Biomineralization Process Inspired In Situ Growth of Calcium Carbonate Nanocrystals in Chitosan Hydrogels
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials and Sample Preparation
2.2. Composition and Structure Characterization
2.3. Mechanical Property Tests
3. Results and Discussions
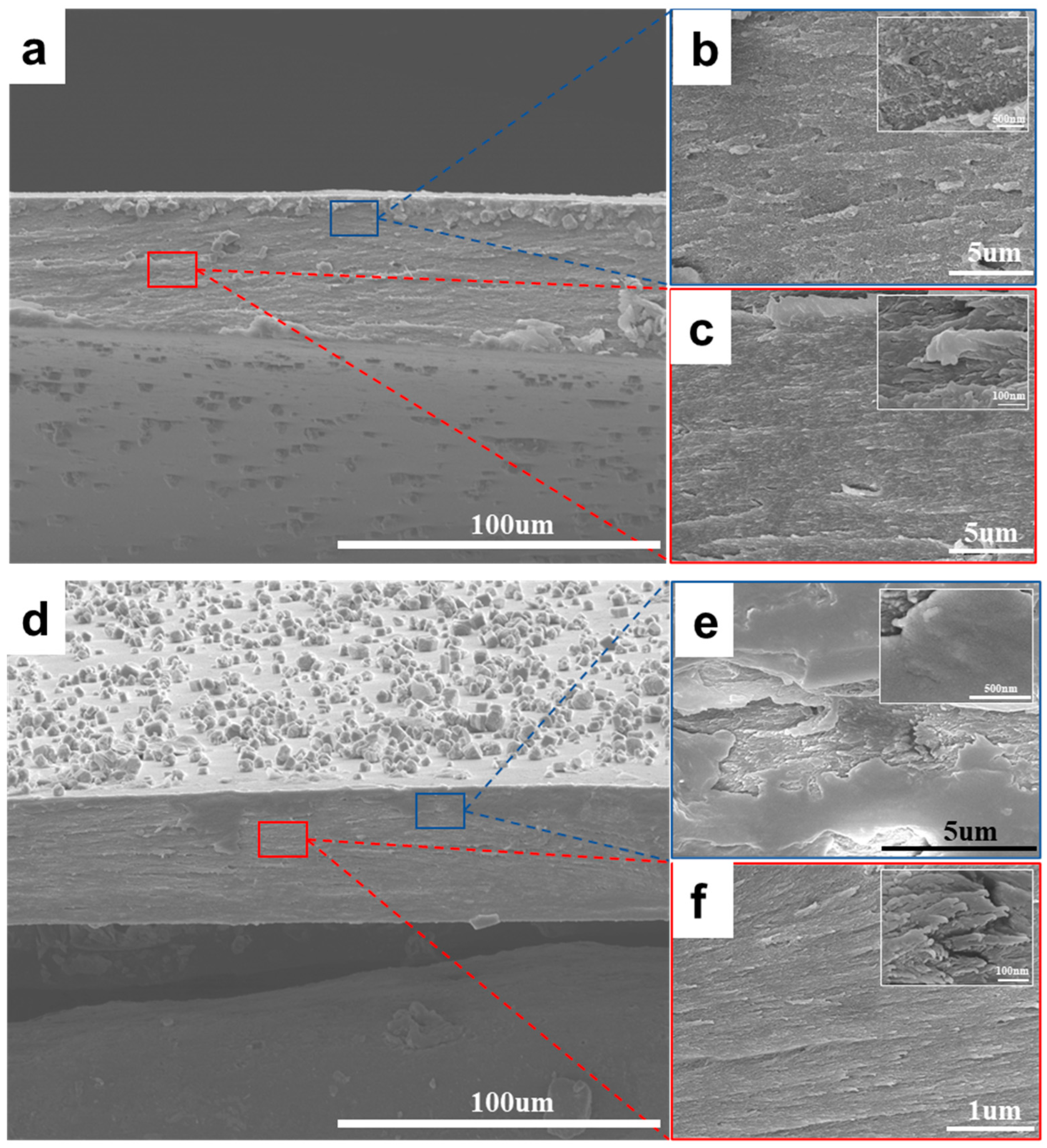
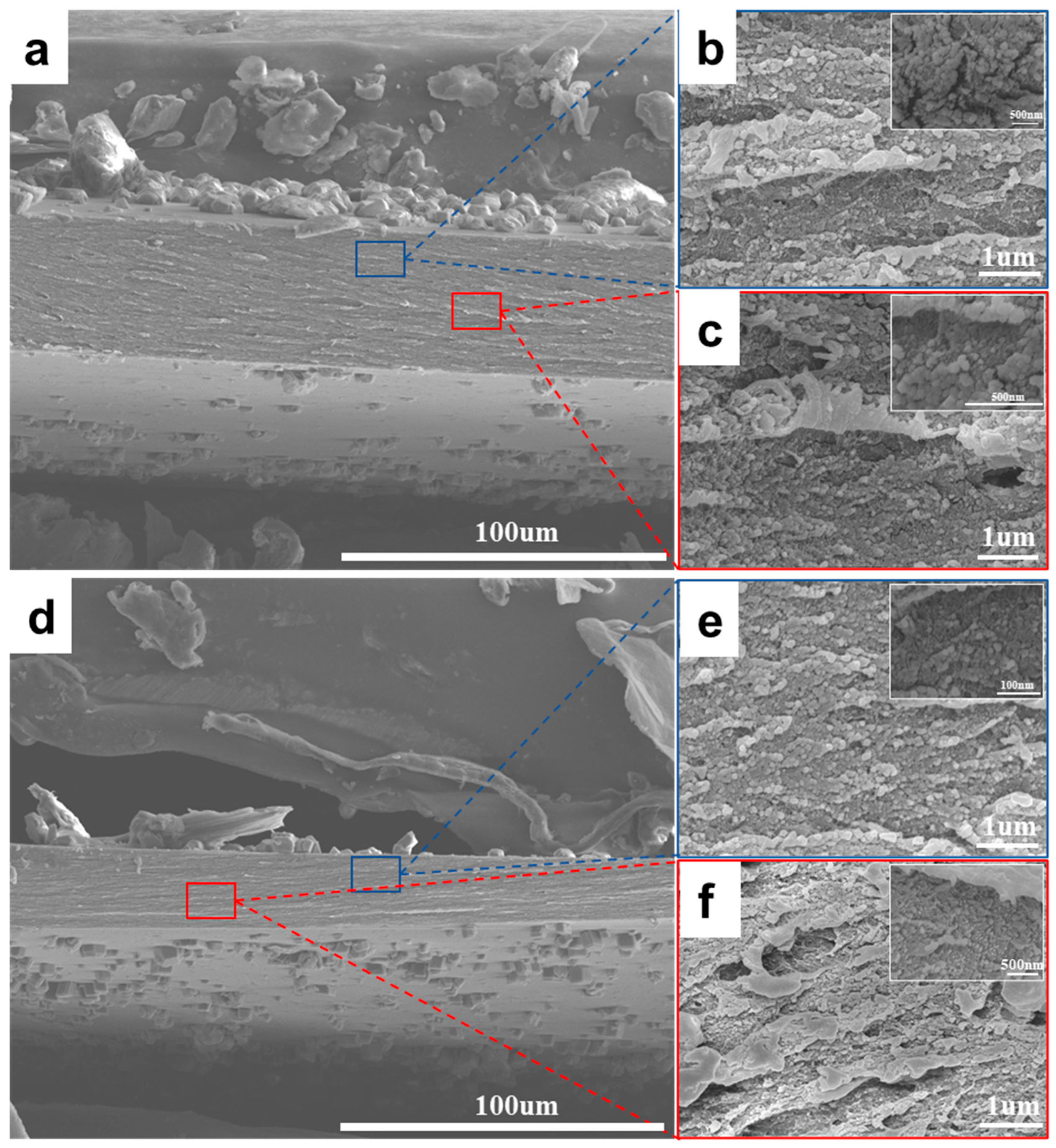
3.1. Structure and Composition of Chitosan/CaCO3 Composites
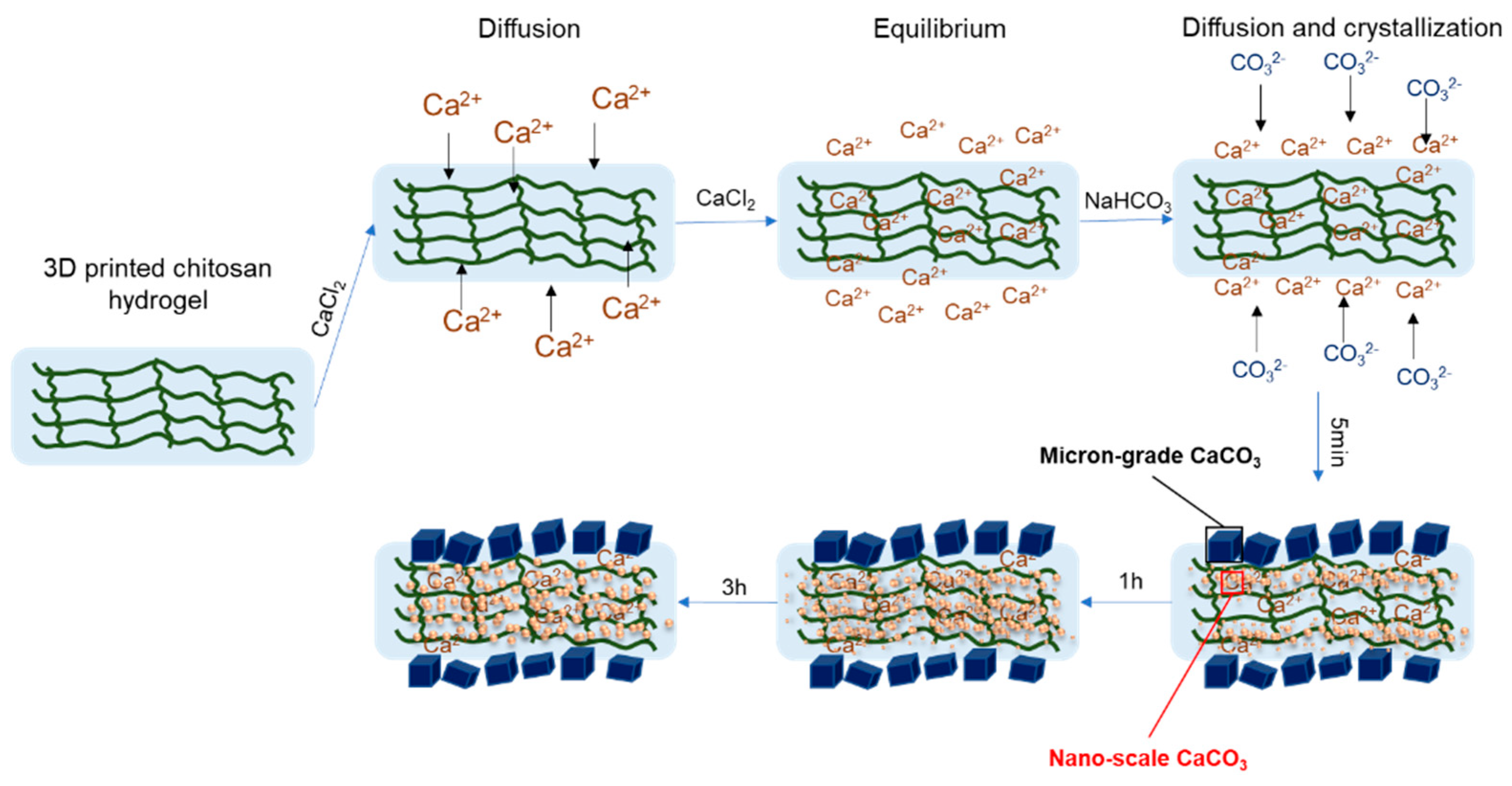
3.2. Crystal Growth Mechanisms of CaCO3 Nanoparticles in Chitosan Hydrogels
3.3. Mechanical Properties of Chitosan/CaCO3 Composites
4. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, B.; Yuan, X.; Zhou, A.; Zhang, H.; Jiang, D. Designer functionalised self-assembling peptide nanofibre scaffolds for cartilage tissue engineering. Expert Rev. Mol. Med. 2014, 16, e12. [Google Scholar] [CrossRef]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M.; et al. Bioactive Materials for Bone Regeneration: Biomolecules and Delivery Systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef] [PubMed]
- Armengol, E.S.; Hock, N.; Saribal, S.; To, D.; Summonte, S.; Veider, F.; Kali, G.; Bernkop-Schnürch, A.; Laffleur, F. Unveiling the potential of biomaterials and their synergistic fusion in tissue engineering. Eur. J. Pharm. Sci. 2024, 196, 106761. [Google Scholar] [CrossRef] [PubMed]
- Asadi, N.; Del Bakhshayesh, A.R.; Davaran, S.; Akbarzadeh, A. Common biocompatible polymeric materials for tissue engineering and regenerative medicine. Mater. Chem. Phys. 2020, 242, 122528. [Google Scholar] [CrossRef]
