Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apple Juice Preparation
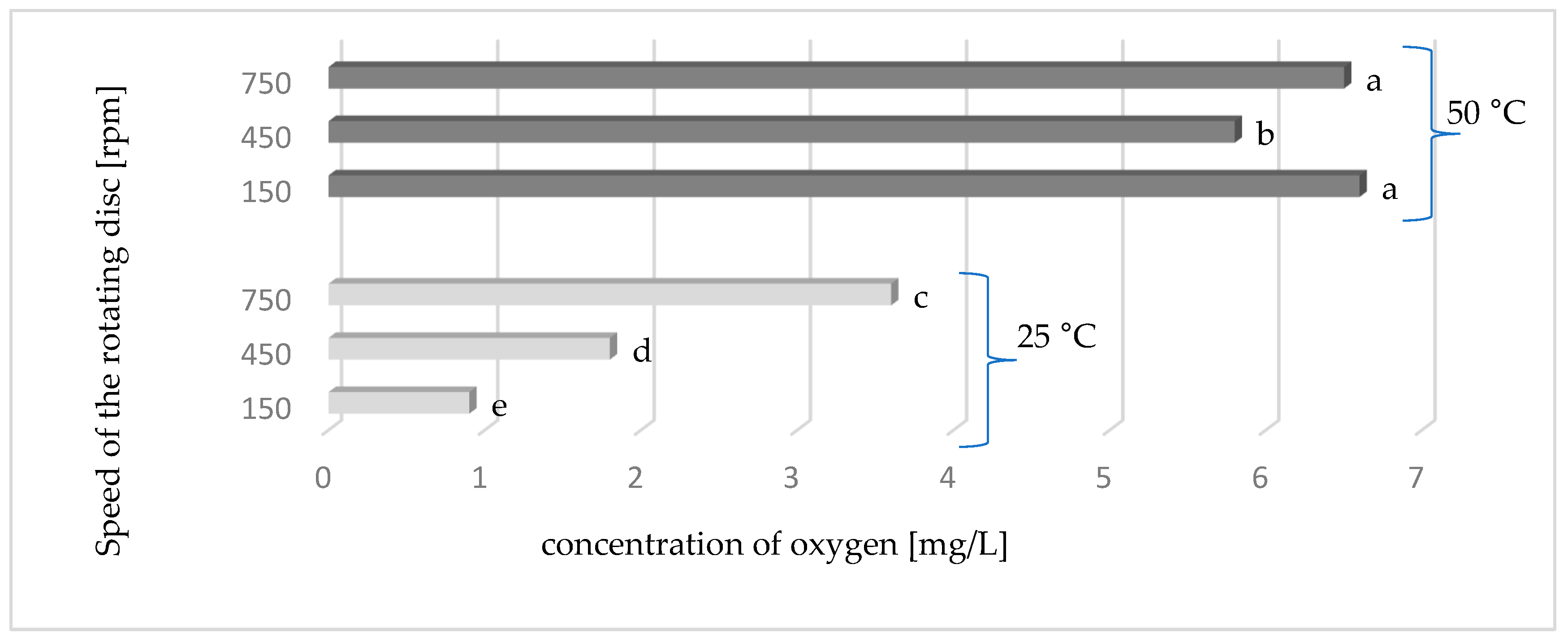
2.2. Deaeration of Juice
2.3. Pasteurization Process
2.4. Microbiology
2.5. Enzyme Activities
2.6. Polyphenol Content (TPC and Poyphenols Profile)
2.6.1. Extraction
2.6.2. Total Polyphenol Content (TPC)
2.6.3. HPLC Analysis of the Polyphenols
2.7. Antioxidant Activity (DPPH•)
2.8. Color Parameters
2.9. Sensory Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. Deaeration of Juice
3.2. Microbiology and Enzymatic Activity
3.3. Polyphenols Content and Antioxidant Activity
3.4. Color Change and Sensory Evaluation
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdullah, S.A.; Lee, S.H.; Cho, I.K.; Li, Q.X.; Jun, S.; Choi, W. Pasteurization of kava juice using novel continuous flow microwave heating technique. Food Sci. Biotechnol. 2013, 22, 961–966. [Google Scholar] [CrossRef]
- Abdullah, N.; Chin, N.L. Application of thermosonication treatment in processing and production of high quality and safe-to-drink fruit juices. Agric. Agric. Sci. Procedia 2014, 2, 320–327. [Google Scholar] [CrossRef]
- Espachs-Barroso, A.; Barbosa-Cánovas, G.V.; Martín-Belloso, O. Microbial and enzymatic changes in fruit juice induced by high-intensity pulsed electric fields. Food Rev. Int. 2003, 19, 253–273. [Google Scholar] [CrossRef]
- Augusto, P.E.D.; Vitali, A.A. Assessing Juice Quality: Advances in the Determination of Rheological Properties of Fruit Juices and Derivatives. In Juice Processing: Quality, Safety and Value-Added Opportunities, 1st ed.; Falguera, V., Ibarz, A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 100–109. [Google Scholar] [CrossRef]
- Riahi, E.; Ramaswamy, H.S. High pressure inactivation kinetics of amylase in apple juice. J. Food Eng. 2004, 64, 151–160. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.A.; Aguayo, E. Continuous microwave pasteurization of a vegetable smoothie improves its physical quality and hinders detrimental enzyme activity. Food Sci. Technol. Int. 2016, 23, 36–45. [Google Scholar] [CrossRef]
- Perek, A.; Dolata, W. Zastosowanie mikrofal do obróbki cieplnej żywności. Postępy Tech. Przetwórstwa Spożywczego 2009, 2, 103–108. [Google Scholar]
