1. Introduction
Endodontic treatment is primarily aimed at thoroughly chemo-mechanically disinfecting and obturation the root canal completely [
1]. In this process, the smear layer and debris are removed from the wall of the root canal, as they can serve to prevent canal irrigation solutions and root canal sealers from penetrating dentinal tubules, which, in turn, could cause microbial microleakage and failure of the root canal treatment [
2].
Employing conventional needle irrigation alone is insufficient for complete chemo-mechanical preparation. Thus, a variety of different manual and machine-assisted irrigation methods were introduced to enhance the effectiveness of chemo-mechanical disinfection, such as endo brushes, dynamic gutta-percha technique activation, sonic, ultrasonic, and laser devices [
3,
4]. However, the EndoActivator (EA) system constitutes one of the devices most frequently used for activating irrigation. EA is a sonic activation technique that comprises a handpiece along with three flexible non-cutting polymer tips. This device, when run in the root canal, produces vigorous intracanal fluid agitation, which in turn activates the irrigation solution [
5]. Ultrasonic devices are also used for irrigation activation, which is an effective method for cleaning residual debridement within the root canal and increasing canal disinfection. The operating frequency at which the majority of ultrasonic devices function for passive ultrasonic irrigation (PUI) is 25–30 kHz. A non-cutting tool is positioned within the root canal at its working length. This tool then transmits energy via PUI (passive ultrasonic irrigation) to the irrigation solution inside the canal. This process aids in cleaning and disinfecting the canal by utilizing ultrasonic energy to agitate the solution, effectively removing debris and bacteria from difficult-to-reach areas [
6].
A different method that has recently been proposed for disinfecting the root canal and efficiently removing debris and the smear layer is laser-activated irrigation (LAI). Laser devices such as erbium, chromium:yttriumscandium-gallium-garnet (Er,Cr:YSGG) as well as erbium:yttrium-aluminum-garnet (Er:YAG), generate explosive vapor bubbles that have a secondary cavitation effect as the laser energy is highly absorbed by water [
7,
8]. The wavelength of Er,Cr:YSGG is 2780 nm, which is delivered to the canal at different output powers by radial firing tips (RFTs). In comparison to alternative laser devices, the use of such a laser enables superior cleaning of the root canal due to its strong affinity to hydroxyapatite and water in addition to its minimal thermal damage [
9,
10]. On the other hand, shockwave-enhanced emission of photoacoustic streaming (SWEEPS) constitutes a novel LAI concept that was introduced according to use of the Er:YAG laser (2940 nm wavelength) with the irrigation solution. This technique’s unique mechanism is that a pair of ultrashort sub-ablative energy pulses (20 mJ) are emitted, where the second of these pulses occurs immediately before the bubble caused by the first bubble collapses. Consequently, the pulse peak power generated is extremely high (800 W) and secondary cavitation is amplified, which disseminates to the extreme regions of the root canal [
11].
Another crucial goal in root canal treatment is to employ biocompatible materials to achieve a tightly sealed and three-dimensionally filled root canal. The canal is sealed laterally and apically using root canal sealers, which facilitate the effective adaptation between the filling materials and canal walls. This can be achieved via the penetration of the sealer into the canal wall’s dentine tubules, which causes the sealing ability to be enhanced and the sealer to be retained [
12]. Additionally, bacterial colonization and reinfection of the root canal can be prevented via the penetration of the sealer into the dentine tubules via its antibacterial activity [
13]. The AH-Plus root canal sealer (Dentsply DeTrey, Konstanz, Germany) is a frequently employed epoxy sealer based on resin that is considered a “Gold Standard” in endodontic research. It has superior dentine tubular penetration and adaptation, high biocompatibility, and is dimensionally stable [
13,
14]. However, AH-Plus Bioceramic (Dentsply Sirona, Charlotte, NC, USA) was recently proposed as a novel premixed tricalcium silicate-based sealer. Based on information provided by the manufacturer, its set time is rapid and can be predicted, and it has a low solubility, increased washout resistance, biocompatibility, a lower film thickness and better radiopacity, no tooth discoloration, and it can even be removed once set using an NiTi file or general hand file. The components of this sealer include thickening agents, lithium carbonate, dimethyl sulfoxide, tricalcium silicate, and zirconium dioxide [
15,
16]. However, in the literature, only one study was found that evaluated the AH-Plus Bioceramic sealer in terms of dentin tubule penetration [
17].