- Polishchuk, I.; Bracha, A.A.; Bloch, L.; Levy, D.; Kozachkevich, S.; Etinger-Geller, Y.; Kauffmann, Y.; Burghammer, M.; Giacobbe, C.; Villanova, J.; et al. Coherently aligned nanoparticles within a biogenic single crystal: A biological prestressing strategy. Science 2017, 358, 1294–1298. [Google Scholar] [CrossRef]
- Kunitake, M.E.; Mangano, L.M.; Peloquin, J.M.; Baker, S.P.; Estroff, L.A. Evaluation of strengthening mechanisms in calcite single crystals from mollusk shells. Acta Biomater. 2013, 9, 5353–5359. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Jia, Y.; Liu, K.; Zhang, X.S. Modulate stress distribution with bio-inspired irregular architected materials towards optimal tissue support. Nat. Commun. 2024, 15, 4072. [Google Scholar] [CrossRef]
- Xia, Y.; Gu, Y.; Zhou, X.; Xu, H.; Zhao, X.; Yaseen, M.; Lu, J.R. Controllable Stabilization of Poly (N-isopropylacrylamide)-Based Microgel Films through Biomimetic Mineralization of Calcium Carbonate. Biomacromolecules 2012, 13, 2299–2308. [Google Scholar] [CrossRef]
- McNally, E.A.; Schwarcz, H.P.; Botton, G.A.; Arsenault, A.L. A model for the ultrastructure of bone based on electron microscopy of ion-milled sections. PLoS ONE 2012, 7, e29258. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Zanghellini, E.; Matic, A.; Thomsen, P.; Palmquist, A. The Orientation of Nanoscale Apatite Platelets in Relation to Osteoblastic–Osteocyte Lacunae on Trabecular Bone Surface. Calcif. Tissue Int. 2016, 98, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Rho, J.-Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Arash, A.; Dehgan, F.; Benisi, S.Z.; Jafari-Nodoushan, M.; Pezeshki-Modaress, M. Polysaccharide base electrospun nanofibrous scaffolds for cartilage tissue engineering: Challenges and opportunities. Int. J. Biol. Macromol. 2024, 277, 134054. [Google Scholar] [CrossRef]
- Hoemann, C.D.; Lafantaisie-Favreau, C.-H.; Lascau-Coman, V.; Chen, G.; Guzmán-Morales, J. The Cartilage-Bone Interface. J. Knee Surg. 2012, 25, 85–98. [Google Scholar] [CrossRef]
- Huang, W.; Shishehbor, M.; Guarín-Zapata, N.; Kirchhofer, N.D.; Li, J.; Cruz, L.; Wang, T.; Bhowmick, S.; Stauffer, D.; Manimunda, P.; et al. A natural impact-resistant bicontinuous composite nanoparticle coating. Nat. Mater. 2020, 19, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Fiedler, I.A.; Busse, B. Breaking new ground in mineralized tissue: Assessing tissue quality in clinical and laboratory studies. J. Mech. Behav. Biomed. Mater. 2021, 113, 104138. [Google Scholar] [CrossRef]
- Fukao, K.; Tanaka, K.; Kiyama, R.; Nonoyama, T.; Gong, J.P. Hydrogels toughened by biominerals providing energy-dissipative sacrificial bonds. J. Mater. Chem. B 2020, 8, 5184–5188. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Bi, W.; Sun, Y.; Wang, L.; Yu, X.; Cheng, R.; Yu, Y.; Cui, W. Biomimetic or-ganic-inorganic hybrid hydrogel electrospinning periosteum for accelerating bone regeneration. Mater. Sci. Eng. C 2020, 110, 110670. [Google Scholar] [CrossRef]
