Fenugreek: New Therapeutic Resource or Emerging Allergen?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Allergological Work-Up
2.3. Statistical Analysis
3. Results
3.1. Patients with a History of Spice Allergy
3.2. Patients with a History of Legumes Allergy
3.3. Other Sensitizations in Patients with SPT Positive for Fenugreek and Legumes
4. Discussion
4.1. Fenugreek Is a Pharmaceutical Product
4.2. Allergic Reactions to Fenugreek
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Case | Age Sex | Symptoms Associated with Fenugreek | Symptoms Associated with Pistachio | Symptoms Associated with Other Legumes and Foods | Skin Prick Test for Food Allergens | Inhalant Sensitizations | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| 1 | 37 M | Nasal obstruction, rhinorrhea, palmoplantar itching, and difficulty breathing after chicken curry ingestion. | Anaphylaxis after consuming pistachio ice cream and slush. | / | Fenugreek, pistachio (Figure 2 and Figure 3), curry mix, cumin, mango, grapes, fennel, and celery. | Chicken, coconut milk, peanuts, and lupin. | Pellitory, grass, mugwort, plantain, ragweed, cat fur, dog coat, and house dust mites. |
| 2 | 36 F | Itchy rash or urticaria, sometimes accompanied by lip angioedema after the ingestion of spice mixes. | / | Itchy rash or urticaria sometimes accompanied by lip angioedema after the ingestion of various foods on separate occasions (pomegranate, raw and cooked tomato, pumpkin, shellfish, chestnuts, mozzarella, aubergine, melon, watermelon, zucchini, nuts). | Fenugreek, pistachio (Figure 2), cumin, coriander, cashew, peanut, watermelon, mango, lupine, pomegranate, fennel, celery, pumpkin seed, yellow melon. | Other suspected food allergens. | Grass, mugwort, olea, plantain, and birch. |
| 3 | 42 F | Sneezing blank, rhinorrhea, nasal obstruction, tightness in throat, tongue swelling, extremity itching after ingestion of soy snack (containing spices). | / | Sneezing blank, rhinorrhea, nasal obstruction, tight throat, tongue swelling, extremity itching after corn and onion ingestion. | Fenugreek, cumin, peanuts, and pomegranate. | Soy, corn. | Pellitory, grass, mugwort, olea, plantain, ragweed, and birch. |
| ISAC test: Ara h6. Ara h9. | |||||||
| 4 | 26 F | / | / | Episodes of lingual oedema, velvety, and urticarial rash after the ingestion of peach, melon, watermelon, and cherry juice; episodes of velvety oral cavity and pharynx after the ingestion of peanuts, walnuts, and fennel. | Fenugreek, pistachio, Peanuts, mango, strawberries, pomegranate fennel, celery, and yellow melon. | Other main suspected food allergens. | Pellitory, grass, mugwort, olea, cat fur, house dust mites. |
