Sustainable Recovery of an Agricultural Area Impacted by an Oil Spill Using Enhanced Phytoremediation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Soil Characterization
2.3. Hydrocarbon Oxidizing Bacteria Isolation and Characterization
2.4. In Vitro Evaluation of PGP Properties
2.5. Microcosm Tests
- Four soil types: A, B, C and D (as described in Table 1), with 500 g of contaminated soil per pot;
- Three plant species: Medicago sativa (alfalfa)—0.8 g seeds, Zea mays (corn)—5 seeds, Lupinus albus (lupine)—6 seeds;
- Three different tests per plant and soil type: a control (CT) using neighboring uncontaminated agricultural land with similar characteristics, a “base” test using contaminated soil, and an additional test by adding PGPB inoculum to the contaminated soil.
2.6. Mesocosm Tests
2.7. GC-MS Analysis of Samples
- Thermal gradient of 40 °C (isothermal for 2 min) with a ramp of 7 °C/min up to 270 °C;
- Tamp of 15 °C/min up to 320 °C;
- A 320 °C isotherm for 10 min;
- “SCAN” mode (mass from 50 to 600).
2.8. Statistical Analysis
3. Results and Discussion
3.1. Properties of the PGPB Inoculum
3.2. Plant Response at Microcosm Scale
3.3. Contaminants Biodegradation at Microcosm Scale
3.4. PAHs Evolution in Plants and Soils
3.5. Mesocosm Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Little, D.I.; Sheppard, S.R.; Hulme, D. A perspective on oil spills: What we should have learned about global warming. Ocean Coast. Manag. 2021, 202, 105509. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Bhaskarwar, A.N. A review on sorbent devices for oil-spill control. Environ. Pollut. 2018, 243, 1758–1771. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; De Folly D’Auris, A.; Reverberi, A.P. A novel graphene-based sorbent for oil spill cleanup. Materials 2022, 5, 609. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, G.; Doulia, D. Morphology of polymeric resins in adsorption of organic pesticides. Fresenius Environ. Bull. 2007, 16, 731–734. [Google Scholar]
- Pietrelli, L.; Ferro, S.; Reverberi, A.P.; Vocciante, M. Removal of polyethylene glycols from wastewater: A comparison of different approaches. Chemosphere 2021, 273, 129725. [Google Scholar] [CrossRef] [PubMed]
- de Folly d’Auris, A.; Rubertelli, F.; Taini, A.; Vocciante, M. A novel polyurethane-based sorbent material for oil spills management. J. Environ. Chem. Eng. 2023, 11, 111386. [Google Scholar] [CrossRef]
- Yati, I.; Aydin, G.O.; Sonmez, H.B. Cross-linked poly (tetrahydrofuran) as promising sorbent for organic solvent/oil spill. J. Hazard. Mater. 2016, 309, 210–218. [Google Scholar] [CrossRef]
- Pavel, L.V.; Gavrilescu, M. Overview of ex situ decontamination techniques for soil cleanup. Environ. Eng. Manag. J. 2008, 7, 815–834. [Google Scholar] [CrossRef]
- Liu, J.W.; Wei, K.H.; Xu, S.W.; Cui, J.; Ma, J.; Xiao, X.L.; Xi, B.D.; He, X.S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent developments and prospects of sustainable remediation treatments for major contaminants in soil: A review. Sci. Total Environ. 2023, 912, 168769. [Google Scholar] [CrossRef]
- Hou, D.; Bolan, N.S.; Tsang, D.C.W.; Kirkham, M.B.; O’Connor, D. Sustainable soil use and management: An interdisciplinary and systematic approach. Sci. Total Environ. 2020, 729, 138961. [Google Scholar] [CrossRef] [PubMed]
- Vocciante, M.; De Follis D’Auris, A.; Franchi, E.; Petruzzelli, G.; Ferro, S. CO2 footprint analysis of consolidated and innovative technologies in remediation activities. J. Clean. Prod. 2021, 297, 126723. [Google Scholar] [CrossRef]
- Conte, A.; Chiaberge, S.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Vocciante, M.; Franchi, E.; Pietrini, I. Dealing with complex contamination: A novel approach with a combined bio-phytoremediation strategy and effective analytical techniques. J. Environ. Manag. 2021, 288, 112381. [Google Scholar] [CrossRef]
- Chojnacka, K.; Moustakas, K.; Mikulewicz, M. The combined rhizoremediation by a triad: Plant-microorganism-functional materials. Environ. Sci. Pollut. Res. Int. 2023, 30, 90500–90521. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Cirrincione, L.; La Gennusa, M.; Peri, G.; Scaccianoce, G. Green Roofs’ End of Life: A Literature Review. Energies 2023, 16, 596. [Google Scholar] [CrossRef]
- Llorach-Massana, P.; Cirrincione, L.; Sierra-Perez, J.; Scaccianoce, G.; La Gennusa, M.; Peña, J.; Rieradevall, J. Environmental assessment of a new building envelope material derived from urban agriculture wastes: The case of the tomato plants stems. Int. J. Life Cycle Assess. 2023, 28, 813–827. [Google Scholar] [CrossRef]
- Pandey, V.C.; Gajic, G.; Sharma, P.; Roy, M. Adaptive phytoremediation practices for sustaining ecosystem services. In Adaptive Phytoremediation Practices: Resilience to Climate Change; Elsevier: Amsterdam, The Netherlands, 2022; pp. 181–225. [Google Scholar] [CrossRef]
