The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge
Abstract
:1. Introduction
1.1. The End-Stage Kidney Disease and the History of Kidney Replacement Therapy
1.2. Current Extracorporeal Purification between Limitations and New Perspectives
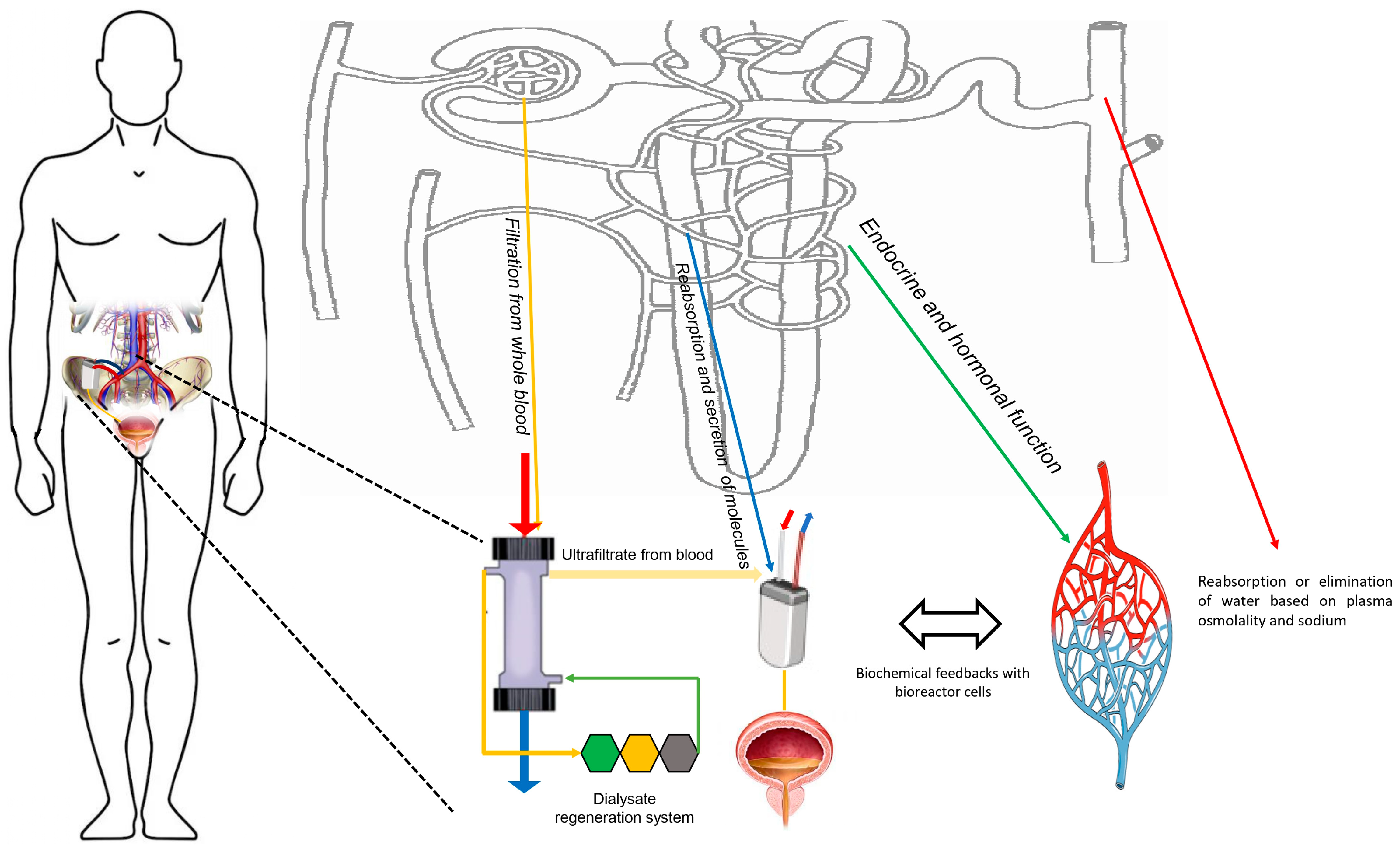
1.3. Bioreactors for Artificial Kidneys
2. Future Perspective beyond Current Technical Limitations
3. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Kolff, W.J. First Clinical Experience with the Artificial Kidney. Ann. Intern. Med. 1965, 62, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Kurkus, J.; Ostrowski, J. Nils Alwall and his artificial kidneys: Seventieth anniversary of the start of serial production. Artif. Organs 2019, 43, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.S.; Tsai, Y.C.; Chen, T.W.; Li, S.Y. Artificial Kidney Engineering: The Development of Dialysis Membranes for Blood Purification. Membranes 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]
- Jean, G.; Souberbielle, J.C.; Chazot, C. Vitamin D in Chronic Kidney Disease and Dialysis Patients. Nutrients 2017, 9, 328. [Google Scholar] [CrossRef] [PubMed]
- Wesson, D.E.; Buysse, J.M.; Bushinsky, D.A. Mechanisms of Metabolic Acidosis-Induced Kidney Injury in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.D.; Verlander, J.W. Renal ammonia metabolism and transport. Compr. Physiol. 2013, 3, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.A.; Bridges, R.J.; Meister, A. Extracellular metabolism of glutathione accounts for its disappearance from the basolateral circulation of the kidney. J. Biol. Chem. 1984, 259, 15393–15400. [Google Scholar] [CrossRef]
- Jelkmann, W. Regulation of erythropoietin production. J. Physiol. 2011, 589, 1251–1258. [Google Scholar] [CrossRef]
- Fissell, W.H.; Dyke, D.B.; Weitzel, W.F.; Buffington, D.A.; Westover, A.J.; MacKay, S.M.; Gutierrez, J.M.; Humes, H.D. Bioartificial kidney alters cytokine response and hemodynamics in endotoxin-challenged uremic animals. Blood Purif. 2002, 20, 55–60. [Google Scholar] [CrossRef]
- Yang, F.; Liao, M.; Wang, P.; Yang, Z.; Liu, Y. The Cost-Effectiveness of Kidney Replacement Therapy Modalities: A Systematic Review of Full Economic Evaluations. Appl. Health Econ. Health Policy 2021, 19, 163–180. [Google Scholar] [CrossRef]
- Bonenkamp, A.A.; van Eck van der Sluijs, A.; Hoekstra, T.; Verhaar, M.C.; van Ittersum, F.J.; Abrahams, A.C.; van Jaarsveld, B.C. Health-Related Quality of Life in Home Dialysis Patients Compared to In-Center Hemodialysis Patients: A Systematic Review and Meta-analysis. Kidney Med. 2020, 2, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Filipčič, T.; Bogataj, Š.; Pajek, J.; Pajek, M. Physical Activity and Quality of Life in Hemodialysis Patients and Healthy Controls: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; McLeggon, J.A.; Mehta, N.; Leung, S.; Barta, V.; McGinn, T.; Nesrallah, G. Mortality and Hospitalizations in Intensive Dialysis: A Systematic Review and Meta-Analysis. Can. J. Kidney Health Dis. 2018, 5, 2054358117749531. [Google Scholar] [CrossRef] [PubMed]
