Removal of Cochineal Dye Color through Atmospheric Pressure Plasma Discharge Jet
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cochineal Dye Preparation
2.3. Mealybug Dye Treatment by APPJ Discharge
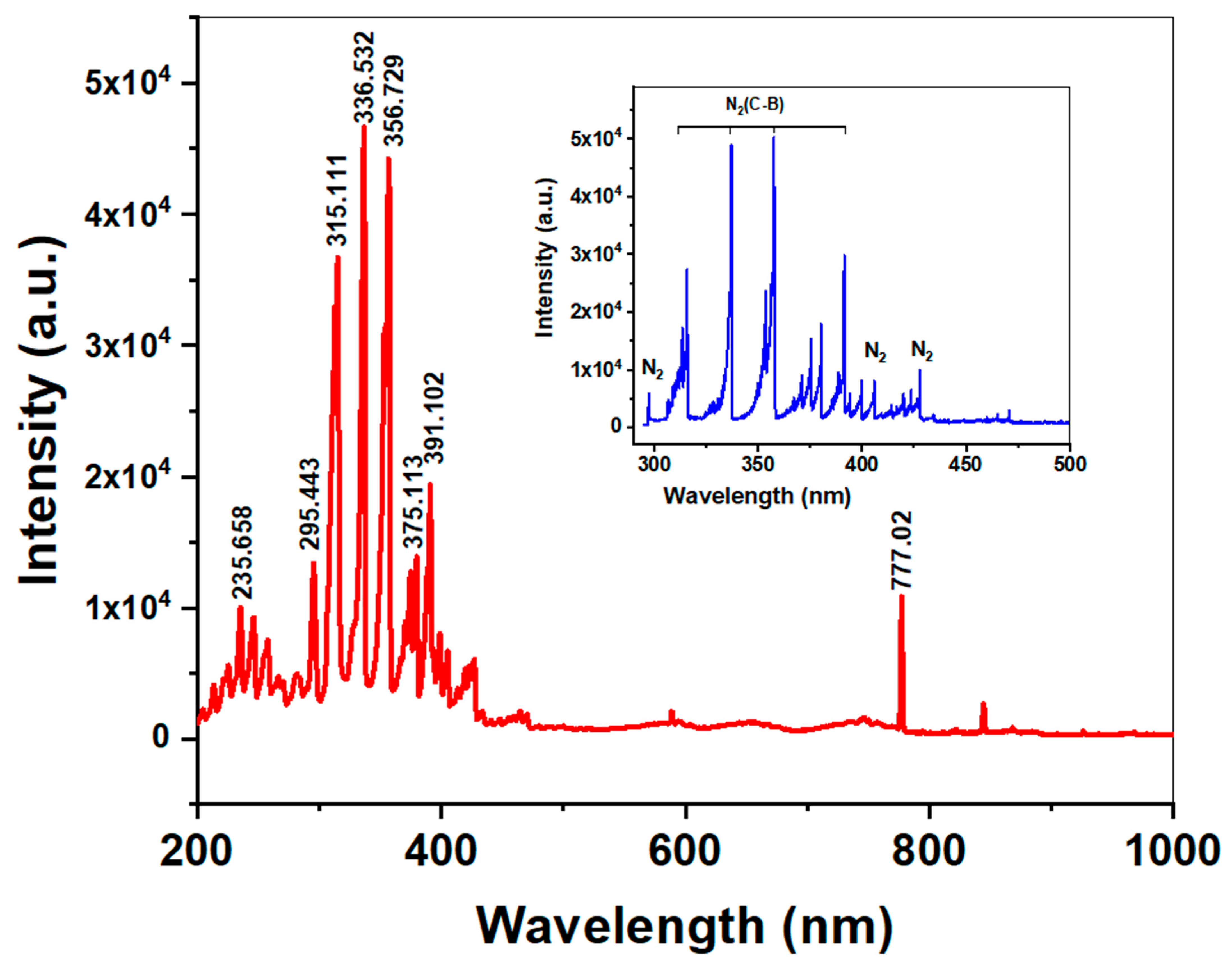
2.4. Analysis of Plasma Composition Using Optical Emission Spectroscopy
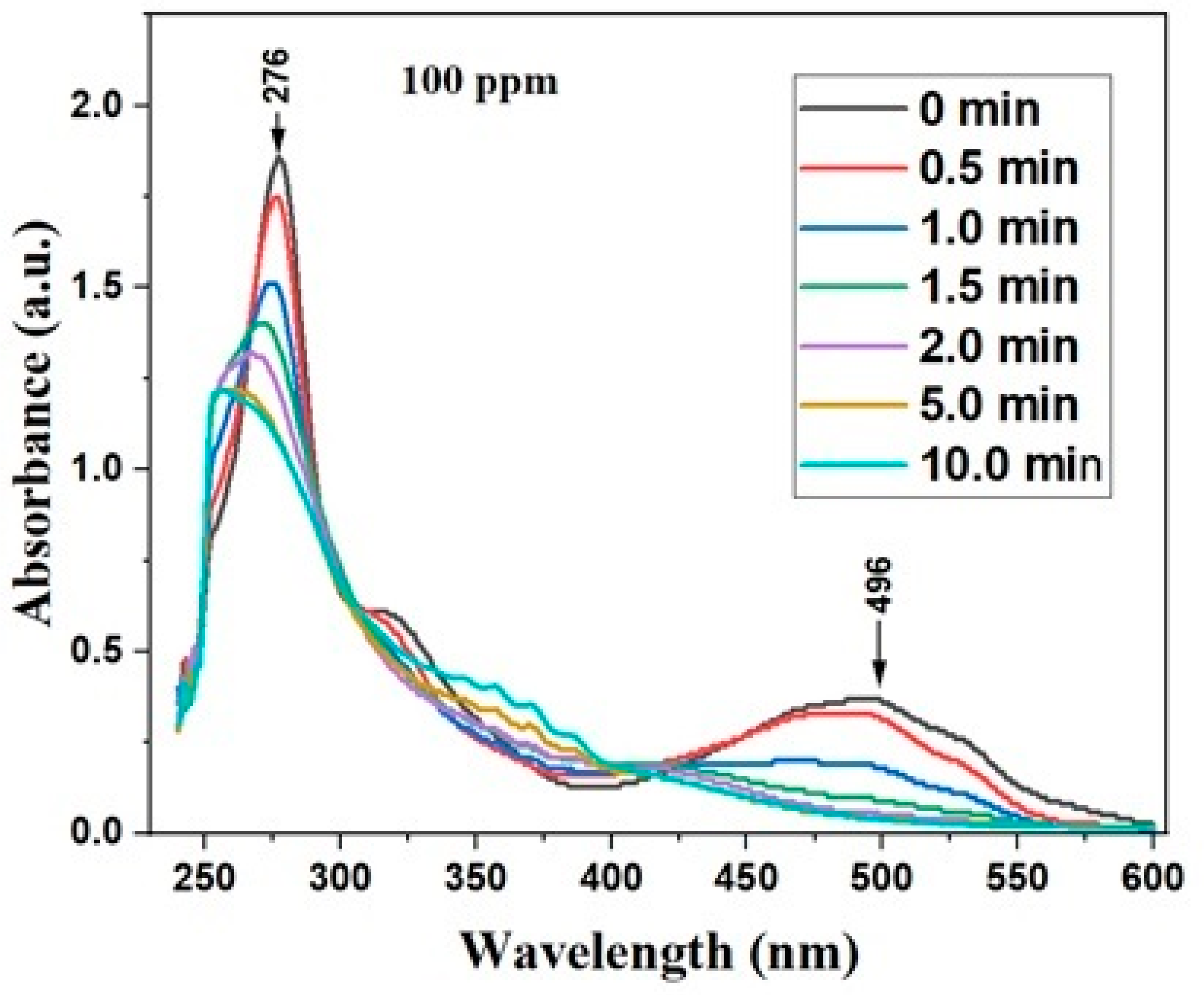
2.5. The Kinetics and Efficiency of the Decolorization of Cochineal Dye through the Use of Absorption Spectroscopy
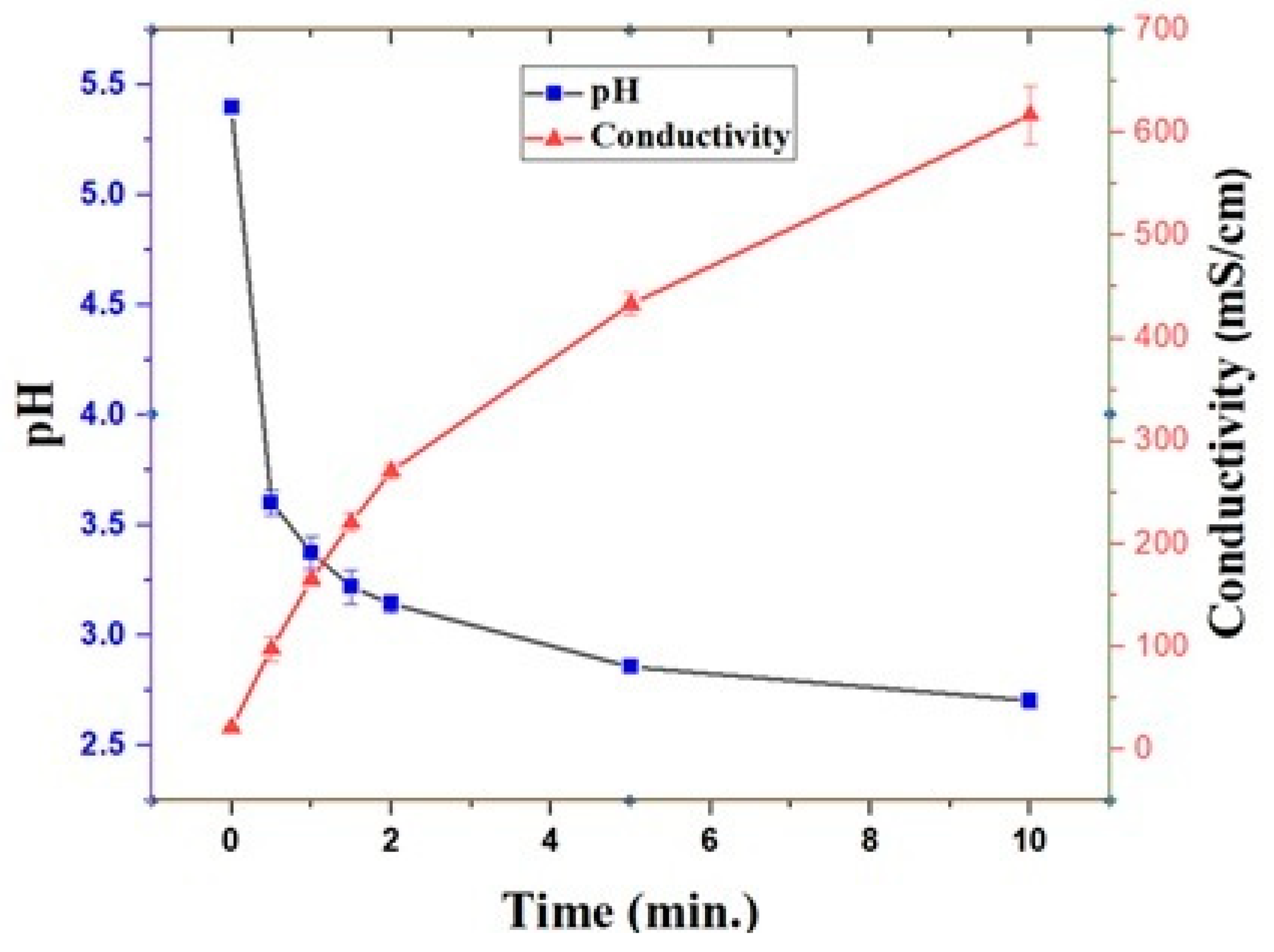
2.6. pH and Electrical Conductivity Measurements
2.7. Concentration of Reactive Nitrogen Species (RNS)
3. Results and Discussion
3.1. Analysis of Plasma Composition
3.2. Effects of Plasma Treatment on pH and Electrical Conductivity during Dye Degradation
3.3. Measurement of the Concentration of Nitrites, Nitrates, and Their Role in the Dye Bleaching Process
3.4. UV-Visible Spectral Analysis
3.5. Kinetic of Cochineal Dye Color Remotion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanafi, M.F.; Sapawe, N. A review on the water problem associate with organic pollutants derived from phenol, methyl orange, and remazol brilliant blue dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Ben Slama, H.; Bouket, A.C.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Berradi, M.; Hsissou, R.; Khudhair, M.; Assouag, M.; Cherkaoui, O.; El Bachiri, A.; El Harfi, A. Textile finishing dyes and their impact on aquatic environs. Heliyon 2019, 5, e02711. [Google Scholar] [CrossRef] [PubMed]
- Roopadevi, H.; Somashekar, R.K. Assessment of the toxicity of waste water from a textile industry to Cyprinus carpio. J. Environ. Biol. 2012, 33, 167–171. [Google Scholar] [PubMed]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Saratale, R.G.; Ferreira, L.F.R.; Bilal, M.; Chandra, R.; Bharagava, R.N. Ecotoxicological and health concerns of persistent coloring pollutants of textile industry wastewater and treatment approaches for environmental safety. J. Environ. Chem. Eng. 2021, 9, 105012. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental Impact and Remediation. In Environmental Protection Strategies for Sustainable Development; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2012; pp. 111–162. [Google Scholar] [CrossRef]
- Elsahida, K.; Fauzi, A.M.; Sailah, I.; Siregar, I.Z. Sustainability of the use of natural dyes in the textile industry. IOP Conf. Series Earth Environ. Sci. 2019, 399, 012065. [Google Scholar] [CrossRef]
- Muthu, S.S. Roadmap to Sustainable Textiles and Clothing: Eco-friendly Raw Materials, technologies, and Processing Methods; Springer Science and Business Media LLC: Dordrecht, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Müller, J.; Gras, C. The carmine problem and potential alternatives. In Handbook on Natural Pigments in Food and Beverages; Springer: Berlin/Heidelberg, Germany, 2020; pp. 385–428. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total. Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Flores-Alatorre, H.L.; Abrego-Reyes, V.; Reyes-Esparza, J.A.; Angeles, E.; Alba-Hurtado, F. Variation in the Concentration of Carminic Acid Produced by Dactylopius coccus (Hemiptera: Dactylopidae) at Various Maturation Stages. J. Econ. Èntomol. 2014, 107, 1700–1705. [Google Scholar] [CrossRef]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 78, 1477–1491. [Google Scholar] [CrossRef]
- Gibaja, S. Pigmentos Naturales Qinónicos, 1st ed.; Fondo Editorial UNMSM: Lima, Peru, 1998; p. 277. [Google Scholar]