- Peri, G.; Licciardi, G.R.; Matera, N.; Mazzeo, D.; Cirrincione, L.; Scaccianoce, G. Disposal of green roofs: A contribution to identifying an “Allowed by legislation” end–of–life scenario and facilitating their environmental analysis. Build. Environ. 2022, 226, 109739. [Google Scholar] [CrossRef]
- Capitano, C.; Cirrincione, L.; Peri, G.; Rizzo, G.; Scaccianoce, G. A simplified method for the indirect evaluation of the “embodied pollution” of natural stones (marble) working chain to be applied for achieving the Ecolabel brand of the product. J. Clean. Prod. 2022, 362, 132576. [Google Scholar] [CrossRef]
- Ashraf, S.S.; Ali, Q.; Zahir, Z.A.; Ashraf, S.S.; Asghar, H.N. Phytoremediation: Environmentally sustainable way for reclamation of heavy metal polluted soils. Ecotoxicol. Environ. Saf. 2019, 174, 714–727. [Google Scholar] [CrossRef]
- Nawrot, N.; Wojciechowska, E.; Mohsin, M.; Kuittinen, S.; Pappinen, A.; Matej-Łukowicz, K.; Szczepańska, K.; Cichowska, A.; Irshad, M.A.; Tack, F.M. Chromium (III) removal by perennial emerging macrophytes in floating treatment wetlands. Sci. Rep. 2023, 13, 22417. [Google Scholar] [CrossRef]
- Pedron, F.; Grifoni, M.; Barbafieri, M.; Petruzzelli, G.; Rosellini, I.; Franchi, E.; Bagatin, R.; Vocciante, M. Applicability of a Freundlich-like model for plant uptake at an industrial contaminated site with a high variable arsenic concentration. Environments 2017, 4, 67. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Hosseinniaee, S.; Jafari, M.; Tavili, A.; Zare, S.; Cappai, G. Chelate facilitated phytoextraction of Pb, Cd, and Zn from a lead-zinc mine contaminated soil by three accumulator plants. Sci. Rep. 2023, 13, 21185. [Google Scholar] [CrossRef]
- Kotoky, R.; Pandey, P. Rhizosphere mediated biodegradation of benzo(A)pyrene by surfactin producing soil bacilli applied through Melia azedarach rhizosphere. Int. J. Phytoremed. 2020, 22, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Kamran, M.; Javed, M.T.; Hayat, K.; Farooq, M.A.; Ali, N.; Ali, M.; Manghwar, H.; Jan, F.; Chaudhary, H.J. Individual and combinatorial application of Kocuria rhizophila and citric acid on phytoextraction of multi-metal contaminated soils by Glycine max L. Environ. Exp. Bot. 2019, 159, 23–33. [Google Scholar] [CrossRef]
- Manoj, S.R.; Karthik, C.; Kadirvelu, K.; Arulselvi, P.I.; Shanmugasundaram, T.; Bruno, B.; Rajkumar, M. Understanding the molecular mechanisms for the enhanced phytoremediation of heavy metals through plant growth promoting rhizobacteria: A review. J. Environ. Manag. 2020, 254, 109779. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Butt, T.A.; Naqvi, S.T.A.; Yousaf, S.; Qureshi, M.K.; Zafar, M.I.; Farooq, G.; Nawaz, I.; Iqbal, M. Lead tolerant endophyte Trametes hirsuta improved the growth and lead accumulation in the vegetative parts of Triticum aestivum L. Heliyon 2020, 6, e04188. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh-Dahaji, P.; Omidvari, M.; Ghorbanpour, M. Increasing phytoremediation efficiency of heavy metal-contaminated soil using PGPR for sustainable agriculture. In Plant-Microbe Interaction: An Approach to Sustainable Agriculture; Choudhary, D.K., Varma, A., Narendra, T., Eds.; Springer: Singapore, 2017; pp. 187–204. [Google Scholar] [CrossRef]
- Franchi, E.; Barbafieri, M.; Petruzzelli, G.; Ferro, S.; Vocciante, M. Screening of plants and indigenous bacteria to improve arsenic phytoextraction. Appl. Sci. 2022, 12, 7267. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; He, X.; Li, B.; Du, S. Plant growth-promoting rhizobacteria: A good companion for heavy metal phytoremediation. Chemosphere 2023, 338, 139475. [Google Scholar] [CrossRef]
- Wyszkowski, M.; Sivitskaya, V. Changes in the content of organic carbon and available forms of macronutrients in soil under the influence of soil contamination with fuel oil and application of different substances. J. Elem. 2012, 17, 139–148. [Google Scholar] [CrossRef]
- Zhang, S.; Yao, H.; Lu, Y.; Yu, X.; Wang, J.; Sun, S.; Liu, M.; Li, D.; Li, Y.F.; Zhang, D. Uptake and translocation of polycyclic aromatic hydrocarbons (PAHs) and heavy metals by maize from soil irrigated with wastewater. Sci. Rep. 2017, 7, 12165. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.F.; da Cunha, D.L.; Daflon, S.D.A.; Machado, A.R.; Gaudie-Ley, L.W.; de Mattos, J., Jr.; da Fonseca, E.M. Bioaccumulation of PAHs in marine bivalves of the Santos Estuary (Brazil) associated with the evaluation of human consumption. Mar. Pollut. Bull. 2023, 199, 115900. [Google Scholar] [CrossRef] [PubMed]
- U.S. EPA. Available online: https://www.epa.gov/sites/default/files/2015-11/documents/pah-rpfs.pdf (accessed on 28 December 2023).
