Abstract
Dye-sensitized solar cells (DSSCs) are a type of thin-film solar cell that has been extensively studied for more than two decades due to their low manufacturing cost, flexibility and ability to operate under low-light conditions. However, there are some challenges that need to be addressed, such as energy losses, material integration, weak photocurrent generation and stability, to enhance the performance of DSSCs. One of the approaches to enhance the performance of DSSCs is the use of luminescent materials. These are materials that can absorb light and re-emit at different wavelengths, allowing the conversion of ultraviolet (UV) and near-infrared (NIR) light, which DSSCs do not efficiently utilize, into visible light that can be absorbed. The main objective of this article is to provide an in-depth review of the impact of luminescent materials in DSSCs. Research interest on luminescent materials, particularly down conversion, up-conversion and quantum dots, was analyzed using data from the “Web of Science”. It revealed a remarkable number of over 200,000 publications in the past decade. Therefore, the state of the art of luminescent materials for enhancing the performance of the solar cells was reviewed, which showed significant potential in enhancing the performance of DSSCs.
1. Introduction
The growing energy demand, coupled with the concerns about climate change and environmental degradation due to the burning of fossil fuels, has increased the urgent need for sustainable and renewable energy sources. Renewable energy is acquired from sources that are constantly replenishable by natural elements like sunlight, wind, water, etc. Solar energy, being abundant and clean, stands out a pivotal component of the future energy landscape. The primary advantage about solar energy is that it does not lead to environmental pollution. Different photovoltaic (PV) technologies such as monocrystalline silicon solar cells, polycrystalline silicon solar cells, Copper Indium Gallium Selenide (CIGS), amorphous silicon solar cells, Cadmium Telluride (CdTe) [1,2,3,4,5], etc., have garnered significant attention in recent years as a promising solution to address climate change and reduce the reliance on fossil fuels. However, the high production and deployment costs associated with PV cell technologies have posed a significant barrier to their widespread adoption. These costs can be attributed to the materials and manufacturing processes involved in their fabrication. For instance, the high purity silicon required for PV cell production is a costly material, and the manufacturing processes involved in creating PV cells can be complex and capital-intensive. In comparison, dye-sensitized solar cells (DSSCs) offer unique advantages such as low production costs, flexibility and the ability to perform under low-light conditions, making them particularly suitable for integrated and flexible applications [6,7,8].
DSSCs represent a class of third-generation solar cells that have attracted significant attention due to their potential for cost-effective and flexible photovoltaic applications. First introduced by Michael Grätzel and Brian O’Regan in 1991, DSSCs have been recognized for their ability to efficiently convert sunlight into electrical energy using a mechanism that mimics photosynthesis [7]. Unlike conventional silicon-based solar cells, DSSCs utilize organic dye molecules to capture light and generate excitons, which are then converted into an electrical current through a series of electrochemical processes [8]. DSSCs operate on a fundamentally different principle compared to traditional photovoltaic cells. They are composed of a photo-anode, typically made from titanium dioxide (TiO2) and coated with a layer of light-absorbing dye molecules. The photo-anode receives a few drops of an electrolyte solution containing a redox mediator, usually an iodide/tri-iodide couple, and paired with a counter electrode, often made of platinum or carbon-based materials [9]. The unique structure and working mechanism of DSSCs enable them to achieve high efficiencies under diverse lighting conditions, paving the way for their use in various innovative applications such as off-grid power systems and the powering of portable device like cell phones [10].
The journey of DSSCs began with fundamental research into photo-electrochemical cells in the 1960s and 1970s. Early studies explored the potential of various semiconductor materials and dye molecules for light-induced electron transfer processes. It was not until 1991 that Grätzel and O’Regan successfully demonstrated a practical DSSC with significant efficiency, marking a breakthrough in the field [7,10]. Since the pioneering work of Grätzel and O’Regan, the development of DSSCs has seen numerous milestones. The key advancements include the introduction of new dye sensitizers with broader absorption spectra, the optimization of TiO2 nanoparticle films for improved electron transport, and the development of more stable and efficient electrolytes. These milestones have collectively contributed to a gradual improvement in the performance and stability of DSSCs, with some laboratory-scale cells achieving efficiencies of about 14% [11]. Today, DSSCs are recognized as a mature technology with several commercial applications. They are used in various niche markets such as portable electronics (powering of cell phones and cameras), building-integrated photovoltaics (BIPV), and off-grid power systems. However, despite their advantages, DSSCs still face several challenges and gaps that hinder their widespread adoption and commercialization. One of the main challenges is the stability of the dye used in these cells, as many organic dyes degrade rapidly under prolonged exposure to sunlight, limiting the cell’s lifespan and efficiency [12,13]. Additionally, the low conversion efficiency of DSSCs compared to other types of solar cells, such as silicon-based solar cells, remains a major gap that needs to be addressed to enhance the advancement of DSSCs in the market [14,15]. Another significant challenge of DSSCs is their scalability and manufacturing processes [16,17]. The complex and delicate nature of assembling DSSCs makes mass production difficult and costly, hindering their commercial viability [18,19].
Due to the problem of the efficiency and stability of the DSSCs, ongoing research continues to focus on addressing these challenges and exploring new materials and cell architectures to further improve performance [20]. The performance of conventional DSSCs is limited by factors such as narrow absorption spectra and charge recombination losses, which hamper the device’s performance. The energy loss in the device encompasses two ways: the first is the charge carrier recombination, which occurs during the electron transport that leads to photoelectron loss, thereby generating a weak photocurrent. The second is the spectral mismatch between the solar irradiation and the dye, which leads to the thermalization of the device by photons of high energy. Current efforts to improve the DSSC performance focus on engineering the nanostructured photo-anode” with luminescent materials [21,22,23,24,25,26,27,28].
The need to develop strong luminescent materials and approaches to improve the DSSCs’ performance is crucial [29,30,31,32,33,34,35,36]. They have good absorption, especially in the UV and NIR wavelengths where the DSSC has a low absorption of light, and they emit visible wavelengths, where the dye sensitizers have effective light absorption [37,38,39,40]. The emitted photons can be re-absorbed by the DSSC device to enhance the light harvesting efficiency of the cells. Basically, there is a gap in research concerning the exploration of alternative luminescent nanomaterials that can extend the absorption spectrum of DSSCs into the UV and near-infrared (NIR) ranges while simultaneously improving charge carrier dynamics and reducing recombination losses. The commonly used dye sensitizers have absorption band ranging from around 400–600 nm, which shows that photons <400 nm and >600 nm are not absorbed by the dye-sensitizers. The need to extend the absorption spectrum of the device to the UV and NIR is crucial to address such spectral mismatch in order to convert the incidence photons in such regions into usable energy [41,42,43,44]. In this review, three luminescent materials, namely down-conversion, up-conversion and quantum dots were elucidated, each contributing to the optimization of the DSSCs due to the good optical properties and wide absorption spectrum in the region where the DSSC has a weak spectral responsivity. The article is composed of the following sections: Section 1 introduces the paper. Section 2 elaborates the fundamental principles of DSSC. Section 3 highlights the methods. The luminescent materials in DSSCs are deliberated in Section 4. Section 5 expounds the discussion based on challenges and recommendations of the luminescent materials in DSSCs. Finally, Section 6 concludes the paper.
2. Fundamental Principles
2.1. Working Operation
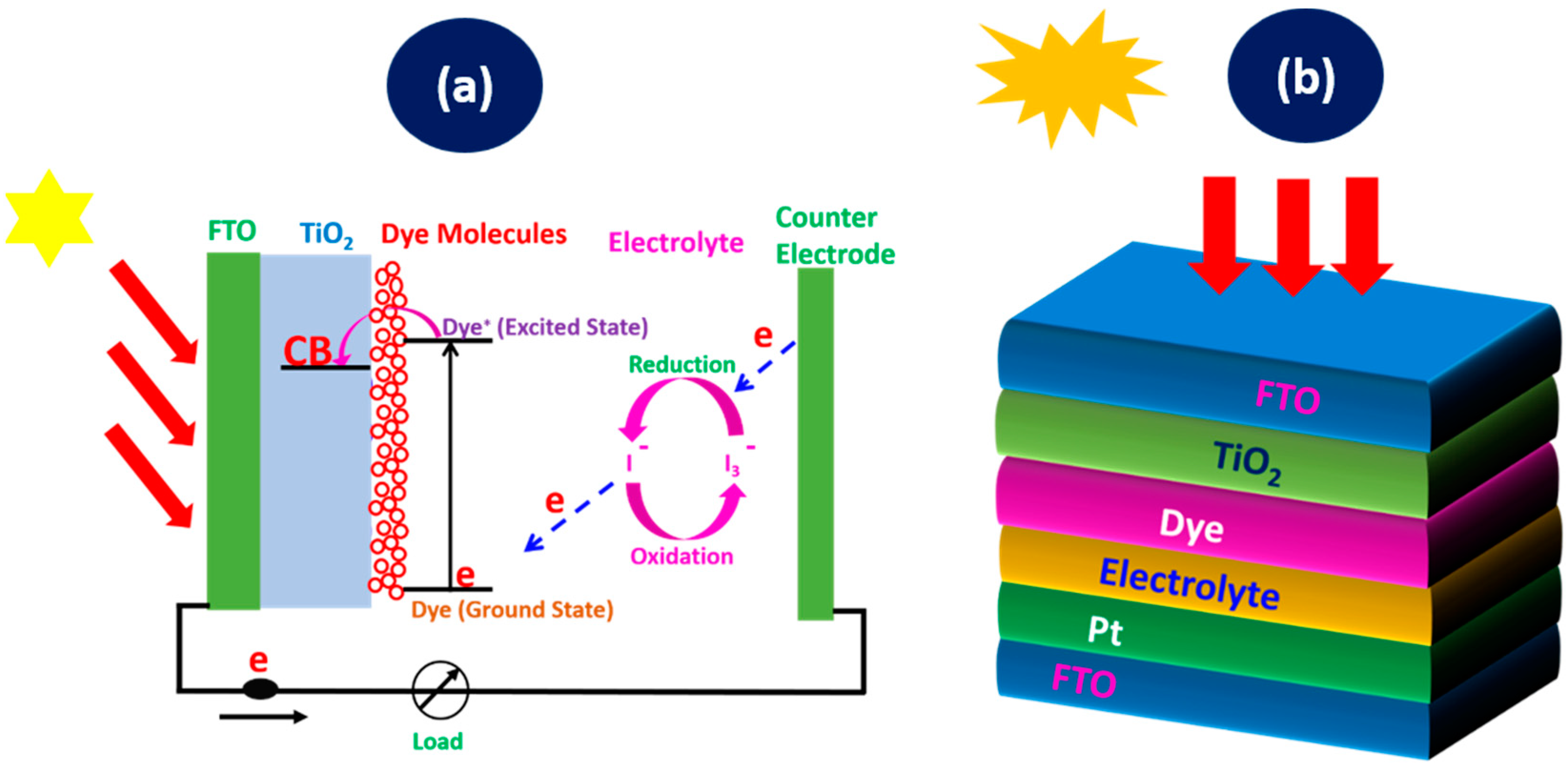
DSSCs consist of several key components such as a photo-anode, a dye sensitizer, an electrolyte, and a counter electrode. The photo-anode is composed of fluorine-doped tin oxide (FTO), coated with a mesoporous semiconductor layer, such as titanium dioxide (TiO2), which provides a sufficient surface area for the dye molecules to be adsorbed. The operation of DSSCs relies on a series of electrochemical processes that collectively convert light into electricity. When sunlight strikes the dye molecules adsorbed on the surface of the TiO2 photo-anode, it excites electrons to a higher energy state. These excited electrons are rapidly injected into the conduction band (CB) of the TiO₂, where they diffuse through the nanoporous structure to the back contact and eventually flow through the external circuit, generating an electric current [45]. Meanwhile, the oxidized dye molecules are regenerated by electrons from the electrolyte, which undergoes a redox reaction. The iodide ions (I−) in the electrolyte donate electrons to the oxidized dye, converting back to tri-iodide ions (), which diffuse to the counter electrode. At the counter electrode, electrons from the external circuit reduce the back to I−, completing the cycle. The working operation is presented in Figure 1a. The seamless flow of electrons through the various components of the DSSC forms the basis of its photovoltaic operation [46,47]. The performance of DSSC relies on factors such as the sufficient surface area of the coated TiO2 for dye adsorption, effective absorption of light by the dye, the efficient injection of electrons into the TiO2, and the rapid regeneration of the dye and electrolyte. It can be fabricated on flexible substrates and operate efficiently under low-light conditions, making it suitable for both indoor and outdoor applications.