- Monavari, M.; Homaeigohar, S.; Fuentes-Chandía, M.; Nawaz, Q.; Monavari, M.; Venkatraman, A.; Boccaccini, A.R. 3D printing of alginate dialdehyde-gelatin (ADA-GEL) hydrogels incorporating phytotherapeutic icariin loaded mesoporous SiO2-CaO nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2021, 131, 112470. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, G.; Shi, W.; Ma, X.; Yang, X.; Yang, H.; Guo, Y.; Yang, L. In situ generation of biocompatible amorphous calcium carbonate onto cell membrane to block membrane transport protein—A new strategy for cancer therapy via mimicking abnormal mineralization. J. Colloid Interface Sci. 2019, 541, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z. Chitosan-calcium carbonate scaffold with high mineral content and hierarchical structure for bone regeneration. Smart Mater. Med. 2023, 4, 552–561. [Google Scholar] [CrossRef]
- Fu, J.; Leo, C.P.; Show, P.L. Recent advances in the synthesis and applications of pH-responsive CaCO3. Biochem. Eng. J. 2022, 187, 108446. [Google Scholar] [CrossRef]
- Asenath-Smith, E.; Li, H.; Keene, E.C.; Seh, Z.W.; Estroff, L.A. Crystal Growth of Calcium Carbonate in Hydrogels as a Model of Biomineralization. Adv. Funct. Mater. 2012, 22, 2891–2914. [Google Scholar] [CrossRef]
- Damian, P.; Style, R.W.; Zlopaša, J.; Petrozzini, J.J.; Pfeifer, M.A.; Jonkers, H.M.; Dufresne, E.R.; Estroff, L.A. Forming Anisotropic Crystal Composites: Assessing the Mechanical Translation of Gel Network An-isotropy to Calcite Crystal Form. J. Am. Chem. Soc. 2021, 143, 3439–3447. [Google Scholar]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tis-sue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Deepthi, S.; Venkatesan, J.; Kim, S.