- Kjellstrand, C.M.; Buoncristiani, U.; Ting, G.; Traeger, J.; Piccoli, G.B.; Sibai-Galland, R.; Young, B.A.; Blagg, C.R. Short daily hemodialysis: Survival in 415 patients treated for 1006 patient-years. Nephrol. Dial. Transplant 2008, 23, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Groth, T.; Stegmayr, B.G.; Ash, S.R.; Kuchinka, J.; Wieringa, F.P.; Fissell, W.H.; Roy, S. Wearable and implantable artificial kidney devices for end-stage kidney disease treatment: Current status and review. Artif. Organs 2023, 47, 649–666. [Google Scholar] [CrossRef] [PubMed]
- Gura, V.; Rivara, M.B.; Bieber, S.; Munshi, R.; Smith, N.C.; Linke, L.; Kundzins, J.; Beizai, M.; Ezon, C.; Kessler, L.; et al. A wearable artificial kidney for patients with end-stage renal disease. JCI Insight 2016, 1, e86397. [Google Scholar] [CrossRef]
- Wester, M.; Gerritsen, K.G.; Simonis, F.; Boer, W.H.; Hazenbrink, D.H.; Vaessen, K.R.; Verhaar, M.C.; Joles, J.A. A regenerable potassium and phosphate sorbent system to enhance dialysis efficacy and device portability: A study in awake goats. Nephrol. Dial. Transplant 2017, 32, 951–959. [Google Scholar] [CrossRef]
- Lee, S.; Sirich, T.L.; Blanco, I.J.; Plummer, N.S.; Meyer, T.W. Removal of Uremic Solutes from Dialysate by Activated Carbon. Clin. J. Am. Soc. Nephrol. 2022, 17, 1168–1175. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef]
- Blumenkrantz, M.J.; Gordon, A.; Roberts, M.; Lewin, A.J.; Pecker, E.A.; Moran, J.K.; Coburn, J.W.; Maxwell, M.H. Applications of the Redy sorbent system to hemodialysis and peritoneal dialysis. Artif. Organs 1979, 3, 230–236. [Google Scholar] [CrossRef]
- van Gelder, M.K.; Jong, J.A.W.; Folkertsma, L.; Guo, Y.; Blüchel, C.; Verhaar, M.C.; Odijk, M.; Van Nostrum, C.F.; Hennink, W.E.; Gerritsen, K.G.F. Urea removal strategies for dialysate regeneration in a wearable artificial kidney. Biomaterials 2020, 234, 119735. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.H.; Cheah, W.K.; Sim, Y.L.; Pung, S.Y.; Yeoh, F.Y. Conversion and characterization of activated carbon fiber derived from palm empty fruit bunch waste and its kinetic study on urea adsorption. J. Environ. Manag. 2017, 197, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ramada, D.L.; de Vries, J.; Vollenbroek, J.; Noor, N.; Ter Beek, O.; Mihăilă, S.M.; Wieringa, F.; Masereeuw, R.; Gerritsen, K.; Stamatialis, D. Portable, wearable and implantable artificial kidney systems: Needs, opportunities and challenges. Nat. Rev. Nephrol. 2023, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Storr, M.; Ward, R.A. Membrane innovation: Closer to native kidneys. Nephrol. Dial. Transpl. 2018, 33, iii22–iii27. [Google Scholar] [CrossRef]