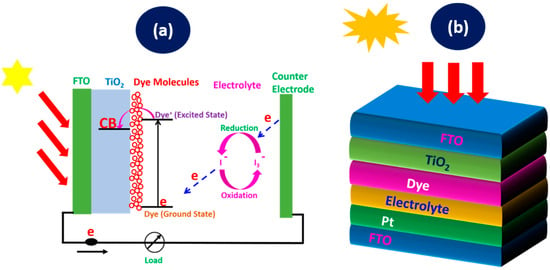
Figure 1.
(a) Working operation of a DSSC and (b) DSSC schematic diagram of the layers.
2.2. Photovoltaic Effect in DSSCs
The photovoltaic effect in DSSCs is driven by the absorption of photons by the dye sensitizer and the subsequent injection of electrons into the TiO₂. The excited electrons with a lifetime within a nanosecond range are injected into the conduction band of the TiO2 semiconductor, which lies below the excited state of the dye where the TiO2 absorbs a small fraction of the solar photons from the UV region [48]. This is accompanied by the electron transport through the external circuit to the counter electrode. Then, the dye undergoes regeneration, where the excited dye returns to the ground state, and, finally, the electrolyte undergoes regeneration, where the is reduced to the I−. The schematic diagram in Figure 1b shows the representation of the assembled DSSCs, which encompasses the dye molecules adsorbed into the TiO2 and coated on the transparent conductive oxide glass, like the FTO. The dye molecules harvest photons and yield the excited electrons from its highest occupied molecular orbital (HOMO) in the ground state to the lowest unoccupied molecular orbital (LUMO) in the excited state. The electrolyte is for redox mediation and the platinum coated on the FTO to provide counter electrode. The processes involved in the working operation can be broken down into various key steps:
- I.
- Light Absorption: The dye molecules absorb incident photons, leading to their excitation:The excited has an electron promoted to a higher energy state.
- II.
- Electron Injection: The excited electron is injected into the conduction band of TiO2:This leaves the dye in an oxidized state (Dye⁺).
- III.
- Electron Transport: The injected electrons diffuse through the TiO2 nanoparticles to the fluorine-doped tin oxide (FTO) layer and flow through an external circuit, generating electrical power:Electrons travel through the TiO2, move to the FTO, flow through the external circuit, and finally reach the counter electrode.
- IV.
- Dye Regeneration: The oxidized dye is reduced back to its ground state by the redox mediator in the electrolyte:
- V.
- Electrolyte Regeneration: The tri-iodide () formed is reduced back to iodide ions (I−) through gaining electrons at the counter electrode:
This completes the cycle, allowing continuous operation of the cell. These steps collectively result in the generation of electrical power, with the efficiency of the DSSC being influenced by factors such as the light absorption properties of the dye, the electron transport efficiency in the TiO2 and the regeneration kinetics of the redox mediator [47].
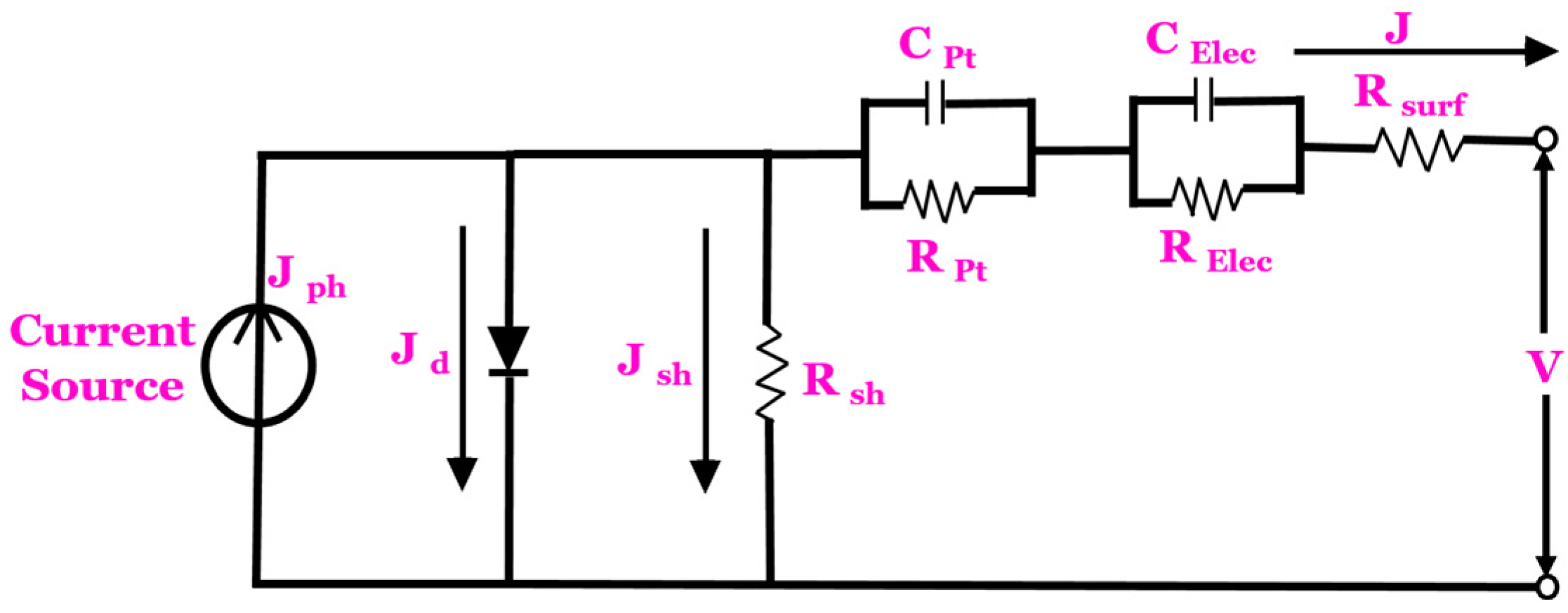
2.3. Equivalent Circuit Model of DSSCs
The equivalent circuit of the DSSC consists of the current source, diode, capacitances, series resistance and the shunt resistance . “The current source creates the photo-generated current density parallel to the diode, which is proportional to the sunlight irradiation. The diode represents the interface between the dye-coated semiconductor (the TiO2) and the electrolyte. It accounts for the non-linear behaviour at this interface and is typically represented by a diode equation. The series resistance accounts for the resistance encountered by the flow of electrons within the device’s components, including the TiO2 layer, conducting substrates and contacts. The shunt resistance represents any unintended paths (e.g., leakage currents) that allow the current to bypass the photovoltaic cell, reducing overall efficiency. The is the surface resistance. The and are the impedance through carrier transport on the surface of the counter electrode. The and are the impedance to carrier transport through the ions in the electrolyte with regard to the dye–electrolyte interface.
The equivalent circuit model of the DSSC is shown in Figure 2. The capacitance element can be neglected since the capacitance at the dye–electrolyte interface tends to be relatively small compared to other factors affecting the performance of the device. Kirchhoff’s current law is used and expressed in Equation (1).
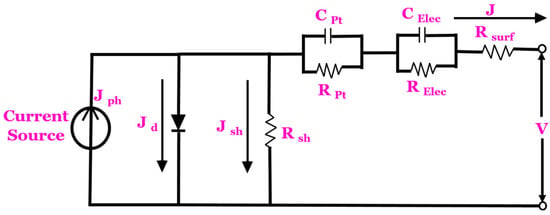
Figure 2.
Equivalent circuit model of a DSSC.
The current density flowing through the diode is governed by
Equation (3) represents the current flowing through the shunt resistance. The Rsurf, RElec and Rpt are resolved as .
The output current density (J) in the circuit can be expressed as
The short-circuit current density in a dye-sensitized solar cell (DSSC) represents the maximum current flowing through the cell when the voltage across it is zero (short-circuited). This current is solely due to the photo-generated carriers in the cell and is not influenced by the external load resistance (external circuit is removed). The expression for the Jsc in a DSSC can be derived from the diode equation itself under the condition that the voltage across the cell is zero. Hence, the photo-generated current density becomes equal to the Jsc.
The open-circuit voltage of a dye-sensitized solar cell (DSSC) is the voltage across the terminals of the cell when no external load is connected, i.e., when the J flowing through the cell is zero. In the equivalent circuit model of a DSSC, the open-circuit condition occurs when the J is zero, meaning that the diode equation simplifies to
The fill factor (FF) quantifies how closely the solar cell’s (J-V) curve approaches the rectangular shape of an ideal diode’s J-V curve. A higher fill factor indicates a more efficient conversion of light into electrical power and a better utilization of the cell’s active area. It is defined as the ratio of the maximum power output of the solar cell to the product of the and .
The efficiency (η) of the DSSC is an expression that measures how effectively the DSSC converts incident sunlight into usable electrical power and is commonly used to evaluate the performance of DSSCs. The expression is given as
where
Isun = the intensity of the sunlight and A = the active surface area of the DSSC.
