Biomechanical Characterization of Abdominal Aortic Aneurysm: The Rupture Mechanism
Abstract
:1. Introduction
2. Material and Methods
2.1. Mouse Model of AAA
2.2. Aorta Sample Preparation
2.3. Histological and Immunofluorescence Analysis
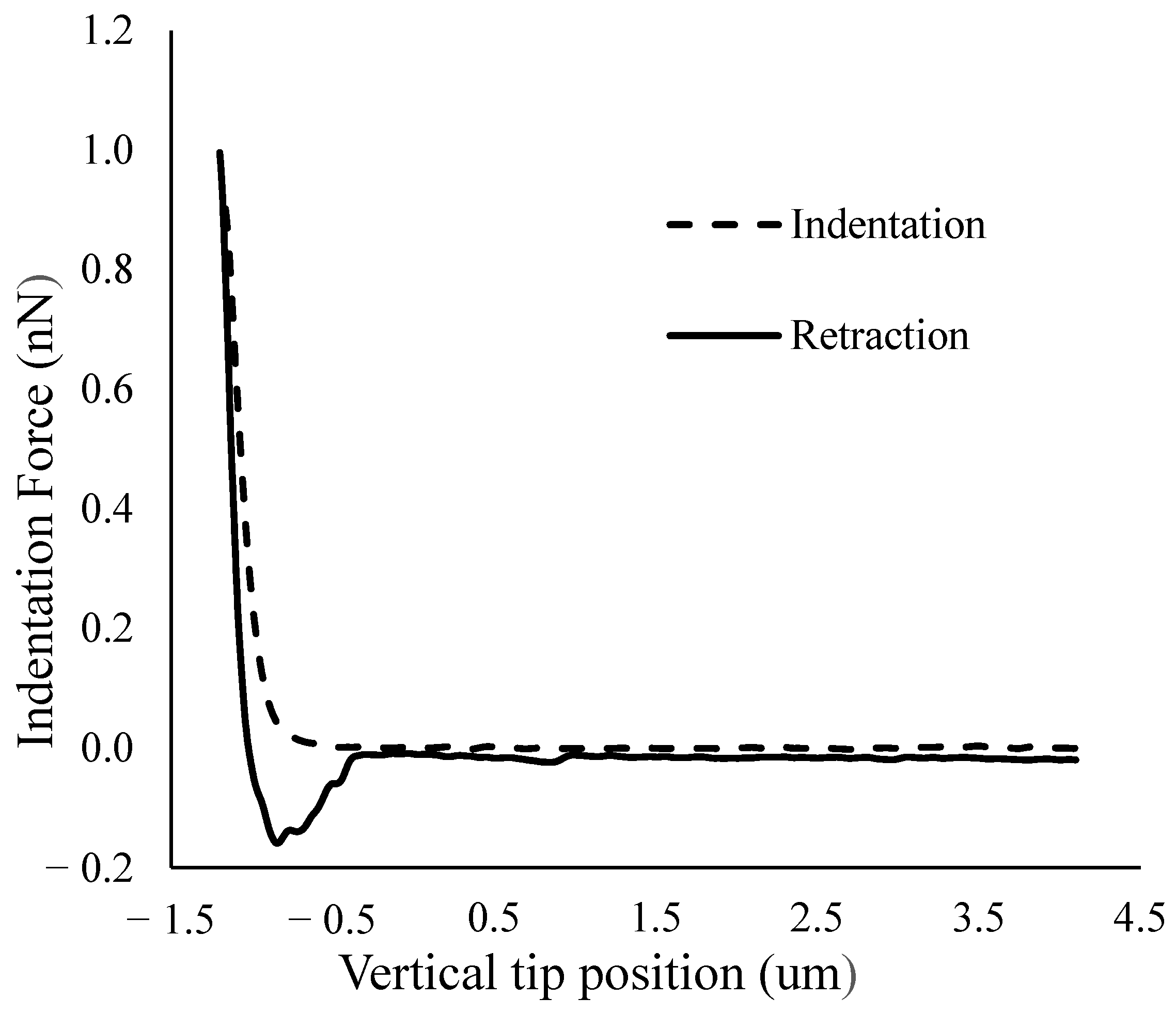
2.4. AFM Indentation Test
2.5. Data Processing
2.6. Statistical Method
3. Results
3.1. Histological Analysis
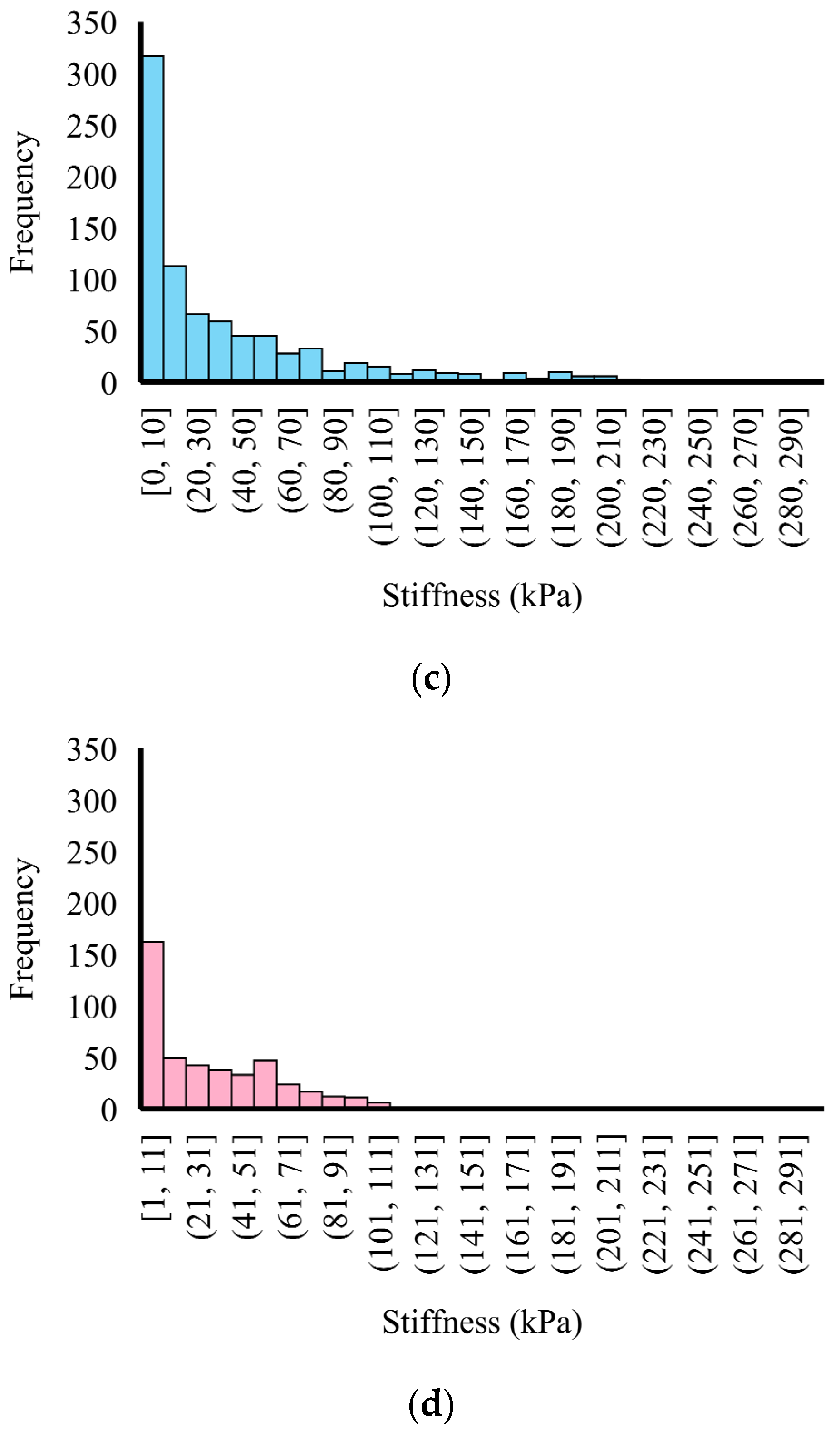
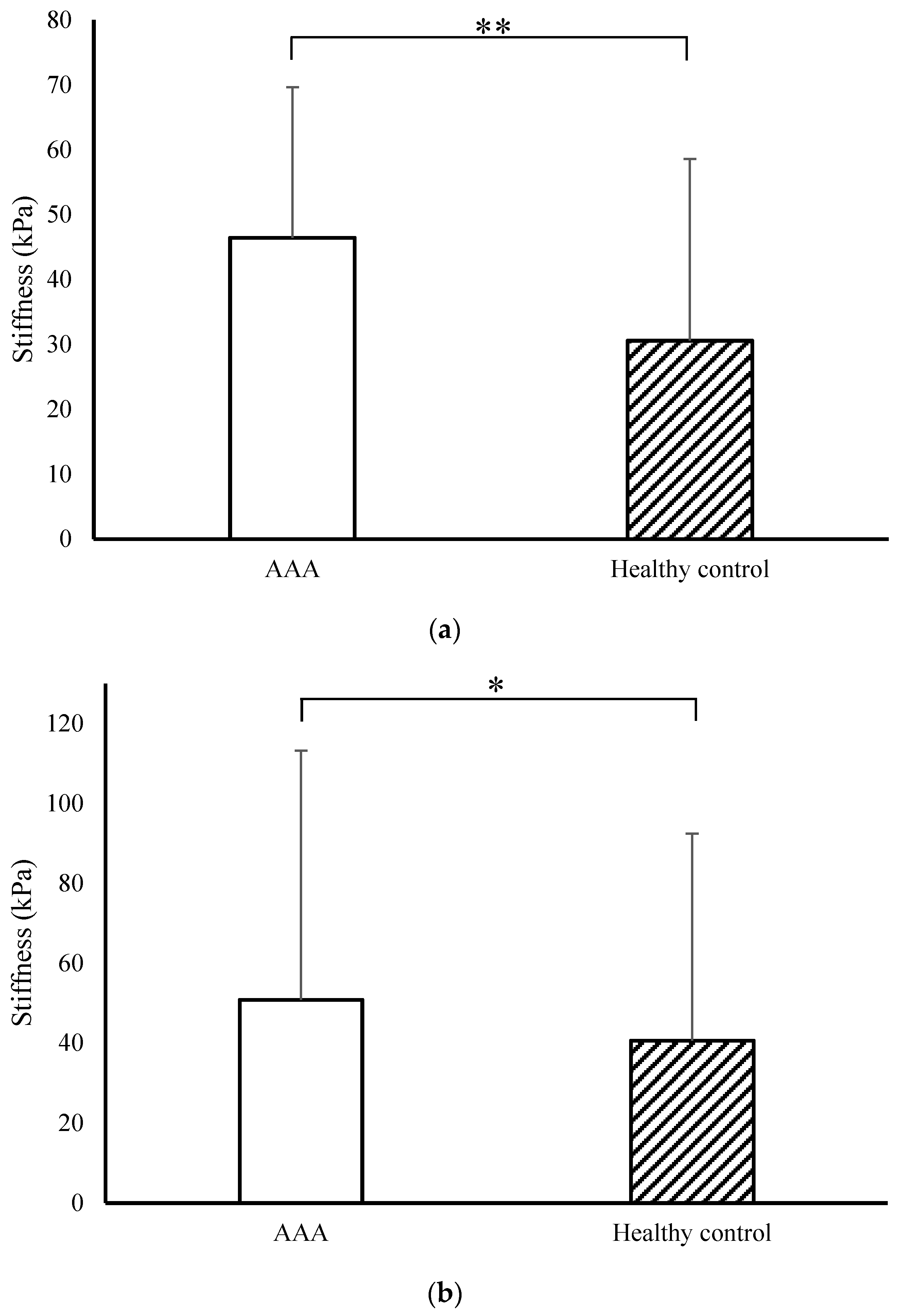
3.2. Micromechanical Properties of the Mice Artery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, M.H.; Appoo, J.J.; Saczkowski, R.; Smith, H.N.; Ouzounian, M.; Gregory, A.J.; Herget, E.J.; Boodhwani, M. Association of Mortality and Acute Aortic Events With Ascending Aortic Aneurysm: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2018, 1, e181281. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, T.F.X.; Landon, B.E.; Schermerhorn, M.L. AAA Screening Should Be Expanded. Circulation 2019, 140, 889–890. [Google Scholar] [CrossRef] [PubMed]
- Al-Balah, A.; Goodall, R.; Salciccioli, J.D.; Marshall, D.C.; Shalhoub, J. Mortality from Abdominal Aortic Aneurysm: Trends in European Union 15+ Countries from 1990 to 2017. J. Br. Surg. 2020, 107, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Groeneveld, M.E.; Meekel, J.P.; Rubinstein, S.M.; Merkestein, L.R.; Tangelder, G.J.; Wisselink, W.; Truijers, M.; Yeung, K.K. Systematic Review of Circulating, Biomechanical, and Genetic Markers for the Prediction of Abdominal Aortic Aneurysm Growth and Rupture. J. Am. Heart Assoc. 2018, 7, e007791. [Google Scholar] [CrossRef] [PubMed]
- Lederle, F.A.; Johnson, G.R.; Wilson, S.E.; Ballard, D.J.; Jordan, J.; William, D.; Blebea, J.; Littooy, F.N.; Freischlag, J.A.; Bandyk, D.; et al. Rupture Rate of Large Abdominal Aortic Aneurysms in Patients Refusing or Unfit for Elective Repair. JAMA 2002, 287, 2968–2972. [Google Scholar] [CrossRef]