- Geremia, I.; Stamatialis, D. Innovations in dialysis membranes for improved kidney replacement therapy. Nat. Rev. Nephrol. 2020, 16, 550–551. [Google Scholar] [CrossRef] [PubMed]
- Gura, K.M.; Mulberg, A.E.; Mitchell, P.D.; Yap, J.; Kim, C.Y.; Chen, M.; Potemkin, A.; Puder, M. Pediatric Intestinal Failure-Associated Liver Disease: Challenges in Identifying Clinically Relevant Biomarkers. JPEN J. Parenter. Enteral. Nutr. 2016, 42, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Chen, C.; Gologorsky, R.; Santandreu, A.; Torres, A.; Wright, N.; Goodin, M.S.; Moyer, J.; Chui, B.W.; Blaha, C.; et al. Feasibility of an implantable bioreactor for renal cell therapy using silicon nanopore membranes. Nat. Commun. 2023, 14, 4890. [Google Scholar] [CrossRef]
- Zawada, A.M.; Melchior, P.; Erlenkötter, A.; Delinski, D.; Stauss-Grabo, M.; Kennedy, J.P. Polyvinylpyrrolidone in hemodialysis membranes: Impact on platelet loss during hemodialysis. Hemodial. Int. 2021, 25, 498–506. [Google Scholar] [CrossRef]
- Namekawa, K.; Matsuda, M.; Fukuda, M.; Kaneko, A.; Sakai, K. Poly(N-vinyl-2-pyrrolidone) elution from polysulfone dialysis membranes by varying solvent and wall shear stress. J. Artif. Organs 2012, 15, 185–192. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, X.; Yang, S.; Zhang, Q.; Zhao, L. Preparation of anticoagulant polyvinylidene fluoride hollow fiber hemodialysis membranes. Biomed Tech. 2017, 62, 57–65. [Google Scholar] [CrossRef]
- Fu, X.; Ning, J.P. Synthesis and biocompatibility of an argatroban-modified polysulfone membrane that directly inhibits thrombosis. J. Mater. Sci. Mater. Med. 2018, 29, 66. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Thodi, F.V.; Sengupta, S.; Kannan, S.; Krishnan, L.; Bhattacharya, E. Correction to: Effective clearance of uremic toxins using functionalised silicon Nanoporous membranes. Biomed. Microdevices 2021, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Fissell, W.H.; Dubnisheva, A.; Eldridge, A.N.; Fleischman, A.J.; Zydney, A.L.; Roy, S. High-Performance Silicon Nanopore Hemofiltration Membranes. J. Memb. Sci. 2009, 326, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Kensinger, C.; Karp, S.; Kant, R.; Chui, B.W.; Goldman, K.; Yeager, T.; Gould, E.R.; Buck, A.; Laneve, D.C.; Groszek, J.J.; et al. First Implantation of Silicon Nanopore Membrane Hemofilters. Asaio J. 2016, 62, 491–495. [Google Scholar] [CrossRef]
