Potential of Proton-Exchange Membrane Fuel-Cell System with On-Board O2-Enriched Air Generation
Abstract
:1. Introduction
- Quantify the effect of O enrichment on a PEMFC stack efficiency and mass flow with constant O and H excess ratio.
- Evaluate the power consumption linked to the operation of a polymeric membrane for O and N separation.
- Propose a novel balance for a plant layout for on-board O enrichment and identify the potential sources for energy recovery.
- Analyze the sensitivity of the new system efficiency regarding its main design and operational parameters to evaluate its potential with respect to a standard architecture.
2. Approach to Fuel-Cell System Analysis
2.1. Fuel Cell Stack
2.2. N2–O2 Separation
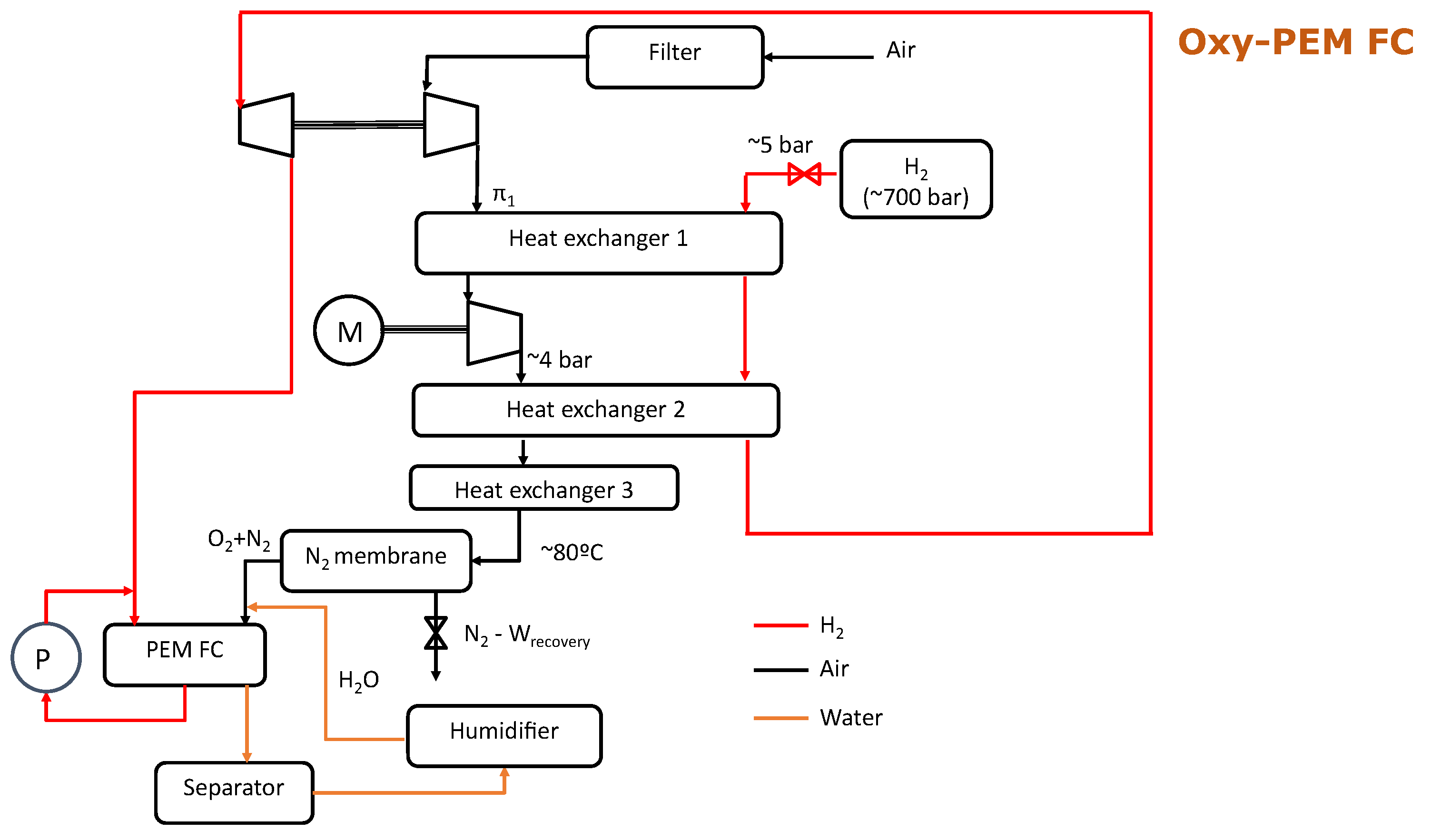
2.3. Balance of Plant Description
2.4. Summary of Main Assumptions
- For all operating conditions, the sensitivity of the fuel cell stack polarization curve with respect to the oxygen molar fraction is assumed to be equal to the data obtained from the study of Fournier et al. [26].
