Non-Surgical Treatment for Hepatocellular Carcinoma: What to Expect at Follow-Up Magnetic Resonance Imaging—A Pictorial Review
Abstract
:1. Introduction
2. Minimally Invasive Therapies for HCC and Their Tissular Effects
2.1. Thermal Ablative Therapies
2.1.1. Radiofrequency Ablation
2.1.2. Microwave Ablation
2.1.3. Laser Ablation
2.1.4. High-Intensity Focused Ultrasound
2.1.5. Cryoablation
2.2. Non-Thermal Ablative Therapies
2.2.1. Percutaneous Ethanol Injection
2.2.2. Irreversible Electroporation
2.2.3. Transarterial Chemoembolization
2.2.4. Yttrium-90 Radioembolization
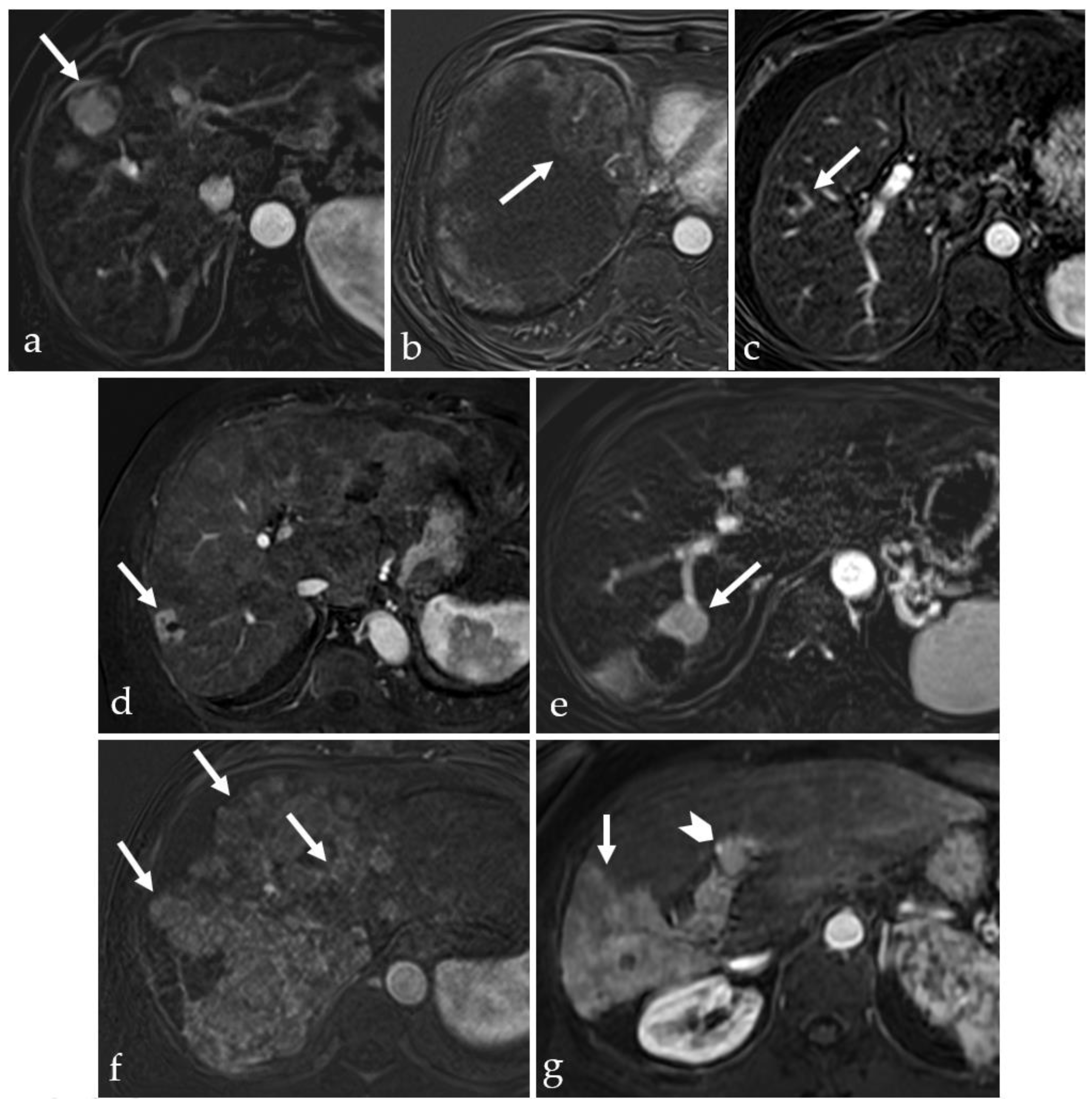
3. Imaging in the Follow-Up of Treated HCC
3.1. Considerations on the MRI Scanning Protocol in HCC Follow-Up
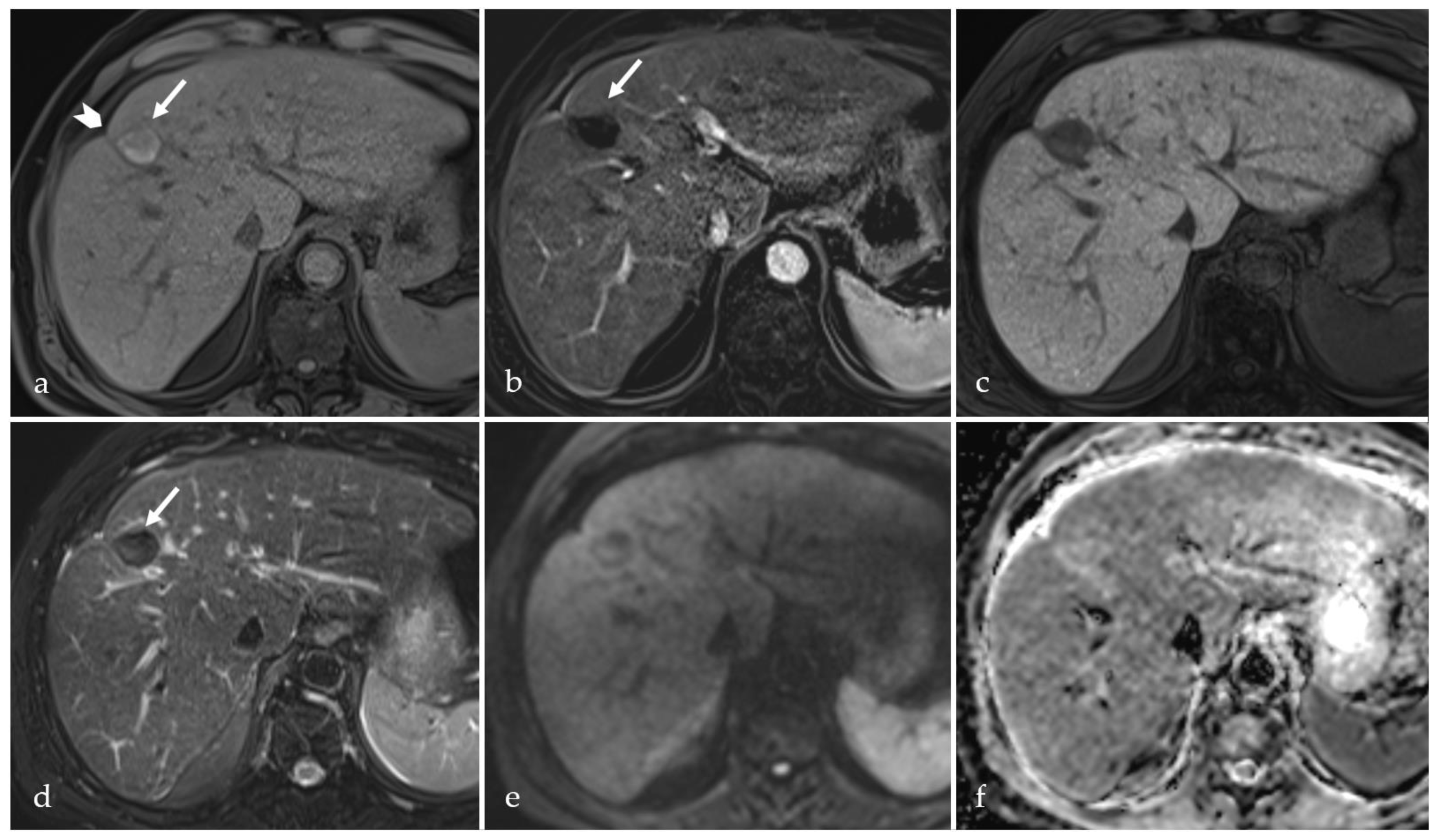
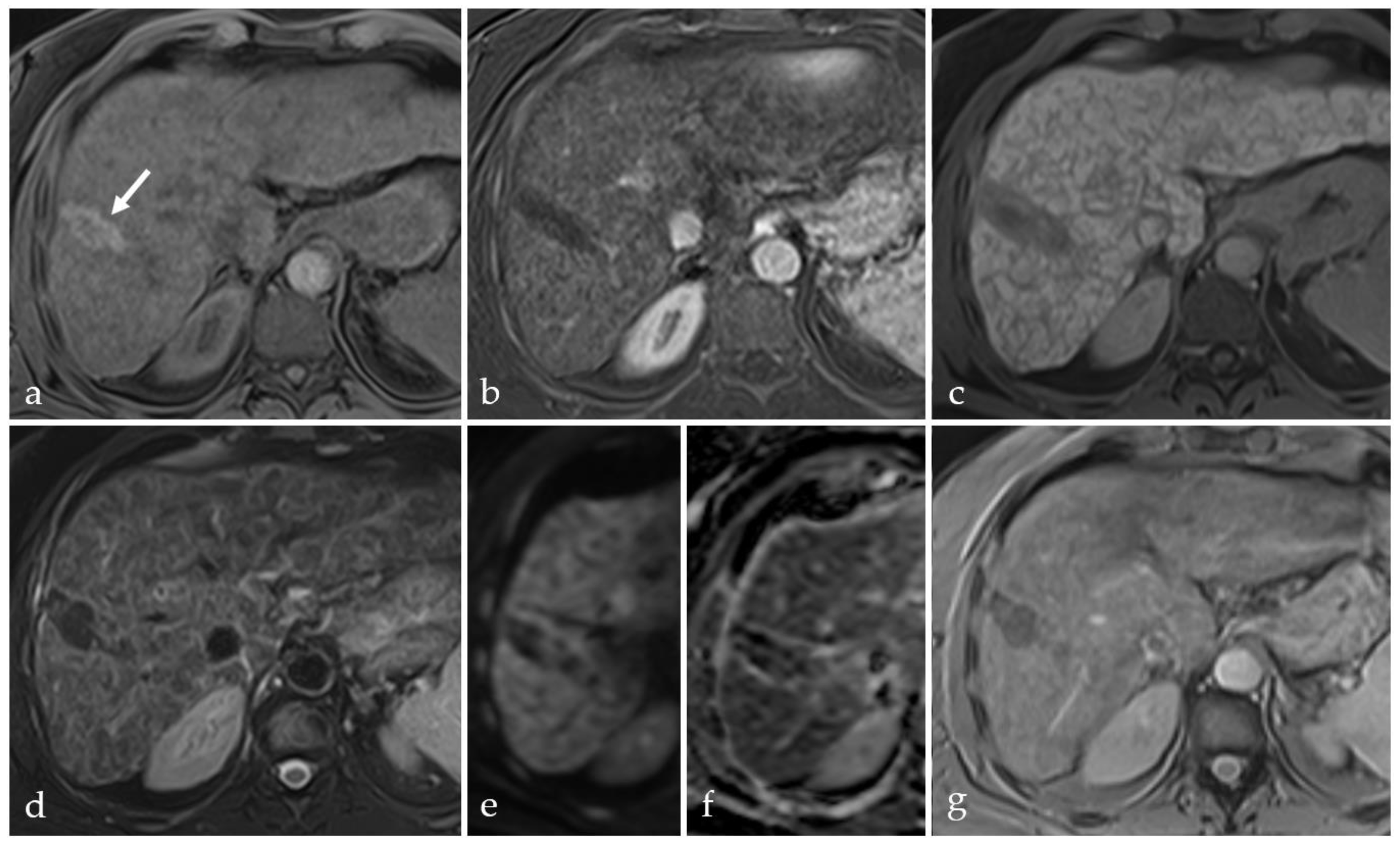
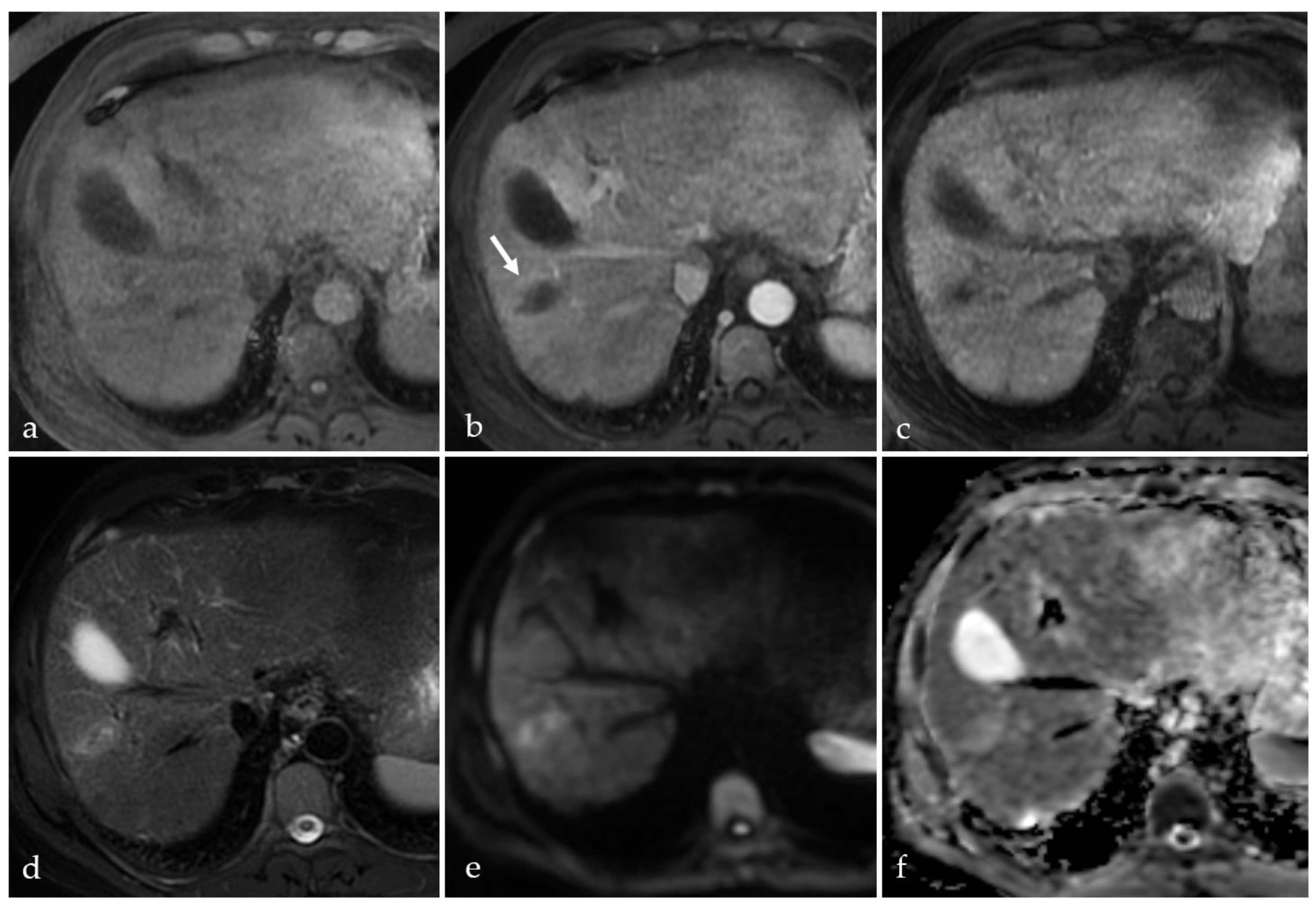
3.2. Expected Post-Treatment Imaging Features in the Absence of Viable Tumor Tissue
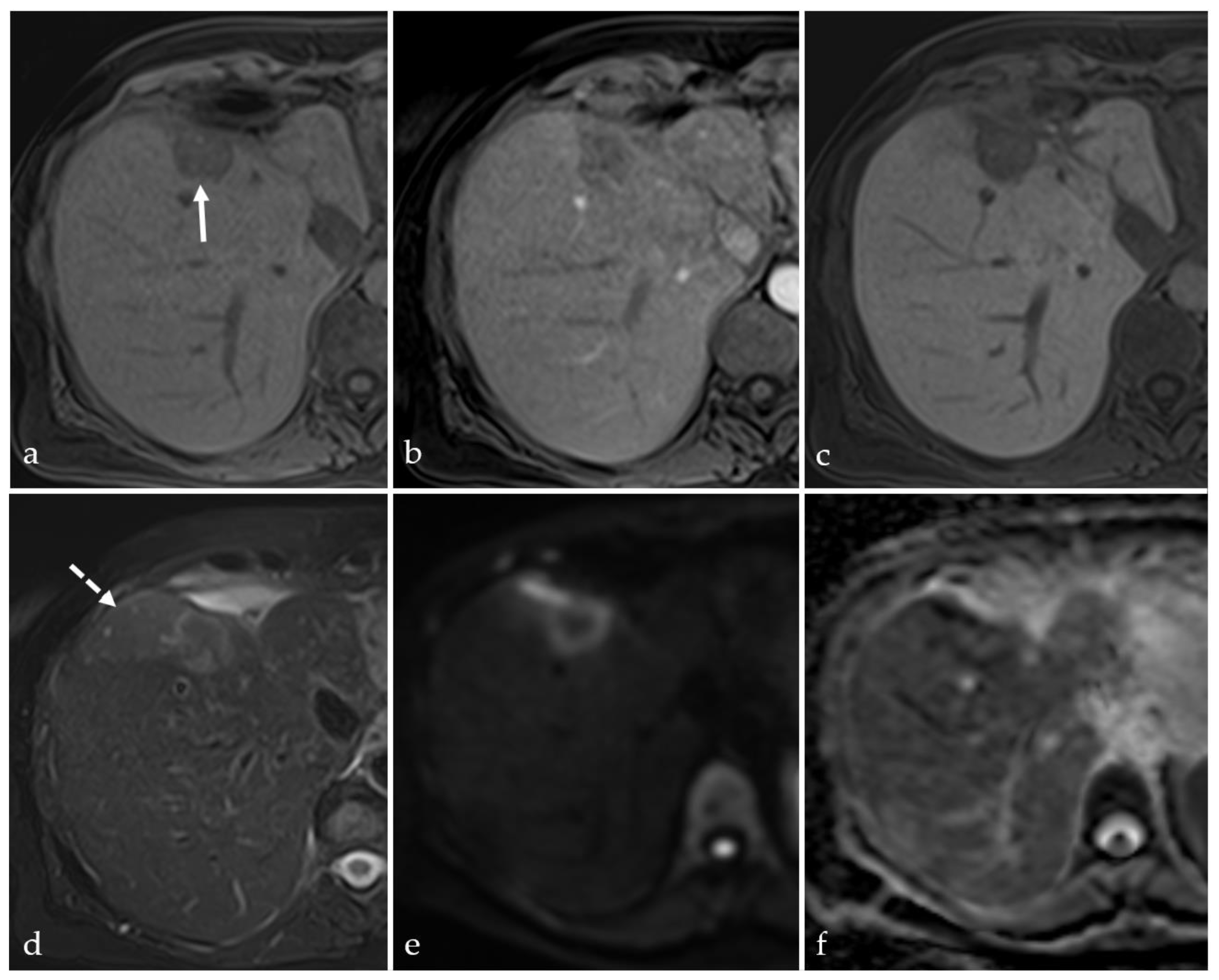
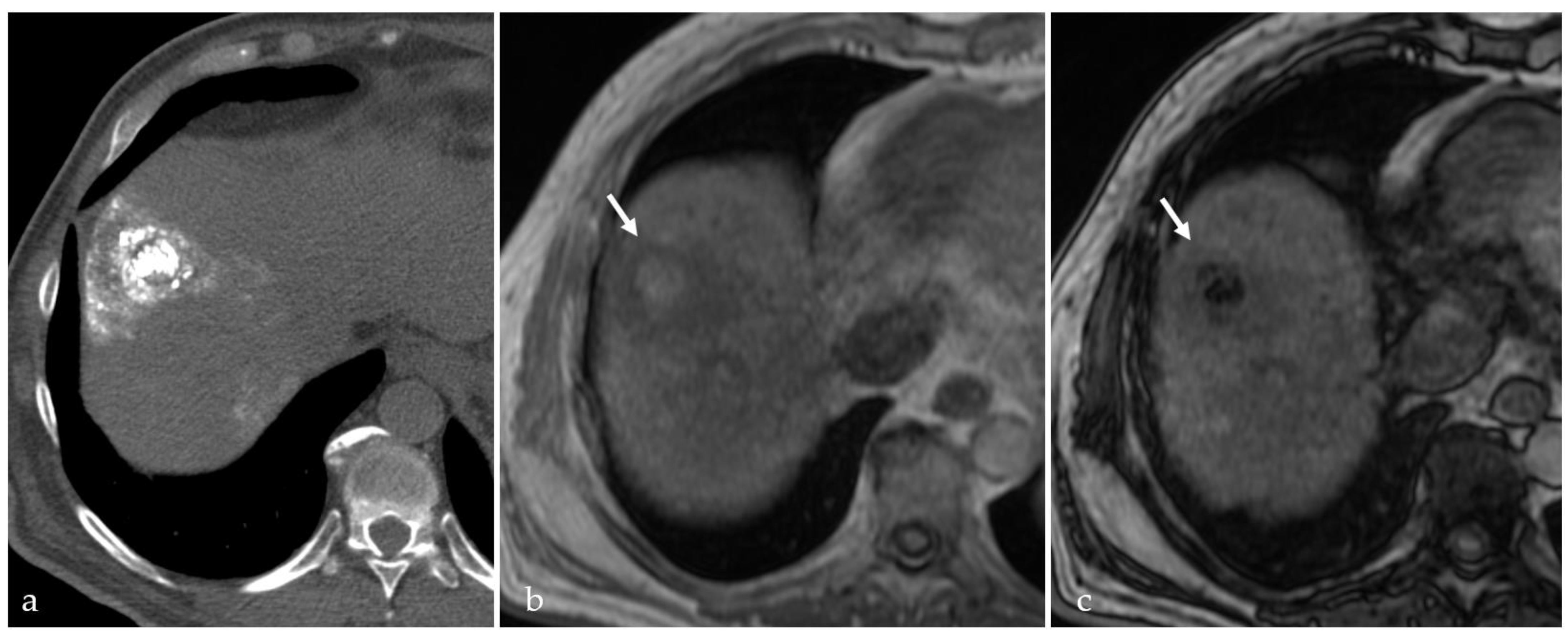
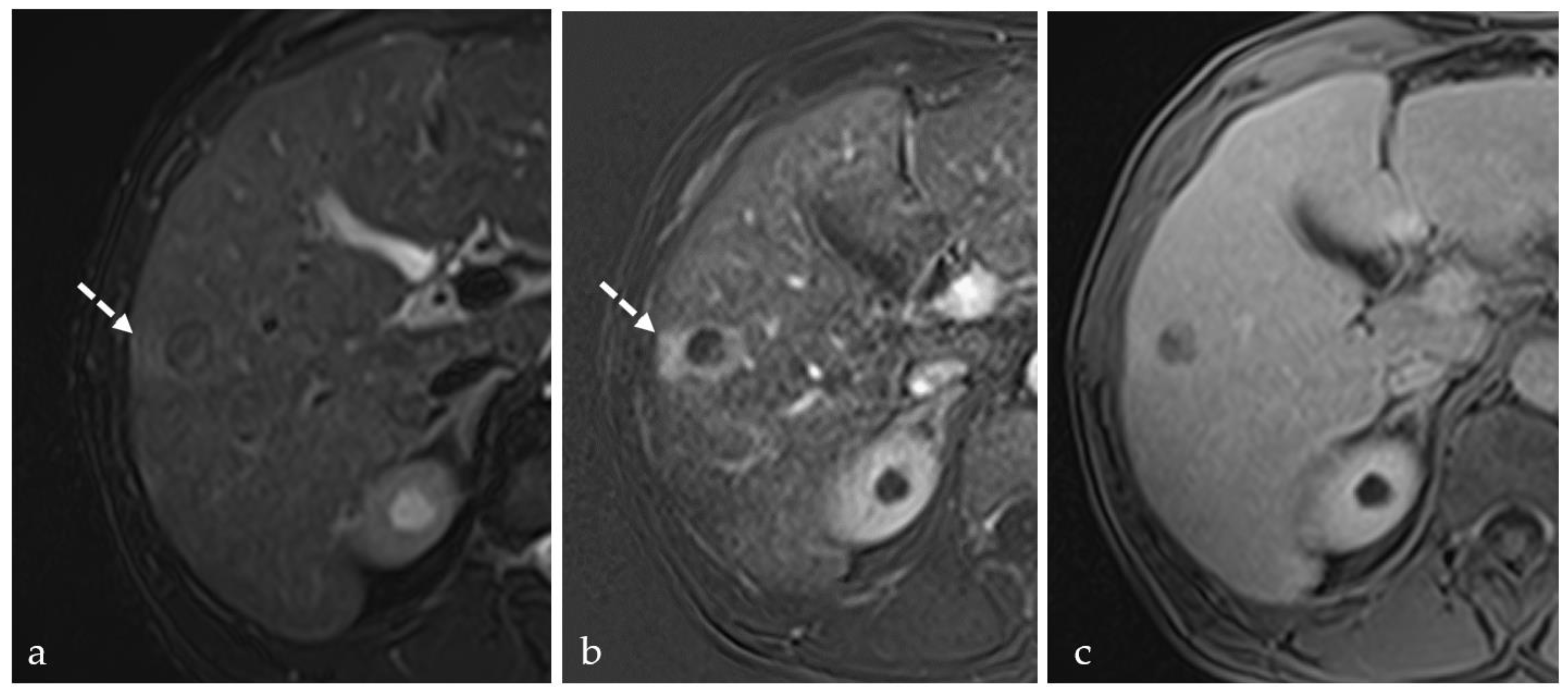
3.3. Transient Hyperemia
3.4. TACE-Associated Necrosis
3.5. Post-Treatment Imaging Features of Tumor Viability
3.6. Current and Future Perspectives in Targeting Tumor Nodules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. S1), 4–13. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, C.; Rimola, J.; Vilana, R.; Burrel, M.; Darnell, A.; García-Criado, Á.; Bianchi, L.; Belmonte, E.; Caparroz, C.; Barrufet, M.; et al. Diagnosis and staging of hepatocellular carcinoma (HCC): Current guidelines. Eur. J. Radiol. 2018, 101, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S. The Role of Liver Biopsy in Hepatocellular Carcinoma. Gastroenterol. Hepatol. 2016, 12, 628–630. [Google Scholar]
- Scheau, A.E.; Scheau, C.; Lupescu, I.G. Nodule-in-Nodule Imaging Pattern in Hepatocellular Carcinoma Treated by Transarterial Chemoembolization—A Multiparametric Magnetic Resonance Imaging Study. J. Gastrointest. Liver Dis. 2017, 26, 387–393. [Google Scholar] [CrossRef]
- Wege, H.; Schulze, K.; von Felden, J.; Calderaro, J.; Reig, M. Rare variants of primary liver cancer: Fibrolamellar, combined, and sarcomatoid hepatocellular carcinomas. Eur. J. Med. Genet. 2021, 64, 104313. [Google Scholar] [CrossRef]
- Huang, S.C.; Liao, S.H.; Su, T.H.; Jeng, Y.M.; Kao, J.H. Clinical manifestations and outcomes of patients with scirrhous hepatocellular carcinoma. Hepatol. Int. 2021, 15, 472–481. [Google Scholar] [CrossRef]
- Nevola, R.; Ruocco, R.; Criscuolo, L.; Villani, A.; Alfano, M.; Beccia, D.; Imbriani, S.; Claar, E.; Cozzolino, D.; Sasso, F.C.; et al. Predictors of early and late hepatocellular carcinoma recurrence. World J. Gastroenterol. 2023, 29, 1243–1260. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Singal, A.G.; Llovet, J.M.; Yarchoan, M.; Mehta, N.; Heimbach, J.K.; Dawson, L.A.; Jou, J.H.; Kulik, L.M.; Agopian, V.G.; Marrero, J.A.; et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology 2023, 78, 1922–1965. [Google Scholar] [CrossRef] [PubMed]
- Chernyak, V.; Fowler, K.J.; Kamaya, A.; Kielar, A.Z.; Elsayes, K.M.; Bashir, M.R.; Kono, Y.; Do, R.K.; Mitchell, D.G.; Singal, A.G.; et al. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology 2018, 289, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, M.E.; Murthy, R.; Avritscher, R.; Mahvash, A.; Wallace, M.J.; Kaseb, A.O.; Odisio, B.C. Minimally invasive image-guided therapies for hepatocellular carcinoma. J. Hepatocell Carcinoma 2016, 3, 55–61. [Google Scholar] [CrossRef]
- Arora, A.; Kumar, A. Treatment Response Evaluation and Follow-up in Hepatocellular Carcinoma. J. Clin. Exp. Hepatol. 2014, 4, S126–S129. [Google Scholar] [CrossRef]
- Crocetti, L.; Bargellini, I.; Cioni, R. Loco-regional treatment of HCC: Current status. Clin. Radiol. 2017, 72, 626–635. [Google Scholar] [CrossRef]
- Han, K.; Kim, J.H. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J. Gastroenterol. 2015, 21, 10327–10335. [Google Scholar] [CrossRef]
- Faivre, S.; Rimassa, L.; Finn, R.S. Molecular therapies for HCC: Looking outside the box. J. Hepatol. 2020, 72, 342–352. [Google Scholar] [CrossRef]
- Liu, D.; Song, T. Changes in and challenges regarding the surgical treatment of hepatocellular carcinoma in China. Biosci. Trends 2021, 15, 142–147. [Google Scholar] [CrossRef]
- Bouda, D.; Lagadec, M.; Alba, C.G.; Barrau, V.; Dioguardi Burgio, M.; Moussa, N.; Vilgrain, V.; Ronot, M. Imaging review of hepatocellular carcinoma after thermal ablation: The good, the bad, and the ugly. J. Magn. Reson. Imaging 2016, 44, 1070–1090. [Google Scholar] [CrossRef]
- Wu, Z.J.; Xie, Y.F.; Chang, X.; Zhang, L.; Wu, H.Y.; Liu, J.B.; Zhang, J.X.; Sun, P. Type of Necrosis Influences Prognosis in Hepatocellular Carcinoma After the First Transarterial Chemoembolization. Med. Sci. Monit. 2021, 27, e929884. [Google Scholar] [CrossRef] [PubMed]
- Sindram, D.; Lau, K.N.; Martinie, J.B.; Iannitti, D.A. Hepatic tumor ablation. Surg. Clin. N. Am. 2010, 90, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, D.J.; Simo, K.A.; Iannitti, D.A.; McKillop, I.H. Ablation therapy for hepatocellular carcinoma: Past, present and future perspectives. Hepat. Oncol. 2014, 1, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Shiina, S.; Tateishi, R.; Arano, T.; Uchino, K.; Enooku, K.; Nakagawa, H.; Asaoka, Y.; Sato, T.; Masuzaki, R.; Kondo, Y.; et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am. J. Gastroenterol. 2012, 107, 569–577, quiz 578. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yan, L.; Cheng, Z.; Wu, H.; Du, L.; Wang, J.; Xu, Y.; Zeng, Y. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann. Surg. 2010, 252, 903–912. [Google Scholar] [CrossRef]
- Feng, K.; Yan, J.; Li, X.; Xia, F.; Ma, K.; Wang, S.; Bie, P.; Dong, J. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J. Hepatol. 2012, 57, 794–802. [Google Scholar] [CrossRef]
- Crocetti, L.; de Baere, T.; Lencioni, R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc. Interv. Radiol. 2010, 33, 11–17. [Google Scholar] [CrossRef]