3. Methods
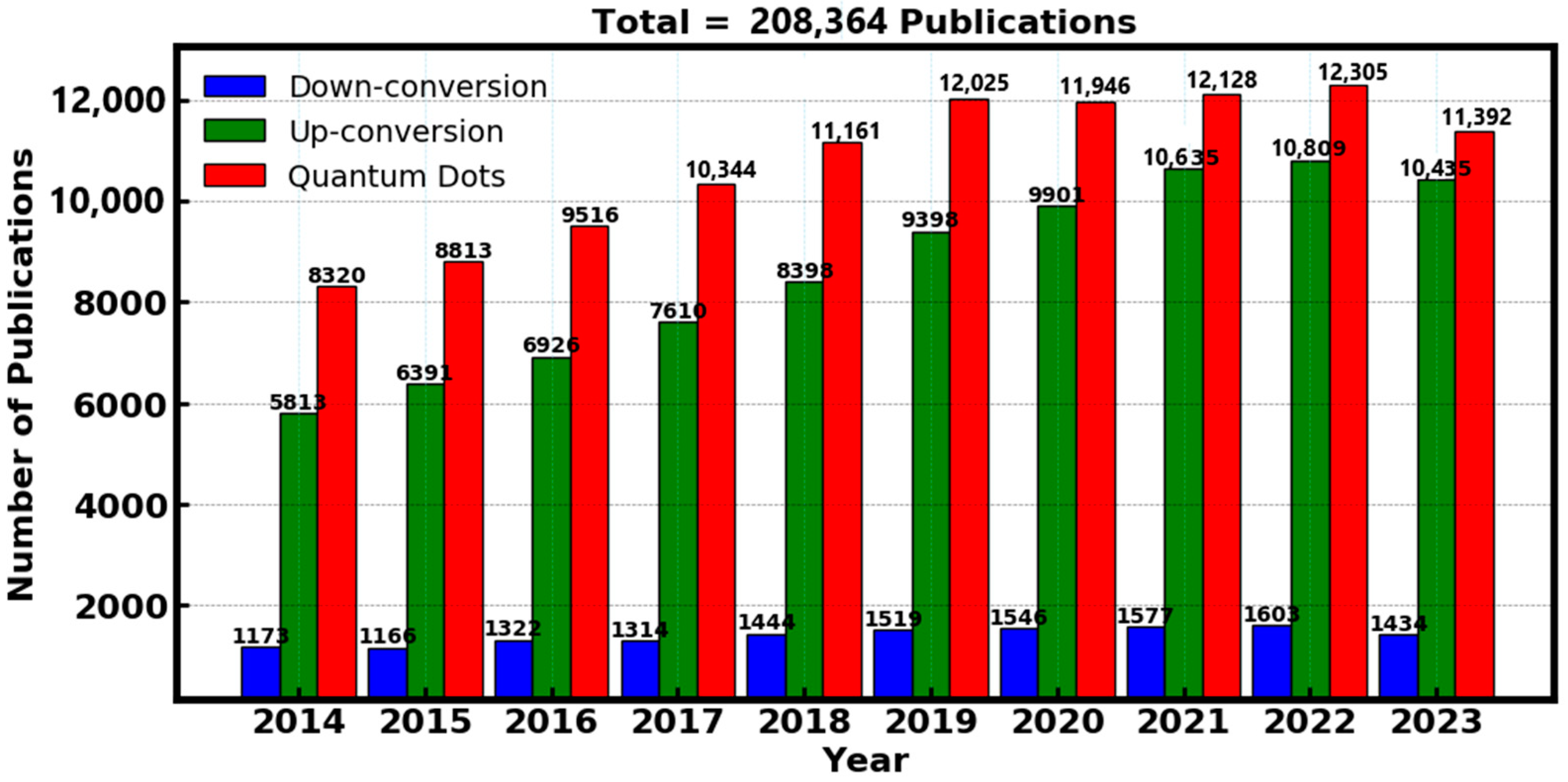
To find prominent scientific data on luminescent materials, a search was conducted on the “Web of Science” database, which was selected due to its simplicity of undertaking a methodological assessment of the literature and gives access to research articles and journals from prestigious academic publishers. The focus of the search was on a careful examination of the literature released a decade ago to ensure the study’s relevance and incorporation of the most recent materials. Particular keywords were entered such as “down conversion”, “up conversion” and “quantum dots” in order to conduct the search. The outcome of the data was analyzed in-terms of the number of publications with respect to the corresponding year, which showed a remarkable research output of the luminescent materials over a decade ago. However, the advantage of luminescent materials for various applications paved the way for high research interest, which has garnered a significant increase of over 200,000 publications in the past decade as shown in Figure 3, specifically the down-conversion, up-conversion and quantum dots materials.
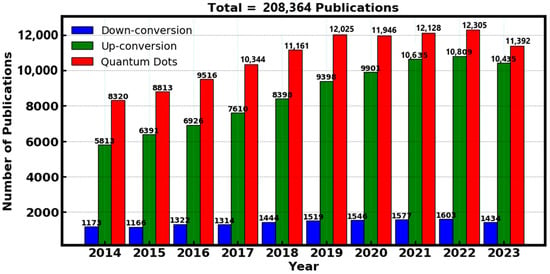
Figure 3.
Analysis of the number of publications related to down-conversion, up-conversion and quantum dots in the past decade. Data obtained from the “Web of Science”.
4. Luminescent Materials in DSSCs
Luminescent phosphors are materials that can absorb light and re-emit it at different wavelengths. These materials can be used to convert UV and NIR light, which are not effectively utilized by DSSCs, into visible light that can be absorbed by the dye sensitizer. They include down-conversion and up-conversion materials and quantum dots, each contributing uniquely to enhancing DSSC efficiency.
4.1. Up-Conversion
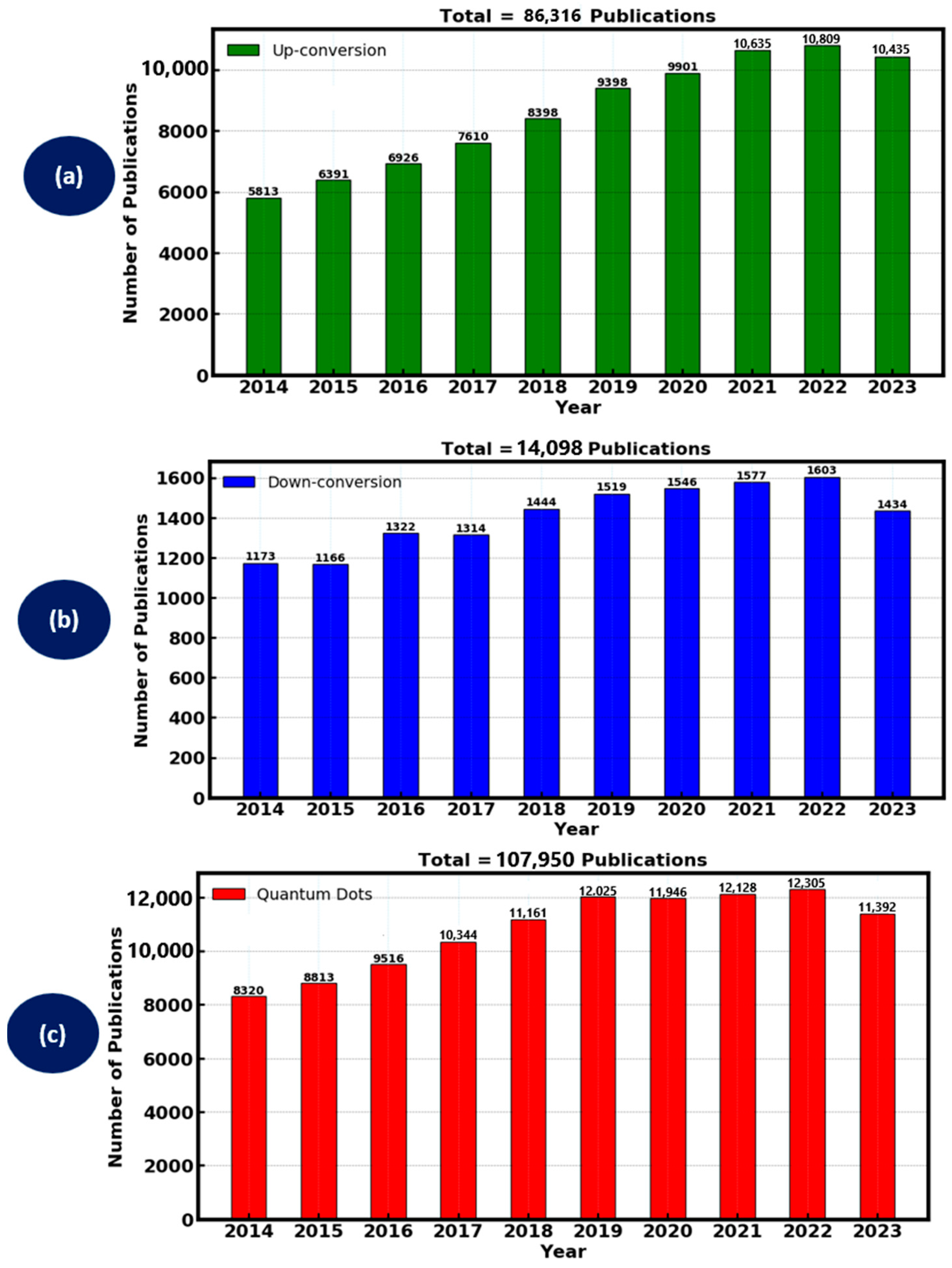
Up-conversion materials absorb two or more lower-energy photons and emit a single higher-energy photon. The research interest of up-conversion materials in the past decade from the Web of Science is depicted in Figure 4a. The year 2022 had the most research interest, followed by 2021 and 2023, with a nearly similar research interest. In the recent decade, up-conversion materials yielded a total of 86,316 publications. However, the properties of up-conversion luminescent material are advantageous for DSSCs as it allows the conversion of near-infrared (NIR) light, which is not typically absorbed by the dye, into visible light that can be used more effectively. Lanthanide-doped nanoparticles, such as those containing Yb3+ and Er3+ ions, are commonly used for their efficient up-conversion capabilities due to their desirable photon modulation properties of converting photons of NIR wavelengths to visible wavelengths so that the DSSC can convert them into usable electrical energy. These materials can be synthesized most often by hydrothermal synthesis and sol–gel processes. The up-conversion process involves energy transfer between dopant ions within a host lattice, typically through sequential absorption or energy transfer up-conversion (ETU) mechanisms. In DSSCs, up-conversion materials are typically integrated into the photo-anode or incorporated into the dye-sensitizer. For instance, embedding NaYF4:Yb3+ and Er3+ up-conversion nanoparticles into TiO2 photo-anodes has been shown to improve light absorption in the NIR region, thereby enhancing photocurrent and overall cell efficiency. This mechanism extends the range of usable solar spectrum, addressing the limitations of traditional dyes, which primarily absorb in the visible range [49].