- Ławniczak, Ł.; Woźniak-Karczewska, M.; Loibner, A.P.; Heipieper, H.J.; Chrzanowski, Ł. Microbial Degradation of Hydrocarbons-Basic Principles for Bioremediation: A Review. Molecules 2020, 25, 856. [Google Scholar] [CrossRef]
- Omrani, R.; Spini, G.; Puglisi, E.; Saidane, D. Modulation of microbial consortia enriched from different polluted environments during petroleum biodegradation. Biodegradation 2018, 29, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, S.; Tecon, R.; Borer, B.; Or, D. The engineering of spatially linked microbial consortia—Potential and perspectives. Curr. Opin. Biotechnol. 2020, 62, 137–145. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Devi, A.; Kalita, M.C.; Deka, S. Uptake of total petroleum hydrocarbon (TPH) and polycyclic aromatic hydrocarbons (PAHs) by Oryza sativa L. Grown in soil contaminated with crude oil. Bull. Environ. Contam. Toxicol. 2017, 98, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Marchal, G.; Smith, K.E.C.; Mayer, P.; de Jonge, L.W.; Karlson, U.G. Impact of soil amendments and the plant rhizosphere on PAH behaviour in soil. Environ. Pollut. 2014, 188, 124–131. [Google Scholar] [CrossRef]
- Li, H.L.; Ma, Y.B. Field study on the uptake, accumulation, translocation and risk assessment of PAHs in a soil-wheat system with amendments of sewage sludge. Sci. Total Environ. 2016, 560, 55–61. [Google Scholar] [CrossRef]
- Dettenmaier, E.M.; Doucette, W.J.; Bugbee, B. Chemical Hydrophobicity and Uptake by Plant Roots. Environ. Sci. Technol. 2009, 43, 324–329. [Google Scholar] [CrossRef]
- Song, C.; Sarpong, C.K.; He, J.; Shen, F.; Zhang, J.; Yang, G.; Long, L.; Tian, D.; Zhu, Y.; Deng, S. Accelerating phytoremediation of degraded agricultural soils utilizing rhizobacteria and endophytes: A review. Environ. Rev. 2020, 28, 115–127. [Google Scholar] [CrossRef]
- Allamin, I.A.; Halmi, M.I.E.; Yasid, N.A.; Ahmad, S.A.; Abdullah, S.R.S.; Shukor, Y. Rhizodegradation of Petroleum Oily Sludge-contaminated Soil Using Cajanus cajan Increases the Diversity of Soil Microbial Community. Sci. Rep. 2020, 10, 4094. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Verma, I.; Banerjee, B.; Saleena, L.M. Unveiling bacterial consortium for xenobiotic biodegradation from Pichavaram mangrove forest soil: A metagenomic approach. Arch. Microbiol. 