- The hydrogen consumption is directly calculated from the anode reaction stoichiometry. At the cathode, the total oxygen mass flow is the same regardless the oxygen molar fraction achieved, while this value is adapted as a function of the current density assuming a constant oxygen excess factor.
- The separation membrane operates at a constant temperature of 80 °C and pressure ratio (nominally, four). The ratio of inlet to permeate (O-enriched) mass flows (stage cut) is extracted from the operating map of a commercial system (model NM C05 of the UBE Corporation, Tokyo, Japan).
- In the retentate flow (nitrogen-enriched), a pressure drop of 5% is assumed for all working conditions. This stream is directed to an electric turbine, characterized by a constant isentropic efficiency. Instead, in the permeate (oxygen-enriched) stream, the outlet pressure is assumed to be equal to 1 bar. For this reason, the operation of the oxygen-enriched concept was compared to an atmospheric fuel cell, also working with 1 bar at the cathode inlet, so that the pressure effect on the fuel cell stack performance is equivalent.
- In order to reach the necessary pressure upstream of the separation membrane, a two-stage boosting system with intermediate cooling is used. A cooling efficiency of 90% is assumed for this intermediate cooling.
- The hydrogen stream is assumed to be provided at 5 bar, controlled by an expansion valve at the hydrogen tank outlet. This stream is heated using the energy available in the cathode stream after compression, increasing the exergy available in the flow. Afterword, the 5-bar heated hydrogen stream is directed to a turbine included in a turbocharger, providing the power for the first compression in the cathode stream with a mechanical efficiency of 97%. Instead, the second compression is directly driven by an electrical compressor, representing the main energy consumption in the proposed system.
- The isentropic efficiency of each compressor stage is assumed to be equal and independent on the working conditions. In the same sense, both nitrogen and hydrogen turbine efficiencies are also constant and equal. The selected ranges for turbine and compressor efficiencies were extracted from the typical values of the radial turbomachinery currently used in automotive applications in the peak efficiency area.
3. Commercial Membrane Analysis
4. Sensitivity Analysis to Air Management Parameters