| 5 | 22 M | / | Erythema on the face and trunk after consuming pistachios and beer. | Vomiting and diarrhea after eating peanuts; gut discomfort after eating lentils and strawberries; oral itching and throat constriction after eating peach and nuts; and rhinitis and erythema on the face and trunk after eating tomato. | Fenugreek, pistachio, tomato, lentil, peach, walnut, hazelnut, almond, mango, strawberries pomegranate, fennel. | Other suspected food allergens. | Pellitory, grass, mugwort. |
| 6 | 28 F | / | / | Lip angioedema after peach ingestion; sore mouth and oral itching after eating hazelnut cream, zucchini, garlic, corn, and dragon fruit; throat constriction after lemon and red berries; episodes of oral itching after eating lettuce, arugula, radicchio, plum, pumpkin, eggplant, parsley, mushrooms, and potato. | Fenugreek, pistachio, mustard, soy, corn, rice, lentil, peach, hazelnut, peanut, strawberry, pomegranate, fennel, and celery. | Other suspected food allergens. | / |
| 7 | 29 F | Nonitchy rash, diarrhea, abdominal cramps after the ingestion of spiced potatoes. | Tight throat, dyspnea, and swelling of the tongue after pistachios ingestion. | Tight throat, dyspnea, and swelling of the tongue after the ingestion of peach, walnut, and almond. | Fenugreek, pistachio (Figure 2 and Figure 3), peach, hazelnut, cashew, mango, and pomegranate. | Other suspected food allergens. | Pellitory, grass, mugwort, and olea. |
| 8 | 31 M | / | Constriction in throat after aperitif with alcohol, nuts and pizza with pistachios. | Nausea and vomiting after apple ingestion, constriction in throat after banana ingestion. | Fenugreek, pistachio, mustard, lentil, apple, mango, grapes, pomegranate, fennel, lupine, anise, banana, zucchini, and eggplant. | Other suspected food allergens. | Cypress. |
| 9 | 36 M | Urticaria after ingestion of rice, mutton, fish, and a spice mixture including turmeric, coriander, ginger, chili pepper and other spices. | / | Angioedema and vomiting after corn ingestion; itching of the oral cavity after eggplant and green peas; urticaria, angioedema, and vomiting after zucchini, tuna, and mozzarella meal. | Fenugreek, cumin, turmeric, coriander, mustard, oat, rice, lentil, peach, peanut, pomegranate, fennel, anise, zucchini, eggplant, and rice. | Other suspected food allergens. | Olea, house dust mites. |
| 10 | 42 F. | / | / | Itchy plantar palm and angioedema after hazelnut snack; anaphylaxis after eating a pizza with zucchini, Parma ham and maybe contaminated with arugula. | Fenugreek, pistachio, peanut, hazelnut, walnut, grape, celery, eggplant, pea, corn, sunflower seed, parsley, songino, onion, garlic, arugula, basil, strawberry, apricot, cherry, and apple. | Other suspected food allergens. | Pellitory, mugwort, hemp, plane tree. |
| ISAC test: Corn, peanut, kiwi, apple, peach, grape, celery, hazelnut, walnut (Par j2, Can S3, Art v3, Pla a3, Zea m14, Ara h9, Act d10, Mal d3, Pru p3, Vit v1, Api g2, Cor a8, Jug r3). | |||||||
