrossRef] [PubMed]
- Klevinskas, A.; Kantminiene, K.; Zmuidzinaviciene, N.; Jonuskiene, I.; Griskonis, E. Microbial Fuel Cell as a Bioelectrochemical Sensor of Nitrite Ions. Processes 2021, 9, 1330. [Google Scholar] [CrossRef]
- Qiu, X.-Y.; Cheng, Y.-Y.; Li, Q.; Yu, Y.-Y.; Xiao, X. An in-field assembled hierarchical mesoporous electroenzymatic sensor for sensitive and real-time monitoring of nitrite. J. Clean. Prod. 2023, 426, 139102. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, X.; Yang, C.; Yang, W.; Liu, G.; Li, Y.; Zhang, G.; Zhao, X. Surfactant-free synthesis of CuBr NPs decorated by Pt for glucose and nitrite sensors. J. Ind. Eng. Chem. 2023, 124, 323–330. [Google Scholar] [CrossRef]
- Koukouviti, E.; Soulis, D.; Economou, A.; Kokkinos, C. Wooden Tongue Depressor Multiplex Saliva Biosensor Fabricated via Diode Laser Engraving. Anal. Chem. 2023, 95, 6765–6768. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.T.; Chou, Y.Y.; Wu, J.-G.; Wang, Y.C.; Tseng, T.-N.; Pan, S.-W.; Luo, S.-C.; Ho, M.-L. An electrochemical conducting polymer-based biosensor for Leukocyte esterase and nitrite detection for diagnosing urinary tract infections: A pilot study. Microchem. J. 2023, 188, 108493. [Google Scholar] [CrossRef]
- de Freitas, R.C.; Orzari, L.O.; de Oliveira, P.R.; Janegitz, B.C. Pd and Ag Binary Nanoparticles Supported on Carbon Black and Tapioca for Nitrite Electrochemical Detection. J. Electrochem. Soc. 2021, 168, 117518. [Google Scholar] [CrossRef]
- Jiang, M.; Yin, C.; Du, J.; Fu, W.; Han, X.; Sun, W. Gold nanorods and tungsten disulfide nanocomposite modified electrode for hemoglobin electrochemical biosensing of trichloroacetic acid and nitrite. Int. J. Electrochem. Sci. 2023, 18, 100371. [Google Scholar] [CrossRef]
- Kaladevi, G.; Wilson, P.; Pandian, K. Simultaneous and Selective Electrochemical Detection of Sulfite and Nitrite in Water Sources Using Homogeneously Dispersed Ag Nanoparticles over PANI/rGO Nanocomposite. J. Electrochem. Soc. 2020, 167, 027514. [Google Scholar] [CrossRef]
- Wu, S.; Wang, H.; Zhao, B.; Cao, T.; Li, L.; Ma, J.; Liu, L.; Ruan, J.; Cao, J.; Tong, Z. Synthesis of strontium niobium-iron porphyrin nanocomposite for nitrite detection in river water. J. Nanoparticle Res. 2021, 23, 151. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Zheng, J. A Novel Non-Enzymatic Sensor Based on Bismuth Molybdate@polydopamine-Gold Nanocomposites for Efficient Nitrite Sensing. J. Electrochem. Soc. 2021, 168, 067519. [Google Scholar] [CrossRef]