2023, 206, 27. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, P.; Schulthess, C.P.; Kuzovkina, Y.A.; Guillard, K. Bioremediation and phytoremediation of total petroleum hydrocarbons (TPH) under various conditions. Int. J. Phytoremed. 2017, 19, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Franchi, E.; Cardaci, A.; Pietrini, I.; Fusini, D.; Conte, A.; De Folly D’Auris, A.; Grifoni, M.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; et al. Nature-based solutions for restoring an agricultural area contaminated by an oil spill. Plants 2022, 11, 2250. [Google Scholar] [CrossRef] [PubMed]
- Gee, G.W.; Bauder, J.W. Particle-size analysis. In Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Agronomy Monograph No. 9, 2nd ed.; Klute, A., Ed.; American Society of Agronomy/Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Franchi, E.; Rolli, E.; Marasco, R.; Agazzi, G.; Borin, S.; Cosmina, P.; Pedron, F.; Rosellini, I.; Barbafieri, M.; Petruzzelli, G. Phytoremediation of a multi contaminated soil: Mercury and arsenic phytoextraction assisted by mobilizing agent and plant growth promoting bacteria. J. Soils Sediments 2017, 17, 1224–1236. [Google Scholar] [CrossRef]
- National Center for Biotechnology (NCBI). Available online: www.ncbi.nlm.nih.gov/blast/Blast.cgi (accessed on 28 December 2023).
- del Carmen Orozco-Mosqueda, M.; Fadiji, A.E.; Babalola, O.O.; Glick, B.R.; Santoyo, G. Rhizobiome engineering: Unveiling complex rhizosphere interactions to enhance plant growth and health. Microbiol. Res. 2022, 263, 127137. [Google Scholar] [CrossRef]
- Costa, O.Y.; Raaijmakers, J.M.; Kuramae, E.E. Microbial extracellular polymeric substances: Ecological function and impact on soil aggregation. Front. Microbiol. 2018, 9, 1636. [Google Scholar] [CrossRef]
- Santaella, C.; Schue, M.; Berge, O.; Heulin, T.; Achouak, W. The exopolysaccharide of Rhizobium sp. YAS34 is not necessary for biofilm formation on Arabidopsis thaliana and Brassica napus roots but contributes to root colonization. Environ. Microbiol. 2008, 10, 2150–2163. [Google Scholar] [CrossRef]