- Geremia, I.; Bansal, R.; Stamatialis, D. In vitro assessment of mixed matrix hemodialysis membrane for achieving endotoxin-free dialysate combined with high removal of uremic toxins from human plasma. Acta Biomater. 2019, 90, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Buffington, D.A.; Pino, C.J.; Chen, L.; Westover, A.J.; Hageman, G.; Humes, H.D. Bioartificial Renal Epithelial Cell System (BRECS): A Compact, Cryopreservable Extracorporeal Renal Replacement Device. Cell Med. 2012, 4, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Schophuizen, C.M.; Wilmer, M.J.; Jansen, J.; Gustavsson, L.; Hilgendorf, C.; Hoenderop, J.G.; van den Heuvel, L.P.; Masereeuw, R. Cationic uremic toxins affect human renal proximal tubule cell functioning through interaction with the organic cation transporter. Pflug. Arch. 2013, 465, 1701–1714. [Google Scholar] [CrossRef] [PubMed]
- Nieskens, T.T.; Peters, J.G.; Schreurs, M.J.; Smits, N.; Woestenenk, R.; Jansen, K.; van der Made, T.K.; Röring, M.; Hilgendorf, C.; Wilmer, M.J.; et al. A Human Renal Proximal Tubule Cell Line with Stable Organic Anion Transporter 1 and 3 Expression Predictive for Antiviral-Induced Toxicity. AAPS J. 2016, 18, 465–475. [Google Scholar] [CrossRef]
- Castro, A.C.; Neri, M.; Nayak Karopadi, A.; Lorenzin, A.; Marchionna, N.; Ronco, C. Wearable artificial kidney and wearable ultrafiltration device vascular access-future directions. Clin. Kidney J. 2019, 12, 300–307. [Google Scholar] [CrossRef]
- Liu, X.; Li, S. An electromagnetic microvalve for pneumatic control of microfluidic systems. J. Lab. Autom. 2014, 19, 444–453. [Google Scholar] [CrossRef]
- Kim, J.C.; Garzotto, F.; Nalesso, F.; Cruz, D.; Kim, J.H.; Kang, E.; Kim, H.C.; Ronco, C. A wearable artificial kidney: Technical requirements and potential solutions. Expert Rev. Med. Devices 2011, 8, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Humes, H.D.; Buffington, D.A.; MacKay, S.M.; Funke, A.J.; Weitzel, W.F. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat. Biotechnol. 1999, 17, 451–455. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, M.K.; Mihaila, S.M.; Jansen, J.; Wester, M.; Verhaar, M.C.; Joles, J.A.; Stamatialis, D.; Masereeuw, R.; Gerritsen, K.G.F. From portable dialysis to a bioengineered kidney. Expert Rev. Med. Devices 2018, 15, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Bonello, M.; House, A.A.; Cruz, D.; Asuman, Y.; Andrikos, E.; Petras, D.; Strazzabosco, M.; Ronco, F.; Brendolan, A.; Crepaldi, C.; et al. Integration of blood volume, blood pressure, heart rate and bioimpedance monitoring for the achievement of optimal dry body weight during chronic hemodialysis. Int. J. Artif. Organs 2007, 30, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Ronco, C.; Kim, J.C.; Garzotto, F.; Galavotti, D.; Bellini, C.; Brolgli, M.; Nalesso, F. Hydrodynamic analysis of the miniaturized hemofilter for a wearable ultrafiltration device. Blood Purif. 2013, 35, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Gura, V.; Ronco, C.; Nalesso, F.; Brendolan, A.; Beizai, M.; Ezon, C.; Davenport, A.; Rambod, E. A wearable hemofilter for continuous ambulatory ultrafiltration. Kidney Int. 2008, 73, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.; Ronco, C.; Gura, V. From wearable ultrafiltration device to wearable artificial kidney. Contrib. Nephrol. 2011, 171, 237–242. [Google Scholar] [CrossRef]