- Hassaan, M.A.; El Nemr, A.; El-Zahhar, A.A.; Idris, A.M.; Alghamdi, M.M.; Sahlabji, T.; Said, T.O. Degradation mechanism of Direct Red 23 dye by advanced oxidation processes: A comparative study. Toxin Rev. 2022, 41, 38–47. [Google Scholar] [CrossRef]
- Martínez-Sánchez, C.; Robles, I.; Godínez, L.A. Review of recent developments in electrochemical advanced oxidation processes: Application to remove dyes, pharmaceuticals, and pesticides. Int. J. Environ. Sci. Technol. 2022, 12, 12611–12678. [Google Scholar] [CrossRef]
- Lu, X.; Naidis, G.V.; Laroussi, M.; Reuter, S.; Graves, D.B.; Ostrikov, K. Reactive species in non-equilibrium atmospheric-pressure plasmas: Generation, transport, and biological effects. Phys. Rep. 2016, 630, 1–84. [Google Scholar] [CrossRef]
- Smirnov, B.M. Theory of Gas Discharge Plasma; Springer International Publishing: Cham, Switzerland, 2015; Volume 84, pp. 394–420. [Google Scholar]
- Barjasteh, A.; Dehghani, Z.; Lamichhane, P.; Kaushik, N.; Choi, E.H.; Kaushik, N.K. Recent Progress in Applications of Non-Thermal Plasma for Water Purification, Bio-Sterilization, and Decontamination. Appl. Sci. 2021, 11, 3372. [Google Scholar] [CrossRef]
- Ghimire, B.; Lamichhane, P.; Lim, J.S.; Min, B.; Paneru, R.; Weltmann, K.-D.; Choi, E.H. An atmospheric pressure plasma jet operated by injecting natural air. Appl. Phys. Lett. 2018, 113, 194101. [Google Scholar] [CrossRef]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species and production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Heberlein, J. New approaches in thermal plasma technology. Pure Appl. Chem. 2002, 74, 327–335. [Google Scholar] [CrossRef]
- Taylor, P.R.; Pirzada, S.A. Thermal plasma processing of materials: A review. Adv. Perform. Mater. 1994, 1, 35–50. [Google Scholar] [CrossRef]
- Honglertkongsakul, K. Optical Emission Spectroscopy of Argon Plasma Jet. Adv. Mater. Res. 2013, 770, 245–248. [Google Scholar] [CrossRef]