- Milagres, A.M.; Machuca, A.; Napoleão, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Shahab, S.; Ahmed, N.; Khan, N.S. Indole acetic acid production and enhanced plant growth promotion by indigenous PSBs. J. Agric. Res. 2009, 4, 1312–1316. [Google Scholar]
- Islam, M.R.; Madhaiyan, M.; Deka Boruah, H.P.; Yim, W.; Lee, G.; Saravanan, V.S.; Fu, Q.; Hu, H.; Sa, T. Characterization of plant growth-promoting traits of free-living diazotrophic bacteria and their inoculation effects on growth and nitrogen uptake of crop plants. J. Microbiol. Biotechnol. 2009, 19, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, R.; Jan Sørensen, J. Multi-target and medium-independent fungal antagonism by hydrolytic enzymes in Paenibacillus polymyxa and Bacillus pumilus strains from barley rhizosphere. FEMS Microbiol. Ecol. 1997, 22, 183–192. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Li, X.; Sun, P.; Zhang, Y.; Jin, C.; Guan, C. A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maise by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 2020, 174, 104023. [Google Scholar] [CrossRef]
- Saran, A.; Imperato, V.; Fernandez, L.; Gkorezis, P.; d’Haen, J.; Merini, L.J.; Vangronsveld, J.; Thijs, S. Phytostabilization of Polluted Military Soil Supported by Bioaugmentation with PGP-Trace Element Tolerant Bacteria Isolated from Helianthus petiolaris. Agronomy 2020, 10, 204. [Google Scholar] [CrossRef]
- Faddetta, T.; Polito, G.; Abbate, L.; Alibrandi, P.; Zerbo, M.; Caldiero, C.; Reina, C.; Puccio, G.; Vaccaro, E.; Abenavoli, M.R.; et al. Bioactive Metabolite Survey of Actinobacteria Showing Plant Growth Promoting Traits to Develop Novel Biofertilizers. Metabolites 2023, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Patel, A.K.; Gupta, D.; Singh, G.; Mishra, V.K. Rhizospheric remediation of organic pollutants from the soil; A green and sustainable technology for soil clean up. In Abatement of Environmental Pollutants; Singh, P., Kumar, A., Borthakur, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 263–286. [Google Scholar] [CrossRef]
- Grifoni, M.; Pedron, F.; Franchi, E.; Fusini, D.; Reverberi, A.P.; Vocciante, M. Green Remediation for the Sustainable Management of Oil Spills in Agricultural Areas. Chem. Eng. Trans. 2022, 94, 829–834. [Google Scholar] [CrossRef]