Thus, this study was aimed at assessing the depth and percentage of penetration of the novel AH-Plus Bioceramic sealer in comparison to the traditional AH-Plus sealer into dentinal tubules subsequent to different irrigation activation protocols, including the conventional needle irrigation, EA, PUI, Er,Cr:YSGG laser, and SWEEPS techniques utilizing confocal laser scanning microscopy (CLSM).
2. Materials and Methods
2.1. Teeth Selection
The ethical approval for this research was gained from the Scientific Research Ethics Evaluation Board with the protocol number YDU/2023/115-1740. For each of the groups, the minimum size of the sample was 15 teeth based on power analysis (G*Power 3.1.9.7 software; Heinrich Heine University, Dusseldorf, Germany), which was employed at a power of 80% and an alpha-type error of 0.05. In the research, 150 extracted single-rooted mandibular premolars were selected. Teeth with fillings, caries immature apical foramen, resorptions, and a curved root canal were not included [
18].
Periapical radiographs were taken for each tooth from the mesial–distal and buccal–lingual directions, thus ensuring that the teeth had only one canal. This was followed by using a 0.1% thymol solution for cleaning of the selected specimens, which were then saved in filtered water at 4 °C prior to usage. Decoronation of the teeth was performed using a precision cutting machine (Isomet 1000; Buehler, Lake Bluff, IL, USA), where water cooling was applied to standardize the root canal length at 17 mm from the apical foramen. To determine the working length (WL) during the endodontic treatment, a #10 K-file from Dentsply Maillefer, Ballaigues, Switzerland, was placed into the root canal. The file was advanced until its tip became visible at the apex, the endpoint of the root. At this point, the file’s length was measured. The working length was then set at 1 mm shorter than this measured distance. The same practitioner performed these procedures along with the subsequent steps.
2.2. Root Canal Preparation
In this study, the root canal preparation followed the crown-down method, utilizing a ProTaper Universal NiTi rotary system (Dentsply Maillefer). A sequence of files—SX, S1, S2, F1, F2, F3, and F4—were employed within the root canals as stipulated by the manufacturer. These files were powered by an X-Smart electric endomotor (Dentsply Maillefer) operating at 250 rpm with a gear reduction of 16:1. Throughout the procedure, canal irrigation between these files was conducted using a total of 20 mL of 2.5% sodium hypochlorite (NaOCl; Wizard; Rehber Chemistry, Istanbul, Turkey). This specific technique and equipment were utilized to ensure comprehensive cleaning and shaping of the root canals. Subsequently, the apical foramen of the teeth was covered by pink wax to prevent irrigations from overflowing beyond the apex.
2.3. Final Irrigation Technique
According to the final irrigation protocol, the teeth were assigned to 5 groups on a random basis, as follows: conventional syringe irrigation (CSI), EA, PUI, Er,Cr:YSGG laser, and SWEEPS.
In the CSI group, an irrigation needle (30-gauge) (Ultradent, South Jordan, UT, USA) was used to administer 5 mL of 2.5% NaOCl and 5 mL of 17% ethylene diamine tetra acetic acid (EDTA) into the canal for 30 s each. The irrigation needle was positioned 1 mm above the established WL and moved in a 1–2 mm motion in an in-and-out direction. Distilled water was used between these irrigation solutions and as a final rinse.