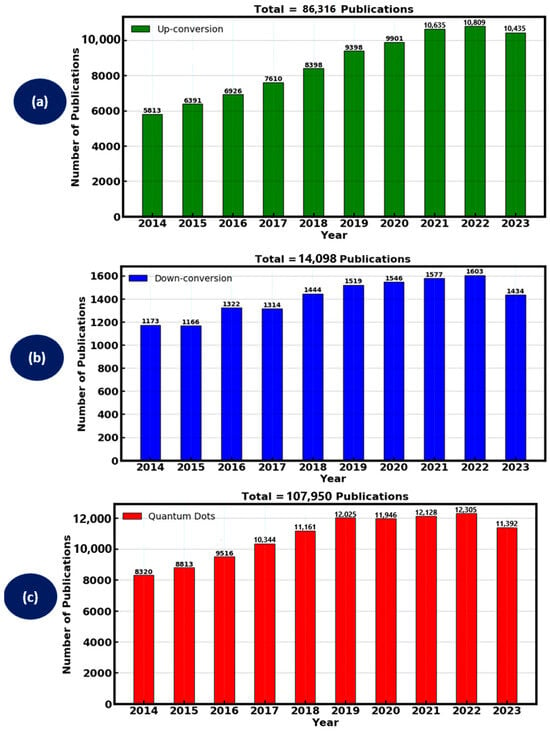
Figure 4.
Luminescent materials research interest in the past decade (a) up-conversion, (b) down-conversion and (c) Quantum dots.
4.2. Down-Conversion
Down-conversion materials absorb high-energy photons and emit two or more lower-energy photons. Figure 4b shows the research interest of down-conversion materials in the past decade, with 2022 having the highest research interest, followed by 2021 and 2020. The total research interest for the material produced 14,098 publications in the past decade. The process of down-conversion luminescent materials allows the conversion of ultraviolet (UV) light, which can degrade organic components in DSSCs, into visible light that is more suitable for energy conversion. Common down-conversion materials include phosphors doped with rare-earth elements like Eu3+ and Tb3+, due to their properties like a wide absorption spectrum in the UV region and the conversion of high energy photons into photons of lower energy to foster sufficient photon absorption and charge carrier generation. Synthesis methods such as solid-state reactions and combustion synthesis are often used to produce these materials. The incorporation of down-conversion phosphors into DSSCs can be achieved by coating the surface of the photo-anode or mixing them with the dye. For example, integrating Gd2O3: Eu3+ down-conversion phosphors with TiO2 photo-anodes has demonstrated improved light harvesting and reduced UV-induced degradation, thereby enhancing both the efficiency and stability of the DSSCs [50].
4.3. Quantum Dots
Quantum dots (QDs) are semiconductor nanoparticles with size-tunable optical properties, allowing them to absorb light over a broad spectrum and emit it at specific wavelengths [51]. Figure 4c shows the research interest of the quantum dots luminescent materials in the past decade. The highest research interest was achieved in 2022, followed by 2021 and 2019. A slightly similar research interest was achieved in 2019 and 2020. Overall research interest yielded 107,950 publications in the past decade for quantum dots. Materials such as CdSe, CdTe and PbS quantum dots have been extensively studied for their potential to enhance DSSC performance due to their tunable emission properties, good quantum yield and broad photon absorption spectrum. Quantum dots are commonly synthesized using colloidal methods, vapour phase synthesis or molecular beam epitaxy and can be integrated into DSSCs by embedding in the photo-anode or mixing with the dye. The primary advantage of quantum dots lies in their high absorption coefficients and potential for multiple exciton generation, which can lead to higher photocurrents and enhanced efficiency.
Generally, up-conversion materials are particularly beneficial for utilizing NIR light, while down-conversion materials enhance the use of UV light, and quantum dots offer broad-spectrum absorption. As elucidated above, up-conversion produced a total of 86,316 publications, down-conversion gave 14,098 publications and quantum dots produced 107,950 publications. These created a remarkable research interest of 208,364 publications all combined in the past decade. Each material type also presents unique challenges, such as the stability of quantum dots and the need for precise synthesis and doping in up-conversion and down-conversion materials.
4.4. Advances and Research
It is possible to improve the efficiency of DSSC using luminescent materials [52,53]. This allows absorbing light in the UV and NIR range, then converting to visible light within the absorption range of the dye sensitizer. Luminescent materials also improve the stability of DSSCs because it prevents the thermal degradation of the dye or the electrolyte due to the energy of the UV light. Wang et al. [54] presented a simple oxalate-assisted hydrothermal method using oxalate assistance to produce nano- and micro-sized β-NaYF4: Er3, Yb3+ (NYFEY) powders with diverse morphologies, which can be applied in DSSCs. The hexagonal rod-shaped NYFEY produced good luminescent properties with a photovoltaic efficiency of 7.31%. Hence, the addition of the hexagonal rod-like NYFEY particles to the photo-anode, improved the performance of DSSCs when compared to those made solely with TiO2 nanoparticles that yielded 5.63% efficiency. This is because the improved photo-anode better absorbs light, therefore increasing the light-harvesting efficiency.
Research by Luo et al. [55] demonstrated an efficient spray pyrolysis-assisted gas-phase aerosol technique to synthesize CaAl2O4:Eu2+, Nd3+ phosphor particles. They successfully doped the CaAl2O4 matrix particles with Eu2+ and Nd3+ after an additional H2 reduction step. With the prominent emission peak observed at a wavelength of around 440 nm, it was discovered through luminescent emission spectrum measurement that the CaAl2O4:Eu2+, Nd3+ phosphor particles created in the study played a crucial role in down-converting the UV radiation into visible blue light. Using DSSC photo-electrodes, they created TiO2 pastes with different phosphor fractions to study the impact of the CaAl2O4:Eu2+, Nd3+ phosphors on photovoltaic performance. With an increase in the CaAl2O4:Eu2+, Nd3+ particle contents (≤5 wt%) in the TiO2 matrix, they observed that the and power conversion efficiency (PCE) of the DSSCs also increased. The photovoltaic performance of the DSSC was shown to be negatively impacted by the addition of an excessive amount of CaAl2O4:Eu2+, Nd3+ phosphors (>5 wt%) to the TiO2 matrix. The negative impact resulted from the faster recombination of photo-generated electrons with holes and the increase in grains and interfaces brought on by the increased phosphor doping, which further perturbed the charge carrier transport, thereby leading to a decrease in the efficiency. Therefore, based upon their research, it was possible to increase the DSSC PCE to 6.61% by adding 5 wt% of the CaAl2O4:Eu2+, Nd3+ phosphors to the TiO2 matrix, but the concentration of the phosphor beyond 5 wt% yielded a negative impact, which decreased the efficiency of the cell to 5.92% and 4.98% for 10 wt% and 20 wt%, respectively.
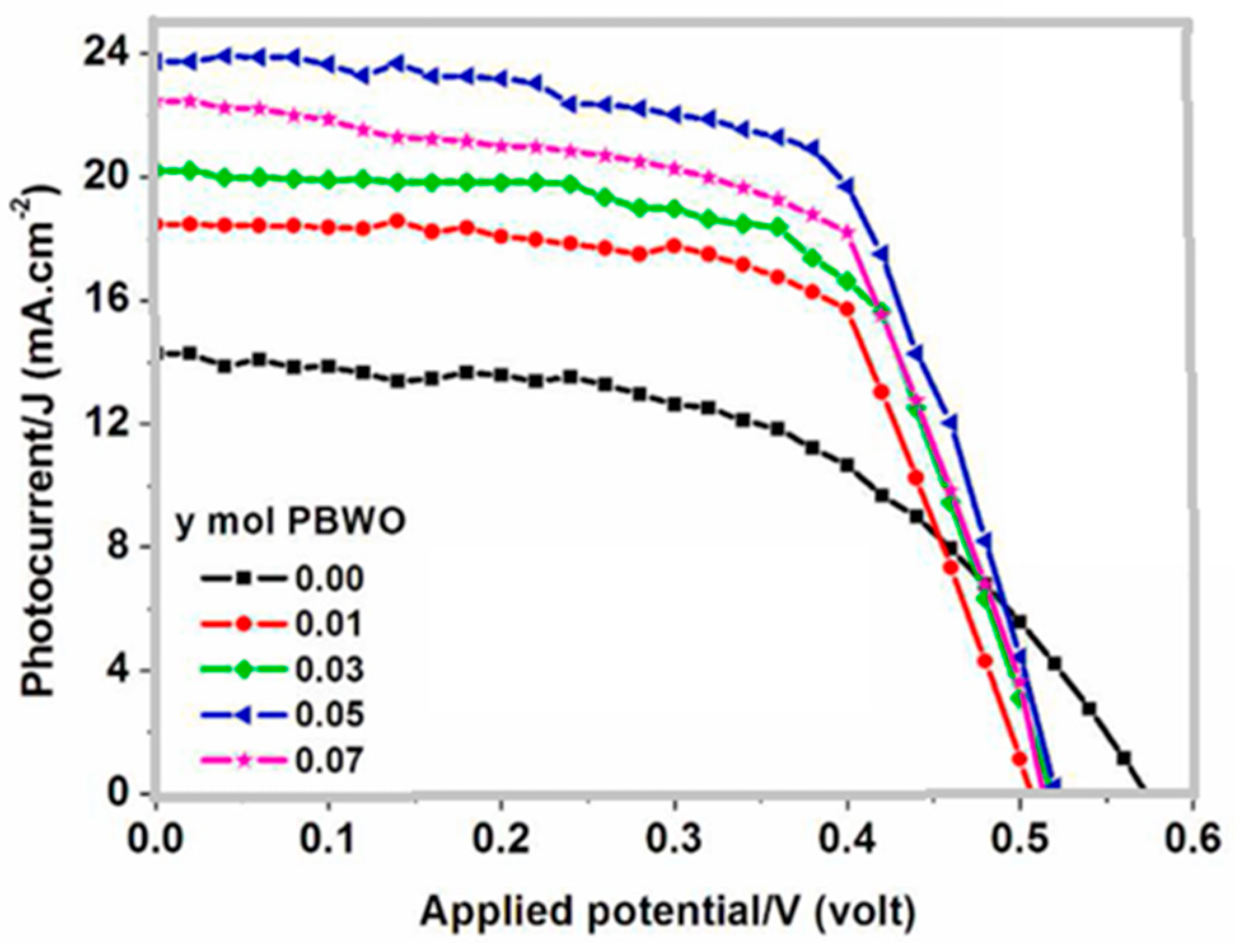
Kamal R. and Hafez H. [56] prepared a novel down-converting single-phase white light Pr3+-doped BaWO4 nanophosphor materials for DSSC applications. The material was synthesized by the hydrothermal ultrasonic-assisted method. The X-ray diffraction (XRD) provided a confirmation of the tetragonal phase. A cubic structure measuring 10 nm in average length and 8 nm in average width was revealed by the transmission electron microscope (TEM) measurement. The values of the optical band gap increased as the Pr3+ ion concentration increased. Three primary emission peaks were observed in the photo-luminescent spectra, which span the white region attributed to the 3P0 → 3F2, 3P1 → 3H5 and 3P0 → 3H4 transitions. When applied as down-converting photoactive electrodes in DSSCs, the overlaid PBWO/TiO2 photoactive anodes demonstrated a notable improvement in the total power conversion efficiency of the developed solar cell to 8.08% for a doping concentration of y = 5 mol % of the Pr3+, as shown in Figure 5. It was shown that the Pr3+ significantly boosted the efficiency of DSSCs. The general photovoltaic performance with and without the down-converting material is presented in Table 1. An asterisk (*) is used to denote the device with the highest efficiency.