| 11 | 26 F. | Episodes of tickling in the pharynx after curry ingestion. | Episodes of dyspnea and constriction in the throat after pistachios ingestion. | Various episodes of dyspnea and constriction in the throat after sesame ingestion. | Fenugreek, pistachio, cumin, coriander, peach, sesame, and pomegranate. | Nuts, mustard, bean, lentils. | Pellitory, grass, house dust mites cat fur, and dog coat. |
| 12 | 20 F. | / | Urticaria and angioedema after pistachios ingestion. | Urticaria and angioedema after ingestion of peanuts, and peach; oral itching after green beans ingestion. | Fenugreek, pistachio, peanuts, bean, lentil, peach, apple, and hazelnut. | Other suspected food allergens. | / |
| 13 | 23 M. | / | Episodes of velvety itchy scalp, and vomiting after pistachio ingestion. | Episodes of velvety itchy scalp, and vomiting after the ingestion of nuts (including peanut, almond, walnut, hazelnut) and apples. | Fenugreek, pistachio (Figure 2 and Figure 3), Apricot, apple, peanut, peach, almond, hazelnut, mango, cashew. | Other suspected food allergens. | Pellitory, grass, house dust mites, cat fur, and dog coat. |
- Case 1
- Case 2
- Case 3
- Case 4
- Case 5
- Case 6
- Case 7
- Case 8
- Case 9
- Case 10
- Case 11
- Case 12
- Case 13
References
- Ouzir, M.; El Bairi, K.; Amzazi, S. Toxicological Properties of Fenugreek (Trigonella Foenum Graecum). Food Chem. Toxicol. 2016, 96, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Narayanan, M.; de Souza, R.J.; van Dam, R.M. Effect of Fenugreek (Trigonella Foenum-Graecum L.) Intake on Glycemia: A Meta-Analysis of Clinical Trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Yadav, U.C.S.; Baquer, N.Z. Pharmacological Effects of Trigonella Foenum-Graecum L. in Health and Disease. Pharm. Biol. 2014, 52, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Nagulapalli Venkata, K.C.; Swaroop, A.; Bagchi, D.; Bishayee, A. A Small Plant with Big Benefits: Fenugreek (Trigonella Foenum-graecum Linn.) for Disease Prevention and Health Promotion. Mol. Nutr. Food Res. 2017, 61, 1600950. [Google Scholar] [CrossRef]
- Caserta, S.; Genovese, C.; Cicero, N.; Toscano, V.; Gangemi, S.; Allegra, A. The Interplay between Medical Plants and Gut Microbiota in Cancer. Nutrients 2023, 15, 3327. [Google Scholar] [CrossRef]
- Li Pomi, F.; Papa, V.; Borgia, F.; Vaccaro, M.; Allegra, A.; Cicero, N.; Gangemi, S. Rosmarinus Officinalis and Skin: Antioxidant Activity and Possible Therapeutical Role in Cutaneous Diseases. Antioxidants 2023, 12, 680. [Google Scholar] [CrossRef]
- Gammeri, L.; Panzera, C.; Calapai, F.; Cicero, N.; Gangemi, S. Asian Herbal Medicine and Chronic Urticaria: Which Are the Therapeutic Perspectives? Nat. Prod. Res. 2023, 37, 1917–1934. [Google Scholar] [CrossRef]