-K.; Bumgardner, J.D.; Jayakumar, R. An overview of chitin or chitosan/nano ceramic composite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2016, 93, 1338–1353. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Maire, M.; Lerouge, S.; Therriault, D.; Heuzey, M.C. 3D Printing of Microstructured and Stretchable Chitosan Hydrogel for Guided Cell Growth. Adv. Biosyst. 2017, 1, 1700058. [Google Scholar] [CrossRef]
- Rivas-Araiza, R.; Alcouffe, P.; Rochas, C.; Montembault, A.; David, L. Micron range mor-phology of physical chitosan hydrogels. Langmuir ACS J. Surf. Colloids 2010, 26, 17495–17504. [Google Scholar] [CrossRef]
- Sereni, N.; Enache, A.; Sudre, G.; Montembault, A.; Rochas, C.; Durand, P.; Perrard, M.-H.; Bozga, G.; Puaux, J.-P.; Delair, T.; et al. Dynamic Structuration of Physical Chitosan Hydrogels. Langmuir 2017, 33, 12697–12707. [Google Scholar] [CrossRef]
- Cai, X.; Chen, L.; Jiang, T.; Shen, X.; Hu, J.; Tong, H. Facile synthesis of anisotropic porous chitosan/hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Chem. 2011, 21, 12015–12025. [Google Scholar] [CrossRef]
- Marsi, T.C.O.; Santos, T.G.; Pacheco-Soares, C.; Corat, E.J.; Marciano, F.R.; Lobo, A.O. Biomineralization of superhydrophilic vertically aligned carbon nanotubes. Langmuir ACS J. Surf. Colloids 2012, 28, 4413–4424. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.-B.; Gao, H.-L.; Yao, H.-B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.-M.; Li, S.-K.; Yan, Y.-X.; Liu, Y.-Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Sugawara-Narutaki, A. Bio-inspired synthesis of polymer–inorganic nanocomposite materials in mild aqueous systems. Polym. J. 2013, 45, 269–276. [Google Scholar] [CrossRef]