- Kontopodis, N.; Pantidis, D.; Dedes, A.; Daskalakis, N.; Ioannou, C.V. The—Not So—Solid 5.5 Cm Threshold for Abdominal Aortic Aneurysm Repair: Facts, Misinterpretations, and Future Directions. Front. Surg. 2016, 3, 1. [Google Scholar] [CrossRef]
- Bellamkonda, K.S.; Nassiri, N.; Sadeghi, M.M.; Zhang, Y.; Guzman, R.J.; Ochoa Chaar, C.I. Characteristics and Outcomes of Small Abdominal Aortic Aneurysm Rupture in the American College of Surgeons National Surgical Quality Improvement Program Database. J. Vasc. Surg. 2021, 74, 729–737. [Google Scholar] [CrossRef]
- Barrett, H.E.; Cunnane, E.M.; Hidayat, H.; O’Brien, J.M.; Moloney, M.A.; Kavanagh, E.G.; Walsh, M.T. On the Influence of Wall Calcification and Intraluminal Thrombus on Prediction of Abdominal Aortic Aneurysm Rupture. J. Vasc. Surg. 2018, 67, 1234–1246.e2. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, E.S.; Bohra, A.; Vande Geest, J.P.; Gupta, N.; Makaroun, M.S.; Vorp, D.A. Biomechanical Properties of Ruptured versus Electively Repaired Abdominal Aortic Aneurysm Wall Tissue. J. Vasc. Surg. 2006, 43, 570–576. [Google Scholar] [CrossRef]
- Vorp, D.A. Biomechanics of abdominal aortic aneurysm. J. Biomech. 2007, 40, 1887–1902. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Tsai, T.T.; Isselbacher, E.M.; Oh, J.K.; O’Gara, P.T.; Evangelista, A.; Fattori, R.; Meinhardt, G.; Trimarchi, S.; Bossone, E.; et al. Aortic Diameter ≥5.5 Cm Is Not a Good Predictor of Type A Aortic Dissection: Observations From the International Registry of Acute Aortic Dissection (IRAD). Circulation 2007, 116, 1120–1127. [Google Scholar] [CrossRef]