- Feng, X.; Han, G.; Cai, J.; Wang, X. Au@Carbon quantum Dots-MXene nanocomposite as an electrochemical sensor for sensitive detection of nitrite. J. Colloid Interface Sci. 2022, 607, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jiang, H.; Gao, J.; Zhang, L.; Zhao, C.; Suo, H. In situ fabrication of copper oxide nanoparticles decorated carbon cloth for efficient electrocatalytic detection of nitrite. Microchem. J. 2023, 194, 109302. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Zou, Y.; Farooq, S.; Li, Y.; Zhang, H. Novel Ag-coated nanofibers prepared by electrospraying as a SERS platform for ultrasensitive and selective detection of nitrite in food. Food Chem. 2023, 412, 135563. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, H.; Ma, J.; Zheng, J. High performance of nitrite electrochemical sensing based on Au-poly (thionine)-tin oxide/graphene nanosheets nanocomposites. Colloids Surf. A-Physicochem. Eng. Asp. 2022, 642, 128582. [Google Scholar] [CrossRef]
- Aslisen, B.; Kocak, S. Preparation of mixed-valent manganese-vanadium oxide and Au nanoparticle modified graphene oxide nanosheets electrodes for the simultaneous determination of hydrazine and nitrite. J. Electroanal. Chem. 2022, 904, 115875. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, Y.; Hasebe, Y.; Zhang, Z. Electrochemical Sensing Platform Based on Lotus Stem-derived Porous Carbon for the Simultaneous Determination of Hydroquinone, Catechol and Nitrite. Electroanalysis 2021, 33, 956–963. [Google Scholar] [CrossRef]
- Wang, F.; Li, Y.; Yan, C.; Ma, Q.; Yang, X.; Peng, H.; Wang, H.; Du, J.; Zheng, B.; Guo, Y. Bismuth-Decorated Honeycomb-like Carbon Nanofibers: An Active Electrocatalyst for the Construction of a Sensitive Nitrite Sensor. Molecules 2023, 28, 3881. [Google Scholar] [CrossRef]
- Yang, Y.; Wei, S.; Wang, J.; Li, J.; Tang, J.; Aaron, A.A.; Cai, Q.; Wang, N.; Li, Z. Highly sensitive and ratiometric detection of nitrite in food based on upconversion-carbon dots nanosensor. Anal. Chim. Acta 2023, 1263, 341245. [Google Scholar] [CrossRef] [PubMed]
- Akbari, A.; Dehghan, P.; Divband, B.; Alipour, E.; Moradi, A.H. Facile Synthesis of a Novel Zr/Fe3O4/GO Nanocomposite and Its Application for Modification of Electrode Surfaces and Voltammetric Determination of Nitrite Ions. J. Anal. Chem. 2023, 78, 1070–1078. [Google Scholar] [CrossRef]
- Wang, S.; Yin, H.; Qu, K.; Wang, L.; Gong, J.; Zhao, S.; Wu, S. Electrodeposition of Au/Ni nanotubes with highly improved electrochemical performance for non-enzymatic nitrite detection. Int. J. Environ. Anal. Chem. 2023. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Guo, W. Electrochemical sensor for sensitive nitrite and sulfite detection in milk based on acid-treated Fe3O4@SiO2 nanoparticles. Food Chem. 2024, 430, 137004. [Google Scholar] [CrossRef]
- Li, Z.; Hu, Y.; Wang, L.; Liu, H.; Ren, T.; Wang, C.; Li, D. Selective and Accurate Detection of Nitrate in Aquaculture Water with Surface-Enhanced Raman Scattering (SERS) Using Gold Nanoparticles Decorated with beta-Cyclodextrins. Sensors 2024, 24, 1093. [Google Scholar] [CrossRef]
- Sudhakara, S.M.; Devendrachari, M.C.; Khan, F.; Thippeshappa, S.; Kotresh, H.M.N. Highly sensitive and selective detection of nitrite by polyaniline linked tetra amino cobalt (II) phthalocyanine surface functionalized ZnO hybrid electrocatalyst. Surf. Interfaces 2023, 36, 102565. [Google Scholar] [CrossRef]