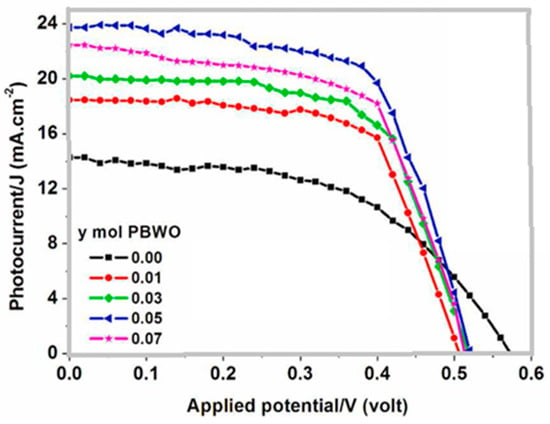
Figure 5.
Photocurrent–voltage (J–V) curves of the DSSC based PBWO/TiO2 down-converting photoactive electrodes [56].

Table 1.
Photovoltaic characteristics of DSSCs based on the different PBWO/TiO2 down-converting photoactive electrodes [56].
Research by Mao et al. [57] proposed a promising new type of up-conversion phosphors (UCPs)-TiO2 composite photo-anode for increased light harvesting in DSSCs. The significantly increased light harvesting was attributed to the outstanding light scattering and the added NIR light effect of the up-conversion of β-NaYF4:Yb3+, Er3+ nanoparticles. However, adding such UCPs to photo-anodes caused the charge recombination at the photo-anode–electrolyte interface to aggravate. The undesirable effect was reduced by coating UCPs with a thin layer of TiO2. The weight ratio of 15% was the optimum concentration for UCPs/TiO2 to produce high performance in the DSSC. The associated DSSC produced a power conversion efficiency of up to 7.17%, which is much better than 5.45% for the bare TiO2 nanoparticle-based device.
Dutta and Rai [58] demonstrated the impact of the BiYO3:Er3+/Yb3+ up-converting (UC) nanoparticles in enhancing the performance of DSSCs. The power conversion efficiency of the DSSCs with the incorporation of the UC was raised from 5.73% to 6.20%. The primary cause of the improvement was the ability of the UC to convert NIR light to visible light. According to the findings of the study, it is feasible to add UC nanophosphors to DSSC in order to enhance their photovoltaic capabilities and the dye capability for light harvesting.
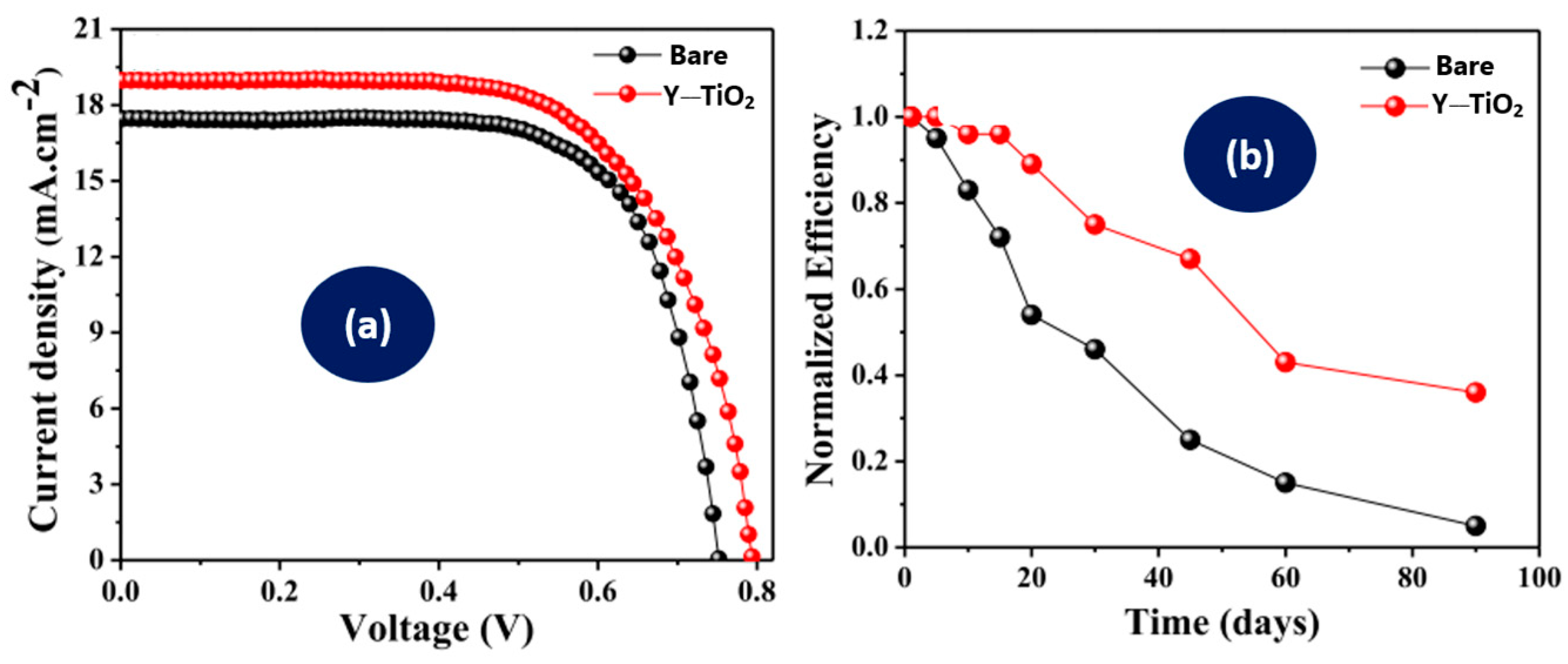
Tadge et al. [59] incorporated Y2O3:Ho3+/Yb3+ into TiO2 to build DSSCs and a comparison of their photovoltaic performance was performed. The ability of the UC phosphor to harness NIR–visible light has been observed to increase the power conversion efficiency of the DSSC by 10.33% when compared to the bare TiO2 DSSC. The efficiency was raised from 8.9% to 9.82% by the integration of the UC phosphors, as shown in Figure 6a. This means that the efficiency of the bare DSSC was 8.9% and the integration of the UC phosphor enhanced the efficiency to 9.82%. The efficiency was enhanced as a result of the rise in the N719 spectral sensitivity. Compared to the bare TiO2-based cell, the DSSC integrated with the UC showed better durability, as presented in Figure 6b. The higher durability was perceived due to the effect generated by the incorporation of UC phosphors into the TiO2, leading to a higher infiltration of electrolytes and prevented the evaporation of electrolytes from the device. The performance parameters of the photovoltaic cells are presented in Table 2. An asterisk (*) is used to denote the device with higher efficiency.
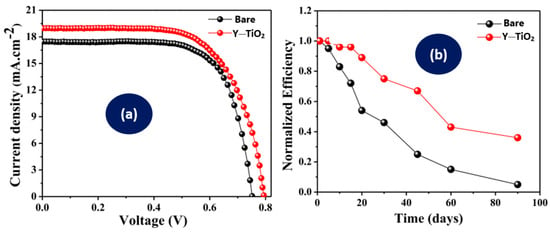
Figure 6.
Photovoltaic parameters [59], (a) current voltage characteristics and (b) time variations in normalized efficiencies of bare and UC-based dye-sensitized solar cells.

Table 2.
Photovoltaic parameters of bare as well as UC-based Dye-sensitized Solar Cells [59].
Frang et al. [60] created graphene quantum dots (GQDs) integrated into dye-sensitized TiO2 photo-electrodes for solar cell application. Transmission electron microscope was used to give the structural analysis of the GQDs. The GQDs were incorporated into TiO2 films, resulting in various GQD-enhanced photo-electrodes and their corresponding dye-sensitized solar cells (DSSCs). Their studies revealed that with increasing the amount of GQDs in the photo-electrodes, the dye adsorption initially decreased and then increased, whereas the of the DSSCs first increased and then decreased. Among all the DSSCs, the cell with an optimal amount of GQDs showed the best performance with a minimum dye-adsorption with the maximum Jsc of 14.07 ± 0.02 mA/cm2 and η of 6.10 ± 0.01%, higher than those of the bare DSSC by 30.9% and 19.6%, respectively. The better performance with minimum dye use was largely due to the unique photoexcitation response of the GQDs and the injection of hot electrons from GQDs into TiO2. The research demonstrated that introducing GQDs can significantly enhance DSSC performance while reducing dye consumption, offering economic and environmental benefits.