- McDermott, S.; Gervais, D.A. Radiofrequency ablation of liver tumors. Semin Interv. Radiol. 2013, 30, 49–55. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L. Radiofrequency ablation of liver cancer. Tech. Vasc. Interv. Radiol. 2007, 10, 38–46. [Google Scholar] [CrossRef]
- Lee, J.D.; Lee, J.M.; Kim, S.W.; Kim, C.S.; Mun, W.S. MR imaging-histopathologic correlation of radiofrequency thermal ablation lesion in a rabbit liver model: Observation during acute and chronic stages. Korean J. Radiol. 2001, 2, 151–158. [Google Scholar] [CrossRef]
- McGahan, J.P.; Brock, J.M.; Tesluk, H.; Gu, W.Z.; Schneider, P.; Browning, P.D. Hepatic ablation with use of radio-frequency electrocautery in the animal model. J. Vasc. Interv. Radiol. 1992, 3, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Rozenblum, N.; Zeira, E.; Bulvik, B.; Gourevitch, S.; Yotvat, H.; Galun, E.; Goldberg, S.N. Radiofrequency Ablation: Inflammatory Changes in the Periablative Zone Can Induce Global Organ Effects, including Liver Regeneration. Radiology 2015, 276, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Rhim, H.; Lim, H.K.; Choi, D.; Lee, M.W.; Park, M.J. Coagulation necrosis induced by radiofrequency ablation in the liver: Histopathologic and radiologic review of usual to extremely rare changes. Radiographics 2011, 31, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.; Montagna, B.; Di Cesare, P.; Riva, G.; Bernardo, G.; Mazzucco, M.; Riccardi, A. Microwave ablation of hepatocellular carcinoma using a new percutaneous device: Preliminary results. Anticancer Res. 2013, 33, 1221–1227. [Google Scholar]
- Sun, A.X.; Cheng, Z.L.; Wu, P.P.; Sheng, Y.H.; Qu, X.J.; Lu, W.; Zhao, C.G.; Qian, G.J. Clinical outcome of medium-sized hepatocellular carcinoma treated with microwave ablation. World J. Gastroenterol. 2015, 21, 2997–3004. [Google Scholar] [CrossRef]
- Yin, X.Y.; Xie, X.Y.; Lu, M.D.; Xu, H.X.; Xu, Z.F.; Kuang, M.; Liu, G.J.; Liang, J.Y.; Lau, W.Y. Percutaneous thermal ablation of medium and large hepatocellular carcinoma: Long-term outcome and prognostic factors. Cancer 2009, 115, 1914–1923. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X.-J.; Chi, J.-C.; Ding, M.; Zhang, Y.; Tang, X.-Y.; Li, P.; Zhang, L.; Zhang, X.-Y.; Zhai, B. Microwave ablation of hepatocellular carcinoma as first-line treatment: Long term outcomes and prognostic factors in 221 patients. Sci. Rep. 2016, 6, 32728. [Google Scholar] [CrossRef]
- Simon, C.J.; Dupuy, D.E.; Mayo-Smith, W.W. Microwave ablation: Principles and applications. Radiographics 2005, 25 (Suppl. S1), S69–S83. [Google Scholar] [CrossRef]
- Habibollahi, P.; Sheth, R.A.; Cressman, E.N.K. Histological Correlation for Radiofrequency and Microwave Ablation in the Local Control of Hepatocellular Carcinoma (HCC) before Liver Transplantation: A Comprehensive Review. Cancers 2020, 13, 104. [Google Scholar] [CrossRef]
- Gravante, G.; Ong, S.L.; Metcalfe, M.S.; Strickland, A.; Dennison, A.R.; Lloyd, D.M. Hepatic microwave ablation: A review of the histological changes following thermal damage. Liver Int. 2008, 28, 911–921. [Google Scholar] [CrossRef]
- Strickland, A.D.; Clegg, P.J.; Cronin, N.J.; Swift, B.; Festing, M.; West, K.P.; Robertson, G.S.; Lloyd, D.M. Experimental study of large-volume microwave ablation in the liver. Br. J. Surg. 2002, 89, 1003–1007. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Nakamura, K.; Hamuro, M.; Sakai, Y.; Nishida, N.; Yamada, R.; Ikura, Y.; Ueda, M.; Inoue, Y. The influence of radiofrequency ablation on hepatic vessels in porcine liver. Hepato-Gastroenterology 2005, 52, 571–574. [Google Scholar] [PubMed]
- Kato, T.; Suto, Y.; Hamazoe, R. Effects of microwave tissue coagulation on the livers of normal rabbits: A comparison of findings of image analysis and histopathological examination. Br. J. Radiol. 1996, 69, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Brace, C.L.; Laeseke, P.F.; Sampson, L.A.; Frey, T.M.; van der Weide, D.W.; Lee, F.T., Jr. Microwave ablation with multiple simultaneously powered small-gauge triaxial antennas: Results from an in vivo swine liver model. Radiology 2007, 244, 151–156. [Google Scholar] [CrossRef]
- Pacella, C.M.; Bizzarri, G.; Magnolfi, F.; Cecconi, P.; Caspani, B.; Anelli, V.; Bianchini, A.; Valle, D.; Pacella, S.; Manenti, G.; et al. Laser thermal ablation in the treatment of small hepatocellular carcinoma: Results in 74 patients. Radiology 2001, 221, 712–720. [Google Scholar] [CrossRef]
- Pacella, C.M.; Francica, G.; Di Lascio, F.M.; Arienti, V.; Antico, E.; Caspani, B.; Magnolfi, F.; Megna, A.S.; Pretolani, S.; Regine, R.; et al. Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: A retrospective analysis. J. Clin. Oncol. 2009, 27, 2615–2621. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; Francica, G.; Pacella, C.M. Laser ablation for small hepatocellular carcinoma: State of the art and future perspectives. World J. Hepatol. 2014, 6, 704–715. [Google Scholar] [CrossRef]
- Gough-Palmer, A.L.; Gedroyc, W.M. Laser ablation of hepatocellular carcinoma—A review. World J. Gastroenterol. 2008, 14, 7170–7174. [Google Scholar] [CrossRef]
- Adwan, H.; Vogl, T.J.; Balaban, Ü.; Nour-Eldin, N.-E.A. Percutaneous Thermal Ablation Therapy of Hepatocellular Carcinoma (HCC): Microwave Ablation (MWA) versus Laser-Induced Thermotherapy (LITT). Diagnostics 2022, 12, 564. [Google Scholar] [CrossRef]
- Arienti, V.; Pretolani, S.; Pacella, C.M.; Magnolfi, F.; Caspani, B.; Francica, G.; Megna, A.S.; Regine, R.; Sponza, M.; Antico, E.; et al. Complications of Laser Ablation for Hepatocellular Carcinoma: A Multicenter Study. Radiology 2008, 246, 947–955. [Google Scholar] [CrossRef]