- Saeb, E.; Asadpour-Zeynali, K. A novel ZIF-8@ZIF-67/Au core-shell metal organic framework nanocomposite as a highly sensitive electrochemical sensor for nitrite determination. Electrochim. Acta 2022, 417, 140278. [Google Scholar] [CrossRef]
- Ranjani, B.; Pandian, K.; Gopinath, S.C.B. Hemin-Modified Halloysite Nanotube as Electrocatalyst for the Enhanced Electrochemical Determination of Nitrite. J. Electrochem. Soc. 2022, 169, 057528. [Google Scholar] [CrossRef]
- Kumar, C.R.R.; Betageri, V.S.; Nagaraju, G.; Suma, B.P.; Kiran, M.S.; Pujar, G.H.; Letha, M.S. One-Pot Synthesis of ZnO Nanoparticles for Nitrite Sensing, Photocatalytic and Antibacterial Studies. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3476–3486. [Google Scholar] [CrossRef]
- Azhdeh, A.; Mashhadizadeh, M.H.; Buhl, K.B. A visualization method for quickly detecting nitrite ions in breath condensate using a portable closed bipolar electrochemical sensor. Analyst 2024, 149, 1825–1836. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Q.; Zhao, Y.; Ge, C.; Lin, S.; Liao, J. Cost-effective, wireless, and portable smartphone-based electrochemical system for on-site monitoring and spatial mapping of the nitrite contamination in water. Sens. Actuators B-Chem. 2020, 319, 128221. [Google Scholar] [CrossRef]
- Akhter, F.; Siddiquei, H.R.; Alahi, M.E.E.; Mukhopadhyay, S.C. An IoT-enabled portable sensing system with MWCNTs/PDMS sensor for nitrate detection in water. Measurement 2021, 178, 109424. [Google Scholar] [CrossRef]
- Gonzalez, P.; Perez, N.; Knochen, M. Design and construction of a low-cost, in-situ analyzer for nutrients in surface waters, based on open-source hardware and software. Microchem. J. 2022, 175, 107134. [Google Scholar] [CrossRef]
- Xiao, J.; Tang, J.; Chen, J.; Li, L.; Zhang, S.; Xiong, X.; Zou, Z. Rapid and selective detection of nitrite in ham sausage and water samples by a portable gas pressure meter. Sens. Actuators B-Chem. 2024, 400, 134914. [Google Scholar] [CrossRef]
- Li, Q.; Liu, R.; Shang, Y.; Wei, Y.; Cui, H. A stacked extreme learning machines model on detection of nitrite-nitrogen concentration in surface water with ultraviolet-visible spectroscopy. Int. J. Environ. Sci. Technol. 2024, 21, 6653–6662. [Google Scholar] [CrossRef]
- Cheng, C.; Jiang, L.; Li, X.; Song, H.; Fang, W. Can natural preservatives serve as a new line of protective technology against bacterial pathogens in meat and meat products? Food Qual. Saf. 2024, 8, fyad049. [Google Scholar] [CrossRef]
- Ma, G.; Wang, Z.; Yu, Q.; Han, L.; Chen, C.; Guo, Z. Effects of low-dose sodium nitrite on the structure of yak meat myoglobin during wet curing. Food Chem.-X 2022, 15, 100434. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Jia, J.; Peng, H.; Qian, Q.; Pan, Z.; Liu, D. Nitrite and nitrate in meat processing: Functions and alternatives. Curr. Res. Food Sci. 2023, 6, 100470. [Google Scholar] [CrossRef]