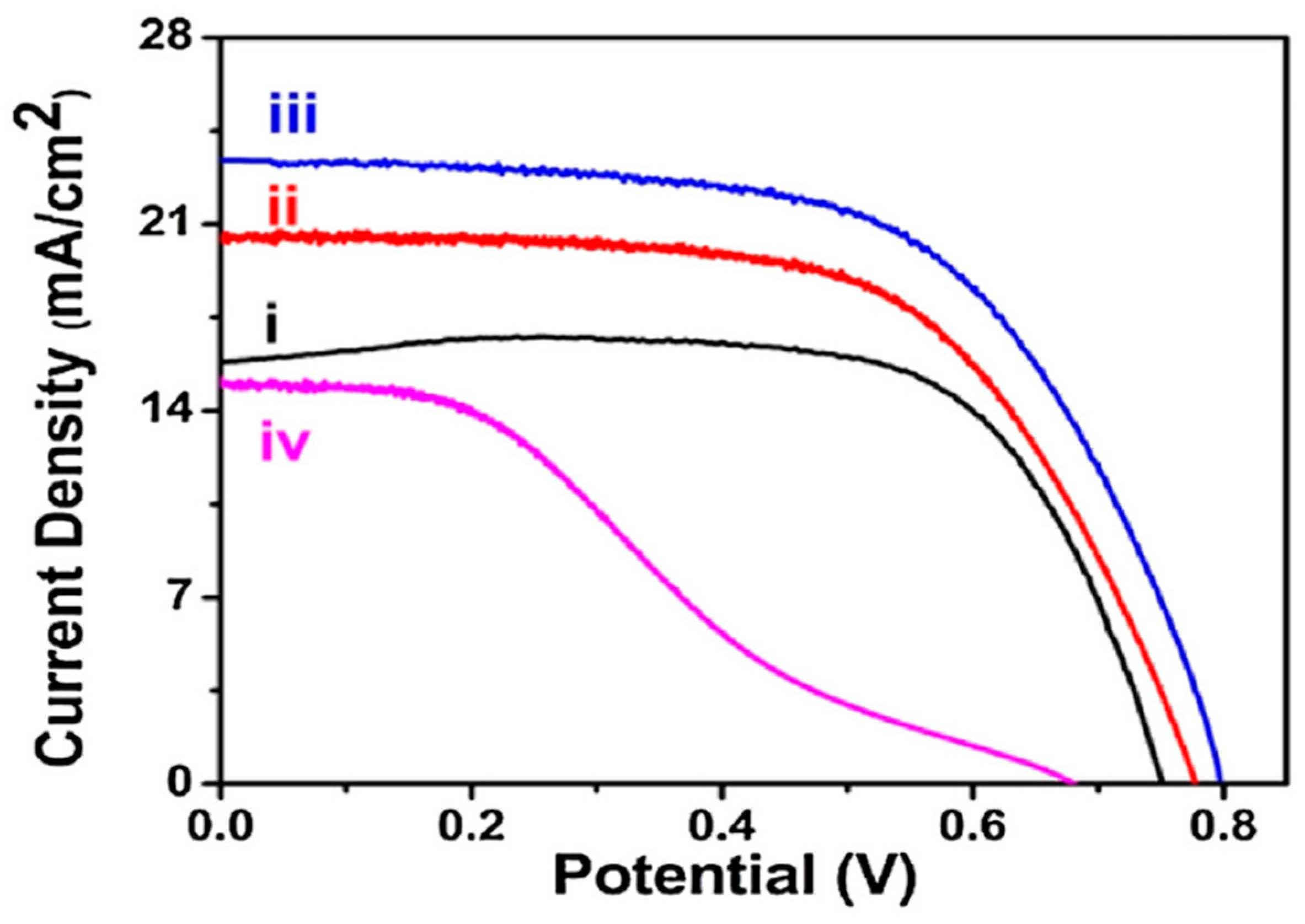
Kundu et al. [61] indicated that modifying TiO2 photo-anodes with co-doped graphene quantum dots (GQDs) offers valuable insights for enhancing the performance of DSSCs. By integrating doped GQDs, they achieved a significant increase in efficiency up to 11.7% with an excellent fill factor (functional area = 0.16 cm2), as shown in Figure 7. Photovoltaic performance parameters are presented in Table 3. An asterisk (*) is used to represent the device with the highest efficiency. The improvement was attributed to the ability of the doped GQDs to prevent the back electron transfer from TiO2 and enhance light absorption. After one month (following electrolyte refilling), the DSSCs still maintained an efficiency of approximately 10%, demonstrating their stability within a laboratory scale measurement. Beyond advancing QD-sensitized solar cell technology, their findings underscore the environmental benefits of using eco-friendly materials, contrasting with conventional QDs. They anticipated that further advancements in cell fabrication and material science will pave the way for next-generation and eco-friendly solar cells with even higher conversion efficiencies.
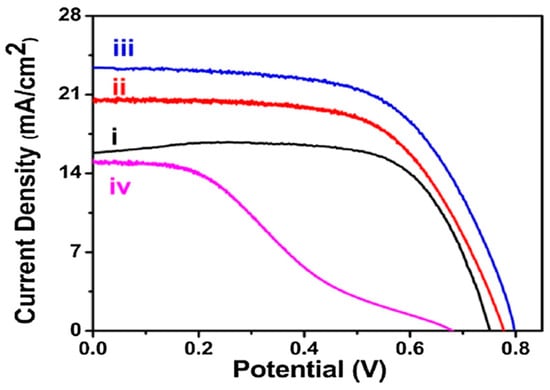
Figure 7.
(Variation of I–V characteristics of only TiO2 with dye which shows η = 7.48% (FF = 63%) (i), NFS-GQDs-TiO2-N719 dye with respect to different doped GQDs loading corresponding to different time interval (3 h: η = 9.7% (FF = 71.70%) (ii), 8 h: η = 11.7% (FF = 71%) (iii), and 12 h: η = 5.4% (FF = 50%) (iv)) [61].

Table 3.
I-V data at a glance [61].
Chou et al. [62] demonstrated that both GQDs and N719 dye were absorbed into the photo-anode film using a simple preparation process. The GQDs solution was adsorbed into the photo-anode film by the spin coating technique. Next, the GQDs adsorbed photo-anode film was totally immersed in 719 dye for 24 h. Introducing GQDs as auxiliary sensitizers to achieve co-sensitization with N719 dye can enhance the photovoltaic performance of DSSCs. They evaluated the photo-response characteristics of DSSCs under different sensitization conditions using the incident photon-to-current conversion efficiency (IPCE). Their result indicated that the photoelectric conversion efficiency of DSSCs using GQDs + N719 co-sensitized photo-anode increased to 5.14%, making a 20% enhancement as compared with the 4.28% of only N719 dye. GQDs enhanced the photo-response of N719 dye particularly within the visible spectral range due to increased overlap between the IPCE spectra of GQD + N719 and the charge transport properties of graphene itself in the GQDs. Hence, GQDs serve as effective co-sensitizers for enhancing the light absorption capability of DSSCs.
Table 4, Table 5 and Table 6 show the photovoltaic performance parameters of the best DSSCs enhanced with different luminescent materials categorized as up-conversion, down-conversion and quantum dots. The of the luminescent enhanced DSSCs were recorded from the corresponding studies. The material with the highest efficiency is denoted with asterisk (*) in each category.

Table 4.
Photovoltaic parameters of best DSSCs with different types of up-conversion materials.

Table 5.
Photovoltaic parameters of best DSSCs with different types of down-conversion materials.

Table 6.
Photovoltaic parameters of best DSSCs with different types of quantum dots materials.
4.5. Energy Transfer
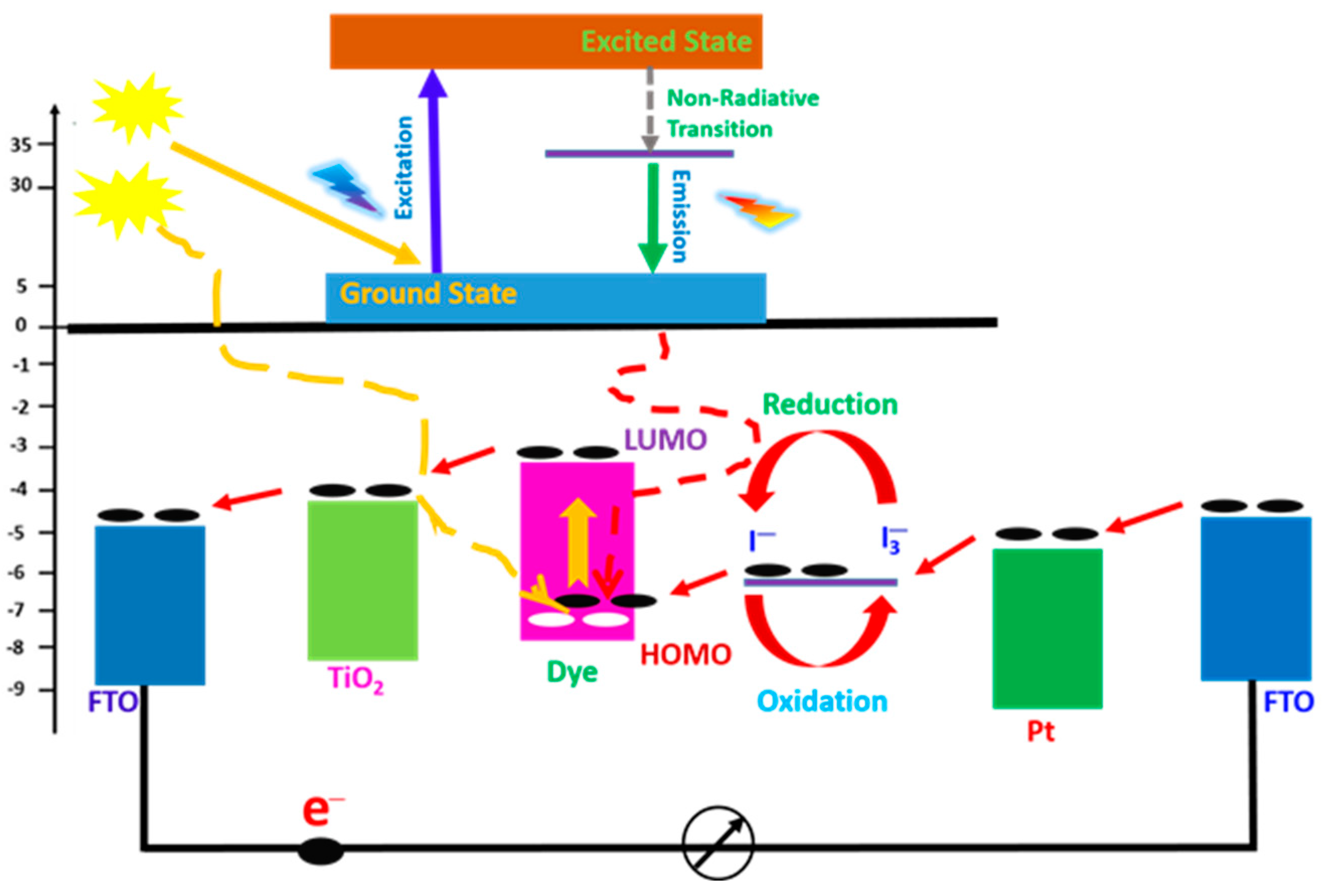
Energy transfer from luminescent materials to dye molecules can occur through mechanisms such as Förster resonance energy transfer (FRET). This process enhances the excitation of dye molecules, boosting the efficiency of the DSSC. The FRET may be used in DSSCs to connect two or more materials in order to enhance strong absorption across a wide range of the solar spectrum and improve device performance [87,88]. Luminescent nanoparticles and organic dyes are particularly effective in facilitating these energy transfer processes. Figure 8 depicts the energy level diagram of the energy transfer from the luminescent material to the dye molecules. Energy from the emission transition of the luminescent material is transferred to the dye molecules. The photon absorption of the dye molecules produces excited electrons from its highest occupied molecular orbital (HOMO) in the ground state to the lowest unoccupied molecular orbital (LUMO) in the excited state. The excited electrons are rapidly injected into the conduction band of the TiO2, which lies below the excited state of the dye, where they diffuse through the nano-porous structure to the FTO back contact and eventually flow through the external circuit.
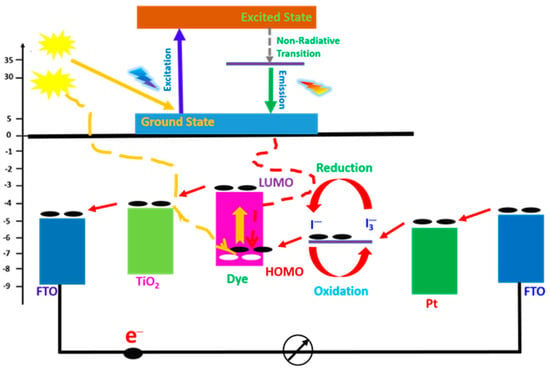
Figure 8.
Energy level diagram of DSSCs.