- Nahak, B.K.; Mishra, A.; Preetam, S.; Tiwari, A. Advances in Organ-on-a-Chip Materials and Devices. ACS Appl. Bio Mater. 2022, 5, 3576–3607. [Google Scholar] [CrossRef]
- Huff, C. How artificial kidneys and miniaturized dialysis could save millions of lives. Nature 2020, 579, 186–188. [Google Scholar] [CrossRef]
- Li, L.; Marchant, R.E.; Dubnisheva, A.; Roy, S.; Fissell, W.H. Anti-biofouling Sulfobetaine Polymer Thin Films on Silicon and Silicon Nanopore Membranes. J. Biomater. Sci. Polym. Ed. 2011, 22, 91–106. [Google Scholar] [CrossRef]
- Armignacco, P.; Lorenzin, A.; Neri, M.; Nalesso, F.; Garzotto, F.; Ronco, C. Wearable devices for blood purification: Principles, miniaturization, and technical challenges. Semin. Dial. 2015, 28, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Fransen, M.F.J.; Addario, G.; Bouten, C.V.C.; Halary, F.; Moroni, L.; Mota, C. Bioprinting of kidney in vitro models: Cells, biomaterials, and manufacturing techniques. Essays Biochem. 2021, 65, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Nishinakamura, R. Advances and challenges toward developing kidney organoids for clinical applications. Cell Stem Cell 2023, 30, 1017–1027. [Google Scholar] [CrossRef]
| Device | Type of Device | Mechanism of Purification | Limits | Future Developments | Integration in IBAK Implantable |
|---|---|---|---|---|---|
| Battery | Source of electric power | device pumps support | time-limited duration | long-lasting durability | Yes |
| Pumps | Peristaltic pumps or other pumps | maintaining the flow of fluids involved in purification | energy consumption, range of fluid flows achievable | improved energy, mechanical, and miniaturization efficiency | Yes |
| Bags of fluids | Fluid for hemodiafiltration | diffusion and/or convection | limited usable volume in implantable devices | on-line fluid regeneration systems | No, required closed dialysate circuits or closed ultrafiltration/reinfusion circuits after fluid regeneration |
| Filter for HD | Filter for diffusion | diffusion of molecules | range of molecules removed (molecular weight below albumin) | greater selectivity for molecules with possible adsorption on the membrane surface | Yes, with limitations for the spectrum of diffusible molecules and the generation of fresh dialysate |
| Filter for HF | Filter for ultrafiltration | convection of molecules | range of molecules removed (molecular weight below albumin) | greater selectivity for molecules with possible adsorption on the membrane surface | Yes, with limitations for the generation of infusion fluids |
| Filter for HDF | Filter for hemodiafiltration | diffusion and convection of molecules | range of molecules removed (molecular weight below albumin) | greater selectivity for molecules with possible adsorption on the membrane surface | Yes, with limitations for the generation of infusion and dialysate fluids |