- The compatibility of H with some of the most common materials used in this type of turbomachinery, especially stainless steel or cast iron.
- The high diffusivity of H, which makes it necessary to optimize interstitial spaces and sealing systems in order to achieve high target performance.
- The relatively high expected expansion ratio (around five), which makes mechanical design and reduction of axial stresses in the turbocharger bearings difficult.
- In order to ensure acceptable turbine performance, it is necessary to ensure a relatively high inlet flow temperature. In the proposed installation, it is very difficult to achieve these conditions, so it would be necessary to introduce a temperature conditioner at the inlet.
5. Conclusions
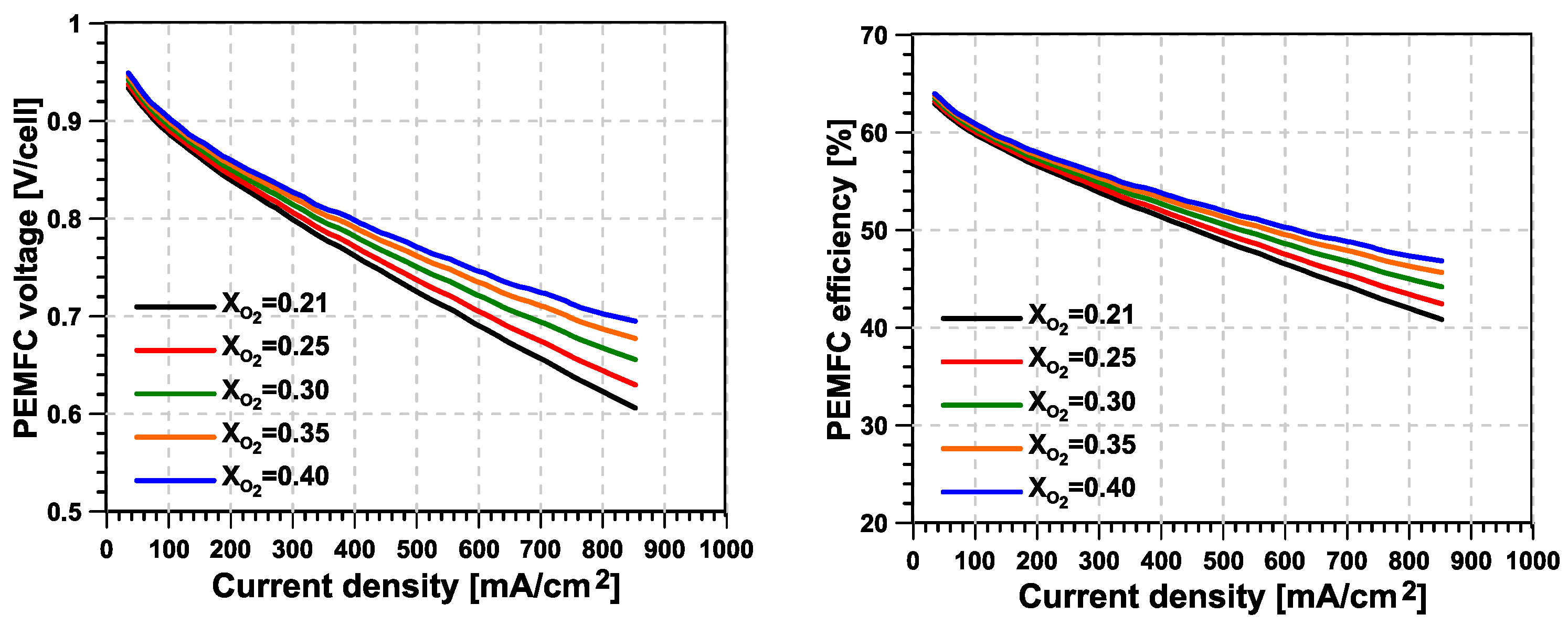
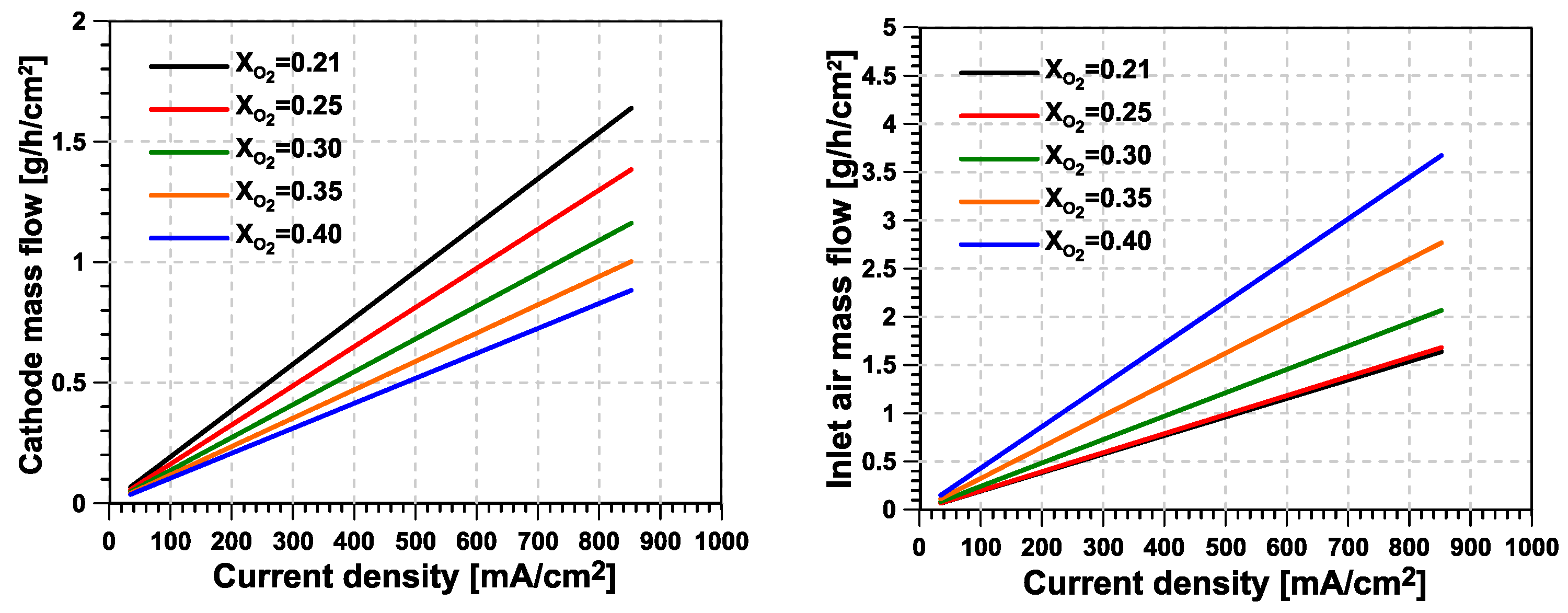
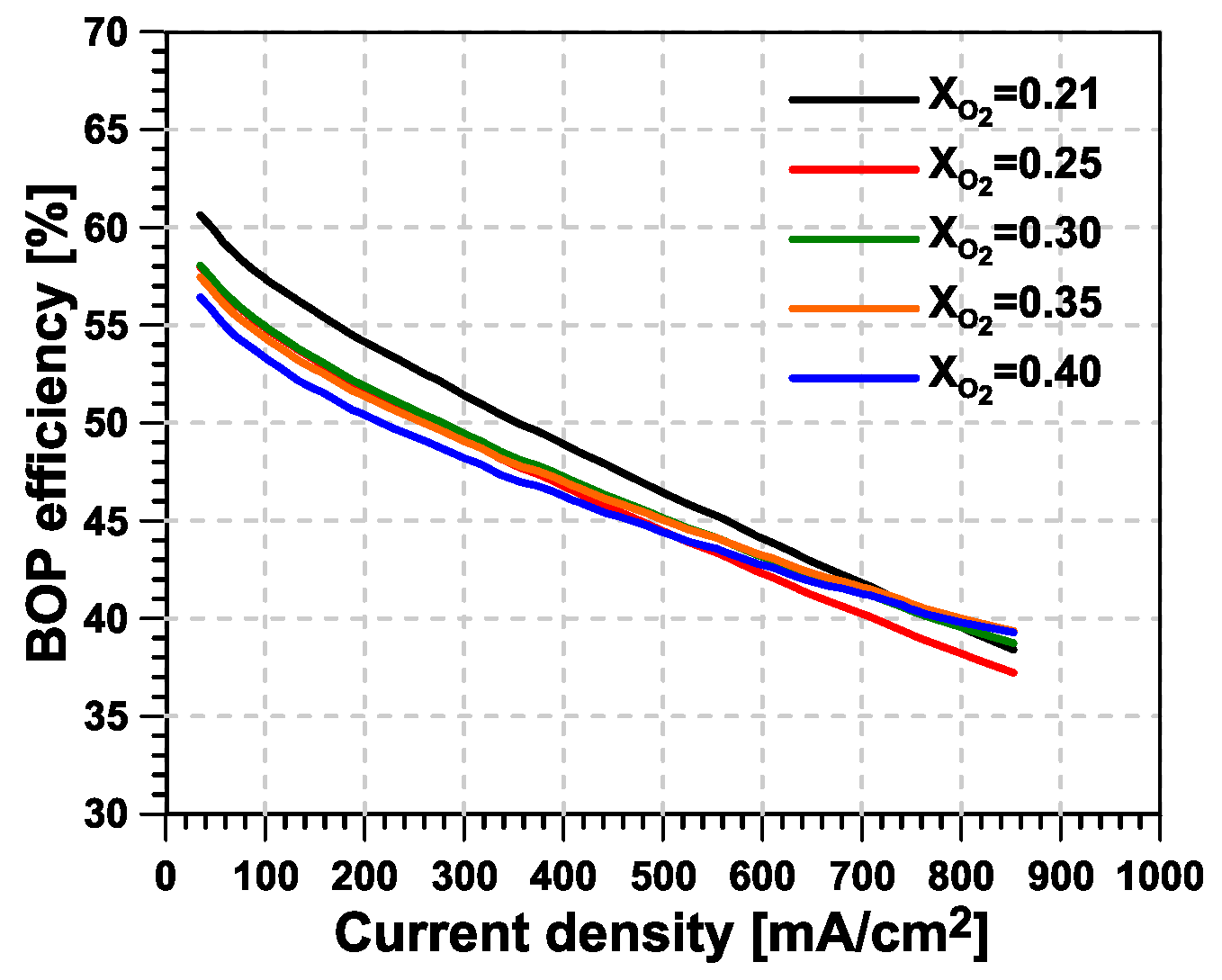
- The main advantage from O enrichment in terms of fuel cell stack efficiency is found at medium-to-high current intensity levels, where a higher O concentration helps to reduce the losses related to diffusion and chemical kinetic aspects at the cathode side. In contrast, the margin for improvement is very small at low current intensity values. Additionally, the mass flow per unit of active area to be supplied to the fuel cell cathode can be reduced if the same level of excess O ratio is applied.