- Dale, M.A.; Suh, M.K.; Zhao, S.; Meisinger, T.; Gu, L.; Swier, V.J.; Agrawal, D.K.; Greiner, T.C.; Carson, J.S.; Baxter, B.T.; et al. Background Differences in Baseline and Stimulated MMP Levels Influence Abdominal Aortic Aneurysm Susceptibility. Atherosclerosis 2015, 243, 621–629. [Google Scholar] [CrossRef]
- Zhao, S.; Li, W.; Gu, L. Biomechanical Prediction of Abdominal Aortic Aneurysm Rupture Risk: Sensitivity Analysis. J. Biomed. Sci. Eng. 2012, 5, 664–671. [Google Scholar] [CrossRef]
- Chen, H.; Bi, Y.; Ju, S.; Gu, L.; Zhu, X.; Han, X. Hemodynamics and Pathology of an Enlarging Abdominal Aortic Aneurysm Model in Rabbits. PLoS ONE 2018, 13, e0205366. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Han, X.; Bi, Y.; Ju, S.; Gu, L. Fluid-Structure Interaction in Abdominal Aortic Aneurysm: Effect of Modeling Techniques. BioMed Res. Int. 2017, 2017, 7023078. [Google Scholar] [CrossRef] [PubMed]
- Shum, J.; DiMartino, E.S.; Goldhammer, A.; Goldman, D.H.; Acker, L.C.; Patel, G.; Ng, J.H.; Martufi, G.; Finol, E.A. Semiautomatic Vessel Wall Detection and Quantification of Wall Thickness in Computed Tomography Images of Human Abdominal Aortic Aneurysms. Med. Phys. 2010, 37, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, M.L.; Kratzberg, J.; Castro De Tolosa, E.M.; Hanaoka, M.M.; Walker, P.; Da Silva, E.S. Regional Distribution of Wall Thickness and Failure Properties of Human Abdominal Aortic Aneurysm. J. Biomech. 2006, 39, 3010–3016. [Google Scholar] [CrossRef]
- Sakalihasan, N.; Limet, R.; Defawe, O.D. Abdominal Aortic Aneurysm. Lancet 2005, 365, 1577–1589. [Google Scholar] [CrossRef]
- Bhamidipati, C.M.; Mehta, G.S.; Lu, G.; Moehle, C.W.; Barbery, C.; DiMusto, P.D.; Laser, A.; Kron, I.L.; Upchurch, G.R.; Ailawadi, G. Development of a Novel Murine Model of Aortic Aneurysms Using Peri-Adventitial Elastase. Surgery 2012, 152, 238–246. [Google Scholar] [CrossRef]