- Engeln, R.; Klarenaar, B.; Guaitella, O. Foundations of optical diagnostics in low-temperature plasmas. Plasma Sources Sci. Technol. 2020, 29, 063001. [Google Scholar] [CrossRef]
- Naz, M.Y.; Shukrullah, S.; Rehman, S.U.; Khan, Y.; Al-Arainy, A.A.; Meer, R. Optical characterization of non-thermal plasma jet energy carriers for effective catalytic processing of industrial wastewaters. Sci. Rep. 2021, 11, 2896. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of reactive species using various gas plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Sarangapani, C.; Misra, N.; Milosavljevic, V.; Bourke, P.; O’regan, F.; Cullen, P. Pesticide degradation in water using atmospheric air cold plasma. J. Water Process. Eng. 2016, 9, 225–232. [Google Scholar] [CrossRef]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Oehmigen, K.; Winter, J.; Hähnel, M.; Wilke, C.; Brandenburg, R.; Weltmann, K.; von Woedtke, T. Estimation of Possible Mechanisms of Escherichia coli Inactivation by Plasma Treated Sodium Chloride Solution. Plasma Process. Polym. 2011, 8, 904–913. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D Appl. Phys. 2011, 44, 3–7. [Google Scholar] [CrossRef]
- Naitali, M.; Kamgang-Youbi, G.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Brisset, J.-L. Combined Effects of Long-Living Chemical Species during Microbial Inactivation Using Atmospheric Plasma-Treated Water. Appl. Environ. Microbiol. 2010, 76, 7662–7664. [Google Scholar] [CrossRef] [PubMed]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S.; Gürses, A.; Açıkyıldız, M.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties; Springer International: Cham, Switzerland, 2016; pp. 13–29. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, U.; Singh, K.; Gupta, S.P.; Beg, M.S.J. Structure and properties of dyes and pigments. In Dyes and Pigments; IntechOpen Limited: London, UK, 2021; pp. 1–19. [Google Scholar]