- Ongaratto, G.C.; Oro, G.; Kalschne, D.L.; Trindade Cursino, A.C.; Canan, C. Cochineal carmine adsorbed on layered zinc hydroxide salt applied on mortadella to improve color stability. Curr. Res. Food Sci. 2021, 4, 758–764. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q. Isolation of Antibacterial, Nitrosylmyoglobin Forming Lactic Acid Bacteria and Their Potential Use in Meat Processing. Front. Microbiol. 2020, 11, 1315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ge, X.; Jiao, Y.; Liu, Y. The protective effects of black tea as nitrite replacer on the oxidation, physicochemical and sensory properties of steamed beef. LWT-Food Sci. Technol. 2023, 188, 115375. [Google Scholar] [CrossRef]
- Nissen, L.; Casciano, F.; Di Nunzio, M.; Galaverna, G.; Bordoni, A.; Gianotti, A. Effects of the replacement of nitrates/nitrites in salami by plant extracts on colon microbiota. Food Biosci. 2023, 53, 102568. [Google Scholar] [CrossRef]
- Sucu, C.; Turp, G.Y. The investigation of the use of beetroot powder in Turkish fermented beef sausage (sucuk) as nitrite alternative. Meat Sci. 2018, 140, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Y.; Wang, F.H.; Wang, F.H.; Wang, Y.H. Effects of partial replacement of nitrite with different fruit and vegetable powder on physicochemical and sensory aspects of fried beef meatballs. Int. Food Res. J. 2023, 30, 964–977. [Google Scholar] [CrossRef]
- Tan, X.; Cui, F.; Wang, D.; Lv, X.; Li, X.; Li, J. Fermented Vegetables: Health Benefits, Defects, and Current Technological Solutions. Foods 2024, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Udayasurian, S.R.; Li, T. Recent research progress on building C-N bonds via electrochemical NOx reduction. Nanoscale 2024, 16, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, J.; Qu, Z.; Liu, W.; Wang, A. What innovative nitrite furnishing processes can be coupled with anammox for excellent nitrogen removal? Crit. Rev. Environ. Sci. Technol. 2024, 54, 1195–1217. [Google Scholar] [CrossRef]
- Yu, N.-N.; Park, G. Nitric Oxide in Fungi: Production and Function. J. Fungi 2024, 10, 155. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Cui, H.; Bai, Y.; Yin, Z.; Qu, K. Hazardous substances and their removal in recirculating aquaculture systems: A review. Aquaculture 2023, 569, 73939. [Google Scholar] [CrossRef]


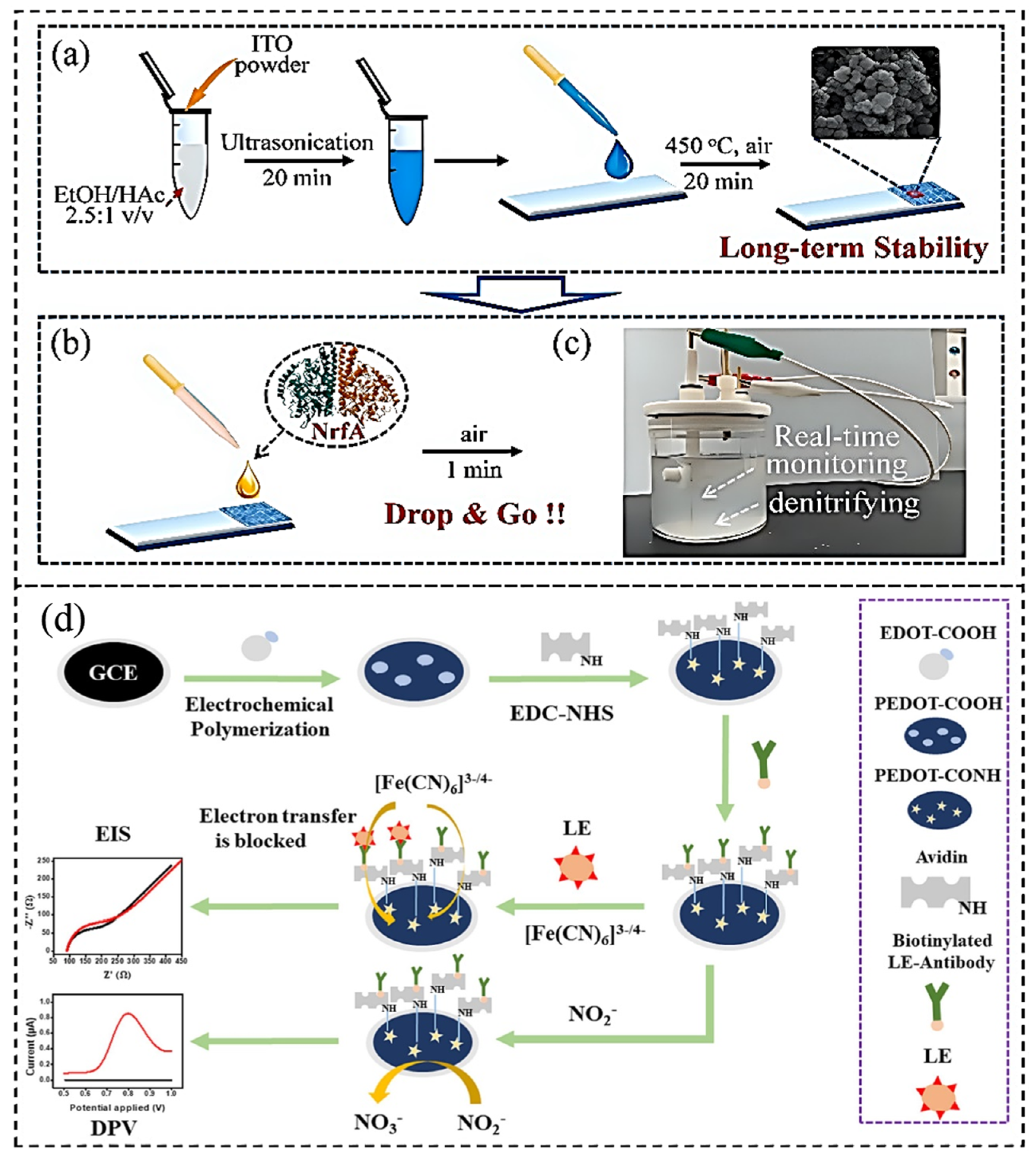
| Detection Methods | Material or Equipment | Detection Range | Detection Limit | References |
|---|---|---|---|---|
| Spectrophotometry | 3-Aph/ADC | 0.1–50 μM | 0.0246 μM | [21] |
| Spectrophotometry | PAD | 0.09–1.47 μg/mL | 0.053 μg/mL | [27] |
| Spectrophotometry |
UV–Vis Spectrophotometer or smartphone | 0.2–1.1 mg/L (spectroscopic) 0.05–0.8 mg/L (smartphone) | 0.050 mg/L (spectroscopic) 0.017 mg/L (smartphone) | [31] |