- Pacella, C.M.; Bizzarri, G.; Francica, G.; Bianchini, A.; De Nuntis, S.; Pacella, S.; Crescenzi, A.; Taccogna, S.; Forlini, G.; Rossi, Z.; et al. Percutaneous laser ablation in the treatment of hepatocellular carcinoma with small tumors: Analysis of factors affecting the achievement of tumor necrosis. J. Vasc. Interv. Radiol. 2005, 16, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Francica, G.; Iodice, G.; Delle Cave, M.; Sarrantonio, R.; Lapiccirella, G.; Molese, V.; Smeraldo, D.; Scarano, F.; De Marino, F. Factors Predicting Complete Necrosis Rate after Ultrasound-Guided Percutaneous Laser Thermoablation of Small Hepatocellular Carcinoma Tumors in Cirrhotic Patients: A Multivariate Analysis. Acta Radiol. 2007, 48, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Morisco, F.; Camera, S.; Guarino, M.; Tortora, R.; Cossiga, V.; Vitiello, A.; Cordone, G.; Caporaso, N.; Di Costanzo, G.G.; group, I.L.C. Laser ablation is superior to TACE in large-sized hepatocellular carcinoma: A pilot case–control study. Oncotarget 2018, 9, 17483–17490. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.M.; Callstrom, M.R.; Butters, K.A.; Knudsen, B.; Grande, J.P.; Roberts, L.R.; Woodrum, D.A. Heat stress induced cell death mechanisms in hepatocytes and hepatocellular carcinoma: In vitro and in vivo study. Lasers Surg. Med. 2014, 46, 290–301. [Google Scholar] [CrossRef]
- Jondal, D.E.; Thompson, S.M.; Butters, K.A.; Knudsen, B.E.; Anderson, J.L.; Carter, R.E.; Roberts, L.R.; Callstrom, M.R.; Woodrum, D.A. Heat Stress and Hepatic Laser Thermal Ablation Induce Hepatocellular Carcinoma Growth: Role of PI3K/mTOR/AKT Signaling. Radiology 2018, 288, 730–738. [Google Scholar] [CrossRef]
- Diana, M.; Schiraldi, L.; Liu, Y.Y.; Memeo, R.; Mutter, D.; Pessaux, P.; Marescaux, J. High intensity focused ultrasound (HIFU) applied to hepato-bilio-pancreatic and the digestive system-current state of the art and future perspectives. Hepatobiliary Surg. Nutr. 2016, 5, 329–344. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, H.; Jin, C.; Zhou, K.; Li, K.; Su, H.; Chen, W.; Bai, J.; Wang, Z. High-intensity focused ultrasound (HIFU): Effective and safe therapy for hepatocellular carcinoma adjacent to major hepatic veins. Eur. Radiol. 2009, 19, 437–445. [Google Scholar] [CrossRef]
- Orsi, F.; Zhang, L.; Arnone, P.; Orgera, G.; Bonomo, G.; Vigna, P.D.; Monfardini, L.; Zhou, K.; Chen, W.; Wang, Z.; et al. High-intensity focused ultrasound ablation: Effective and safe therapy for solid tumors in difficult locations. AJR Am. J. Roentgenol. 2010, 195, W245–W252. [Google Scholar] [CrossRef]
- Hutchinson, L. HIFU is effective for unresectable HCC. Nat. Rev. Clin. Oncol. 2011, 8, 385. [Google Scholar] [CrossRef]
- Mearini, L. High intensity focused ultrasound, liver disease and bridging therapy. World J. Gastroenterol. 2013, 19, 7494–7499. [Google Scholar] [CrossRef]
- Tsang, S.H.; Ma, K.W.; She, W.H.; Chu, F.; Lau, V.; Lam, S.W.; Cheung, T.T.; Lo, C.M. High-intensity focused ultrasound ablation of liver tumors in difficult locations. Int. J. Hyperth. 2021, 38, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; König, A.M.; Figiel, J.H. Current Technique and Application of Percutaneous Cryotherapy. RöFo—Fortschr. Geb. Röntgenstrahlen Bildgeb. Verfahr. 2018, 190, 836–846. [Google Scholar] [CrossRef]
- Niu, L.Z.; Li, J.L.; Xu, K.C. Percutaneous Cryoablation for Liver Cancer. J. Clin. Transl. Hepatol. 2014, 2, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Yang, W.; Hu, K.; Xie, H.; Hu, K.Q.; Bai, W.; Dong, Z.; Lu, Y.; Zeng, Z.; et al. Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 2015, 61, 1579–1590. [Google Scholar] [CrossRef] [PubMed]
- Orlacchio, A.; Bazzocchi, G.; Pastorelli, D.; Bolacchi, F.; Angelico, M.; Almerighi, C.; Masala, S.; Simonetti, G. Percutaneous cryoablation of small hepatocellular carcinoma with US guidance and CT monitoring: Initial experience. Cardiovasc. Interv. Radiol. 2008, 31, 587–594. [Google Scholar] [CrossRef]
- McWilliams, J.P.; Yamamoto, S.; Raman, S.S.; Loh, C.T.; Lee, E.W.; Liu, D.M.; Kee, S.T. Percutaneous ablation of hepatocellular carcinoma: Current status. J. Vasc. Interv. Radiol. 2010, 21, S204–S213. [Google Scholar] [CrossRef]
- Hoffmann, N.E.; Coad, J.E.; Huot, C.S.; Swanlund, D.J.; Bischof, J.C. Investigation of the mechanism and the effect of cryoimmunology in the Copenhagen rat. Cryobiology 2001, 42, 59–68. [Google Scholar] [CrossRef]
- Sabel, M.S. Cryo-immunology: A review of the literature and proposed mechanisms for stimulatory versus suppressive immune responses. Cryobiology 2009, 58, 1–11. [Google Scholar] [CrossRef]
- Sala, M.; Llovet, J.M.; Vilana, R.; Bianchi, L.; Solé, M.; Ayuso, C.; Brú, C.; Bruix, J. Initial response to percutaneous ablation predicts survival in patients with hepatocellular carcinoma. Hepatology 2004, 40, 1352–1360. [Google Scholar] [CrossRef]
- Branco, F.; Brú, C.; Vilana, R.; Bianchi, L.; Alves de Mattos, A. Percutaneous ethanol injection before liver transplantation in the hepatocellular carcinoma. Ann. Hepatol. 2009, 8, 220–227. [Google Scholar] [CrossRef]
- Lee, M.J.; Mueller, P.R.; Dawson, S.L.; Gazelle, S.G.; Hahn, P.F.; Goldberg, M.A.; Boland, G.W. Percutaneous ethanol injection for the treatment of hepatic tumors: Indications, mechanism of action, technique, and efficacy. AJR Am. J. Roentgenol. 1995, 164, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Ohyama, N.; Ito, S.; Fujiwara, K. Small hepatocellular carcinoma: Treatment with US-guided intratumoral injection of acetic acid. Radiology 1994, 193, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, T.; Giorgio, A.; Marin, G.; Salmi, A.; de Sio, I.; Bolondi, L.; Pompili, M.; Brunello, F.; Lazzaroni, S.; Torzilli, G.; et al. Hepatocellular carcinoma and cirrhosis in 746 patients: Long-term results of percutaneous ethanol injection. Radiology 1995, 197, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Ding, X.; Long, H.; Ye, J.; Huang, T.; Lin, Y.; Lv, M.; Xie, X.; Huang, G. Percutaneous ethanol injection enhanced the efficacy of radiofrequency ablation in the treatment of HCC: An insight into the mechanism of ethanol action. Int. J. Hyperth. 2021, 38, 1394–1400. [Google Scholar] [CrossRef]