- Cicero, N.; Gangemi, S.; Allegra, A. Natural Products and Oxidative Stress: Potential Agents against Multiple Myeloma. Nat. Prod. Res. 2023, 37, 687–690. [Google Scholar] [CrossRef]
- Cicero, N.; Gervasi, T.; Durazzo, A.; Lucarini, M.; Macrì, A.; Nava, V.; Giarratana, F.; Tardugno, R.; Vadalà, R.; Santini, A. Mineral and Microbiological Analysis of Spices and Aromatic Herbs. Foods 2022, 11, 548. [Google Scholar] [CrossRef]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can Nutraceuticals Assist Treatment and Improve COVID-19 Symptoms? Nat. Prod. Res. 2022, 36, 2672–2691. [Google Scholar] [CrossRef] [PubMed]
- Tardugno, R.; Cicero, N.; Costa, R.; Nava, V.; Vadalà, R. Exploring Lignans, a Class of Health Promoting Compounds, in a Variety of Edible Oils from Brazil. Foods 2022, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Nicosia, N.; Fumia, A.; Giorgianni, F.; Santini, A.; Cicero, N. Resveratrol and Immune Cells: A Link to Improve Human Health. Molecules 2022, 27, 424. [Google Scholar] [CrossRef] [PubMed]
- Yao, D.; Zhang, B.; Zhu, J.; Zhang, Q.; Hu, Y.; Wang, S.; Wang, Y.; Cao, H.; Xiao, J. Advances on Application of Fenugreek Seeds as Functional Foods: Pharmacology, Clinical Application, Products, Patents and Market. Crit. Rev. Food Sci. Nutr. 2020, 60, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, M.; Shirzad, H.; Mirhosseini, M.; Mesripour, A.; Rafieian-Kopaei, M. A Review on Ethnobotanical and Therapeutic Uses of Fenugreek (Trigonella Foenum-Graceum L). J. Evid. Based Complement. Altern. Med. 2016, 21, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Therapeutic Applications of Fenugreek. Altern. Med. Rev. 2003, 8, 20–27. [Google Scholar]
- European Medicines Agency. Assessment Report on Trigonella Foenum-Graecum L., Semen; European Medicines Agency: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Minciullo, P.L.; Calapai, G.; Miroddi, M.; Mannucci, C.; Chinou, I.; Gangemi, S.; Schmidt, R.J. Contact Dermatitis as an Adverse Reaction to Some Topically Used E Uropean Herbal Medicinal Products–Part 4: S Olidago Virgaurea–V Itis Vinifera. Contact Dermat. 2017, 77, 67–87. [Google Scholar] [CrossRef]
- Che, C.T.; Douglas, L.; Liem, J. Case Reports of Peanut-Fenugreek and Cashew-Sumac Cross-Reactivity. J. Allergy Clin. Immunol. Pract. 2017, 5, 510–511. [Google Scholar] [CrossRef]
- Vinje, N.E.; Namork, E.; Løvik, M. Cross-allergic Reactions to Legumes in Lupin and Fenugreek-sensitized Mice. Scand. J. Immunol. 2012, 76, 387–397. [Google Scholar] [CrossRef]
- Zeng, W.-Y.; Tan, L.; Han, C.; Zheng, Z.-Y.; Wu, G.-S.; Luo, H.-R.; Li, S.-L. Trigonelline Extends the Lifespan of C. Elegans and Delays the Progression of Age-Related Diseases by Activating AMPK, DAF-16, and HSF-1. Oxid. Med. Cell. Longev. 2021, 2021, 7656834. [Google Scholar] [CrossRef]