- Guo, C.; Mujumdar, A.S.; Zhang, M. New development in radio frequency heating for fresh food processing: A review. Food Eng. Rev. 2019, 11, 29–43. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Basak, T. A comprehensive analysis on the effect of shape on the microwave heating dynamics of food materials. Innov. Food Sci. Emerg. Technol. 2017, 39, 247–266. [Google Scholar] [CrossRef]
- Sumnu, S.G.; Sahin, S. Microwave Heating. In Thermal Food Processing: New Technologies and Quality Issues, 2nd ed.; Sun, D.W., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 555–581. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; dos Santos Funcia, E.; Pires, M.N.; Gut, J.A.W. Modeling of time-temperature history and enzymatic inactivation of cloudy apple juice in continuous flow microwave assisted pasteurization. Food Bioprod. Process. 2018, 111, 45–53. [Google Scholar] [CrossRef]
- Aghajanzadeh, S.; Ziaiifar, A.M. A review of pectin methylesterase inactivation in citrus juice during pasteurization. Trends Food Sci. Technol. 2018, 71, 1–12. [Google Scholar] [CrossRef]
- Samani, B.H.; Khoshtaghaza, M.H.; Lorigooini, Z.; Minaei, S.; Zareiforoush, H. Analysis of the combinative effect of ultrasound and microwave power on Saccharomyces cerevisiae in orange juice processing. Innov. Food Sci. Emerg. Technol. 2015, 32, 110–115. [Google Scholar] [CrossRef]
- Marszałek, K.; Mitek, M.; Skąpska, S. Effect of continuous flow microwave and conventional heating on the bioactive compounds, colour, enzymes activity, microbial and sensory quality of strawberry purée. Food Bioprocess Technol. 2015, 8, 1864–1876. [Google Scholar] [CrossRef]
- Gentry, T.S.; Roberts, J.S. Design and evaluation of a continuous flow microwave pasteurization system for apple cider. LWT Food Sci. Technol. 2005, 38, 227–238. [Google Scholar] [CrossRef]
- Kernou, O.N.; Belbahi, A.; Kaanin-Boudraa, G.; Adel, K.; Madani, P.K. A Review: Ultrasound-Microwave Technologies as Alternative Methods for Inactivation Bacterias in Fruit Juice. Int. J. Anal. Appl. Chem. 2022, 8, 31–40. [Google Scholar]
- Kammoun Bejar, A.; Kechaou, N.; Boudhrioua Mihoubi, N. Effect of microwave treatment on physical and functional properties of orange (Citrus sinensis) peel and leaves. J. Food Process. Technol. 2011, 2, 109–116. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Kubo, M.T.; Siguemoto, É.S.; Funcia, E.S.; Augusto, P.E.; Curet, S.; Boillereaux, L.; Gut, J.A. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar] [CrossRef]