- Festi, D.; Monti, F.; Casanova, S.; Livraghi, T.; Frabboni, R.; Roversi, C.A.; Bertoli, D.; Borelli, G.; Mazzella, G.; Bazzoli, F.; et al. Morphological and biochemical effects of intrahepatic alcohol injection in the rabbit. J. Gastroenterol. Hepatol. 1990, 5, 402–406. [Google Scholar] [CrossRef]
- Zimmerman, A.; Grand, D.; Charpentier, K.P. Irreversible electroporation of hepatocellular carcinoma: Patient selection and perspectives. J. Hepatocell Carcinoma 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Scheffer, H.J.; Nielsen, K.; de Jong, M.C.; van Tilborg, A.A.; Vieveen, J.M.; Bouwman, A.R.; Meijer, S.; van Kuijk, C.; van den Tol, P.M.; Meijerink, M.R. Irreversible electroporation for nonthermal tumor ablation in the clinical setting: A systematic review of safety and efficacy. J. Vasc. Interv. Radiol. 2014, 25, 997–1011, quiz 1011. [Google Scholar] [CrossRef]
- Kalra, N.; Gupta, P.; Gorsi, U.; Bhujade, H.; Chaluvashetty, S.B.; Duseja, A.; Singh, V.; Dhiman, R.K.; Chawla, Y.K.; Khandelwal, N. Irreversible Electroporation for Unresectable Hepatocellular Carcinoma: Initial Experience. Cardiovasc. Interv. Radiol. 2019, 42, 584–590. [Google Scholar] [CrossRef]
- Niessen, C.; Thumann, S.; Beyer, L.; Pregler, B.; Kramer, J.; Lang, S.; Teufel, A.; Jung, E.M.; Stroszczynski, C.; Wiggermann, P. Percutaneous Irreversible Electroporation: Long-term survival analysis of 71 patients with inoperable malignant hepatic tumors. Sci. Rep. 2017, 7, 43687. [Google Scholar] [CrossRef]
- Narayanan, G.; Froud, T.; Suthar, R.; Barbery, K. Irreversible electroporation of hepatic malignancy. Semin Interv. Radiol 2013, 30, 67–73. [Google Scholar] [CrossRef]
- Sugimoto, K.; Abe, M.; Yoshimasu, Y.; Takeuchi, H.; Kasai, Y.; Itoi, T. Irreversible electroporation of hepatocellular carcinoma: The role of ultrasonography. Ultrasonography 2020, 39, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Deipolyi, A.R.; Golberg, A.; Yarmush, M.L.; Arellano, R.S.; Oklu, R. Irreversible electroporation: Evolution of a laboratory technique in interventional oncology. Diagn. Interv. Radiol. 2014, 20, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, Z.; Zhu, T.; Zhang, X.; Peng, Z.; Xie, H.; Zhou, L.; Yin, S.; Sun, J.; Zheng, S. Electric Ablation with Irreversible Electroporation (IRE) in Vital Hepatic Structures and Follow-up Investigation. Sci. Rep. 2015, 5, 16233. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, M.P.; De Chiara, M.; Reginelli, A.; Clemente, A.; Urraro, F.; Grassi, R.; Belfiore, G.; Cappabianca, S. An overview of the irreversible electroporation for the treatment of liver metastases: When to use it. Front. Oncol. 2022, 12, 943176. [Google Scholar] [CrossRef]
- Guo, X.; Du, F.; Liu, Q.; Guo, Y.; Wang, Q.; Huang, W.; Wang, Z.; Ding, X.; Wu, Z. Immunological effect of irreversible electroporation on hepatocellular carcinoma. BMC Cancer 2021, 21, 443. [Google Scholar] [CrossRef]
- Lencioni, R.; Petruzzi, P.; Crocetti, L. Chemoembolization of hepatocellular carcinoma. Semin Interv. Radiol. 2013, 30, 3–11. [Google Scholar] [CrossRef]
- Raoul, J.L.; Forner, A.; Bolondi, L.; Cheung, T.T.; Kloeckner, R.; de Baere, T. Updated use of TACE for hepatocellular carcinoma treatment: How and when to use it based on clinical evidence. Cancer Treat. Rev. 2019, 72, 28–36. [Google Scholar] [CrossRef]
- Bzeizi, K.I.; Arabi, M.; Jamshidi, N.; Albenmousa, A.; Sanai, F.M.; Al-Hamoudi, W.; Alghamdi, S.; Broering, D.; Alqahtani, S.A. Conventional Transarterial Chemoembolization Versus Drug-Eluting Beads in Patients with Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 6172. [Google Scholar] [CrossRef]
- Golfieri, R.; Cappelli, A.; Cucchetti, A.; Piscaglia, F.; Carpenzano, M.; Peri, E.; Ravaioli, M.; D’Errico-Grigioni, A.; Pinna, A.D.; Bolondi, L. Efficacy of selective transarterial chemoembolization in inducing tumor necrosis in small (<5 cm) hepatocellular carcinomas. Hepatology 2011, 53, 1580–1589. [Google Scholar] [CrossRef]
- Hsu, H.C.; Wei, T.C.; Tsang, Y.M.; Wu, M.Z.; Lin, Y.H.; Chuang, S.M. Histologic assessment of resected hepatocellular carcinoma after transcatheter hepatic arterial embolization. Cancer 1986, 57, 1184–1191. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Wei, Z.; Wang, T.; Yang, S.; Xiang, C.; Wang, X.; Gong, L.; Dong, J.; Lu, Q.; et al. Exploratory study of microparticle transcatheter arterial chemoembolization combined with resection for huge hepatocellular carcinoma. iLIVER 2022, 1, 35–42. [Google Scholar] [CrossRef]
- Molvar, C.; Lewandowski, R. Yttrium-90 Radioembolization of Hepatocellular Carcinoma-Performance, Technical Advances, and Future Concepts. Semin Interv. Radiol. 2015, 32, 388–397. [Google Scholar] [CrossRef]
- Sato, K.; Lewandowski, R.J.; Bui, J.T.; Omary, R.; Hunter, R.D.; Kulik, L.; Mulcahy, M.; Liu, D.; Chrisman, H.; Resnick, S.; et al. Treatment of unresectable primary and metastatic liver cancer with yttrium-90 microspheres (TheraSphere): Assessment of hepatic arterial embolization. Cardiovasc. Interv. Radiol. 2006, 29, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Lewandowski, R.J. Chemoembolization and radioembolization for hepatocellular carcinoma. Clin. Gastroenterol. Hepatol. 2013, 11, 604–611, quiz e643–604. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Johnson, G.E.; Kim, E.; Riaz, A.; Bishay, V.; Boucher, E.; Fowers, K.; Lewandowski, R.; Padia, S.A. Yttrium-90 Radioembolization for the Treatment of Solitary, Unresectable HCC: The LEGACY Study. Hepatology 2021, 74, 2342–2352. [Google Scholar] [CrossRef]
- Salem, R.; Gordon, A.C.; Mouli, S.; Hickey, R.; Kallini, J.; Gabr, A.; Mulcahy, M.F.; Baker, T.; Abecassis, M.; Miller, F.H.; et al. Y90 Radioembolization Significantly Prolongs Time to Progression Compared with Chemoembolization in Patients with Hepatocellular Carcinoma. Gastroenterology 2016, 151, 1155–1163.e1152. [Google Scholar] [CrossRef]