- Weiner, S.; Wagner, H.D. The Material Bone: Structure-Mechanical Function Relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Baht, G.S.; Hunter, G.K.; Goldberg, H.A. Bone sialoprotein–collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008, 27, 600–608. [Google Scholar] [CrossRef]
- McKee, M.D.; Buss, D.J.; Reznikov, N. Mineral tessellation in bone and the Stenciling Principle for extracellular matrix mineralization. J. Struct. Biol. 2021, 241, 107823. [Google Scholar] [CrossRef]
- Gebauer, D.; Gale, J.D.; Cölfen, H. Crystal Nucleation and Growth of Inorganic Ionic Materials from Aqueous Solution: Selected Recent Developments, and Implications. Small 2022, 18, e2107735. [Google Scholar] [CrossRef]
- Nicolin, D.J.; Rossoni, D.F.; Mario, L.; Jorge, M. Study of uncertainty in the fitting of diffusivity of Fick’s Second Law of Diffusion with the use of Bootstrap Method. J. Food Eng. 2016, 184, 63–68. [Google Scholar] [CrossRef]
- Hashim, F.A.; Mostafa, R.R.; Hussien, A.G.; Mirjalili, S.; Sallam, K.M. Fick’s Law Al-gorithm: A physical law-based algorithm for numerical optimization. Knowl. Based Syst. 2023, 260, 110146. [Google Scholar] [CrossRef]
- Mauro, J.C. Fick’s Laws of Diffusion. In Materials Kinetics: Transport and Rate Phenomena; Mauro, J.C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 3; pp. 39–58. [Google Scholar]