- Esmaeili, A.; Knox, O.; Juhasz, A.; Wilson, S.C. Advancing prediction of polycyclic aromatic hydrocarbon bioaccumulation in plants for historically contaminated soils using Lolium multiflorum and simple chemical in-vitro methodologies. Sci. Total Environ. 2021, 772, 144783. [Google Scholar] [CrossRef]
- Jia, J.; Bi, C.; Guo, X.; Wang, X.; Zhou, X.; Chen, Z. Characteristics, identification, and potential risk of polycyclic aromatic hydrocarbons in road dusts and agricultural soils from industrial sites in Shanghai, China. Environ. Sci. Pollut. Res. 2017, 24, 605–615. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Lü, H.; Tang, G.X.; Huang, Y.H.; Mo, C.H.; Zhao, H.M.; Xiang, L.; Li, Y.W.; Li, H.; Cai, Q.Y.; Li, Q.X. Response and adaptation of rhizosphere microbiome to organic pollutants with enriching pollutant-degraders and genes for bioremediation: A critical review. Sci. Total Environ. 2023, 912, 169425. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, I.; Race, M.; Papirio, S.; Esposito, G. Phytoremediation of pyrene-contaminated soils: A critical review of the key factors affecting the fate of pyrene. J. Environ. Manag. 2021, 293, 112805. [Google Scholar] [CrossRef] [PubMed]

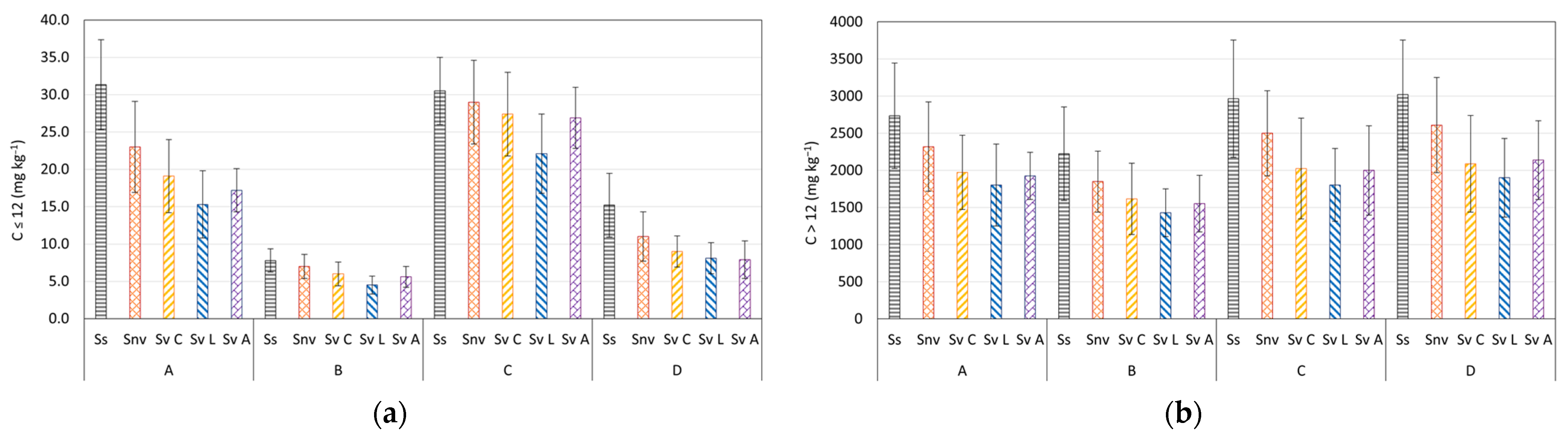
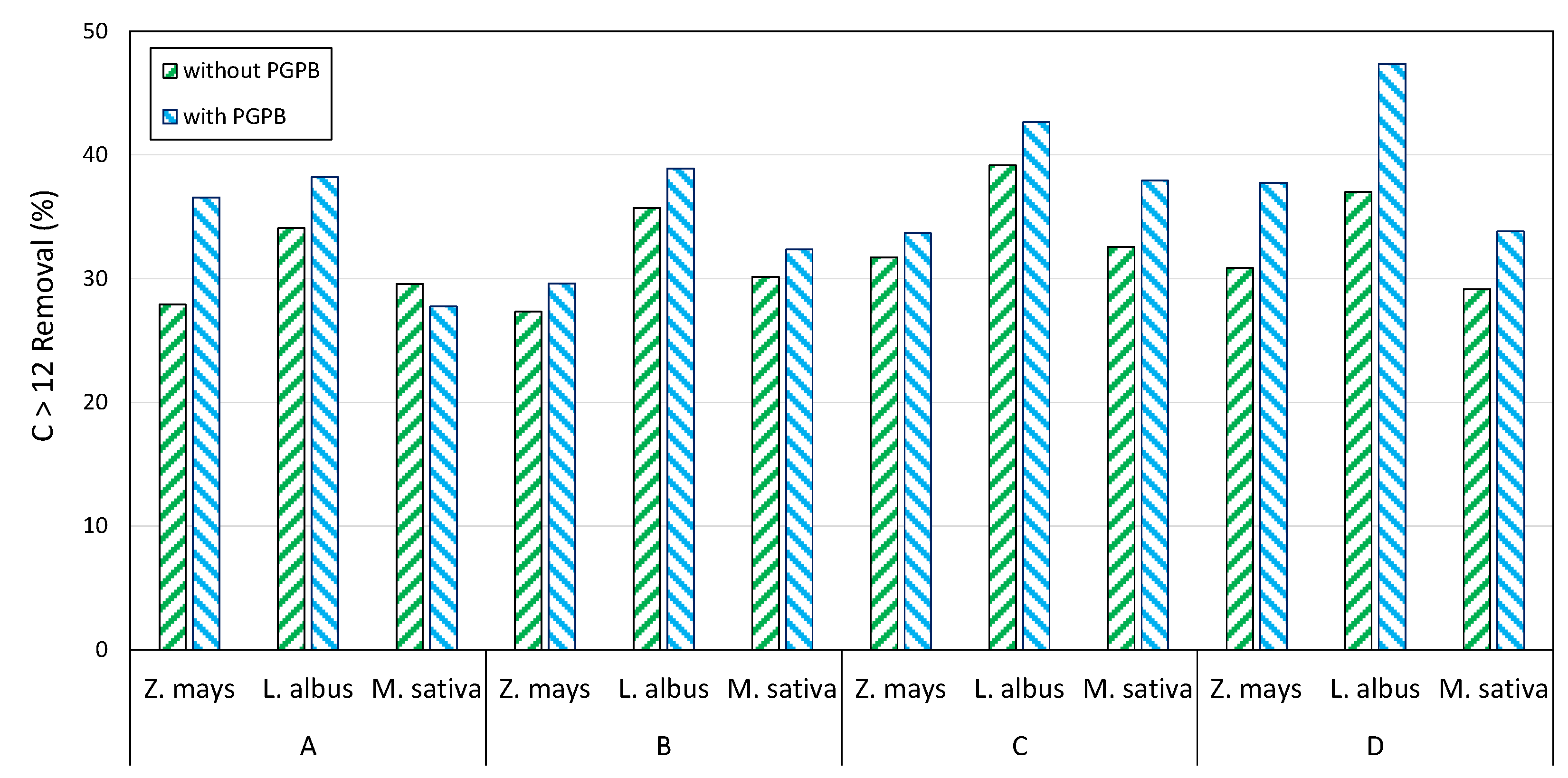
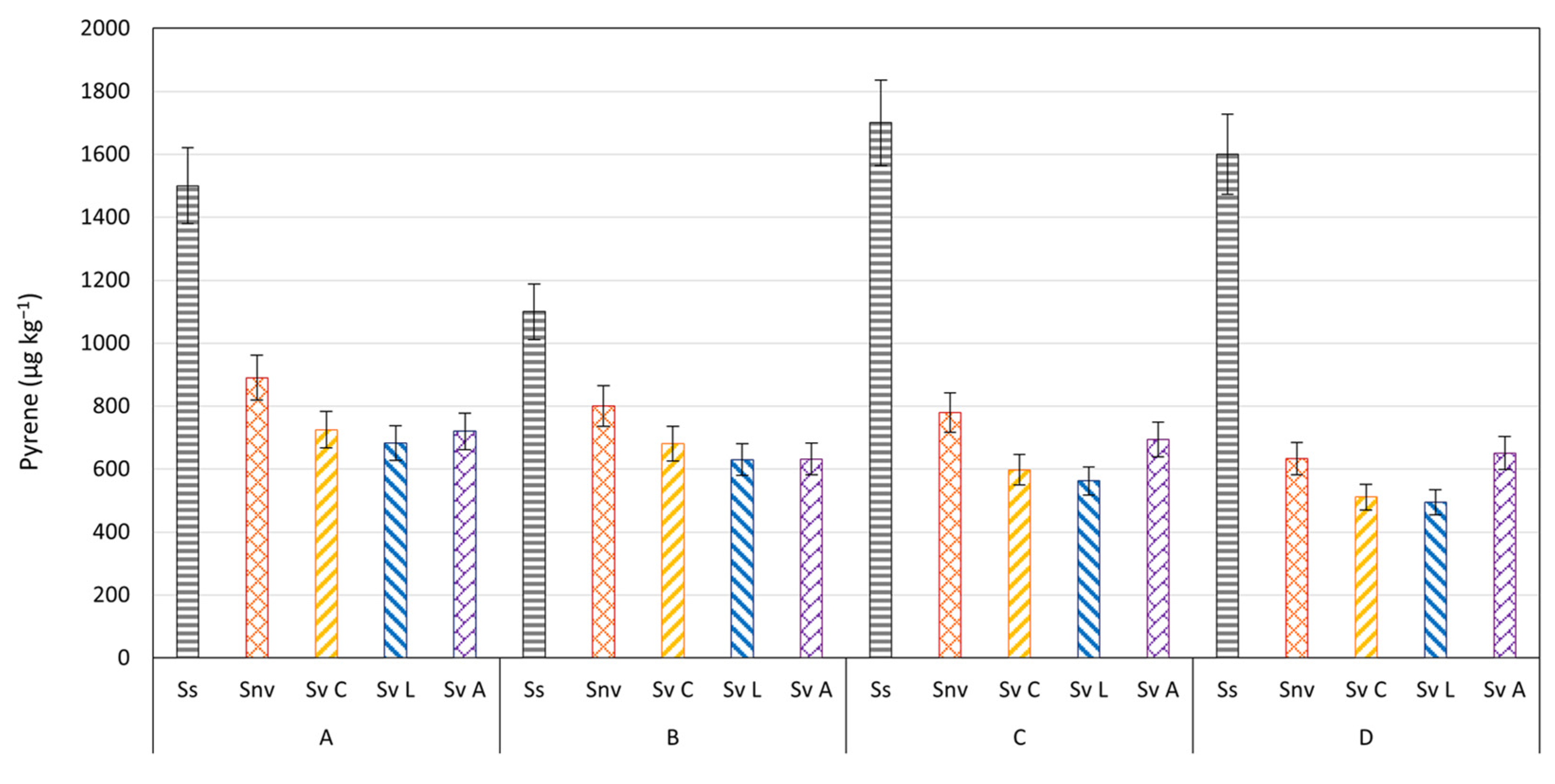
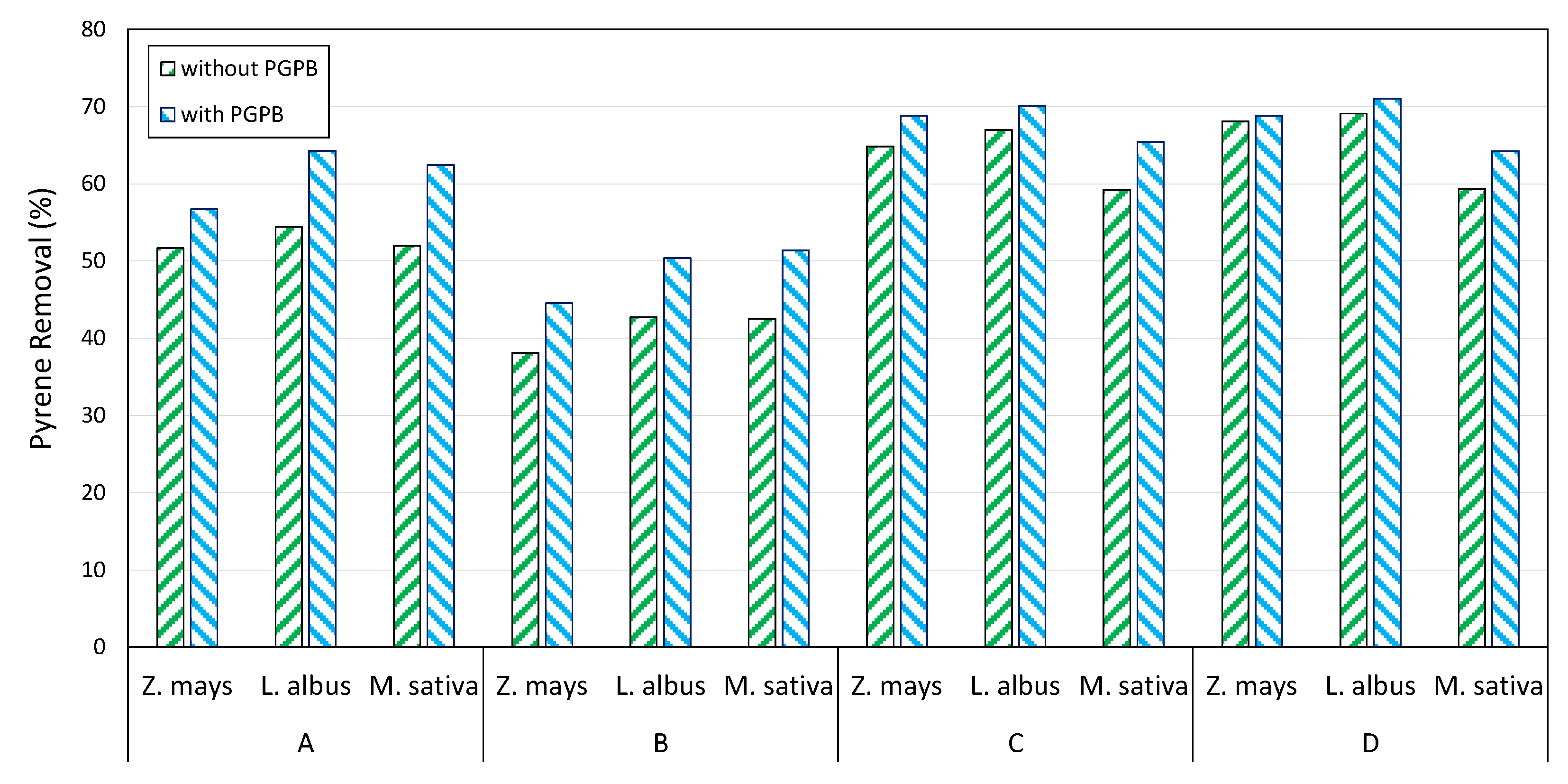
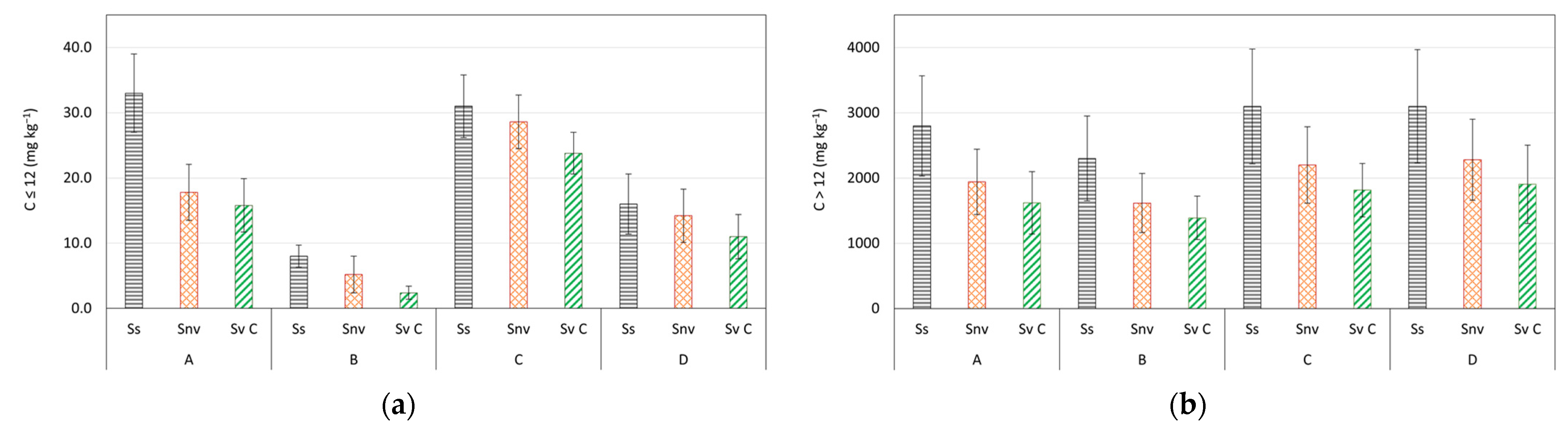
| Depth (m) | C ≤ 12 (mg kg−1) | C > 12 (mg kg−1) | Aromatics (mg kg−1) | Sand (%) | Clay (%) | Silt (%) | |
|---|---|---|---|---|---|---|---|
| A (BH3 + CO8 + PZ8) | 0–1 | 79.3 ± 3.7 | 3800 ± 97 | 1.41 ± 0.14 | 86.1 | 5.1 | 8.8 |
| B (CO5 + PZ9 + PZ7 + C13 + C41) | 0–1 | 50.6 ± 2.4 | 1820 ± 48 | 0.89 ± 0.09 | 88.6 | 4.3 | 7.1 |
| C (BH3 + PZ7 + C13 + C41) | 1–2 | 79.5 ± 3.5 | 3475 ± 85 | 2.89 ± 0.25 | 88.1 | 4.1 | 7.8 |
| D (CO5 + PZ9 + CO8 + PZ8) | 1–2 | 113.8 ± 5.1 | 4800 ± 115 | 5.55 ± 0.48 | 92.6 | 1.8 | 5.6 |
| Zea mays | Medicago sativa | Lupinus albus | ||||
|---|---|---|---|---|---|---|
| C ≤ 12 | C > 12 | C ≤ 12 | C > 12 | C ≤ 12 | C > 12 | |
| A | 0.91 ± 0.16 | 215 ± 100 | 4.10 ± 0.9 | 931 ± 217 | 0.83 ± 0.28 | 227 ± 51.6 |
| A+ | 1.07 ± 0.49 | 207 ± 35.3 | 4.30 ± 1.59 | 988 ± 144 | 1.16 ± 0.53 | 240 ± 42.1 |
| B | 0.98 ± 0.18 | 185 ± 117 | 3.40 ± 0.7 | 675 ± 149 | 1.10 ± 0.25 | 167 ± 85.9 |