- Singh, P.; Anil, G. Yttrium-90 radioembolization of liver tumors: What do the images tell us? Cancer Imaging 2014, 13, 645–657. [Google Scholar] [CrossRef]
- Keppke, A.L.; Salem, R.; Reddy, D.; Huang, J.; Jin, J.; Larson, A.C.; Miller, F.H. Imaging of hepatocellular carcinoma after treatment with yttrium-90 microspheres. AJR Am. J. Roentgenol. 2007, 188, 768–775. [Google Scholar] [CrossRef]
- Atassi, B.; Bangash, A.K.; Bahrani, A.; Pizzi, G.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; Gates, V.L.; Mulcahy, M.F.; Kulik, L.; et al. Multimodality imaging following 90Y radioembolization: A comprehensive review and pictorial essay. Radiographics 2008, 28, 81–99. [Google Scholar] [CrossRef]
- Adwan, H.; Hammann, L.; Vogl, T.J. Microwave Ablation of Recurrent Hepatocellular Carcinoma after Curative Surgical Resection. J. Clin. Med. 2023, 12, 2560. [Google Scholar] [CrossRef]
- Sun, Q.; Shi, J.; Ren, C.; Du, Z.; Shu, G.; Wang, Y. Survival analysis following microwave ablation or surgical resection in patients with hepatocellular carcinoma conforming to the Milan criteria. Oncol. Lett. 2020, 19, 4066–4076. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; De Baere, T.; Kulik, L.; Haber, P.K.; Greten, T.F.; Meyer, T.; Lencioni, R. Locoregional therapies in the era of molecular and immune treatments for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 293–313. [Google Scholar] [CrossRef] [PubMed]
- Rong, G.; Bai, W.; Dong, Z.; Wang, C.; Lu, Y.; Zeng, Z.; Qu, J.; Lou, M.; Wang, H.; Gao, X.; et al. Long-Term Outcomes of Percutaneous Cryoablation for Patients with Hepatocellular Carcinoma within Milan Criteria. PLoS ONE 2015, 10, e0123065. [Google Scholar] [CrossRef] [PubMed]
- Sehmbi, A.S.; Froghi, S.; Oliveira de Andrade, M.; Saffari, N.; Fuller, B.; Quaglia, A.; Davidson, B. Systematic review of the role of high intensity focused ultrasound (HIFU) in treating malignant lesions of the hepatobiliary system. HPB 2021, 23, 187–196. [Google Scholar] [CrossRef]
- Frühling, P.; Stillström, D.; Holmquist, F.; Nilsson, A.; Freedman, J. Irreversible electroporation of hepatocellular carcinoma and colorectal cancer liver metastases: A nationwide multicenter study with short- and long-term follow-up. Eur. J. Surg. Oncol. 2023, 49, 107046. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Lin, C.-Y.; Chuang, M.-T.; Lin, C.-Y.; Tsai, Y.-S.; Wang, C.-K.; Ou, M.-C. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018, 18, 124. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, Y.Y.; Lee, B.C.; Shin, S.S.; Heo, S.H.; Kim, H.O.; Park, C.; Jeong, W.G. Drug-Eluting Bead Transarterial Chemoembolization Versus Radiofrequency Ablation as an Initial Treatment of Single Small (≤3 cm) Hepatocellular Carcinoma. J. Korean Med. Sci. 2023, 38, e362. [Google Scholar] [CrossRef]
- Ma, Z.; Zhou, W.; Huang, H.; Yao, Y. Prognosis comparison between hepatocellular carcinoma patients with microvascular invasion who received hepatectomy alone and those who underwent early PA-TACE: A retrospective cohort study. J. Gastrointest. Oncol. 2024, 15, 1112–1121. [Google Scholar] [CrossRef]
- Teyateeti, A.; Mahvash, A.; Long, J.P.; Abdelsalam, M.E.; Avritscher, R.; Chasen, B.; Kaseb, A.O.; Kuban, J.D.; Murthy, R.; Odisio, B.C.; et al. Survival Outcomes for Yttrium-90 Transarterial Radioembolization with and without Sorafenib for Unresectable Hepatocellular Carcinoma Patients. J. Hepatocell. Carcinoma 2020, 7, 117–131. [Google Scholar] [CrossRef]
- Horvat, N.; de Oliveira, A.I.; Clemente de Oliveira, B.; Araujo-Filho, J.A.B.; El Homsi, M.; Elsakka, A.; Bajwa, R.; Martins, G.L.P.; Elsayes, K.M.; Menezes, M.R. Local-Regional Treatment of Hepatocellular Carcinoma: A Primer for Radiologists. Radiographics 2022, 42, 1670–1689. [Google Scholar] [CrossRef]
- Ahmed, M.; Goldberg, S.N. Thermal Ablation Therapy for Hepatocellular Carcinoma. J. Vasc. Interv. Radiol. 2002, 13, S231–S243. [Google Scholar] [CrossRef] [PubMed]
- Khatri, G.; Pedrosa, I.; Ananthakrishnan, L.; de Leon, A.D.; Fetzer, D.T.; Leyendecker, J.; Singal, A.G.; Xi, Y.; Yopp, A.; Yokoo, T. Abbreviated-protocol screening MRI vs. complete-protocol diagnostic MRI for detection of hepatocellular carcinoma in patients with cirrhosis: An equivalence study using LI-RADS v2018. J. Magn. Reson. Imaging 2020, 51, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.A.; Al Azzazy, M.Z.; Ahmed, A.F.; Yousef, H.Y.; Shehata, S.M.; El Sammak, D.; Fathy, T.; Obaya, A.A.; Abdelbary, E.H. Does a combined CT and MRI protocol enhance the diagnostic efficacy of LI-RADS in the categorization of hepatic observations? A prospective comparative study. Eur. Radiol. 2018, 28, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Kamal, O.; Sy, E.; Chernyak, V.; Gupta, A.; Yaghmai, V.; Fowler, K.; Karampinos, D.; Shanbhogue, K.; Miller, F.H.; Kambadakone, A.; et al. Optional MRI sequences for LI-RADS: Why, what, and how? Abdom. Radiol. 2023, 48, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Stocker, D.; Hectors, S.; Bane, O.; Vietti-Violi, N.; Said, D.; Kennedy, P.; Cuevas, J.; Cunha, G.M.; Sirlin, C.B.; Fowler, K.J.; et al. Dynamic contrast-enhanced MRI perfusion quantification in hepatocellular carcinoma: Comparison of gadoxetate disodium and gadobenate dimeglumine. Eur. Radiol. 2021, 31, 9306–9315. [Google Scholar] [CrossRef]
- Zech, C.J.; Schwenke, C.; Endrikat, J. Diagnostic Efficacy and Safety of Gadoxetate Disodium vs Gadobenate Dimeglumine in Patients with Known or Suspected Focal Liver Lesions: Results of a Clinical Phase III Study. Magn. Reason. Insights 2019, 12, 1178623x19827976. [Google Scholar] [CrossRef]
- Tirkes, T.; Mehta, P.; Aisen, A.M.; Lall, C.; Akisik, F. Comparison of Dynamic Phase Enhancement of Hepatocellular Carcinoma Using Gadoxetate Disodium vs Gadobenate Dimeglumine. J. Comput. Assist. Tomogr. 2015, 39, 479–482. [Google Scholar] [CrossRef]