- Fernandes, F.; Barroso, M.F.; De Simone, A.; Emriková, E.; Dias-Teixeira, M.; Pereira, J.P.; Chlebek, J.; Fernandes, V.C.; Rodrigues, F.; Andrisano, V. Multi-Target Neuroprotective Effects of Herbal Medicines for Alzheimer’s Disease. J. Ethnopharmacol. 2022, 290, 115107. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.-C.; Jiang, J.-G. Effects of Diosgenin and Its Derivatives on Atherosclerosis. Food Funct. 2019, 10, 7022–7036. [Google Scholar] [CrossRef] [PubMed]
- El Bairi, K.; Ouzir, M.; Agnieszka, N.; Khalki, L. Anticancer Potential of Trigonella Foenum Graecum: Cellular and Molecular Targets. Biomed. Pharmacother. 2017, 90, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Alshatwi, A.A.; Shafi, G.; Hasan, T.N.; Syed, N.A.; Khoja, K.K. Fenugreek Induced Apoptosis in Breast Cancer MCF-7 Cells Mediated Independently by Fas Receptor Change. Asian Pac. J. Cancer Prev. 2013, 14, 5783–5788. [Google Scholar] [CrossRef] [PubMed]
- Allaoui, A.; Gascón, S.; Benomar, S.; Quero, J.; Osada, J.; Nasri, M.; Rodríguez-Yoldi, M.J.; Boualga, A. Protein Hydrolysates from Fenugreek (Trigonella Foenum Graecum) as Nutraceutical Molecules in Colon Cancer Treatment. Nutrients 2019, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Suresh, S.; Debnath, A.; Jha, S. Trigonella Seed Extract Ameliorates Inflammation via Regulation of the Inflammasome Adaptor Protein, ASC. Front. Biosci. 2017, 9, 246–257. [Google Scholar]
- Pradeep, S.R.; Srinivasan, K. Amelioration of Oxidative Stress by Dietary Fenugreek (Trigonella Foenum-Graecum L.) Seeds is Potentiated by Onion (Allium Cepa L.) in Streptozotocin-Induced Diabetic Rats. Appl. Physiol. Nutr. Metab. 2017, 42, 816–828. [Google Scholar] [CrossRef]
- Pradeep, S.R.; Srinivasan, K. Alleviation of Oxidative Stress-Mediated Nephropathy by Dietary Fenugreek (Trigonella Foenum-Graecum) Seeds and Onion (Allium Cepa) in Streptozotocin-Induced Diabetic Rats. Food Funct. 2018, 9, 134–148. [Google Scholar] [CrossRef]
- Hadi, A.; Arab, A.; Hajianfar, H.; Talaei, B.; Miraghajani, M.; Babajafari, S.; Marx, W.; Tavakoly, R. The Effect of Fenugreek Seed Supplementation on Serum Irisin Levels, Blood Pressure, and Liver and Kidney Function in Patients with Type 2 Diabetes Mellitus: A Parallel Randomized Clinical Trial. Complement. Ther. Med. 2020, 49, 102315. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic Effect of Fenugreek Seed Powder Solution (Trigonella Foenum-Graecum L.) on Hyperlipidemia in Diabetic Patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef]
- Najdi, R.A.; Hagras, M.M.; Kamel, F.O.; Magadmi, R.M. A Randomized Controlled Clinical Trial Evaluating the Effect of Trigonella Foenum-Graecum (Fenugreek) versus Glibenclamide in Patients with Diabetes. Afr. Health Sci. 2019, 19, 1594–1601. [Google Scholar] [CrossRef] [PubMed]