| B+ | 1.07 ± 0.18 | 162 ± 53.6 | 2.50 ± 0.46 | 664 ± 81.5 | 0.91 ± 0.30 | 144 ± 51.6 |
| C | 0.90 ± 0.22 | 228 ± 133 | 4.60 ± 1.0 | 1050 ± 340 | 0.87 ± 0.46 | 273 ± 45 |
| C+ | 0.92 ± 0.21 | 238 ± 47.0 | 4.40 ± 1.28 | 1073 ± 239 | 0.95 ± 0.49 | 260 ± 97.0 |
| D | 0.85 ± 0.10 | 183 ± 111 | 4.30 ± 1.0 | 1271 ± 381 | 0.84 ± 0.33 | 264 ± 68.6 |
| D+ | 1.01 ± 0.14 | 180 ± 39.7 | 5.10 ± 0.35 | 1266 ± 302 | 0.88 ± 0.27 | 274 ± 49.1 |
| CT | n.d. | 122 ± 67.0 | 1.23 ± 0.81 | 253 ± 136 | 4.00 ± 1.56 | 265 ± 75 |
| A | B | |||||||||||||||
| Z. mays | L. albus | M. sativa | Z. mays | L. albus | M. sativa | |||||||||||
| Ss | Snv | Sv C | Sv C+ | Sv L | Sv L+ | Sv A | Sv A+ | Ss | Snv | Sv C | Sv C+ | Sv L | Sv L+ | Sv A | Sv A+ | |
| Benzo(a)pyrene | 0.74 c | 0.70 b | nd | nd | nd | nd | 0.65 ab | 0.58 a | 0.38 a | 0.74 b | nd | nd | nd | nd | nd | nd |
| Benzo(b)fluoranthene | 3.1 | nd | nd | nd | nd | nd | nd | nd | 2.1 | nd | nd | nd | nd | nd | nd | nd |
| Benzo(k)fluoranthene | 1.7 | nd | nd | nd | nd | nd | nd | nd | 1.0 | nd | nd | nd | nd | nd | nd | nd |
| Benzo(g,h,i)perylene | 8.6 e | 7.2 d | 6.2 c | 5.7 c | 4.2 b | 3.1 a | 6.5 c | 5.5 c | 5.4 b | 6.8 c | 5.2 b | 5.1 b | 3.4 a | 2.9 a | 5.2 b | 5.0 b |
| Dibenzo(a,i)pyrene | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Chrysene | 28.0 c | 21.0 b | 17.8 a | 16.4 a | 16.5 a | 15.4 a | 18.5 a | 16.8 a | 26 d | 22 c | 18.1 b | 14.8 a | 15.4 a | 13.9 a | 20.0 b | 15.0 a |
| Indeno(1,2,3-c,d)pyrene | 0.81 a | 0.82 a | nd | nd | nd | nd | nd | nd | 0.45 a | 0.43 a | nd | nd | nd | nd | nd | nd |
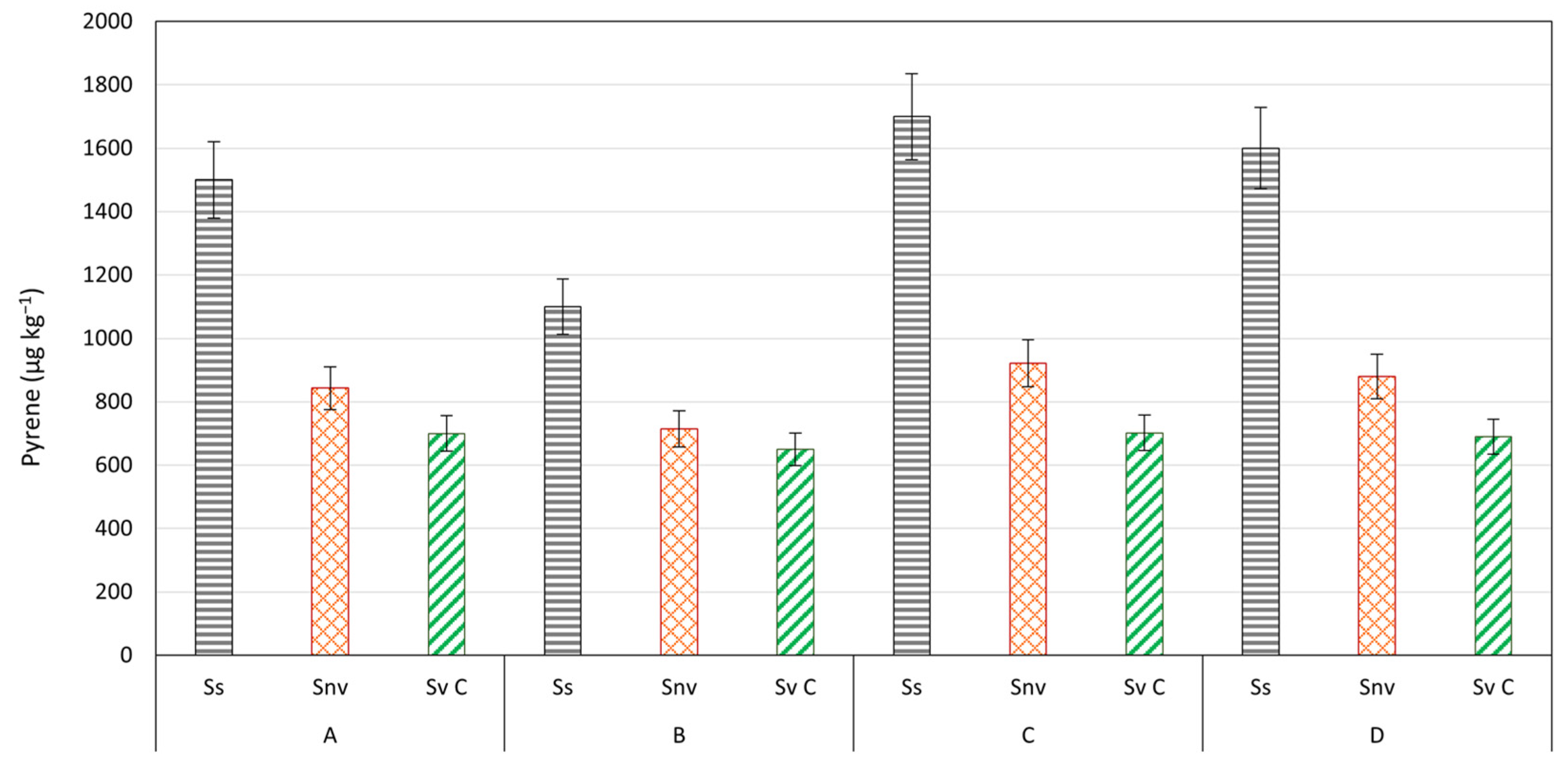
| Pyrene | 1500 e | 890 d | 725 c | 650 b | 683 b | 536 a | 720 b | 564 a | 1100 e | 800 d | 681 c | 610 b | 630 b | 546 a | 632 b | 535 a |
| C | D | |||||||||||||||
| Z. mays | L. albus | M. sativa | Z. mays | L. albus | M. sativa | |||||||||||
| Ss | Snv | Sv C | Sv C+ | Sv L | Sv L+ | Sv A | Sv A+ | Ss | Snv | Sv C | Sv C+ | Sv L | Sv L+ | Sv A | Sv A+ | |
| Benzo(a)pyrene | 0.66 a | 1.2 b | nd | nd | nd | nd | 0.81 b | 0.5 a | 0.78 a | 0.81 a | nd | nd | nd | nd | 0.68 | 0.55 |
| Benzo(b)fluoranthene | 3.3 | nd | nd | nd | nd | nd | nd | nd | 2.7 | nd | nd | nd | nd | nd | nd | nd |
| Benzo(k)fluoranthene | 1.6 | nd | nd | nd | nd | nd | nd | nd | 1.7 | nd | nd | nd | nd | nd | nd | nd |