In the quest to enhance the efficiency of dye-sensitized solar cells (DSSCs), the concept of energy transfer from luminescent materials to dye molecules has gained significant attention. This process can improve light harvesting and facilitate better utilization of the solar spectrum, particularly by extending the absorption range from the visible to the UV and NIR regions where the device has weak spectral responsivity, thereby enhancing the photon absorption of the dye molecules and increasing charge carrier generation. This section explains the mechanisms of energy transfer, the types of luminescent materials involved and their impact on DSSC performance.
4.6. Mechanism of Energy Transfer
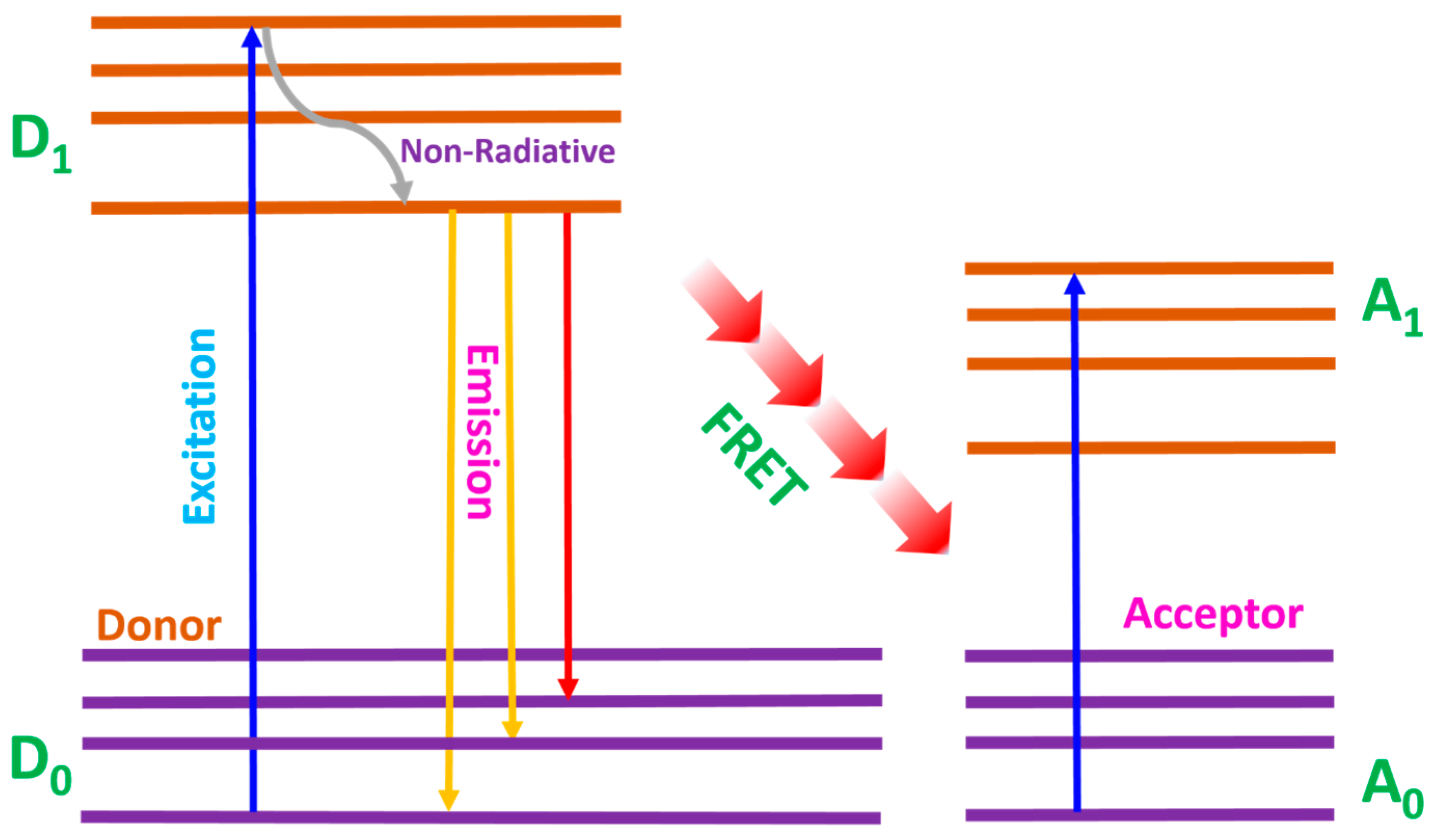
Energy transfer from luminescent materials to dye molecules in DSSCs primarily occurs through Förster resonance energy transfer (FRET). FRET is a non-radiative process where energy is transferred from a donor (luminescent material) to an acceptor (dye molecule) through dipole–dipole interactions. FRET analysis is crucial in measuring changes in the donor and acceptor spectra, which is effective in analyzing energy transfer efficiency [89,90]. This mechanism is highly dependent on the distance between the donor and acceptor, typically occurring over a range of 1–10 nm. Figure 9 shows the FRET mechanism, where the donor transitions from the ground state (D0) to the excited state (D1). Non-radiative relaxation occurred within the lower level of the excited state, which could be phonon-assisted, then the emission and energy transfer to the acceptor via FRET mediation. The excited molecules transition from the ground state (A0) to the excited state (A1) and this process allows continuous energy transfer.
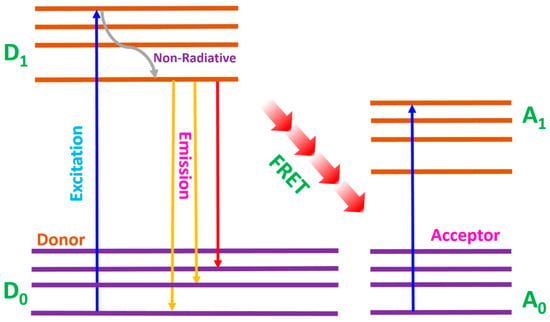
Figure 9.
Förster resonance energy transfer mechanism.
For effective FRET, there must be a spectral overlap between the emission spectrum of the donor and the absorption spectrum of the acceptor. The overlap integral is governed by the following equation [89]:
where FD is the normalized emission spectrum of the donor, a dimensionless entity, ∈A (λ) is the molar absorption or extinction coefficient of the acceptor in M−1cm−1 and λ is the wavelength of the incident light in nm.
The distance between the donor and acceptor molecules as well as the strength of spectral overlap determines the efficiency of energy transfer. The J—overlap integral can be expressed conveniently in terms of the Förster distance. The efficiency of energy transfer via FRET can be expressed generally as [87]:
where R is the distance between the donor and acceptor molecules and R0 is the Förster distance upon which the energy transfer efficiency is 50%. Forster distance in Å is expressed as [87]
where k2 is the relative orientation of the transition dipoles of the donors and acceptors generally given as two-thirds, QY is the quantum yield of the donor in the absence of the acceptor, n is the refractive index of the medium containing the donor and acceptor molecules and J (λ) is the spectral overlap between the donor and the acceptor molecule.
In the context of DSSCs, luminescent nanoparticles or quantum dots can act as donors, transferring their absorbed energy to the dye molecules close to the TiO2 surface, thereby enhancing the dye’s photo-excitation.
- Quantum Dots as Donors: Quantum dots (QDs), due to their size-tunable emission properties, are excellent candidates for FRET applications in DSSCs. For instance, CdSe QDs can be engineered to emit light in the visible spectrum, overlapping well with the absorption spectra of many commonly used dyes, such as N719 or perovskites [91]. This overlap facilitates efficient energy transfer, enhancing the photoexcitation of the dye molecules and potentially increasing the photocurrent.
- Lanthanide-Doped Nanoparticles: Lanthanide-doped nanoparticles exhibit sharp emission peaks and long-lived excited states, making them suitable for FRET applications. Materials doped with lanthanides like Eu3+ or Tb3+ can emit visible light after absorbing UV light, effectively transferring energy to the dye molecules in DSSCs [92].
4.7. How to Incorporate Luminescent Materials in DSSCs
The general approach in integrating luminescent materials in DSSCs follows some careful sequential steps and involves photo-anode preparation, the sensitization of the photo-anode with dye molecules, and the assembly of the cell. The following activities depict the procedural approach of incorporating luminescent materials in DSSCs.
- Activity 1: Preparation of Photo-anode
- Mix the TiO2 nanomaterial with a suitable solvent to synthesize TiO2 paste.
- Incorporate the synthesized luminescent material to the TiO2 paste (either in varying concentrations or just the optimum concentration of the luminescent material) to study its effect on the photo-anode performance.
- Apply the TiO2 mixed with the luminescent material onto fluorine-doped tin oxide (FTO) glass substrates using any appropriate material deposition technique to form a photo-anode [93,94,95].
- Anneal the photo-anode at appropriate temperatures to remove the binder and enhance adhesion.
- Activity 2: Sensitization with Dye
- Prepare a dye solution, typically containing a ruthenium-based dye or organic dye, known for its high absorption in the visible range [96,97].
- Immerse the annealed photo-anode in the dye solution for a few hours to allow thorough dye adsorption.
- Depending on the focus of the study, you could optimize dye loading conditions, such as concentration and immersion time, to maximize the light absorption capabilities.
- Activity 3: Cell Assembly
- Pair the sensitized photo-anode with a platinum-coated counter electrode to form a sandwich.
- Fill the inter-electrode space with a liquid electrolyte containing iodide/tri-iodide redox couple.
- Seal the cells to prevent electrolyte leakage and ensure stable operation.
- Finally, measure the photovoltaic current density—voltage performance of the device under standard solar illumination (Air Mass = 1.5 G) to determine the photovoltaic power conversion efficiency of the device.
5. Discussion
In the preceding section, we have seen that integrating luminescent materials into DSSCs offers substantial potential for boosting their efficiency and enhancing the overall performance of the device. The scope of this section is to discuss some challenges arising from the incorporation of luminescent materials in DSSCs and possible recommendations to mitigate them. One major issue is the stability of luminescent materials. Many such materials such as QDs and organic dyes degrade when exposed to prolonged light and operational heat, degrading the cells’ long-term efficiency and lifespan [91,98]. Ensuring these materials remain stable under operational conditions is critical for consistent performance.
Energy transfer efficiency within DSSCs is another significant challenge. Luminescent materials need to absorb light effectively and re-emit it in a way that enhances the cell’s performance. However, low luminescent efficiency and re-absorption of emitted photons can limit their effectiveness [99]. Moreover, integrating these materials with existing DSSC components is complex. It is essential that luminescent materials are compatible with the cell’s sensitizers and do not chemically degrade or react with other parts of the cell, such as electrodes or electrolytes [100].
The cost and scalability of incorporating luminescent materials into DSSCs also present some hurdles. High-quality luminescent materials, like rare-earth elements and certain QDs, can be expensive and challenging to produce in large quantities. The integration process can add complexity to the manufacturing of DSSCs, potentially increasing production costs and hindering widespread adoption. Additionally, there are environmental and health concerns particularly regarding the toxicity of some luminescent materials. This raises questions about their safety and environmental impact, especially if the cells are damaged or improperly disposed of [101].