- Xue, Q.; He, Y.; Zhang, X.; Zhang, X.; Cai, M.; Guo, C.F.; Yang, C. Strong Interfaces Enable Efficient Load Transfer for Strong, Tough, and Impact-Resistant Hydrogel Composites. ACS Appl. Mater. Interfaces 2022, 14, 33797–33805. [Google Scholar] [CrossRef] [PubMed]




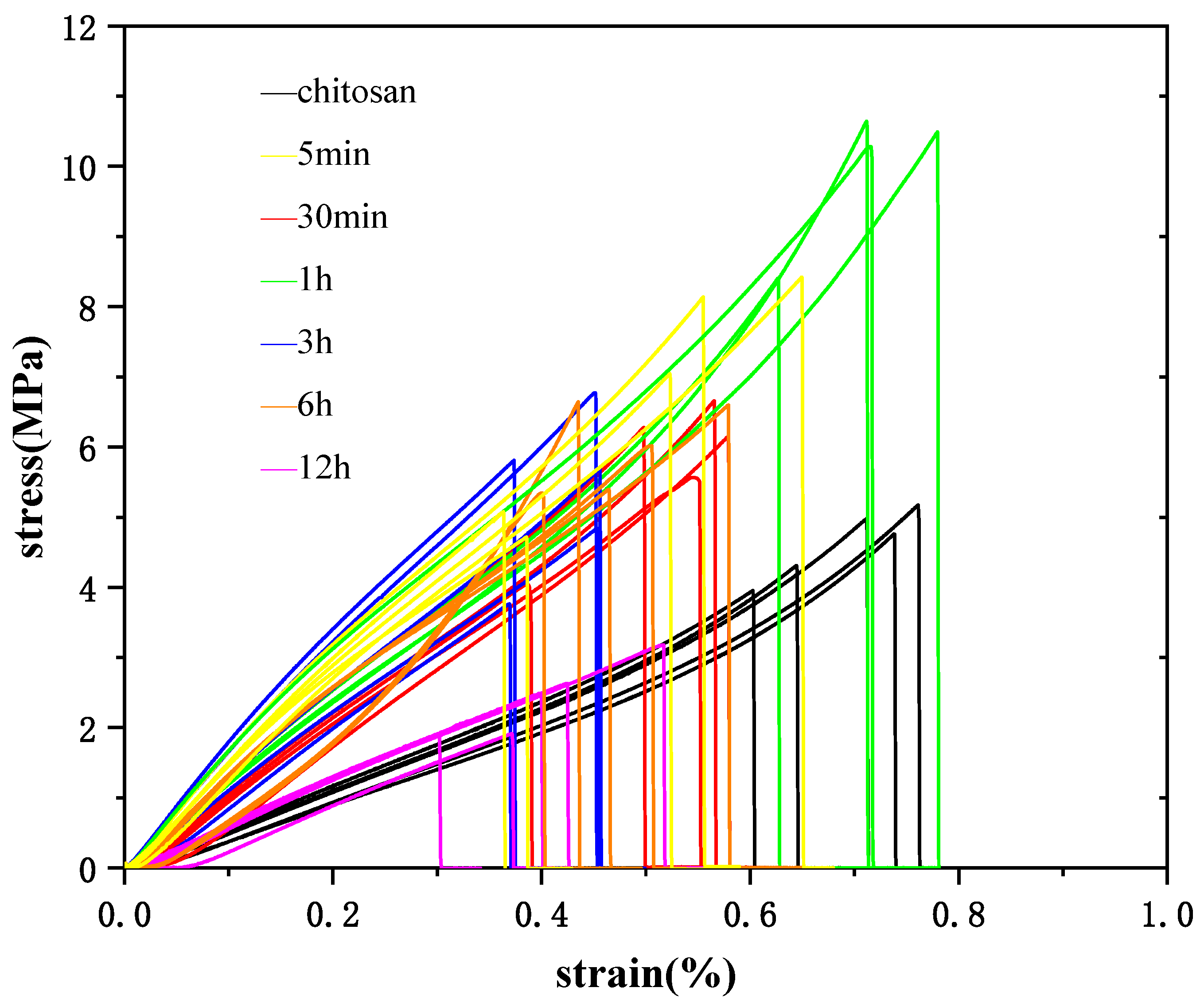
| Mineralization Time | Young’s Moduli E [MPa] | Tensile Strength σm [MPa] | Ultimate Strain εb [%] |
|---|---|---|---|
| 0 min | 5.59 ± 0.34 | 4.64 ± 0.50 | 73.64 ± 6.68 |
| 5 min | 13.84 ± 2.15 | 6.68 ± 1.71 | 54.87 ± 9.67 |
| 30 min | 10.91 ± 0.86 | 5.74 ± 1.03 | 57.68 ± 7.21 |
| 1 h | 11.68 ± 1.38 | 8.72 ± 2.90 | 68.05 ± 19.15 |
| 3 h | 13.88 ± 3.11 | 5.37 ± 1.13 | 46.34 ± 3.41 |
| 6 h | 12.15 ± 1.57 | 6.00 ± 0.63 | 52.12 ± 8.04 |
| 12 h | 6.96 ± 0.21 | 2.42 ± 0.54 | 44.47 ± 8.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zhu, Z.; Chang, W.; Wu, B.; Huang, W. Biomineralization Process Inspired In Situ Growth of Calcium Carbonate Nanocrystals in Chitosan Hydrogels. Appl. Sci. 2024, 14, 9193. https://doi.org/10.3390/app14209193
Zeng X, Zhu Z, Chang W, Wu B, Huang W. Biomineralization Process Inspired In Situ Growth of Calcium Carbonate Nanocrystals in Chitosan Hydrogels. Applied Sciences. 2024; 14(20):9193. https://doi.org/10.3390/app14209193
Chicago/Turabian StyleZeng, Xinyue, Zheng Zhu, Wei Chang, Bin Wu, and Wei Huang. 2024. "Biomineralization Process Inspired In Situ Growth of Calcium Carbonate Nanocrystals in Chitosan Hydrogels" Applied Sciences 14, no. 20: 9193. https://doi.org/10.3390/app14209193