In the second experimental group, an EA device (Dentsply Tulsa Dental Specialities, Tulsa, OK, USA) was employed to activate the NaOCl and EDTA irrigation solutions. Each irrigation solution, totaling 5 mL, was introduced into the canal and then activated using the EA handpiece set at 10,000 cycles/min, utilizing a medium polymer tip (size 25, taper 0.04). The tip was positioned 2 mm away from the WL and moved vertically in a 2–3 mm range from the root apex towards the crown for a duration of 20 s. This entire procedure was repeated three times for each irrigant. Then, distilled water was used between both solutions and as a final rinse.
In the PUI group, the same irrigation solutions and volume that were used in the previous group were activated by PUI (Endo Soft Instruments (ESI); EMS, Le Sentier, Switzerland). This involved the insertion of a stainless-steel tip no. 20, 0.02 taper (IrriSafe tip, Satelec Acteon Group, Merignac, France), into the canal by 2 mm such that it was shorter than WL, which was followed by the activation of the PUI device at a frequency of 40 kHz for 20 s without touching the canal walls. Gentle irrigation was continued throughout the activation procedure. The process was conducted thrice for each irrigant. Distilled water was used to wash the canal between and after the PUI activation.
In samples taken from the fourth group, the application involved the use of an Er,Cr:YSGG laser (Waterlase MD; Biolase, Irvine, CA, USA) emitting light at a 2780 nm wavelength. To activate the previously mentioned irrigation solutions, an RFT2 fiber tip (diameter of 275 lm and length of 21 mm; Endolase; Biolase Technology, San Clemente, CA, USA) was utilized. The setting used for the laser device was the hard tissue mode, and the parameters utilized were as follows: pulse duration of 140 µs duration, 1 W of output power, 10 Hz repetition rate of 20 Hz, and 100 mJ of energy per pulse. As the process of activation occurred, the gold handpiece’s coaxial water spray (Biolase Technology, Irvine, CA, USA) was deactivated. The RFT2 tip was only inserted into the coronal reservoir, and activation of the Er,Cr:YSGG laser lasted for 30 s, with a subsequent period of 30 s of rest, followed by 30 s of activation to end the procedure. Gentle irrigation was continued throughout the activation procedure. However, washing of the canal between and after the laser was performed using distilled water.
In the final group, both irrigation solutions underwent activation using an Er:YAG laser employing a flat-end fiber tip known as SWEEPS (SWEEPS 600; Fotona, Ljubljana, Slovenia). The tip was positioned in the coronal reservoir of the canals, and the Er:YAG laser settings were configured at 0.3 W, 20 mJ/pulse, 15 Hz, and 50 µs pulse duration. The air-and-water system was deactivated during this process. The activation duration was 30 s for each solution, and gentle irrigation was continued throughout the activation procedure. Finally, distilled water was used between these irrigation solutions and as a final rinse.
2.4. Root Canal Obturation
Subsequent to using distilled water to rinse the canals, the pink wax was extracted from the apical region, followed by drying with F4 absorbent paper points (Dentsply Maillefer). The teeth in each group were separated into 2 sub-groups on the basis of the type of root canal sealer utilized: AH-Plus (AHP) and AH-Plus Bioceramic (AHPB) sealers (n = 15). Both sealers were mixed with 0.1% rhodamine B fluorescent dye (Merck, Darmstadt, Germany) before canal filling prior to subsequent evaluation with confocal laser scanning microscopy.
The single-cone obturation technique was used in this study. Sealer was introduced into the root canals, after which the F4 gutta-percha was coated with the sealer before being inserted into the canal at the WL. An instrument with a heated tip was utilized to trim off any surplus gutta-percha, and then a plugger was used to compact and condense the material. After that, moistened cotton was used to clean the excess sealer in the coronal access, and teeth sealing was performed using a temporary filling (3M, St. Paul, MN, USA). Thereafter, an incubator set at 37 °C and at a humidity of 100% was used for storing each of the samples for 10 days to ensure a full set of sealers.