- Allen, B.C.; Ho, L.M.; Jaffe, T.A.; Miller, C.M.; Mazurowski, M.A.; Bashir, M.R. Comparison of Visualization Rates of LI-RADS Version 2014 Major Features with IV Gadobenate Dimeglumine or Gadoxetate Disodium in Patients at Risk for Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2018, 210, 1266–1272. [Google Scholar] [CrossRef]
- Song, M.; Cho, H.J.; Cho, Y.K.; Kim, M.Y.; Noh, S.I.; Yang, S.H. Detecting hepatocellular carcinoma in gadoxetic-acid-enhanced hepatobiliary-phase MR imaging at 3T: Comparing high and low flip angle. Jpn. J. Radiol. 2013, 31, 803–811. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, Y.K.; Lee, M.W.; Lee, W.J.; Kim, Y.-S.; Kim, S.H.; Choi, D.; Rhim, H. Small Hepatocellular Carcinomas: Improved Sensitivity by Combining Gadoxetic Acid–enhanced and Diffusion-weighted MR Imaging Patterns. Radiology 2012, 264, 761–770. [Google Scholar] [CrossRef]
- Mannelli, L.; Kim, S.; Hajdu, C.H.; Babb, J.S.; Taouli, B. Serial diffusion-weighted MRI in patients with hepatocellular carcinoma: Prediction and assessment of response to transarterial chemoembolization. Preliminary experience. Eur. J. Radiol. 2013, 82, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Corona-Villalobos, C.P.; Halappa, V.G.; Bonekamp, S.; Eng, J.; Reyes, D.; Cosgrove, D.; Rastegar, N.; Pan, L.; Pawlik, T.M.; Kamel, I.R. Functional magnetic resonance imaging response of targeted tumor burden and its impact on survival in patients with hepatocellular carcinoma. Investig. Radiol. 2015, 50, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Gluskin, J.S.; Chegai, F.; Monti, S.; Squillaci, E.; Mannelli, L. Hepatocellular Carcinoma and Diffusion-Weighted MRI: Detection and Evaluation of Treatment Response. J. Cancer 2016, 7, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Dromain, C.; de Baere, T.; Elias, D.; Kuoch, V.; Ducreux, M.; Boige, V.; Petrow, P.; Roche, A.; Sigal, R. Hepatic tumors treated with percutaneous radio-frequency ablation: CT and MR imaging follow-up. Radiology 2002, 223, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Gabr, A.E.; Mikhael, H.S.W.; El-Maadawy, S.M. Comparison between subtraction and dynamic MRI in assessing treatment response following radiofrequency ablation in patients with hepatocellular carcinoma. Egypt. J. Radiol. Nucl. Med. 2021, 52, 285. [Google Scholar] [CrossRef]
- El-Assaly, H.; Abdallah, M.F.H.; Mohamed, W.M.; Youssef, M.I. Additive role of dynamic subtraction MRI in assessment of unresolved HCC post-radiofrequency ablation. Egypt. J. Radiol. Nucl. Med. 2021, 52, 257. [Google Scholar] [CrossRef]
- Illing, R.O.; Kennedy, J.E.; Wu, F.; ter Haar, G.R.; Protheroe, A.S.; Friend, P.J.; Gleeson, F.V.; Cranston, D.W.; Phillips, R.R.; Middleton, M.R. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef]
- Gatti, M.; Maino, C.; Darvizeh, F.; Serafini, A.; Tricarico, E.; Guarneri, A.; Inchingolo, R.; Ippolito, D.; Ricardi, U.; Fonio, P.; et al. Role of gadoxetic acid-enhanced liver magnetic resonance imaging in the evaluation of hepatocellular carcinoma after locoregional treatment. World J. Gastroenterol. 2022, 28, 3116–3131. [Google Scholar] [CrossRef]
- Ratanaprasatporn, L.; Sainani, N.; Duda, J.B.; Aghayev, A.; Tatli, S.; Silverman, S.G.; Shyn, P.B. Imaging findings during and after percutaneous cryoablation of hepatic tumors. Abdom. Radiol. 2019, 44, 2602–2626. [Google Scholar] [CrossRef]
- Kim, A.Y.; Rhim, H.; Park, M.; Lee, M.W.; Kim, Y.S.; Choi, D.; Lim, H.K. Venous thrombosis after radiofrequency ablation for hepatocellular carcinoma. AJR Am. J. Roentgenol. 2011, 197, 1474–1480. [Google Scholar] [CrossRef]
- Shyn, P.B.; Mauri, G.; Alencar, R.O.; Tatli, S.; Shah, S.H.; Morrison, P.R.; Catalano, P.J.; Silverman, S.G. Percutaneous imaging-guided cryoablation of liver tumors: Predicting local progression on 24-hour MRI. AJR Am. J. Roentgenol. 2014, 203, W181–W191. [Google Scholar] [CrossRef] [PubMed]
- Shyn, P.B.; Oliva, M.R.; Shah, S.H.; Tatli, S.; Catalano, P.J.; Silverman, S.G. MRI contrast enhancement of malignant liver tumours following successful cryoablation. Eur. Radiol. 2012, 22, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Honjo, K.; Ito, K.; Arita, T.; Koike, S.; Takano, K.; Tamura, S.; Matsumoto, T.; Matsunaga, N. Fan-shaped hepatic parenchymal damage after ethanol injection therapy for hepatocellular carcinoma: MRI appearances. Abdom. Imaging 1999, 24, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Burgener, F.A.; Steinmetz, S.D. Treatment of experimental adenocarcinomas by percutaneous intratumoral injection of absolute ethanol. Investig. Radiol. 1987, 22, 472–478. [Google Scholar] [CrossRef]
- Guan, Y.S.; Sun, L.; Zhou, X.P.; Li, X.; Zheng, X.H. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J. Gastroenterol. 2004, 10, 3543–3548. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Piccirillo, M.; De Bellis, M.; Izzo, F.; Petrillo, A. Percutaneous ablation therapy of hepatocellular carcinoma with irreversible electroporation: MRI findings. AJR Am. J. Roentgenol. 2015, 204, 1000–1007. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Nijm, G.M.; Sahakian, A.V.; Yang, G.Y.; Omary, R.A.; Larson, A.C. Irreversible electroporation in the liver: Contrast-enhanced inversion-recovery MR imaging approaches to differentiate reversibly electroporated penumbra from irreversibly electroporated ablation zones. Radiology 2011, 258, 461–468. [Google Scholar] [CrossRef]
- Paturneau-Jouas, M.; Parzy, E.; Vidal, G.; Carlier, P.G.; Wary, C.; Vilquin, J.T.; de Kerviler, E.; Schwartz, K.; Leroy-Willig, A. Electrotransfer at MR imaging: Tool for optimization of gene transfer protocols--feasibility study in mice. Radiology 2003, 228, 768–775. [Google Scholar] [CrossRef]
- Faron, A.; Sprinkart, A.M.; Pieper, C.C.; Kuetting, D.L.R.; Fimmers, R.; Block, W.; Meyer, C.; Thomas, D.; Attenberger, U.; Luetkens, J.A. Yttrium-90 radioembolization for hepatocellular carcinoma: Outcome prediction with MRI derived fat-free muscle area. Eur. J. Radiol. 2020, 125, 108889. [Google Scholar] [CrossRef]