- Siguemoto, É.S.; Pereira, L.J.; Gut, J.A.W. Inactivation kinetics of pectin methylesterase, polyphenol oxidase, and peroxidase in cloudy apple juice under microwave and conventional heating to evaluate non-thermal microwave effects. Food Bioprocess Technol. 2018, 11, 1359–1369. [Google Scholar] [CrossRef]
- Latorre, M.E.; Bonelli, P.R.; Rojas, A.M.; Gerschenson, L.N. Microwave inactivation of red beet (Beta vulgaris L. var. conditiva) peroxidase and polyphenoloxidase and the effect of radiation on vegetable tissue quality. J. Food Eng. 2012, 109, 676–684. [Google Scholar] [CrossRef]
- Cywińska-Antonik, M.; Chen, Z.; Groele, B.; Marszałek, K. Application of Emerging Techniques in Reduction of the Sugar Content of Fruit Juice: Current Challenges and Future Perspectives. Foods 2023, 12, 1181. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, I.B.; Miranda, N.G.; Gomes, F.S.; Santos, M.C.; de G.C. Freitas, D.; Tonon, R.V.; Cabral, L.M. Physicochemical and sensory properties of apple juice concentrated by reverse osmosis and osmotic evaporation. Innov. Food Sci. Emerg. Technol. 2012, 16, 137–142. [Google Scholar] [CrossRef]
- Candrawinata, V.I.; Golding, J.B.; Roach, P.D.; Stathopoulos, C.E. From apple to juice—The fate of polyphenolic compounds. Food Rev. Int. 2013, 29, 276–293. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, Q.; Yu, J. Life cycle assessment of concentrated apple juice production in China: Mitigation options to reduce the environmental burden. Sustain. Prod. Consum. 2022, 32, 15–26. [Google Scholar] [CrossRef]
- Wojcik, S.; Jakubowska, M. Deep neural networks in profiling of apple juice adulteration based on voltammetric signal of the iridium quadruple-disk electrode. Chemom. Intell. Lab. Syst. 2021, 209, 104246. [Google Scholar] [CrossRef]
- Will, F.; Roth, M.; Olk, M.; Ludwig, M.; Dietrich, H. Processing and analytical characterisation of pulp-enriched cloudy apple juices. LWT Food Sci. Technol. 2008, 41, 2057–2063. [Google Scholar] [CrossRef]
- Marszałek, K. Method of Producing Juices and Fruit Beverages. Patent 238909, 22 July 2021. [Google Scholar]
- PN-EN ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. Polish Committee for Standardization: Warsaw, Poland, 2013.
- PN-EN ISO 21527-1:2009; Microbiology of Food and Animal Feeding Stuffs. Horizontal Method for the Enumeration of Yeasts and Moulds. Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. Polish Committee for Standardization: Warsaw, Poland, 2009.