2.5. Sample Sectioning and CLSM Analysis
Subsequent to the incubation period, the teeth were embedded into acrylic blocks vertically followed by perpendicular sectioning to their long axis to obtain a pair of sections with respective thicknesses of 1 mm, 6 mm (middle) and 3 mm (apical) from the apical foramen. A precision saw (Isomet 1000; Buehler, Lake Bluff, IL, USA) was utilized for sample cutting with a diamond disc under water cooling at low speed. Silicon carbide abrasive papers were used to polish the coronal surfaces such that any particles of dentin generated by the process of cutting could be removed.
Confocal laser scanning microscopy (CLSM; Leica SPEII; Leica Microsystems, Mannheim, Germany) was employed to scrutinize the sections. Prior to examination, each section was coded and numbered. The CLSM facilitated observation at a 10× magnification under Ar/HeNe laser excitation with a wavelength of 543 nm. After that, Adobe Photoshop was used to combine the multiple images of each sample into an individual completed image (CS3 extended; Adobe Systems, Inc., San Jose, CA, USA). Then, this was followed by importation of the images into ImageJ (ImageJ software; V1.54d, NIH), and the maximum penetration depth (MPD) point in micrometers (µm) and penetration percentage (µm
2) were calculated for each specimen’s image depending on the Gharib method [
19]. The distance tool in the program was used to measure the MPD from the wall of the canal to the deepest level of sealer penetration. This was followed by the determination of the circumference of the canal as well as the regions where the sealer penetrated along the walls of the canal with a freehand line tool. The penetration percentage was calculated by dividing the values of the regions where the sealer penetrated by the circumference of the root canal.
2.6. Statistical Analysis