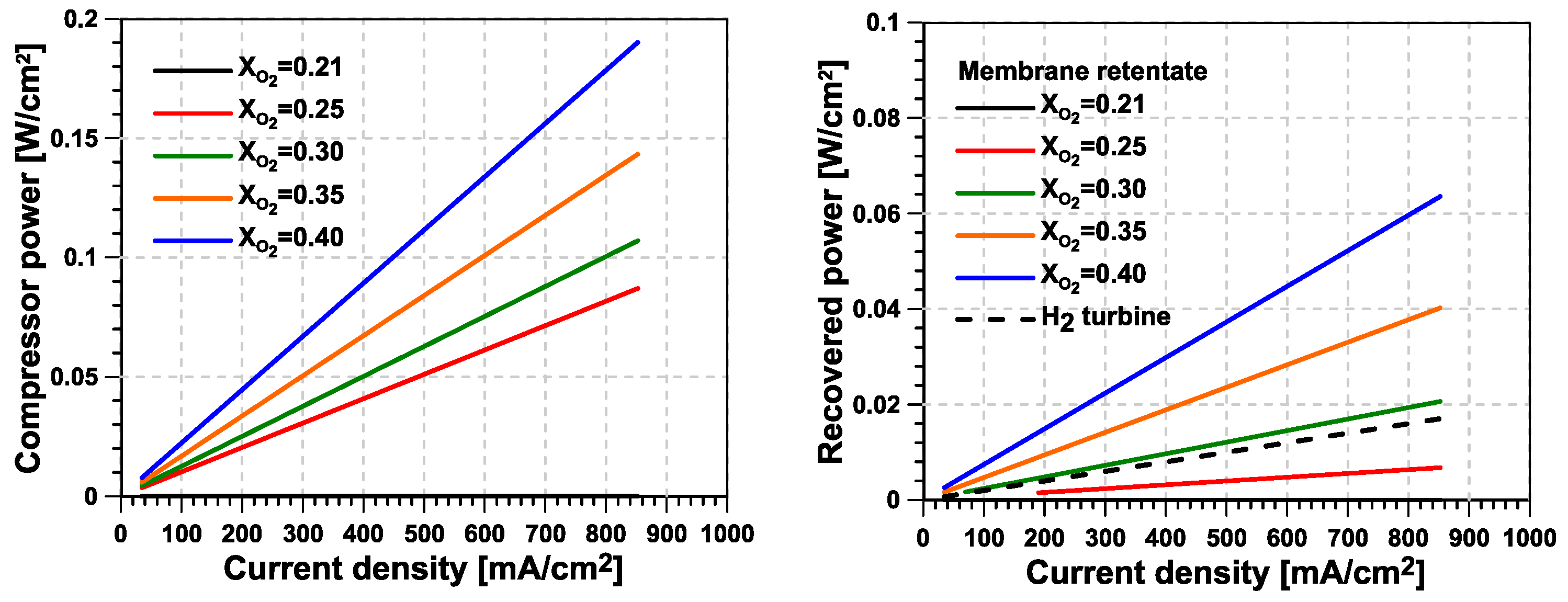
- Higher levels of O enrichment imply the need to supply the O–N separation membrane with a mass flow that is significantly higher than the useful (permeate) O-enriched generated stream. This reverses the reduction in cathode mass flow seen at the stack level, significantly increasing the compressor’s power consumption and limiting the potential advantages of the system compared with a traditional fuel-cell system. As a consequence, the new proposed concept consumes additional power, which is mostly concentrated in the second stage of compression, driven by an electric motor.
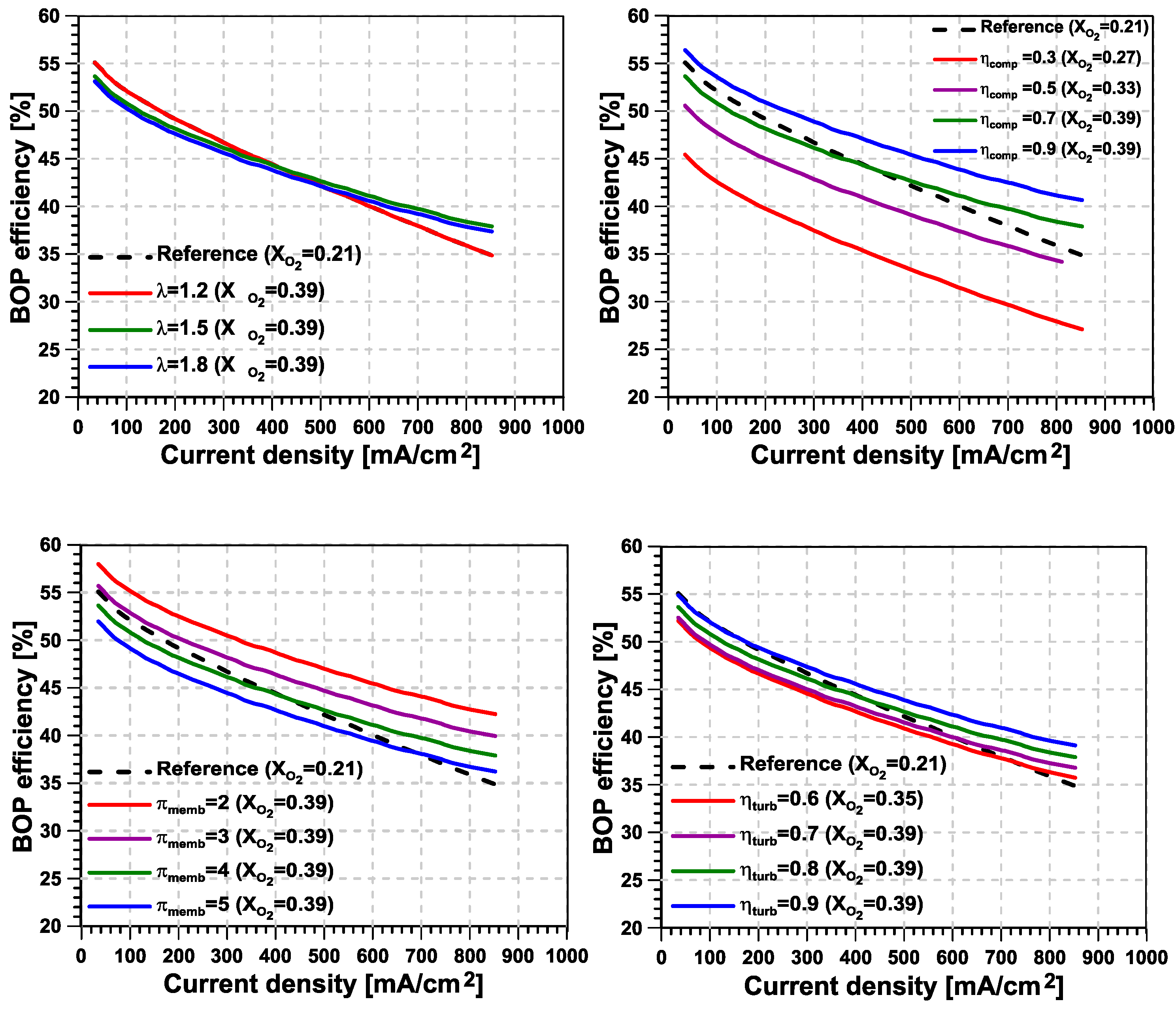
- From the sensitivity analyses performed, it was concluded that the factors that affect the most the system level efficiency are, in this order, the pressure ratio in the membrane and the compressor efficiency. Both are related to the compression work, highlighting the fact that the proposed system’s potential increases as more efficient O–N separation membrane technologies are developed.