| Colorimetric | Acetic acid-capped CuO NPs | 0.01–2.40 μM | 0.2 μM | [32] |
| Colorimetric | Microfluidic paper-based | 1–100 ppm | 1 ppm | [39] |
| Colorimetric | Hydrogel sensor | 10 μM–5 mM | 10 μM | [43] |
| Colorimetric/Fluorescence | r-CDs (dual-mode detection) | 0.193 μM (colorimetric) 0.149 μM (fluorescence) | [44] | |
| Fluorescence | g-CNQDs/BPS-Fe2+ | 2.32–34.8 μM | [58] | |
| Fluorescence | Methanobactin-AuNPs | 0–8.0 μM 8.0–50.0 μM | 16.21 nM | [70] |
| Fluorescence | BDP-OPD | 0–4.0 μM | 0.17 nM | [75] |
| Biosensors | NrfA from S. oneidensis | 1–140 μM | 0.59 μM | [91] |
| Biosensors | LE antibody/Avidin/EDC-NHS/PEDOT-COOH/GCE |
0.2–9.5; 10.1–89.7; 90.3–590 μM | 0.2 μM | [94] |
| Nomenclature | Definitions and Explanations |
|---|---|
| 3-Aph/ADC | 3-aminophenylalanine/antibody coupled drug, a form of drug formed by linking 3-aminophenylalanine to an antibody |
| PAD | Paper-based analytical device |
| UV–Vis | Ultraviolet-Visible Spectroscopy |
| CuO NPs | copper oxide nanoparticles |
| g-CNQDs/BPS-Fe2+ | graphitic carbon nitrite quantum dots/Fe (II)-bathophenanthroline complex |
| BDP-OPD | practical sensing platform based on 8-(o-phenylenediamine)-boron dipyrromethene |
| NrfA | a pentahaem, C2-symmetric nitrite reductase with crystal size around 5.15 × 9.59 × 22.38 nm |
| S. oneidensis | Shewanella oneidensis, a Gram-negative bacterium |
| LE antibody | Leukocyte esterase antibody, which is an antibody that binds specifically to leukocyte esterase |
| Avidin | anti-biotin protein, a protein that binds specifically to biotin |
| EDC-NHS | 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and N-hydroxysuccinimide, commonly used in biocoupling reactions |
| PEDOT-COOH | Poly(3,4-ethylenedioxythiophene)-carboxylic acid, a conductive polymer |
| GCE | Glassy carbon electrode, a commonly used electrode material |
| Substance | Function |
|---|---|
| layered matrices adsorbed with cochineal | Coloring agent, replacing the coloring effect of nitrites |
| Met-Mb (strain) | Natural biological colorants and preservatives |
| black tea broth | Antioxidant, preventing food spoilage |
| BP (beetroot powder) | Adding color and flavor to food |
| Detection Methods | Advantages | Disadvantages |
|---|---|---|
| Ion Chromatography | High sensitivity and selectivity for accurate determination of nitrite in samples. Multiple anions can be determined simultaneously, making it suitable for the analysis of complex samples. High separation efficiency and good reproducibility, making it suitable for the analysis of large quantities of samples. | Instrumentation is expensive, with high operating and maintenance costs. Sample pre-treatment is complex and requires specialized operational skills. The analysis process is relatively slow and not suitable for rapid on-site testing. |