Statistical analysis of the data gathered during the research was conducted using appropriate software (IBM SPSS Statistics, v26; IBM Corp., Armonk, NY, USA). The Shapiro–Wilk test confirmed a normal data distribution, allowing for the selection of parametric tests due to this normal distribution. Because of the data normality, the three-way analysis of variance (ANOVA) test was employed for the statistical analysis (final irrigation protocol, sealer type, and root third). Whenever a statistically significant interaction was found, the post hoc Tukey test was utilized for multiple comparisons in the presence of significance. For all analyses, a 5% level of statistical significance was used.
3. Results
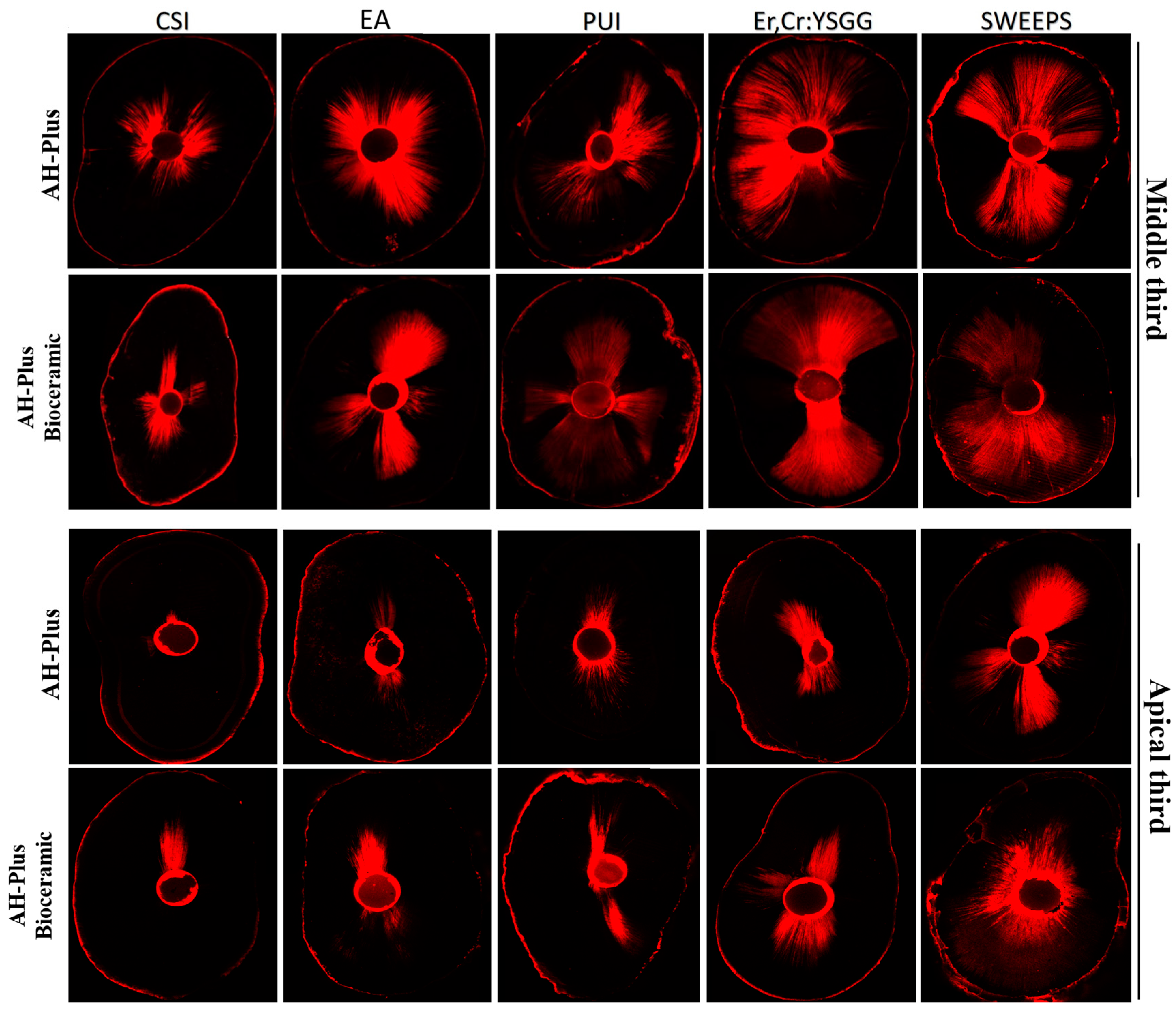
Figure 1 illustrates a sample of a CLSM image of the canal sealer penetration from each group at the root canal’s middle and apical third.
The three-way ANOVA revealed that the tested variables (irrigation activation protocol, sealer type, and root third) had significant effects on the MPD variables and their interactions terms (
p < 0.05) except for the sealer type * root third and irrigation activation protocol * sealer type * root third interactions (
p > 0.05).
Table 1 presents a summary of the values for the mean and standard deviation of the MPD of the sealers for all groups. However, regardless of the irrigation activation protocol, the AHP and AHPB sealers statistically presented similar penetration properties. Regarding the effect of the irrigation activation protocol, the highest MPD values were recorded in the SWEEPS groups (697.90 ± 279.30), while the lowest value was observed in the CSI groups (259.68 ± 83.74). Multiple comparisons indicated significant differences between all irrigation activation protocols (
p < 0.05). Considering the sealer type and irrigation protocol interaction effect, a statistically significant difference was detected between the Er,Cr:YSGG laser and EA groups for the AHPB sealer (
p < 0.05), while it was insignificant for the AHP sealer (
p > 0.05). Considering the irrigation protocol and root third interaction, a significant difference was detected between the maximum depth to which the sealer penetrated between all irrigation protocols groups at the middle third, while the SWEEPS, Er,Cr:YSGG, and EA values were significantly higher compared to the PUI and CSI protocols at the apical third (
p < 0.05).