- Khanna, A.; John, F.; Das, S.; Thomas, J.; Rao, J.; Maliakel, B.; Im, K. Efficacy of a Novel Extract of Fenugreek Seeds in Alleviating Vasomotor Symptoms and Depression in Perimenopausal Women: A Randomized, Double-blinded, Placebo-controlled Study. J. Food Biochem. 2020, 44, e13507. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, A.; Galla, C.; Thummisetti, S.; Marikanty, R.K.; Palanisamy, U.D.; Rao, P.V. Role of Fenugreek in the Prevention of Type 2 Diabetes Mellitus in Prediabetes. J. Diabetes Metab. Disord. 2015, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Niphadkar, P.V.; Bapat, M.M. Allergy to Fenugreek (Trigonella Foenum Graecum). Ann. Allergy Asthma Immunol. 1997, 78, 297–300. [Google Scholar] [CrossRef]
- Joseph, N.I.; Slavin, E.; Peppers, B.P.; Hostoffer, R.W., Jr. Fenugreek Anaphylaxis in a Pediatric Patient. Allergy Rhinol. 2018, 9, 2152656718764134. [Google Scholar] [CrossRef]
- Fæste, C.K.; Namork, E.; Lindvik, H. Allergenicity and Antigenicity of Fenugreek (Trigonella Foenum-Graecum) Proteins in Foods. J. Allergy Clin. Immunol. 2009, 123, 187–194. [Google Scholar] [CrossRef]
- Aurich, S.; Spiric, J.; Engin, A.; Simon, J.C.; Mahler, V.; Treudler, R. Report of a Case of IgE-Mediated Anaphylaxis to Fenugreek. J. Investig. Allergol. Clin. Immunol. 2019, 29, 56–58. [Google Scholar] [CrossRef]
- Ohnuma, N.; Yamaguchi, E.; Kawakami, Y. Anaphylaxis to Curry Powder. Allergy 1998, 53, 452–454. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Mertens, M.H.; Stevens, W.J. Coriander Anaphylaxis in a Spice Grinder with Undetected Occupational Allergy. Acta Clin. Belg. 2006, 61, 152–156. [Google Scholar] [CrossRef]
- Bentele-Jaberg, N.; Guenova, E.; Mehra, T.; Nägeli, M.; Chang, Y.-T.; Cozzio, A.; French, L.E.; Hoetzenecker, W. The Phytotherapeutic Fenugreek as Trigger of Toxic Epidermal Necrolysis. Dermatology 2015, 231, 99–102. [Google Scholar] [CrossRef]
- Namork, E.; Fæste, C.K.; Stensby, B.A.; Egaas, E.; Løvik, M. Severe Allergic Reactions to Food in Norway: A Ten Year Survey of Cases Reported to the Food Allergy Register. Int. J. Environ. Res. Public Health 2011, 8, 3144–3155. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Luc, A.; Adam, T.; Jarlot-Chevaux, S.; Dumond, P.; Schweitzer, C.; Codreanu-Morel, F.; Divaret-Chauveau, A. Relevance of Sensitization to Legumes in Peanut-allergic Children. Pediatr. Allergy Immunol. 2022, 33, e13846. [Google Scholar] [CrossRef] [PubMed]
- Reese, I.; Schäfer, C.; Ballmer-Weber, B.; Beyer, K.; Dölle-Bierke, S.; van Dullemen, S.; Jappe, U.; Müller, S.; Schnadt, S.; Treudler, R. Vegan Diets from an Allergy Point of View–Position Paper of the DGAKI Working Group on Food Allergy. Allergol. Select 2023, 7, 57. [Google Scholar] [CrossRef] [PubMed]