- Chouchene, W.; Bellakhal, N. Humid Air Plasma Treatment of Birnessite Surface: Application to the Removal of Cochineal Red. Mater. Sci. Appl. 2015, 6, 1014–1021. [Google Scholar] [CrossRef]
- Crema, A.P.S.; Borges, L.D.P.; Micke, G.A.; Debacher, N.A. Degradation of indigo carmine in water induced by non-thermal plasma, ozone and hydrogen peroxide: A comparative study and by-product identification. Chemosphere 2020, 244, 125502. [Google Scholar] [CrossRef]
- Abdel-Fattah, E. Atmospheric pressure helium plasma jet and its applications to methylene blue degradation. J. Electrost. 2019, 101, 103360. [Google Scholar] [CrossRef]
- Arroyo-Figueroa, G.; Ruiz-Aguilar, G.M.L.; Cuevas-Rodriguez, G.; Sanchez, G.G. Cotton fabric dyeing with cochineal extract: Influence of mordant concentration. Color. Technol. 2011, 127, 39–46. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Wan, Z.; Colonna, W.; Keener, K.M. Degradation kinetics of organic dyes in water by high voltage atmospheric air and modified air cold plasma. Water Sci. Technol. 2017, 76, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Keener, K.M.; Bourke, P.; Cullen, P.J. Generation of In-Package Cold Plasma and Efficacy Assessment Using Methylene Blue. Plasma Chem. Plasma Process. 2015, 35, 1043–1056. [Google Scholar] [CrossRef]
- Khlyustova, A.; Labay, C.; Machala, Z.; Ginebra, M.-P.; Canal, C. Important parameters in plasma jets for the production of RONS in liquids for plasma medicine: A brief review. Front. Chem. Sci. Eng. 2019, 13, 238–252. [Google Scholar] [CrossRef]
- Vogel, A.I. Quantitative Analytical Chemistry. 1960. Available online: https://biblioteca.uazuay.edu.ec/buscar/item/15750 (accessed on 28 May 2023).