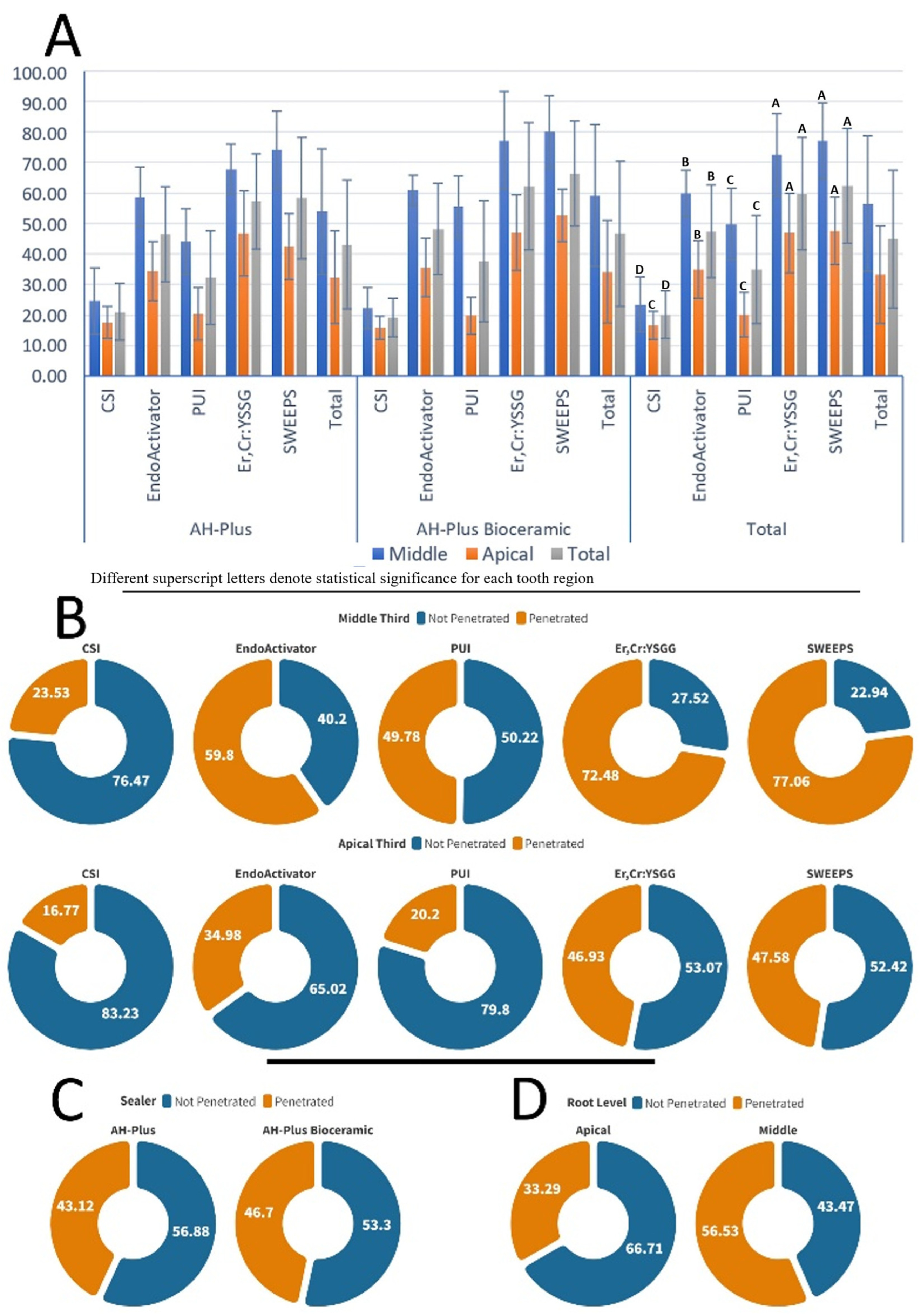
Figure 2A shows the mean penetration percentages with the associated standard deviations, highlighting the intragroup and intergroup efficacy across the various sealers and irrigation techniques.
Figure 2B illustrates the penetration effectiveness of the different irrigation methods at two root levels regardless of the sealer type.
Figure 2C represents the overall penetration performance of the canal sealers independent of the irrigation method and root level.