- Terefe, N.S.; Yang, Y.H.; Knoerzer, K.; Buckow, R.; Versteeg, C. High pressure and thermal inactivation kinetics of polyphenol oxidase and peroxidase in strawberry puree. Innov. Food Sci. Emerg. Technol. 2010, 11, 52–60. [Google Scholar] [CrossRef]
- Szczepańska, J.; Barba, F.J.; Skąpska, S.; Marszałek, K. High pressure processing of carrot juice: Effect of static and multi-pulsed pressure on the polyphenolic profile, oxidoreductases activity and colour. Food Chem. 2020, 307, 125549. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high performance liquid chromatography. J. Chromatogr. 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.-C.; Chen, H.-Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- PN-ISO 4121:1998; Sensory Analysis—Methodology—Evaluation of Food Products by Methods Using Scales. Polish Committee for Standardization: Warsaw, Poland, 1998.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- García-Torres, R.; Ponagandla, N.R.; Rouseff, R.L.; Goodrich-Schneider, R.M.; Reyes-De-Corcuera, J.I. Effects of dissolved oxygen in fruit juices and methods of removal. Compr. Rev. Food Sci. Food Saf. 2009, 8, 409–423. [Google Scholar] [CrossRef]
- Cachon, R.; Alwazeer, D. Quality Performance Assessment of Gas Injection during Juice Processing and Conventional Preservation Technologies. In Value-Added Ingredients and Enrichments of Beverages; Academic Press: Cambridge, MA, USA, 2019; pp. 465–485. [Google Scholar]
- Lin, D.; Long, X.; Huang, Y.; Yang, Y.; Wu, Z.; Chen, H.; Tu, Z. Effects of microbial fermentation and microwave treatment on the composition, structural characteristics, and functional properties of modified okara dietary fiber. LWT 2020, 123, 109059. [Google Scholar] [CrossRef]
- Tajchakavit, S.; Ramaswamy, H.S.; Fustier, P. Enhanced destruction of spoilage microorganisms in apple juice during continuous flow microwave heating. Food Res. Int. 1998, 31, 713–722. [Google Scholar] [CrossRef]
- Nikdel, S.; MacKellar, D.G. A microwave system for continuous pasteurization of orange juice. Proc. Fla. State Hortic. Soc. 1992, 105, 108–109. [Google Scholar]
- Marszałek, K.; Woźniak, Ł.; Skąpska, S.; Mitek, M. High pressure processing and thermal pasteurization of strawberry purée: Quality parameters and shelf life evaluation during cold storage. J. Food Sci. Technol. 2017, 54, 832–841. [Google Scholar] [CrossRef]
- González-Monroy, A.D.; Rodríguez-Hernández, G.; Ozuna, C.; Sosa-Morales, M.E. Microwave-assisted pasteurization of beverages (tamarind and green) and their quality during refrigerated storage. Innov. Food Sci. Emerg. Technol. 2018, 49, 51–57. [Google Scholar] [CrossRef]
- Guo, Q.; Sun, D.W.; Cheng, J.H.; Han, Z. Microwave processing techniques and their recent applications in the food industry. Trends Food Sci. Technol. 2017, 67, 236–247. [Google Scholar] [CrossRef]
- Demianchuk, B.; Guliiev, S.; Ugol’nikov, A.; Kliat, Y.; Kosenko, A. 7 Development of Microwave Technology of Selective Heating the Components of Heterogeneous Media. East. Eur. J. Enterp. Technol. 2022, 1, 115. [Google Scholar]