| Benzo(g,h,i)perylene | 10.0 b | 13.0 d | 9.5 b | 8.5 ab | 8.1 a | 7.6 a | 9.4 b | 7.8 a | 11.1 b | 10 b | 8.3 a | 7.1 a | 8.0 a | 6.8 a | 10.1 b | 8.1 a |
| Dibenzo(a,i)pyrene | 0.62 | nd | nd | nd | nd | nd | nd | nd | 0.66 | nd | nd | nd | nd | nd | nd | nd |
| Chrysene | 52.0 d | 29.1 c | 23.4 b | 20.4 a | 20.1 a | 17.2 a | 30.5 c | 20.4 a | 53.2 e | 42 d | 35.1 c | 23.8 a | 30.4 b | 18.6 a | 39.6 c | 21.4 a |
| Indeno(1,2,3-c,d)pyrene | 0.81 a | 0.92 a | nd | nd | nd | nd | nd | nd | 0.84 a | 0.79 a | nd | nd | nd | nd | nd | nd |
| Pyrene | 1700 e | 780 d | 598 b | 530 a | 562 b | 509 a | 694 c | 588 b | 1600 c | 633 b | 511 a | 500 a | 495 a | 464 a | 651 b | 573 a |
| Zea mays | ||||
|---|---|---|---|---|
| Shoots | Roots | C ≤ 12 | C > 12 | |
| A | 31.3 ± 1.43 | 8.65 ± 1.32 | 1.16 ± 0.56 | 250 ± 53.0 |
| B | 33.8 ± 1.72 | 9.15 ± 1.22 | 1.06 ± 0.58 | 202 ± 70.0 |
| C | 31.4 ± 1.55 | 8.50 ± 1.25 | 2.4 ± 0.89 | 300 ± 52.0 |
| D | 30.7 ± 1.32 | 8.71 ± 1.82 | 2.3 ± 0.76 | 320 ± 60.0 |
| CT | 83.8 ± 2.10 | 11.1 ± 1.70 | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vocciante, M.; Franchi, E.; Fusini, D.; Pedron, F.; Barbafieri, M.; Petruzzelli, G.; Reverberi, A.P. Sustainable Recovery of an Agricultural Area Impacted by an Oil Spill Using Enhanced Phytoremediation. Appl. Sci. 2024, 14, 582. https://doi.org/10.3390/app14020582
Vocciante M, Franchi E, Fusini D, Pedron F, Barbafieri M, Petruzzelli G, Reverberi AP. Sustainable Recovery of an Agricultural Area Impacted by an Oil Spill Using Enhanced Phytoremediation. Applied Sciences. 2024; 14(2):582. https://doi.org/10.3390/app14020582
Chicago/Turabian StyleVocciante, Marco, Elisabetta Franchi, Danilo Fusini, Francesca Pedron, Meri Barbafieri, Gianniantonio Petruzzelli, and Andrea P. Reverberi. 2024. "Sustainable Recovery of an Agricultural Area Impacted by an Oil Spill Using Enhanced Phytoremediation" Applied Sciences 14, no. 2: 582. https://doi.org/10.3390/app14020582
APA StyleVocciante, M., Franchi, E., Fusini, D., Pedron, F., Barbafieri, M., Petruzzelli, G., & Reverberi, A. P. (2024). Sustainable Recovery of an Agricultural Area Impacted by an Oil Spill Using Enhanced Phytoremediation. Applied Sciences, 14(2), 582. https://doi.org/10.3390/app14020582