- Regarding the cathode’s excess O ratio, a higher O partial pressure inside the fuel cell reduces below the value of 1.5 used in the base case. Instead, the penalty induced when working with higher excess O ratio is limited owing to the energy recovery.
- In general, relatively high levels of enrichment are preferable for the technology, despite the increase in compressor power consumption, as long as it can be guaranteed that at least one-third of this consumption can be recovered from the system.
- Conduct an experimental analysis to investigate the effects of the O molar fraction (21–40%) and excess O ratio (1–2) in the cathode stream to ascertain the advantages of O enrichment on cell performance at increased current densities and assess the feasibility of reducing the cathode’s excess O ratio. This research will also aid in developing a 1D fuel cell model with the ability to analyze reactive species depletion, thereby enhancing system efficiency.
- Evaluate other O enrichment separation membrane technologies with higher selectivity and analyze the potential to reduce the membrane working pressure and/or the ratio of inlet to permeate mass flows.
- Integrate the fuel cell and the membrane in a one-dimensional model of the complete balance of plant to study the sizing of the compressor and turbine elements, including preliminary maps of these components to better assess their efficiency as a function of the operating conditions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | |
| BOP | balance of plant |
| PEMFC | proton-exchange membrane fuel cell |
| R | coefficient of determination |
| Latin Letters | |
| F | Faraday constant |
| higher heat value | |
| i | current density |
| N | power |
| average molecular weight of cathode | |
| molecular weight of | |
| molar fraction | |
| Greek Letters | |
| molar flow rate consumption | |
| molar flow reaching the cathode | |
| efficiency | |
| ratio between both mass flow rates | |
| excess ratio | |
| pressure ratio of operation from compressor 1 | |
| expansion ratio in turbine corresponding to H2-fired generator set | |
| level of compression necessary to operate polymeric separation membrane | |
| Subscripts | |
| compressor | |
| net | |
| stack | |
| turbine |
References
- Crowley-Vigneau, A.; Kalyuzhnova, Y.; Ketenci, N. What motivates the ‘green’ transition: Russian and European perspectives. Resour. Policy 2023, 81, 103128. [Google Scholar] [CrossRef]
- Tan, K.M.; Babu, T.S.; Ramachandaramurthy, V.K.; Kasinathan, P.; Solanki, S.G.; Raveendran, S.K. Empowering smart grid: A comprehensive review of energy storage technology and application with renewable energy integration. J. Energy Storage 2021, 39, 102591. [Google Scholar] [CrossRef]
- Carmo, M.; Stolten, D. Energy storage using hydrogen produced from excess renewable electricity: Power to hydrogen. In Science and Engineering of Hydrogen-Based Energy Technologies; Academic Press: Cambridge, MA, USA, 2019; pp. 165–199. [Google Scholar]
- Ogungbemi, E.; Wilberforce, T.; Ijaodola, O.; Thompson, J.; Olabi, A. Selection of proton exchange membrane fuel cell for transportation. Int. J. Hydrogen Energy 2021, 46, 30625–30640. [Google Scholar] [CrossRef]
- Rosli, R.; Sulong, A.; Daud, W.; Zulkifley, M.; Husaini, T.; Rosli, M.; Majlan, E.; Haque, M. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Fathabadi, H. Combining a proton exchange membrane fuel cell (PEMFC) stack with a Li-ion battery to supply the power needs of a hybrid electric vehicle. Renew. Energy 2019, 130, 714–724. [Google Scholar] [CrossRef]
- Alaswad, A.; Baroutaji, A.; Achour, H.; Carton, J.; Al Makky, A.; Olabi, A.G. Developments in fuel cell technologies in the transport sector. Int. J. Hydrogen Energy 2016, 41, 16499–16508. [Google Scholar] [CrossRef]