- Zhou, R.; Zhou, R.; Prasad, K.; Fang, Z.; Speight, R.; Bazaka, K.; Ostrikov, K. Cold atmospheric plasma activated water as a prospective disinfectant: The crucial role of peroxynitrite. Green Chem. 2018, 20, 5276–5284. [Google Scholar] [CrossRef]
- Benetoli, L.O.d.B.; Cadorin, B.M.; Baldissarelli, V.Z.; Geremias, R.; de Souza, I.G.; Debacher, N.A. Pyrite-enhanced methylene blue degradation in non-thermal plasma water treatment reactor. J. Hazard. Mater. 2012, 237–238, 55–62. [Google Scholar] [CrossRef]
- Zuo, M.; Yi, S.; Choi, J. Excellent dye degradation performance of FeSiBP amorphous alloys by Fenton-like process. J. Environ. Sci. 2021, 105, 116–127. [Google Scholar] [CrossRef]


| Treatment Time | Nitrates | Nitrites |
|---|---|---|
| 0 min | 0.892 | 0.058 |
| 10 min | 2.32 | 0.122 |
| Exposure Time | Kinetic Parameters k (min−1) | R2 (adj.) |
|---|---|---|
| 0–2 min | 0.96645 ± 0.10845 | 0.9636 |
| 2–10 min | 0.03919 ± 0.00427 | 0.97684 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe-Quispe, A.; Pérez-Falcón, L.F.; Quispe-Marcatoma, J.; Landauro, C.V.; Peña Rodriguez, V.A. Removal of Cochineal Dye Color through Atmospheric Pressure Plasma Discharge Jet. Appl. Sci. 2024, 14, 680. https://doi.org/10.3390/app14020680
Quispe-Quispe A, Pérez-Falcón LF, Quispe-Marcatoma J, Landauro CV, Peña Rodriguez VA. Removal of Cochineal Dye Color through Atmospheric Pressure Plasma Discharge Jet. Applied Sciences. 2024; 14(2):680. https://doi.org/10.3390/app14020680
Chicago/Turabian StyleQuispe-Quispe, Arturo, Luis F. Pérez-Falcón, Justiniano Quispe-Marcatoma, Carlos V. Landauro, and Victor A. Peña Rodriguez. 2024. "Removal of Cochineal Dye Color through Atmospheric Pressure Plasma Discharge Jet" Applied Sciences 14, no. 2: 680. https://doi.org/10.3390/app14020680