Figure 2D illustrates the sealer penetration at the different root levels irrespective of the sealer type and irrigation method used. The three-way ANOVA test revealed that the variables tested affected the penetration percentage values (
p < 0.05), but the interactions between the variables were insignificant (
p > 0.05) except for irrigation technique * root third interaction (
p < 0.05). Regarding the effect of the irrigation activation protocol, the SWEEPS and Er,Cr:YSGG laser groups exhibited the highest percentage values, while the CSI group had the lowest value. Significant differences were presented between all the irrigation activation protocol groups (
p < 0.05) apart from the difference between the SWEEPS and Er,Cr:YSGG groups (
p > 0.05). Considering the irrigation protocol and root third interaction, a significant difference was detected among the PUI and CSI groups at the middle third of the canal (
p < 0.05), while it was insignificant at the apical third (
p > 0.05).
4. Discussion
According to the results of our study, the highest MPD values were observed in the SWEEPS groups, while the lowest value was in the CSI groups. Considering the sealer type and irrigation protocol interaction effect, there was a significant difference between the Er,Cr:YSGG laser and EA groups for the AHPB sealer, while it was insignificant for the AHP sealer. The penetration percentage values were affected by the tested variables, but the interactions between the variables were insignificant except for irrigation technique * root third interaction. However, the novel AHPB sealer is a recently introduced root canal sealer for endodontic use. Generally, the biocompatibility and bioactive properties of bioceramic sealers are superior in addition to their ability to encourage a surface layer of hydroxyapatite, which could generate a mineral attachment to dentin tissue [
20,
21]. However, the extent to which root canal sealers are capable of effectively sealing the root canal is influenced by many factors, including penetration of the sealer into dentin tubules, the canal irrigation technique used for smear layer removal, and the type of sealer [
22,
23]. Thus, this research aimed to assess the depth and percentage of the novel AHPB sealer compared to the traditional AHP sealer into dentinal tubules subsequent to different irrigation activation protocols using CLSM.
Researchers have previously used CLSM and scanning electron microscopy (SEM) devices to evaluate the extent to which sealers are capable of penetrating into dentin tubules [
22,
24]. In this study, it was preferred to use CLSM due to its superior evaluation properties compared to SEM. A desiccation process should be applied to root sections for SEM evaluation, which may result in sealer being lost and the specimen and materials being deformed [
25]. In addition, a detailed image of the specimen and a quick evaluation of the sealer penetration can be acquired at a lower magnification by CLSM using fluorescent rhodamine B dye without changing the flow characteristics of the sealers [
26,
27].
To minimize the presence of anatomical irregularities and apical delta, which could impact the penetration of the sealer, sample sections were chosen to be taken from 3 and 6 mm distances from the apex in this study, similar to previous studies [
22,
28]. However, in agreement with other papers [
29,
30], our results stated that for both sealers, the sealer penetration values (depth and percentage) in all the irrigation protocol groups were higher in the middle third in comparison to the apical third. These anticipated outcomes could arise from the larger diameter and higher density of the dentinal tubules in the middle region compared to those in the apical area. Furthermore, the task of eliminating the smear layer in the apical third poses greater difficulty, primarily because effectively delivering irrigation solutions to that particular area is more complex and demanding. [
31].
In the current study, irrigation solutions including NaOCl and EDTA were used with different methods of activation for removing the smear layer, which is essential to ensuring that the root canal sealer penetrates the dentinal tubules at a high rate, increasing the sealing ability, and decreasing microbial microleakage [
2,
32]. According to this study’s findings, significant differences were detected between all the irrigation protocol groups regarding MPD, where the order from the highest to the lowest values was as follows: SWEEPS > Er,Cr:YSGG > EA > PUI > CSI. As expected, the CSI group showed the lowest depth and percentage of sealer penetration in comparison to the other groups, in line with the findings of past research [
33,
34]. An explanation of this finding could be that when the CSI method was used, the irrigant reached no further than 2 mm from tip of the needle, meaning that it was incapable of reaching irregularities and the canal’s apical region [
35], although it has been stated in other studies that significant differences did not exist between CSI and other irrigation activation techniques [
36]. However, Lukac et al. [
11] noted that SWEEPS enhances the capacity for debris and smear layer removal. This technique can produce bubbles by activating the Er:YAG laser via the photoacoustic streaming effect. When these bubbles collapse, they generate an intensely swift irrigant jet adjacent to the canal walls, significantly amplifying surface cleaning through acoustic streaming. According to the findings of the study by Ozbay et al. [
37], the Er:YAG laser method presented the greatest efficiency in terms of removing the smear layer, irrespective of which solution was employed.