- Lindeman, J.H.N.; Ashcroft, B.A.; Beenakker, J.-W.M.; Van Es, M.; Koekkoek, N.B.R.; Prins, F.A.; Tielemans, J.F.; Abdul-Hussien, H.; Bank, R.A.; Oosterkamp, T.H. Distinct Defects in Collagen Microarchitecture Underlie Vessel-Wall Failure in Advanced Abdominal Aneurysms and Aneurysms in Marfan Syndrome. Proc. Natl. Acad. Sci. USA 2010, 107, 862–865. [Google Scholar] [CrossRef]
- Thompson, R.W.; Geraghty, P.J.; Lee, J.K. Abdominal Aortic Aneurysms: Basic Mechanisms and Clinical Implications. Curr. Probl. Surg. 2002, 39, 110–230. [Google Scholar] [CrossRef]
- Durmus, I.; Kazaz, Z.; Altun, G.; Cansu, A. Augmentation Index and Aortic Pulse Wave Velocity in Patients with Abdominal Aortic Aneurysms. Int. J. Clin. Exp. Med. 2014, 7, 421–425. [Google Scholar] [PubMed]
- Thubrikar, J.; Labrosse, M.; Robic, F. Mechanical Properties of Abdominal Aortic Aneurysm Wall. J. Med. Eng. Technol. 2001, 25, 133–142. [Google Scholar]
- Raghavan, M.L.; Hanaoka, M.M.; Kratzberg, J.A.; Higuchi, M.D.L.; Da Silva, E.S. Biomechanical Failure Properties and Microstructural Content of Ruptured and Unruptured Abdominal Aortic Aneurysms. J. Biomech. 2011, 44, 2501–2507. [Google Scholar] [CrossRef] [PubMed]
- Fahner, P.J.; Idu, M.M.; Van Gulik, T.M.; Van Wijk, B.; Van Der Wal, A.C.; Legemate, D.A. Glycerol-Preserved Arterial Allografts Evaluated in the Infrarenal Rat Aorta. Eur. Surg. Res. 2009, 42, 78–86. [Google Scholar] [CrossRef]
- Shojaee, M.; Sameti, M.; Vuppuluri, K.; Ziff, M.; Carriero, A.; Bashur, C.A. Design and Characterization of a Porous Pouch to Prevent Peritoneal Adhesions during in Vivo Vascular Graft Maturation. J. Mech. Behav. Biomed. Mater. 