- Bester, L.; Hobbins, P.G.; Wang, S.C.; Salem, R. Imaging characteristics following 90yttrium microsphere treatment for unresectable liver cancer. J. Med. Imaging Radiat. Oncol. 2011, 55, 111–118. [Google Scholar] [CrossRef]
- Semaan, S.; Makkar, J.; Lewis, S.; Chatterji, M.; Kim, E.; Taouli, B. Imaging of Hepatocellular Carcinoma Response After (90)Y Radioembolization. AJR Am. J. Roentgenol. 2017, 209, W263–W276. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, R.; Rempp, H.; Schraml, C.; Schwenzer, N.; Grözinger, G.; Blumenstock, G.; Rothgang, E.; Pereira, P.L.; Claussen, C.D.; Clasen, S. Diffusion-weighted imaging during MR-guided radiofrequency ablation of hepatic malignancies: Analysis of immediate pre- and post-ablative diffusion characteristics. Acta Radiol. 2015, 56, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.M.; Nikolaidis, P.; Miller, F.H.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; Senthilnathan, S.; Riaz, A.; Kulik, L.; Mulcahy, M.F.; et al. Radiologic findings following Y90 radioembolization for primary liver malignancies. Abdom. Imaging 2009, 34, 566–581. [Google Scholar] [CrossRef] [PubMed]
- Shinagawa, Y.; Sakamoto, K.; Fujimitsu, R.; Ida, M.; Urakawa, H.; Kora, S.; Higashihara, H.; Takano, K.; Yoshimitsu, K. Pseudolesion of the liver observed on gadoxetate disodium-enhanced magnetic resonance imaging obtained shortly after transarterial chemoembolization for hepatocellular carcinoma. Jpn. J. Radiol. 2010, 28, 483–488. [Google Scholar] [CrossRef]
- Koda, M.; Ueki, M.; Maeda, Y.; Mimura, K.I.; Okamoto, K.; Matsunaga, Y.; Kawakami, M.; Hosho, K.; Murawaki, Y. The influence on liver parenchymal function and complications of radiofrequency ablation or the combination with transcatheter arterial embolization for hepatocellular carcinoma. Hepatol. Res. 2004, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ebraheem Ebeed, A.; Abd El-hamied Romeih, M.; Mohamed Refat, M.; Hamdy Yossef, M. Role of dynamic contrast-enhanced and diffusion weighted MRI in evaluation of hepatocellular carcinoma after chemoembolization. Egypt. J. Radiol. Nucl. Med. 2017, 48, 807–815. [Google Scholar] [CrossRef]
- Kloeckner, R.; Otto, G.; Biesterfeld, S.; Oberholzer, K.; Dueber, C.; Pitton, M.B. MDCT Versus MRI Assessment of Tumor Response After Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2010, 33, 532–540. [Google Scholar] [CrossRef]
- De Santis, M.; Alborino, S.; Tartoni, P.L.; Torricelli, P.; Casolo, A.; Romagnoli, R. Effects of lipiodol retention on MRI signal intensity from hepatocellular carcinoma and surrounding liver treated by chemoembolization. Eur. Radiol. 1997, 7, 10–16. [Google Scholar] [CrossRef]
- Takayasu, K.; Arii, S.; Matsuo, N.; Yoshikawa, M.; Ryu, M.; Takasaki, K.; Sato, M.; Yamanaka, N.; Shimamura, Y.; Ohto, M. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am. J. Roentgenol. 2000, 175, 699–704. [Google Scholar] [CrossRef]
- Kallini, J.R.; Miller, F.H.; Gabr, A.; Salem, R.; Lewandowski, R.J. Hepatic imaging following intra-arterial embolotherapy. Abdom. Radiol. 2016, 41, 600–616. [Google Scholar] [CrossRef]
- Najmi Varzaneh, F.; Pandey, A.; Aliyari Ghasabeh, M.; Shao, N.; Khoshpouri, P.; Pandey, P.; Zarghampour, M.; Fouladi, D.; Liddell, R.; Anders, R.A.; et al. Prediction of post-TACE necrosis of hepatocellular carcinoma usingvolumetric enhancement on MRI and volumetric oil deposition on CT, with pathological correlation. Eur. Radiol. 2018, 28, 3032–3040. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Koh, D.M.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: Consensus and recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef]
- Vossen, J.A.; Buijs, M.; Kamel, I.R. Assessment of tumor response on MR imaging after locoregional therapy. Tech. Vasc. Interv. Radiol 2006, 9, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hussein, R.S.; Tantawy, W.; Abbas, Y.A. MRI assessment of hepatocellular carcinoma after locoregional therapy. Insights Imaging 2019, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, N.; Camacho, J.C.; Xing, M.; Edalat, F.; Mittal, P.K.; Kim, H.S. Immediate post-doxorubicin drug-eluting beads chemoembolization Mr Apparent diffusion coefficient quantification predicts response in unresectable hepatocellular carcinoma: A pilot study. J. Magn. Reson. Imaging 2015, 42, 981–989. [Google Scholar] [CrossRef]
- Abdelhamed, W.; El-Kassas, M. Hepatocellular carcinoma recurrence: Predictors and management. Liver Res. 2023, 7, 321–332. [Google Scholar] [CrossRef]
- Ogasawara, S.; Chiba, T.; Motoyama, T.; Kanogawa, N.; Saito, T.; Shinozaki, Y.; Suzuki, E.; Ooka, Y.; Tawada, A.; Kato, H.; et al. Prognostic Significance of Concurrent Hypovascular and Hypervascular Nodules in Patients with Hepatocellular Carcinoma. PLoS ONE 2016, 11, e0163119. [Google Scholar] [CrossRef]
- Pecchi, A.; Besutti, G.; De Santis, M.; Del Giovane, C.; Nosseir, S.; Tarantino, G.; Di Benedetto, F.; Torricelli, P. Post-transplantation hepatocellular carcinoma recurrence: Patterns and relation between vascularity and differentiation degree. World J. Hepatol. 2015, 7, 276–284. [Google Scholar] [CrossRef]
- Kojiro, M. ‘Nodule-in-nodule’ appearance in hepatocellular carcinoma: Its significance as a morphologic marker of dedifferentiation. Intervirology 2004, 47, 179–183. [Google Scholar] [CrossRef]
- Takeda, H.; Takai, A.; Kumagai, K.; Iguchi, E.; Arasawa, S.; Eso, Y.; Shimizu, T.; Ueda, Y.; Taura, K.; Uemoto, S.; et al. Multiregional whole-genome sequencing of hepatocellular carcinoma with nodule-in-nodule appearance reveals stepwise cancer evolution. J. Pathol. 2020, 252, 398–410. [Google Scholar] [CrossRef]