- Azencott, H.R.; Peter, G.F.; Prausnitz, M.R. Influence of the cell wall on intracellular delivery to algal cells by electroporation and sonication. Ultrasound Med. Biol. 2007, 33, 1805–1817. [Google Scholar] [CrossRef]
- Vian, A.; Roux, D.; Girard, S.; Bonnet, P.; Paladian, F.; Davies, E.; Ledoigt, G. Microwave irradiation affects gene expression in plants. Plant Signal. Behav. 2006, 1, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Marin-Castro, U.R.; Dominique, P.; García-Alvarado, M.A.; Vargas-Ortiz, M.A.; Salgado-Cervantes, M.A.; Servent, A. Effect of the thermal state during Manila mango processing by mild flash vacuum-expansion on carotenoids and enzymatic activity. Innov. Food Sci. Emerg. Technol. 2022, 75, 102900. [Google Scholar] [CrossRef]
- Matsui, K.N.; Granado, L.M.; De Oliveira, P.V.; Tadini, C.C. Peroxidase and polyphenol oxidase thermal inactivation by microwaves in green coconut water simulated solutions. LWT Food Sci. Technol. 2007, 40, 852–859. [Google Scholar] [CrossRef]
- de Ancos, B.; Cano, M.P.; Hernandez, A.; Monreal, M. Effects of microwave heating on pigment composition and colour of fruit purees. J. Sci. Food Agric. 1999, 79, 663–670. [Google Scholar] [CrossRef]
- Kumar, S.; Khadka, M.; Mishra, R.; Kohli, D.; Upadhaya, S. Effects of conventional and microwave heating pasteurization on physiochemical properties of pomelo (Citrus maxima) juice. J. Food Process. Technol. 2017, 8, 8–11. [Google Scholar] [CrossRef]
- Guiné, R.D.P.F.; Barroca, M.J. Influence of processing and storage on fruit juices phenolic compounds. Int. J. Med. Biol. Front. 2014, 20, 45. [Google Scholar]
- Chedea, V.S.; Pop, R.M. Total Polyphenols Content and Antioxidant DPPH Assays on Biological Samples. In Polyphenols in Plants; Academic Press: Cambridge, MA, USA, 2019; pp. 169–183. [Google Scholar]
- Stasiak, A.; Ulanowska, A. Aktywnosc przeciwutleniajaca nowych odmian fasoli [Phaseolus vulgaris L.]. Żywność Nauka Technol. Jakość 2008, 15, 74–82. [Google Scholar]
- Madhujith, T.; Naczk, M.; Shahidi, F. Antioxidant activity of common beans (Phaseolus vulgaris L.). J. Food Lipids 2004, 11, 220–233. [Google Scholar] [CrossRef]
- Pysz, M.; Bieżanowska, R.; Pisulewski, P. Porównanie wpływu zabiegów termicznych i kiełkowania na skład chemiczny, zawartość substancji nieodżywczych oraz wartość odżywczą białka nasion grochu i soi. Żywność Nauka Technol. Jakość 2001, 1, 85–91. [Google Scholar]
- Troszyńska, A.; Ciska, E. Phenolic compounds of seed coats of white and coloured varieties of pea (Pisum sativum L.) and their total antioxidant activity. J. Food Sci. 2002, 20, 15–22. [Google Scholar] [CrossRef]
- Arjmandi, M.; Otón, M.; Artés, F.; Artés-Hernández, F.; Gómez, P.A.; Aguayo, E. Microwave flow and conventional heating effects on the physicochemical properties, bioactive compounds and enzymatic activity of tomato puree. J. Sci. Food Agric. 2017, 97, 984–990. [Google Scholar] [CrossRef] [PubMed]
- De Souza Comapa, S.; Carvalho, L.M.S.; Lamarão, C.V.; das Chagas do Amaral Souza, F.; Aguiar, J.P.L.; da Silva, L.S.; Mar, J.M.; Sanches, E.A.; dos Santos, F.F.; de Araújo Bezerra, J.; et al. Microwave Processing of Camu-Camu Juices: Physicochemical and Microbiological Parameters. J. Food Process. Preserv. 2019, 43, e13989. [Google Scholar] [CrossRef]