- Fæste, C.K.; Christians, U.; Egaas, E.; Jonscher, K.R. Characterization of Potential Allergens in Fenugreek (Trigonella Foenum-Graecum) Using Patient Sera and MS-Based Proteomic Analysis. J. Proteom. 2010, 73, 1321–1333. [Google Scholar] [CrossRef]
- Vinje, N.E.; Namork, E.; Løvik, M. Anaphylactic Reactions in Mice with Fenugreek Allergy. Scand. J. Immunol. 2011, 74, 342–353. [Google Scholar] [CrossRef]
- Costa, J.; Silva, I.; Vicente, A.A.; Oliveira, M.B.P.P.; Mafra, I. Pistachio Nut Allergy: An Updated Overview. Crit. Rev. Food Sci. Nutr. 2019, 59, 546–562. [Google Scholar] [CrossRef]

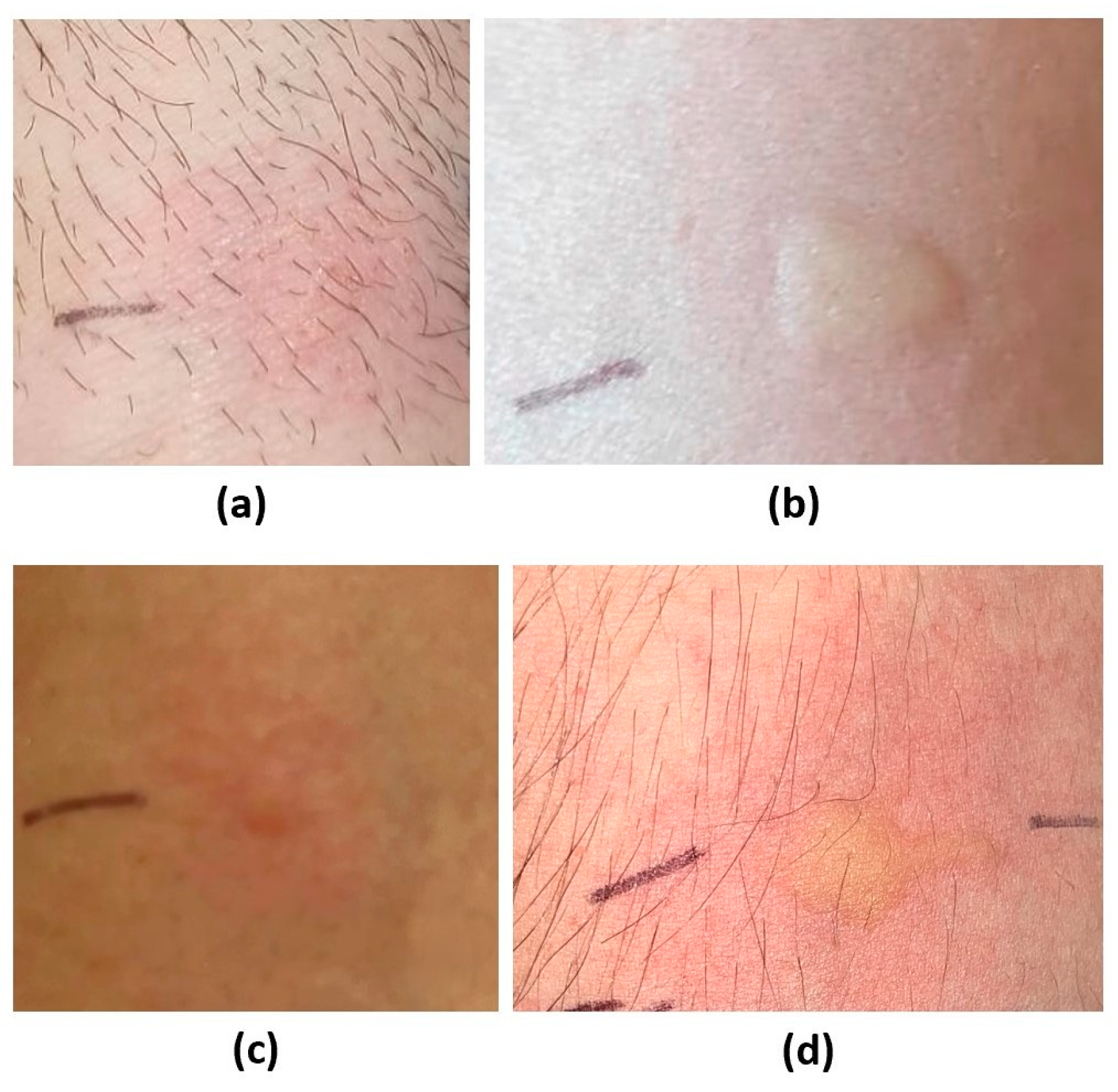
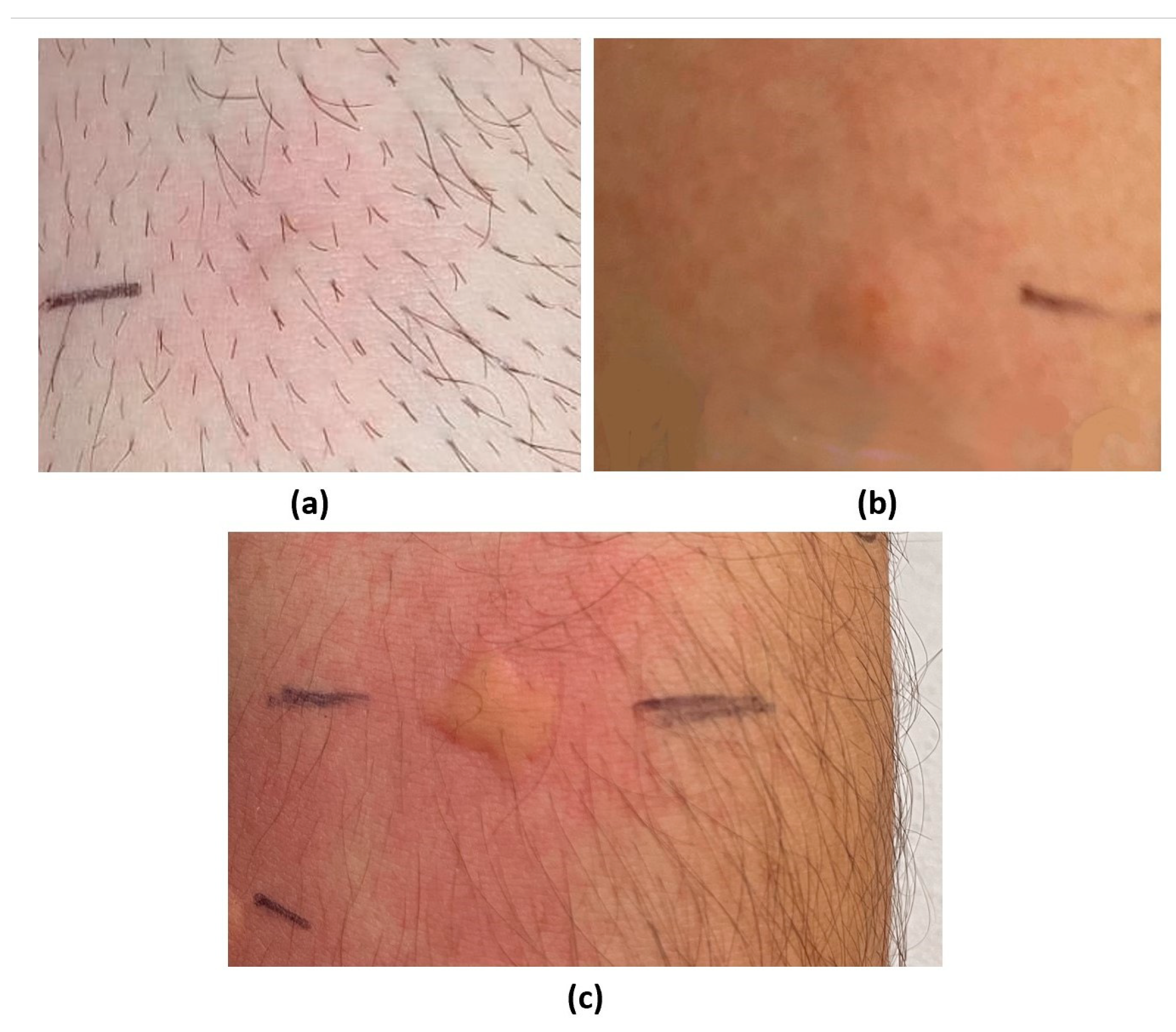
| Case series | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Foods | |||||||||||||
| Fenugreek | ++ | ++++ | ++ | ++ | ++ | ++ | ++ | ++ | ++++ | ++ | +++ | ++++ | ++++ |
| Cashew | - | ++ | <0.3 1 | - | - | - | ++ | - | N.T. 3 | - | - | - | ++ |
| Pistachio | +++ | ++ | - | ++ | ++ | + | +++ | +++ | - | ++++ | ++ | ++++ | ++++ |
| Peanut | - | +++ | + | + | +++ | +++ | - | ++ | + | +++ | - | +++ | + |
| Other legumes | - | Lupine | - | - | Lentil | Lentil, soy | - | Lentil, lupine | Lentil | Pea | - | Bean | - |
| Other nuts | - | - | - | - | Walnut, hazelnut almond | Hazelnut | Hazelnut | Walnut, hazelnut | - | Walnut, hazelnut | - | Hazelnut | Hazelnut, almond |
| Apiaceae | Cumin, fennel, celery | Cumin, fennel, celery, coriander | Cumin | Fennel, celery | Fennel | Fennel, celery | - | Fennel, celery | Cumin, fennel, coriander | Celery | Cumin, coriander | - | - |
| Mustard | - | - | - | N.T. 3 | N.T. 3 | + | - | +++ | + | <0.10 2 | - | - | - |
| Peach | - | - | - | N.T. 3 | +++ | ++++ | ++ | ++ | ++++ | ++ | +++ | ++++ | ++ |
| Inhalants | |||||||||||||
| Mugwort | +++ | ++ | ++ | +++ | +++ | ++ | ++++ | 9.91 2 | - | 0.86 2 | - | - | ++++ |
| Other inhalants | Plantain, cypress | Birch, plantain | Birch, plantain, cypress | - | - | - | - | Cypress | - | - | - | - | Cypress |
| Cases of Fenugreek Hypersensitivity | |||||||
|---|---|---|---|---|---|---|---|
| Author | Article Type | Year | Age | Sex | Symptoms Associated with Fenugreek | Previous Adverse Reactions | Other Cosensitizations |
| Patil SP [35] | Clinical trial | 1997 | 36 | F | Sneezing, rhinorrhea, tearing, coughing, and wheezing after smelling. | Chickpeas ingestion. | / |
| 45 | F | Nasal obstruction, hoarseness, angioedema, and wheezing after cutaneous contact. | Fenugreek ingestion. | / | |||
| Joseph NI [36] | Case report | 2018 | 14 | M | Urticaria, chest tightness, abdominal pain, and emesis after ingestion. | Fava bean and lentils ingestion. | / |
| Faeste CK [37] | Clinical study | 2009 | 11 | M | Anaphylaxis after the ingestion of curry mix. | / | Peanuts, lupin, pea, hazelnut, and almond. |