- Kang, T.W.; Rhim, H.; Song, K.D.; Lee, M.W.; Cha, D.I.; Ha, S.Y.; Ahn, J.H. Radiofrequency Ablation of Hepatocellular Carcinoma with a “Nodule-in-Nodule” Appearance: Long-Term Follow-up and Clinical Implications. Cardiovasc. Interv. Radiol. 2017, 40, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Deng, H.; Hu, H.; Wu, C.; Huang, D.; Fang, C.; Yang, J.; Xiang, N. Laparoscopic Right Posterior Sectionectomy with Preservation of Subsegment Using Augmented Reality Navigation Plus ICG Fluorescence Imaging in Patients with HCC After Conversion Therapy. Ann. Surg. Oncol. 2024, 31, 5642–5644. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yan, W.; Zhao, J.; Ji, Y.; Qian, L.; Ding, H.; Zhao, Z.; Wang, G. Navigate biopsy with ultrasound under augmented reality device: Towards higher system performance. Comput. Biol. Med. 2024, 174, 108453. [Google Scholar] [CrossRef] [PubMed]
- Baz, R.O.; Scheau, C.; Baz, R.A.; Niscoveanu, C. Buhler’s Arc: An Unexpected Finding in a Case of Chronic Abdominal Pain. J. Gastrointest. Liver Dis. 2020, 29, 304. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Bakhshali, M.A.; Mazlooman, S.R.; Aliakbarian, M.; Gholizadeh, F.; Eslami, S.; Modrzejewski, A. Minimally invasive and invasive liver surgery based on augmented reality training: A review of the literature. J. Robot. Surg. 2023, 17, 753–763. [Google Scholar] [CrossRef]
- Hammill, C.W.; Clements, L.W.; Stefansic, J.D.; Wolf, R.F.; Hansen, P.D.; Gerber, D.A. Evaluation of a minimally invasive image-guided surgery system for hepatic ablation procedures. Surg. Innov. 2014, 21, 419–426. [Google Scholar] [CrossRef]
- García Vázquez, A.; Rodríguez-Luna, M.R.; Verde, J.; Piantanida, E.; Alonci, G.; Palermo, M.; Serra, E.; De Cola, L.; Giménez, M.E. Image-Guided Surgical Simulation in Minimally Invasive Liver Procedures: Development of a Liver Tumor Porcine Model Using a Multimodality Imaging Assessment. J. Laparoendosc. Adv. Surg. Tech. Part A 2021, 31, 1097–1103. [Google Scholar] [CrossRef]
- Ravi, T.; Ranganathan, R.; Pugalendhi, A.; Arumugam, S. 3D Printed Patient Specific Models from Medical Imaging—A General Workflow. Mater. Today Proc. 2020, 22, 1237–1243. [Google Scholar] [CrossRef]
- Timofticiuc, I.-A.; Călinescu, O.; Iftime, A.; Dragosloveanu, S.; Caruntu, A.; Scheau, A.-E.; Badarau, I.A.; Didilescu, A.C.; Caruntu, C.; Scheau, C. Biomaterials Adapted to Vat Photopolymerization in 3D Printing: Characteristics and Medical Applications. J. Funct. Biomater. 2024, 15, 7. [Google Scholar] [CrossRef]
- Dragosloveanu, S.; Petre, M.-A.; Capitanu, B.S.; Dragosloveanu, C.D.M.; Cergan, R.; Scheau, C. Initial Learning Curve for Robot-Assisted Total Knee Arthroplasty in a Dedicated Orthopedics Center. J. Clin. Med. 2023, 12, 6950. [Google Scholar] [CrossRef]
- Liu, R.; Abu Hilal, M.; Wakabayashi, G.; Han, H.S.; Palanivelu, C.; Boggi, U.; Hackert, T.; Kim, H.J.; Wang, X.Y.; Hu, M.G.; et al. International experts consensus guidelines on robotic liver resection in 2023. World J. Gastroenterol. 2023, 29, 4815–4830. [Google Scholar] [CrossRef]
- Giannone, F.; Felli, E.; Cherkaoui, Z.; Mascagni, P.; Pessaux, P. Augmented Reality and Image-Guided Robotic Liver Surgery. Cancers 2021, 13, 6268. [Google Scholar] [CrossRef]
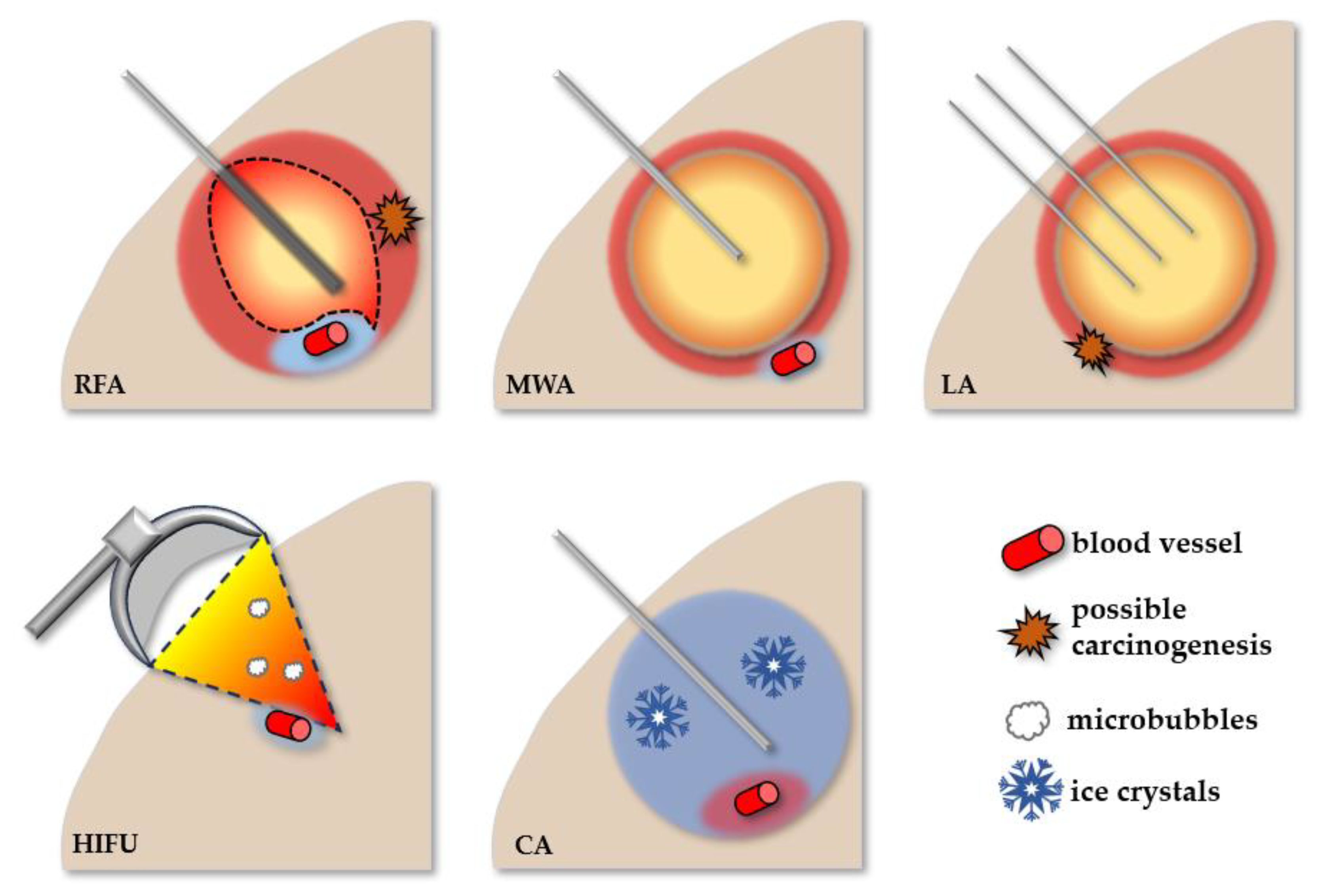
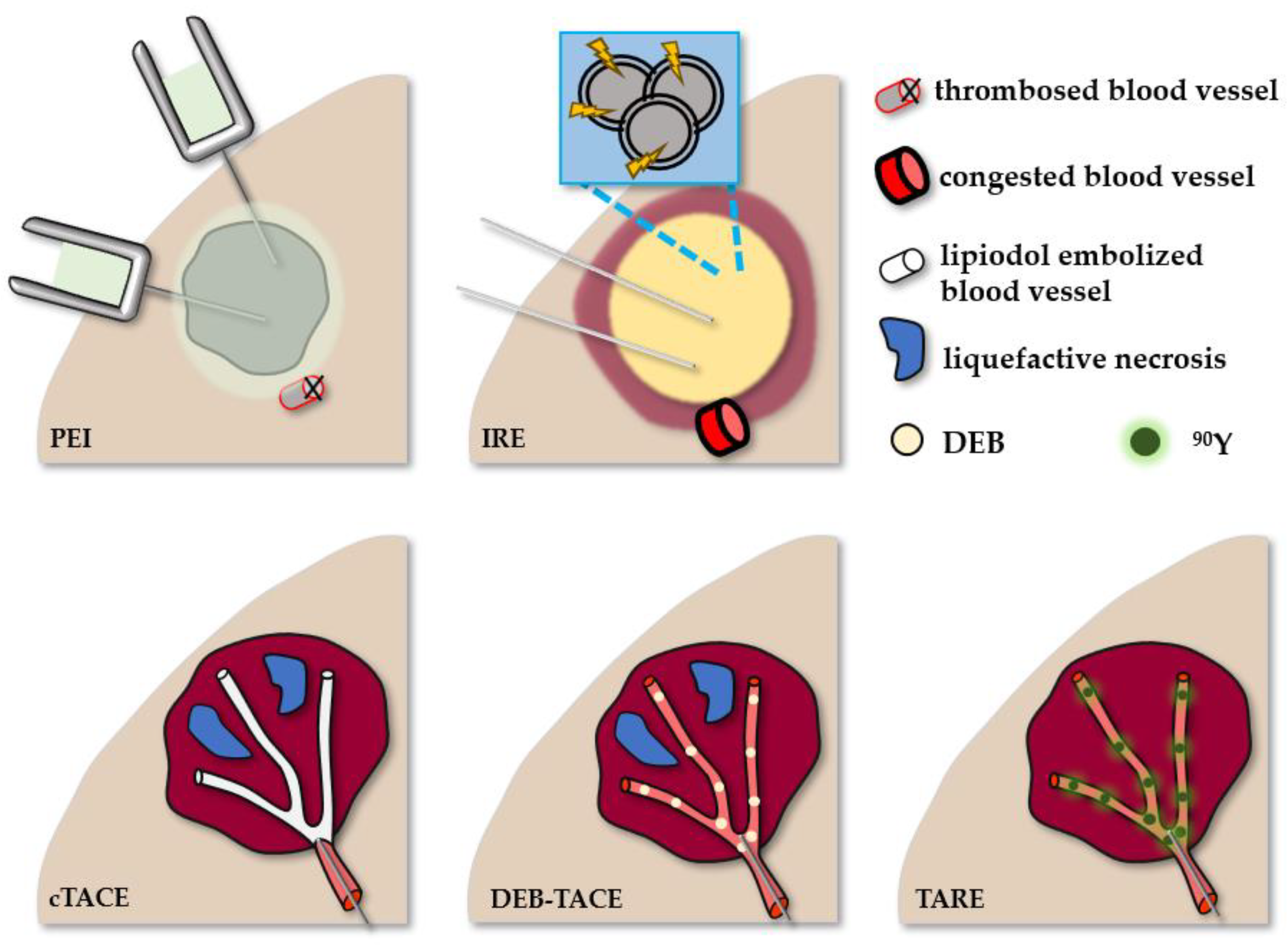

| Ablative Method | BCLC Stage | Advantages | Limitations | Histological (Imaging) Result | Overall Survival at 5 Years |
|---|---|---|---|---|---|
| Thermal ablative methods | |||||
| RFA | 0-A | Better control for larger nodules | Not recommended for superficial or near-hilum lesions Heat sink effect | Coagulation necrosis (H-iso T1, hT2) | 40–68% |
| MWA | 0-A | Higher ablation volume Minimal heat sink effect | Ablation volume may be difficult to estimate More complications in larger nodules | Coagulation necrosis (H-iso T1, hT2) | 50–60% |
| LA | 0-A | Better control for larger nodules Better accessibility to nodules | Relatively small zone of ablation, requiring multiple fibers to achieve sufficient volume | Coagulation necrosis (H-iso T1, hT2) | 15–34% |
| HIFU | 0-A | Minimal heat sink effect Less invasive | Possible damage to adjacent structures Longer procedure time | Coagulation necrosis (H-iso T1, hT2) | 15–60% |
| CA | 0-A | Less painful Area of CA visible on CT/MRI | Cryoshock syndrome is a possible complication | Coagulation necrosis (H-iso T1, hT2) | 23–59% |
| Non-thermal ablative methods | |||||
| PEI | 0-A | Simple, cheap, accessible | Risk of local progression Difficulty to obtain safety margins | Coagulation necrosis (H-iso T1, hT2) | up to 47% |
| IRE | 0-A | No heat sink effect Applicable to near-hilum lesions | Possible technical difficulty in needle positioning Cardiac gating is required | Coagulation necrosis (H-iso T1, hT2) | 14–56% |
| cTACE | 0-B | Combination of local chemotherapy and tumor devascularization | Vascular or biliary complications may occur Difficult in anatomical variants Postembolization syndrome may occur Poor response in hypovascular nodules | Coagulation and liquefactive necrosis (H- and hT1, H- and hT2) | 24–54% |
| DEB-TACE | 0-B | Superior chemotherapeutic effect Fewer adverse effects related to the chemotherapeutic drugs | Coagulation and liquefactive necrosis (H- and hT1, H- and hT2) | 33–61% | |
| TARE | 0-B | Increased radiation dose with curative effect | Radiation-related hepatitis | Coagulation necrosis (H-iso T1, hT2) | up to 40% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheau, A.-E.; Jurca, S.O.; Scheau, C.; Lupescu, I.G. Non-Surgical Treatment for Hepatocellular Carcinoma: What to Expect at Follow-Up Magnetic Resonance Imaging—A Pictorial Review. Appl. Sci. 2024, 14, 9159. https://doi.org/10.3390/app14209159
Scheau A-E, Jurca SO, Scheau C, Lupescu IG. Non-Surgical Treatment for Hepatocellular Carcinoma: What to Expect at Follow-Up Magnetic Resonance Imaging—A Pictorial Review. Applied Sciences. 2024; 14(20):9159. https://doi.org/10.3390/app14209159
Chicago/Turabian StyleScheau, Andreea-Elena, Sandra Oana Jurca, Cristian Scheau, and Ioana Gabriela Lupescu. 2024. "Non-Surgical Treatment for Hepatocellular Carcinoma: What to Expect at Follow-Up Magnetic Resonance Imaging—A Pictorial Review" Applied Sciences 14, no. 20: 9159. https://doi.org/10.3390/app14209159