- Pérez-Grijalva, B.; Herrera-Sotero, M.; Mora-Escobedo, R.; Zebadúa-García, J.C.; Silva-Hernández, E.; Oliart-Ros, R.; Perez-Cruz, C.; Guzmán-Gerónimo, R. Effect of microwaves and ultrasound on bioactive compounds and microbiological quality of blackberry juice. LWT 2018, 87, 47–53. [Google Scholar] [CrossRef]
- Barba, F.J.; Esteve, M.J.; Frigola, A. Physicochemical and nutritional characteristics of blueberry juice after high pressure processing. Food Res. Int. 2013, 50, 545–549. [Google Scholar] [CrossRef]
- Cinquanta, L.; Albanese, D.; Cuccurullo, G.; Di Matteo, M. Effect on orange juice of batch pasteurization in an improved pilot-scale microwave oven. J. Food Sci. 2010, 75, E46–E50. [Google Scholar] [CrossRef]


| TPC [mg GAE /100 mL] | Antioxidant Capacity (DPPH•) [µM/100 mL] | |||
|---|---|---|---|---|
| FJ | 32.5 ± 0.9 h | 51.9 ± 1.4 d | ||
| Storage Time (Month) | TP | MFP | TP | MFP |
| 0 | 38.8 ± 1.2 ef | 73.8 ± 2.3 a | 65.9 ± 2.3 c | 176.8 ± 2.7 a |
| 2 | 38.1 ± 0.4 ef | 67.3 ± 0.6 b | 66.9 ± 3.9 c | 172.3 ± 3.2 a |
| 4 | 36.1 ± 1.1 fg | 59.2 ± 2.1 c | 64.6 ± 2.0 c | 168.7 ± 6.1 a |
| 6 | 34.6 ± 0.5 gh | 43.2 ± 1.3 d | 63.2 ± 1.6 c | 143.1 ± 0.7 b |
| 8 | 33.1 ± 0.4 h | 45.0 ± 1.7 d | 62.1 ± 3.0 c | 146.2 ± 2.6 b |
| 10 | 33.7 ± 0.6 gh | 43.5 ± 0.7 d | 63.4 ± 1.6 c | 143.4 ± 0.7 b |
| 12 | 32.0 ± 0.6 h | 32.0 ± 0.6 h | 63.1 ± 0.8 c | 141.5 ± 3.1 b |
| Floridzin (mg/L) | Epicatechin (mg/L) | Chlorogenic Acid (mg/L) | Caffeic Acid (mg/L) | |||||
|---|---|---|---|---|---|---|---|---|
| FJ | 10.29 ± 0.15 a | 2.67 ± 0.04 d | 27.89 ± 0.05 d | 0.76 ± 0.10 a | ||||
| Storage Time (Month) | TP | MFP | TP | MFP | TP | MFP | TP | MFP |
| 0 | 10.67 ± 0.22 a | 10.70 ± 0.11 a | 2.57 ± 0.08 d | 27.47 ± 0.18 a | 27.50 ± 0.15 d | 150.89 ± 0.02 a | 0.81 ± 0.02 a | 0.60 ± 0.01 b |
| 2 | 7.01 ± 0.12 c | 7.55 ± 0.17 b | 1.35 ± 0.36 e | 6.42 ± 0.28 b | 22.09 ± 0.11 e | 131.37 ± 0.17 b | <LOQ | <LOQ |
| 4 | 5.19 ± 0.21 de | 5.44 ± 0.05 d | <LOQ | 5.61 ± 0.14 c | 21.49 ± 0.03 e | 121.97 ± 1.15 c | <LOQ | <LOQ |
| 6 | 4.98 ± 0.12 e | 3.43 ± 0.08 g | <LOQ | <LOQ | 21.95 ± 0.48 e | 5.46 ± 0.03 g | <LOQ | <LOQ |
| 8 | 4.48 ± 0.05 f | 3.36 ± 0.04 g | <LOQ | <LOQ | 8.51 ± 0.32 f | <LOQ | <LOQ | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosiński, J.; Cywińska-Antonik, M.; Szczepańska-Stolarczyk, J.; Jasińska, U.T.; Woźniak, Ł.; Kaniewska, B.; Marszałek, K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Appl. Sci. 2024, 14, 662. https://doi.org/10.3390/app14020662
Kosiński J, Cywińska-Antonik M, Szczepańska-Stolarczyk J, Jasińska UT, Woźniak Ł, Kaniewska B, Marszałek K. Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice. Applied Sciences. 2024; 14(2):662. https://doi.org/10.3390/app14020662
Chicago/Turabian StyleKosiński, Jakub, Magdalena Cywińska-Antonik, Justyna Szczepańska-Stolarczyk, Urszula T. Jasińska, Łukasz Woźniak, Beata Kaniewska, and Krystian Marszałek. 2024. "Application of an Electromagnetic Field for Extending the Shelf-Life of Not from Concentrate (NFC) Apple Juice" Applied Sciences 14, no. 2: 662. https://doi.org/10.3390/app14020662