Our findings indicated that the sealer MPD of the Er,Cr:YSGG laser was higher in comparison to that of PUI, EA, and CSI but lower than that of SWEEPS. This phenomenon might occur because the laser’s energy absorption by the irrigant leads to the formation of vapor bubbles. These bubbles act as a fluid pump inside the root canal, creating a cavitation effect. Consequently, this process efficiently eliminates the smear layer and facilitates the infiltration of the irrigation solution into the dentinal tubules [
38,
39]. Betancourt et al. [
7] showed that Er,Cr:YSGG had increased effectiveness compared to PUI in improving the antibacterial effect of NaOCl. Conversely, it was demonstrated in another study that SWEEPS was not capable of increasing the sealer penetration compared to PUI [
22]. The differences in these study findings could be associated with the utilization of distinct root canal sealer types in these studies that have various properties.
Considering the penetration percentage, significant differences were detected among all the irrigation activation protocol groups except for the difference between the SWEEPS and Er,Cr:YSGG groups. Nevertheless, as aforementioned, the laser-activated irrigation showed higher activity in terms of cleaning the root canal and removing the smear layer compared with the alternative methods of irrigation.
Interestingly, the EA groups showed a significantly higher sealer penetration depth and percentage than the PUI groups for both sealers used in this research, although it was confirmed by recent reports [
40,
41]. This may possibly be because of the short period of ultrasonic activation and the PUI file and the walls of the canal coming into contact. Furthermore, a downside of PUI was observed in terms of the reduction in its distinctive nodes and antinodes pattern, notably when the instrument made contact with the shaped canal’s lateral walls. Sonic activation functioned with one positive and one negative node. The vibratory sonic tool’s movement was not altered by contact with the lateral wall [
42].
Considering the sealer type and irrigation protocol interaction, a statistically significant difference was found between the Er,Cr:YSGG laser and EA groups for the AHPB sealer, while it was insignificant for the AHP sealer. However, regardless of the irrigation activation protocol, the AHP and AHPB sealers statistically presented similar penetration properties. Although recent studies have reported that the AHPB sealer showed superior properties compared to AHP [
16,
43], our results presented insignificant differences between them in terms of dentin tubule penetration. Shieh et al. [
17] compared AHPB to AH-Plus Jet and EndoSequence BC sealers and stated that the bioceramic-based root canal sealers seemed to exhibit superior performance compared with the epoxy-resin-based sealer with respect to the extent to which they penetrated the dentinal tubules, which was inconsistent with our findings. In their study, Souza et al. [
43] noted that AHPB had a shorter initial setting time and was less radiopaque than AHP. AHP was less soluble than AHPB. AHP’s calcium ion release was lower than AHPB’s. AHPB had a higher flow than AHP. Cell viability after applying the AHPB sealer was higher than with AHP. However, additional studies must be conducted in the future for a more in-depth evaluation and assessment of the properties of AHPB sealer.
This study has specific limitations including the inability to standardize the samples in terms of the distribution and amount of sclerotic dentin and the number of dentinal tubules; additionally, a root canal system in clinical conditions would be much more complex than the single canals used in this study. Nevertheless, this study could serve as a basis for future research exploring teeth with a more intricate anatomy or evaluating root canal sealer penetration under in vivo conditions, comparing it with alternative techniques. Additionally, further assessment of the characteristics of the novel AHPB sealer using diverse materials and methodologies is necessary to delineate its advantages and disadvantages.