- O’hayre, R.; Cha, S.W.; Colella, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Jian, Q.; Zhao, J. Experimental study on spatiotemporal distribution and variation characteristics of temperature in an open cathode proton exchange membrane fuel cell stack. Int. J. Hydrogen Energy 2019, 44, 27079–27093. [Google Scholar] [CrossRef]
- Hao, D.; Shen, J.; Hou, Y.; Zhou, Y.; Wang, H. An improved empirical fuel cell polarization curve model based on review analysis. Int. J. Chem. Eng. 2016, 2016, 4109204. [Google Scholar] [CrossRef]
- Wang, Y.; Northwood, D.O. Effects of O2 and H2 on the corrosion of SS316L metallic bipolar plate materials in simulated anode and cathode environments of PEM fuel cells. Electrochim. Acta 2007, 52, 6793–6798. [Google Scholar] [CrossRef]
- Kannan, V.; Xue, H.; Raman, K.A.; Chen, J.; Fisher, A.; Birgersson, E. Quantifying operating uncertainties of a PEMFC–Monte Carlo-machine learning based approach. Renew. Energy 2020, 158, 343–359. [Google Scholar] [CrossRef]
- Bianchi, F.R.; Bosio, B.; Baldinelli, A.; Barelli, L. Optimization of a reference kinetic model for solid oxide fuel cells. Catalysts 2020, 10, 104. [Google Scholar] [CrossRef]
- Atyabi, S.A.; Afshari, E.; Wongwises, S.; Yan, W.M.; Hadjadj, A.; Shadloo, M.S. Effects of assembly pressure on PEM fuel cell performance by taking into accounts electrical and thermal contact resistances. Energy 2019, 179, 490–501. [Google Scholar] [CrossRef]
- Rahman, M.A.; Mojica, F.; Sarker, M.; Chuang, P.Y.A. Development of 1-D multiphysics PEMFC model with dry limiting current experimental validation. Electrochim. Acta 2019, 320, 134601. [Google Scholar] [CrossRef]
- Sohn, Y.J.; Yim, S.D.; Park, G.G.; Kim, M.; Cha, S.W.; Kim, K. PEMFC modeling based on characterization of effective diffusivity in simulated cathode catalyst layer. Int. J. Hydrogen Energy 2017, 42, 13226–13233. [Google Scholar] [CrossRef]
- Dickinson, E.J.; Hinds, G. The Butler-Volmer equation for polymer electrolyte membrane fuel cell (PEMFC) electrode kinetics: A critical discussion. J. Electrochem. Soc. 2019, 166, F221–F231. [Google Scholar] [CrossRef]
- Ghanbarian, A.; Kermani, M.J. Enhancement of PEM fuel cell performance by flow channel indentation. Energy Convers. Manag. 2016, 110, 356–366. [Google Scholar] [CrossRef]
- Askaripour, H. Effect of operating conditions on the performance of a PEM fuel cell. Int. J. Heat Mass Transf. 2019, 144, 118705. [Google Scholar] [CrossRef]
- Tohidi, M.; Mansouri, S.; Amiri, H. Effect of primary parameters on the performance of PEM fuel cell. Int. J. Hydrogen Energy 2010, 35, 9338–9348. [Google Scholar] [CrossRef]
- He, L.; Han, Z.; Liu, Y.; Niu, Z.; Liu, Z. Effects of gas starvation on performance of a single PEMFC. In Proceedings of the 2017 IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), Harbin, China, 7–10 August 2017; pp. 1–5. [Google Scholar]
- Lee, J.C.; Shay, T. Analysis of fuel cell applied for submarine air independent propulsion (AIP) system. J. Mar. Sci. Technol. 2018, 26, 657–666. [Google Scholar]
- Atlam, O.; Kolhe, M. Performance analysis of PEM fuel cell with varying oxidant supply rates. J. Energy Eng. 2013, 139, 60–63. [Google Scholar] [CrossRef]
- Popat, S.C.; Ki, D.; Young, M.N.; Rittmann, B.E.; Torres, C.I. Buffer pKa and transport govern the concentration overpotential in electrochemical oxygen reduction at neutral pH. ChemElectroChem 2014, 1, 1909–1915. [Google Scholar] [CrossRef]
- Fournier, M.; Hamelin, J.; Agbossou, K.; Bose, T. Fuel cell operation with oxygen enrichment. Fuel Cells 2002, 2, 117–122. [Google Scholar] [CrossRef]
- Kumar, R.; Subramanian, K. Enhancement of efficiency and power output of hydrogen fuelled proton exchange membrane (PEM) fuel cell using oxygen enriched air. Int. J. Hydrogen Energy 2023, 48, 6067–6075. [Google Scholar] [CrossRef]
- Kakizawa, Y.; Schreiber, C.L.; Takamuku, S.; Uchida, M.; Iiyama, A.; Inukai, J. Visualization of the oxygen partial pressure in a proton exchange membrane fuel cell during cell operation with low oxygen concentrations. J. Power Sources 2021, 483, 229193. [Google Scholar] [CrossRef]