| Spectrophotometry | Easy to operate, fast, suitable for rapid on-site testing. The relatively low cost of the equipment makes it easy to popularize and use. Qualitative and quantitative analyses can be performed and are suitable for a wide range of sample types. | Relatively low sensitivity and selectivity and susceptible to interference from other pigments in the sample. Requires the use of toxic color developers, with potential risks to the environment and operators. Analytical results may be affected by experimental conditions such as temperature, pH, etc. |
| Colorimetric | Simple and inexpensive to operate, suitable for initial screening and on-site testing. Qualitative results can be obtained quickly and are suitable for preliminary analysis of large samples. | Low sensitivity and accuracy, not suitable for micro or trace analysis. Affected by sample color and turbidity and may require complex pre-treatment steps. Usually only suitable for specific types of samples and has limited applications. |
| Fluorescence | High sensitivity and low detection limit, suitable for the detection of micro and trace nitrite. Good selectivity reduces interference due to sample complexity. Allows real-time monitoring and imaging, suitable for analyzing dynamic processes. | Equipment is costly and requires specialized operation and maintenance. Sensitive to environmental conditions, e.g., changes in temperature, and pH may affect the fluorescence signal. Requires the use of specific fluorescent probes, which may have stability and toxicity issues. |
| Biosensors | Rapid response allows for real-time monitoring. High sensitivity, can detect low concentrations of nitrite. Portable equipment, suitable for on-site testing and mobile laboratory use. | Sensor stability and repeatability may be challenged. Periodic calibration and sensor replacement may be required. For complex samples, additional sample pre-treatment steps may be required. |
| Electrochemical sensors | High sensitivity and fast response time for rapid on-site detection. Specific electrode materials can be designed to improve selectivity and stability. Relatively low equipment cost and easy to deploy on a large scale. | May be interfered with by other electroactive substances in the sample. The electrode surface may be contaminated and require periodic cleaning and maintenance. For some applications, complex electrode preparation and modification processes may be required. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Song, Y.; Zhou, B.; Xu, H. Nitrite: From Application to Detection and Development. Appl. Sci. 2024, 14, 9027. https://doi.org/10.3390/app14199027
Li H, Song Y, Zhou B, Xu H. Nitrite: From Application to Detection and Development. Applied Sciences. 2024; 14(19):9027. https://doi.org/10.3390/app14199027
Chicago/Turabian StyleLi, Haoneng, Yang Song, Baoqing Zhou, and Hengyi Xu. 2024. "Nitrite: From Application to Detection and Development" Applied Sciences 14, no. 19: 9027. https://doi.org/10.3390/app14199027