2020, 102, 103461. [Google Scholar] [CrossRef]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius Red Staining: A Useful Tool to Appraise Collagen Networks in Normal and Pathological Tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Xue, C.; Zhao, G.; Zhao, Y.; Chen, Y.E.; Zhang, J. Mouse Abdominal Aortic Aneurysm Model Induced by Perivascular Application of Elastase. J. Vis. Exp. 2022, 180, e63608. [Google Scholar]
- Lu, G.; Su, G.; Davis, J.P.; Schaheen, B.; Downs, E.; Roy, R.J.; Ailawadi, G.; Upchurch, G.R. A Novel Chronic Advanced Stage Abdominal Aortic Aneurysm Murine Model. J. Vasc. Surg. 2017, 66, 232–242.e4. [Google Scholar] [CrossRef]
- Liu, X.; Qu, C.; Zhang, Y.; Fang, J.; Teng, L.; Shen, C. Perfusion Pressure of Elastase Impacts the Formation Ratio and Diameters of Abdominal Aortic Aneurysms in Rats. Exp. Ther. Med. 2023, 25, 190. [Google Scholar] [CrossRef]
- Munshaw, S.; Bruche, S.; Redpath, A.N.; Jones, A.; Patel, J.; Dubé, K.N.; Lee, R.; Hester, S.S.; Davies, R.; Neal, G.; et al. Thymosin β4 Protects against Aortic Aneurysm via Endocytic Regulation of Growth Factor Signaling. J. Clin. Investig. 2021, 131, e127884. [Google Scholar] [CrossRef]
- Wołoszko, T.; Skórski, M.; Kwasiborski, P.; Kmin, E.; Gałązka, Z.; Pogorzelski, R. Influence of Selective Biochemical and Morphological Agents on Natural History of Aneurysm of Abdominal Aorta Development. Med. Sci. Monit. 2016, 22, 431–437. [Google Scholar] [CrossRef] [PubMed]
- New Target Identified to Develop Treatment for Abdominal Aortic Aneurysm|University of Oxford. Available online: https://www.ox.ac.uk/news/science-blog/new-target-identified-develop-treatment-abdominal-aortic-aneurysm (accessed on 21 October 2023).
- Darling, R.C.; Messina, C.R.; Brewster, D.C.; Ottinger, L.W. Autopsy Study of Unoperated Abdominal Aortic Aneurysms. The Case for Early Resection. Circulation 1977, 56 (Suppl. 3), II161–II164. [Google Scholar] [PubMed]