- Pukrushpan, J.T.; Stefanopoulou, A.G.; Peng, H. Control of Fuel Cell Power Systems: Principles, Modeling, Analysis and Feedback Design; Springer: Berlin/Heidelberg, Germany, 2004. [Google Scholar]
- Ren, P.; Pei, P.; Li, Y.; Wu, Z.; Chen, D.; Huang, S. Degradation mechanisms of proton exchange membrane fuel cell under typical automotive operating conditions. Prog. Energy Combust. Sci. 2020, 80, 100859. [Google Scholar] [CrossRef]
- Meyers, J.P.; Darling, R.M. Model of carbon corrosion in PEM fuel cells. J. Electrochem. Soc. 2006, 153, A1432–A1442. [Google Scholar] [CrossRef]
- Boyer, C.; Gamburzev, S.; Appleby, A. Evaluation of methods to increase the oxygen partial pressure in PEM fuel cells. J. Appl. Electrochem. 1999, 29, 1095–1102. [Google Scholar] [CrossRef]
- Smith, A.; Klosek, J. A review of air separation technologies and their integration with energy conversion processes. Fuel Process. Technol. 2001, 70, 115–134. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Lee, Y.W.; Liang, K.; Causey, H. Effect of sub-freezing temperatures on a PEM fuel cell performance, startup and fuel cell components. J. Power Sources 2006, 160, 1242–1250. [Google Scholar] [CrossRef]
- Kianfar, E.; Cao, V. Polymeric membranes on base of PolyMethyl methacrylate for air separation: A review. J. Mater. Res. Technol. 2021, 10, 1437–1461. [Google Scholar] [CrossRef]
- Belaissaoui, B.; Le Moullec, Y.; Hagi, H.; Favre, E. Energy efficiency of oxygen enriched air production technologies: Cryogeny vs. membranes. Sep. Purif. Technol. 2014, 125, 142–150. [Google Scholar] [CrossRef]
- Meriläinen, A.; Seppälä, A.; Kauranen, P. Minimizing specific energy consumption of oxygen enrichment in polymeric hollow fiber membrane modules. Appl. Energy 2012, 94, 285–294. [Google Scholar] [CrossRef]
- Hoeflinger, J.; Hofmann, P. Air mass flow and pressure optimisation of a PEM fuel cell range extender system. Int. J. Hydrogen Energy 2020, 45, 29246–29258. [Google Scholar] [CrossRef]
- Santarelli, M.; Torchio, M.F.; Calı, M.; Giaretto, V. Experimental analysis of cathode flow stoichiometry on the electrical performance of a PEMFC stack. Int. J. Hydrogen Energy 2007, 32, 710–716. [Google Scholar] [CrossRef]
- Shah, S.; Shaikh, H.; Hafeez, S.; Malik, M.I. Synthesis of 3-(Trimethoxysilyl) Propyl Methacrylate Functionalized Graphene Oxide Based Mixed Matrix Membrane and Its Application for O2/N2 Separation. Pak. J. Anal. Environ. Chem. 2020, 21, 44–53. [Google Scholar] [CrossRef]
- Serrano, J.R.; García-Cuevas, L.M.; Samala, V.; López-Carrillo, J.A.; Mai, H. Boosting the capabilities of gas stand data acquisition and control systems by using a digital twin based on a holistic turbocharger model. In Proceedings of the Internal Combustion Engine Division Fall Technical Conference. American Society of Mechanical Engineers, Online, 13–15 October 2021; Volume 85512, p. V001T07A002. [Google Scholar]
- Wang, J.; Shi, Z.; Zang, Y.; Jia, H.; Teraguchi, M.; Kaneko, T.; Aoki, T. Macromolecular Design for Oxygen/Nitrogen Permselective Membranes—Top-Performing Polymers in 2020—. Polymers 2021, 13, 3012. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piqueras, P.; de la Morena, J.; Sanchis, E.J.; Lalangui, J.A. Potential of Proton-Exchange Membrane Fuel-Cell System with On-Board O2-Enriched Air Generation. Appl. Sci. 2024, 14, 836. https://doi.org/10.3390/app14020836
Piqueras P, de la Morena J, Sanchis EJ, Lalangui JA. Potential of Proton-Exchange Membrane Fuel-Cell System with On-Board O2-Enriched Air Generation. Applied Sciences. 2024; 14(2):836. https://doi.org/10.3390/app14020836
Chicago/Turabian StylePiqueras, Pedro, Joaquín de la Morena, Enrique J. Sanchis, and José A. Lalangui. 2024. "Potential of Proton-Exchange Membrane Fuel-Cell System with On-Board O2-Enriched Air Generation" Applied Sciences 14, no. 2: 836. https://doi.org/10.3390/app14020836