| Cartridge for adsorption | Adsorption on blood | physisorption, chemisorption | hemocompatibility, saturation, selectivity | greater selectivity, haemocompatibility, and durability; combination of several cartridges | Yes, with limitations for device lifetime and selectivity |
| Bioreactor | Bioreactor with kidney cells | metabolic mechanism, endocrine function, possible reabsorption and processing of useful molecules from the ultrafiltrate | reduced number of usable cells, limited cell life, limited total device lifetime | immortal cell lines, longer overall life of the implanted device | Yes, with a limitation for device lifetime |
| Reinfusion of electrolytes | fluids | correction of electrolyte and acid-base disorders | limited usable volume in implantable devices | regeneration systems based on absorption and chemical reactions | Very difficult for high-volume requirements |
| Device | Filtration Function of Glomerulus | Molecules Reabsorption by Tubular Nephron Cells | Molecule Secretion by Tubular Nephron Cells | Endocrine Function | Water Reabsorption by Distal Nephron | Uremic Toxins Purification |
|---|---|---|---|---|---|---|
| Filter for HD | NO | NO | NO | NO | NO | narrow spectrum |
| Filter for HF | NO | NO | NO | NO | NO | broadened spectrum |
| Filter for HDF | NO | NO | NO | NO | NO | broadened spectrum |
| Cartridge for adsorption | NO | NO | NO | NO | NO | broadened spectrum |
| Bioreactor | YES | YES | YES | NO | NO | |
| Bioreactor combined with filtration system | YES | YES | YES | YES | NO | broadened spectrum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nalesso, F.; Garzotto, F.; Cattarin, L.; Bettin, E.; Cacciapuoti, M.; Silvestre, C.; Stefanelli, L.F.; Furian, L.; Calò, L.A. The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge. Appl. Sci. 2024, 14, 491. https://doi.org/10.3390/app14020491
Nalesso F, Garzotto F, Cattarin L, Bettin E, Cacciapuoti M, Silvestre C, Stefanelli LF, Furian L, Calò LA. The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge. Applied Sciences. 2024; 14(2):491. https://doi.org/10.3390/app14020491
Chicago/Turabian StyleNalesso, Federico, Francesco Garzotto, Leda Cattarin, Elisabetta Bettin, Martina Cacciapuoti, Cristina Silvestre, Lucia F. Stefanelli, Lucrezia Furian, and Lorenzo A. Calò. 2024. "The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge" Applied Sciences 14, no. 2: 491. https://doi.org/10.3390/app14020491
APA StyleNalesso, F., Garzotto, F., Cattarin, L., Bettin, E., Cacciapuoti, M., Silvestre, C., Stefanelli, L. F., Furian, L., & Calò, L. A. (2024). The Future for End-Stage Kidney Disease Treatment: Implantable Bioartificial Kidney Challenge. Applied Sciences, 14(2), 491. https://doi.org/10.3390/app14020491