- Parkinson, F.; Ferguson, S.; Lewis, P.; Williams, I.M.; Twine, C.P. Rupture Rates of Untreated Large Abdominal Aortic Aneurysms in Patients Unfit for Elective Repair. J. Vasc. Surg. 2015, 61, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.T.; Gotensparre, S.M.; Sweeting, M.J.; Brown, L.C.; Fowkes, F.G.R.; Thompson, S.G. Rupture Rates of Small Abdominal Aortic Aneurysms: A Systematic Review of the Literature. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 2–10. [Google Scholar] [CrossRef]
- Vermeulen, J.J.M.; Meijer, M.; de Vries, F.B.G.; Reijnen, M.M.P.J.; Holewijn, S.; Thijssen, D.H.J. A Systematic Review Summarizing Local Vascular Characteristics of Aneurysm Wall to Predict for Progression and Rupture Risk of Abdominal Aortic Aneurysms. J. Vasc. Surg. 2023, 77, 288–298.e2. [Google Scholar] [CrossRef]
- Malayala, S.V.; Raza, A.; Vanaparthy, R. Gender-Based Differences in Abdominal Aortic Aneurysm Rupture: A Retrospective Study. J. Clin. Med. Res. 2020, 12, 794–802. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Delgado, A.I.; Sameti, M.; Dong, P.; Xiong, W.; Bashur, C.A.; Gu, L. Biomechanical Characterization of Abdominal Aortic Aneurysm: The Rupture Mechanism. Appl. Sci. 2024, 14, 613. https://doi.org/10.3390/app14020613
Zhai Y, Delgado AI, Sameti M, Dong P, Xiong W, Bashur CA, Gu L. Biomechanical Characterization of Abdominal Aortic Aneurysm: The Rupture Mechanism. Applied Sciences. 2024; 14(2):613. https://doi.org/10.3390/app14020613
Chicago/Turabian StyleZhai, Yingnan, Ana Isabel Delgado, Mahyar Sameti, Pengfei Dong, Wanfen Xiong, Chris A. Bashur, and Linxia Gu. 2024. "Biomechanical Characterization of Abdominal Aortic Aneurysm: The Rupture Mechanism" Applied Sciences 14, no. 2: 613. https://doi.org/10.3390/app14020613