| 12 | M | Anaphylaxis after eating prepacked Indian food. | / | Peanuts, lupin, pea, soy, hazelnut, and almond. | |||
| Che CT [19] | Case report | 2017 | 14 | M | Itching and lip tingling followed after several hours by chest heaviness, urticaria, and wheezing after ingestion of curry. | Peanuts, lentils, chickpea and peas ingestion. | Peanuts, Ara h2, lentil, chickpea and pea. |
| Aurich S [38] | Case report | 2019 | 34 | F | Flushing, angioedema, dyspnea, nausea, vomiting, and diarrhea after Chinese vegetable soup. Second similar episode after spicy sausage. | Peanuts. | Timothy grass, mugwort, and peanut, Ara h 1, Ara h 2, Ara h 3, Art v 3, and fenugreek seeds. |
| Ohnuma N [39] | Case report | 1998 | 26 | F | Itching, diarrhea and wheezing after curry ingestion | / | Wheat, apples, rice, peanuts, almond, and onions. |
| Ebo DG [40] | Case report | 2006 | 25 | M | Urticaria, conjunctivitis, oropharyngeal angioedema, and bronchospasm after eating pita bread. | Coriander and fenugreek after contact and inhalation at work. | Coriander. |
| Bentele-Jaberg N [41] | Case report | 2015 | 32 | F | Fever, headache and exanthema evolving towards TEN after regular ingestion of an herbal preparation made of pure fenugreek seeds to improve lactation. | / | / |
| Cases of fenugreek hypersensitivity in patients with peanuts allergy | |||||||
| Author | Article type | Year | Outcomes | ||||
| Namork E [42] | Retrospective study | 2011 | The Norwegian Food Allergy Register in 2000–2010 has received 877 reports, of which patients with a known peanut allergy had allergic reactions after spicy sauces and Indian dishes (the reactions were found to be caused by fenugreek seeds, commonly used in curry and other mixed spices). | ||||
| Muller T [43] | Retrospective study | 2022 | A total of 195 children allergic to peanuts showed simultaneous sensitization to at least one other legume (fenugreek, soy, lupin and lentil; n = 122–69.7%); 10% of them were allergic to fenugreek. Main sensitizations were to fenugreek, followed by lentil, soy, pea, lupine, chickpea, broad bean, and bean. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alessandrello, C.; Sanfilippo, S.; Gangemi, S.; Pioggia, G.; Minciullo, P.L. Fenugreek: New Therapeutic Resource or Emerging Allergen? Appl. Sci. 2024, 14, 9195. https://doi.org/10.3390/app14209195
Alessandrello C, Sanfilippo S, Gangemi S, Pioggia G, Minciullo PL. Fenugreek: New Therapeutic Resource or Emerging Allergen? Applied Sciences. 2024; 14(20):9195. https://doi.org/10.3390/app14209195
Chicago/Turabian StyleAlessandrello, Clara, Serena Sanfilippo, Sebastiano Gangemi, Giovanni Pioggia, and Paola Lucia Minciullo. 2024. "Fenugreek: New Therapeutic Resource or Emerging Allergen?" Applied Sciences 14, no. 20: 9195. https://doi.org/10.3390/app14209195