The recommendations to address these challenges encompass developing more stable luminescent materials. Advances in inorganic phosphors, perovskite quantum dots, and improved encapsulation methods can significantly enhance the durability of these materials [102]. Thin layers of alumina (Al2O3) can be integrated on the surface of the TiO2-luminescent layer to improve the stability and resistance to photo-degradation. The incorporation of antioxidants such as Butylated Hydroxytoluene (BHT) and Butylated Hydroxyanisole (BHA), which can be used to protect the dye and electrolyte from oxidative degradation, can foster the stability of the electrolyte and the dye. Improving the efficiency of energy transfer is also a key focus. Leveraging spectral modelling could provide valuable insights into the energy transfer mechanisms to foster the design of more efficient DSSCs. Designing luminescent materials with emission spectra that match the absorption characteristics of DSSC dyes can optimize energy transfer. Additionally, reducing re-absorption losses through strategic positioning and using materials with sharp emission peaks can improve overall cell efficiency [103].
Innovative DSSC designs could be developed to better utilize luminescent materials. Techniques such as luminescent down-conversion, which converts high-energy photons into lower-energy ones, and up-conversion, which transforms lower-energy photons into higher-energy photons, can help capture a broader spectrum of sunlight [104]. Multi-layered structures that incorporate luminescent materials into different layers of the cell can also enhance light management and absorption, boosting the cell’s performance [105].
Researching low-cost luminescent materials, such as organic dyes and polymer-based options, can offer cost effective alternatives materials without sacrificing performance [106]. Simplifying the integration of these materials into DSSCs is crucial for making the technology more commercially viable. This can also be accomplished through the development of multifunctional luminescent materials such as the Boron-Dipyrromethene dyes that can serve dual purposes such as light absorption and charge carrier transport to streamline seamless design of DSSC and improve performance.
Addressing environmental and health concerns is crucial in advancing the development of non-toxic luminescent materials and adopting sustainable manufacturing and disposal practices. Ensuring these materials are safe to use and dispose of is vital to minimizing their environmental impact. Additionally, integrating DSSCs with luminescent materials into hybrid systems, such as combining them with perovskite or organic photovoltaics, would offer a promising avenue for enhancing their efficiency [107]. Applications in smart windows and building-integrated photovoltaics (BIPV) can leverage luminescent materials to improve energy generation while adding aesthetic and functional benefits [108,109,110,111].
However, advancing the use of luminescent materials in DSSCs requires a multidisciplinary approach that combines material science, chemical engineering, and photovoltaic technology. By addressing these challenges and exploring innovative solutions, luminescent materials have the potential to significantly elevate the performance and utility of DSSCs in the renewable energy sector.
6. Conclusions
Luminescent materials present a promising and innovative avenue for enhancing dye-sensitized solar cells (DSSCs). Ongoing research continues to advance the development and integration of these materials, offering significant potential for improving DSSC efficiency, commercialization and deployment. DSSCs offer a clean and sustainable method for generating power without causing environmental pollution. In these systems, the dye sensitizer absorbs photons, while TiO2 facilitates charge carrier transport to the external circuit. DSSCs have gathered considerable attention due to their low cost and compatibility with various materials. However, their power conversion efficiency remains lower than that of traditional silicon-based solar cells. In this review, we focused our insights on the impact of luminescent materials, a vital component that greatly influences both the photovoltaic power conversion performance and the long term durability of the DSSCs.
We identified luminescent materials as down-conversion, up-conversion and quantum dots. These materials attracted a significant research interest of over 200,000 publications, according to the “Web of Science” data. They have the ability to extend the absorption spectrum beyond the visible region of the typical dye sensitizer to enhance the performance of the DSSCs. The key challenges of these materials include stability under operational conditions and energy transfer efficiency.
Improving the energy transfer efficiency between luminescent materials and the dye molecules is one of the recommendations to mitigate these challenges. A better understanding of the spectral alignment between these components is crucial to enhance energy transfer efficiency. Spectral modelling and spectroscopic techniques could provide valuable insights into these mechanisms to foster the design of more efficient DSSCs.
Also, improving the stability of luminescent materials under operational conditions is vital, as long-term durability is crucial for the viability of DSSCs. Future research should prioritize the development materials that resist photo-degradation. Therefore, techniques such as encapsulation that shields other components of DSSCs from UV light and preventing photo-degradation is vital. Also, thin layers of alumina (Al2O3) can be incorporated on the surface of the TiO2 luminescent layer to enhance its durability and resistance to photo-degradation. The addition of an antioxidant such as Butylated Hydroxytoluene (BHT) and Butylated Hydroxyanisole (BHA), which can be used to protect the dye and electrolyte from oxidative degradation, can also stabilize the electrolyte and the dye in DSSCs.
Further, developing multifunctional luminescent materials such as the Boron-Dipyrromethene dyes that can serve dual purposes such as light absorption and charge transport could streamline DSSC design and enhance performance. This could minimize the number of interfaces within the cell, reducing recombination losses and enhancing longevity and efficiency.
Author Contributions
Conceptualization, E.H.O. and N.L.L.; validation, E.H.O., N.L.L. and P.M.; formal analysis, E.H.O. and N.L.L.; data curation, E.H.O.; writing—original draft preparation, E.H.O.; writing—review and editing, E.H.O. and N.L.L.; visualization, E.H.O.; supervision, N.L.L. and P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Niche Area (RNA), Renewable Energy Wind in Govan Mbeki Research and Development Centre (GMRDC), University of Fort Hare, Alice, South Africa.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Acknowledgments
We acknowledge the assistance of the SAMRC Microbial Water Quality Monitoring Centre of the University of Fort Hare, Alice, South Africa.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fébba, D.M.; Rubinger, R.; Oliveira, A.; Bortoni, E. Impacts of temperature and irradiance on polycrystalline silicon solar cells parameters. Sol. Energy 2018, 174, 628–639. [Google Scholar] [CrossRef]
- Isabela, C.B.; Lameirinhas RA, M.; Torres JP, N.; Fernandes, C.A. Comparative study of the copper indium gallium selenide (CIGS) solar cell with other solar technologies. Sustain. Energy Fuels 2021, 5, 2273–2283. [Google Scholar]
- Shah, A. Amorphous silicon solar cells. In Solar Cells and Modules; Springer: Berlin/Heidelberg, Germany, 2020; pp. 139–161. [Google Scholar]
- Park, S.; Simon, J.; Schulte, K.L.; Ptak, A.J.; Wi, J.-S.; Young, D.L.; Oh, J. Germanium-on-nothing for epitaxial liftoff of GaAs solar cells. Joule 2019, 3, 1782–1793. [Google Scholar] [CrossRef]
- Başol, B.M.; McCandless, B. Brief review of cadmium telluride-based photovoltaic technologies. J. Photonics Energy 2014, 4, 040996. [Google Scholar] [CrossRef]
- Kamat, P.V. Meeting the clean energy demand: Nanostructure architectures for solar energy conversion. J. Phys. Chem. C 2008, 112, 18737–18753. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Müller, E.; Liska, P.; Vlachopoulos, N.; Grätzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Mozaffari, S.; Nateghi, M.R.; Zarandi, M.B. An overview of the Challenges in the commercialization of dye sensitized solar cells. Renew. Sustain. Energy Rev. 2017, 71, 675–686. [Google Scholar] [CrossRef]
- Ebenezer Anitha, A.; Dotter, M. A review on liquid electrolyte stability issues for commercialization of dye-sensitized solar cells (DSSC). Energies 2023, 16, 5129. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-sensitized solar cells: Fundamentals and current status. Nanoscale Res. Lett. 2018, 13, 1–46. [Google Scholar] [CrossRef]
- Andualem, A.; Demiss, S. Review on dye-sensitized solar cells (DSSCs). Edelweiss Appl. Sci. Technol. 2018, 2, 145–150. [Google Scholar] [CrossRef]
- Vlachopoulos, N.; Hagfeldt, A.; Benesperi, I.; Freitag, M.; Hashmi, G.; Jia, G.; Dietzek, B. New approaches in component design for dye-sensitized solar cells. Sustain. Energy Fuels 2021, 5, 367–383. [Google Scholar] [CrossRef]
- Jiang, R.; Michaels, H.; Vlachopoulos, N.; Freitag, M. Beyond the limitations of dye-sensitized solar cells. In Dye-Sensitized Solar Cells; Academic Press: Cambridge, MA, USA, 2019; pp. 285–323. [Google Scholar]
- Khir, H.; Pandey, A.K.; Saidur, R.; Ahmad, M.S.; Abd Rahim, N.; Dewika, M.; Samykano, M. Recent advancements and challenges in flexible low temperature dye sensitised solar cells. Sustain. Energy Technol. Assess. 2022, 53, 102745. [Google Scholar] [CrossRef]
- Bandara TM, W.J.; Hansadi JM, C.; Bella, F. A review of textile dye-sensitized solar cells for wearable electronics. Ionics 2022, 28, 2563–2583. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Hosseini, Z.; Huang, W.K.; Tsai, C.M.; Chen, T.M.; Taghavinia, N.; Diau, E.W.G. Enhanced light harvesting with a reflective luminescent down-shifting layer for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 5397–5402. [Google Scholar] [CrossRef]
- Rajeswari, R.; Islavath, N.; Raghavender, M.; Giribabu, L. Recent progress and emerging applications of rare earth doped phosphor materials for dye-sensitized and perovskite solar cells: A review. Chem. Rec. 2020, 20, 65–88. [Google Scholar] [CrossRef]
- Park, C.-H.; Kim, T.H.; Yonesaki, Y.; Kumada, N. A re-investigation of the crystal structure and luminescence of BaCa2MgSi2O8: Eu2+. J. Solid State Chem. 2011, 184, 1566–1570. [Google Scholar] [CrossRef]
- Kim, K.; Nam, S.K.; Moon, J.H. Dual-band luminescent solar converter-coupled dye-sensitized solar cells for high-performance semitransparent photovoltaic device. ACS Appl. Energy Mater. 2020, 3, 5277–5284. [Google Scholar] [CrossRef]
- Yao, N.; Huang, J.; Fu, K.; Deng, X.; Ding, M.; Xu, X. Rare earth ion doped phosphors for dye-sensitized solar cells applications. RSC Adv. 2016, 6, 17546–17559. [Google Scholar] [CrossRef]
- Xie, G.; Lin, J.; Wu, J.; Lan, Z.; Li, Q.; Xiao, Y.; Yue, G.T.; Yue, H.F.; Huang, M. Application of upconversion luminescence in dye-sensitive solar cells. Chin. Sci. Bull. 2011, 56, 96–101. [Google Scholar] [CrossRef]
- Bella, F.; Griffini, G.; Gerosa, M.; Turri, S.; Bongiovanni, R. Performance and stability improvements for dye-sensitized solar cells in the presence of luminescent coatings. J. Power Sources 2015, 283, 195–203. [Google Scholar] [CrossRef]
- Hosseini, Z.; Taghavinia, N.; Wei-Guang Diau, E. Luminescent Spectral Conversion to Improve the Performance of Dye-Sensitized Solar Cells. ChemPhysChem 2017, 18, 3292–3308. [Google Scholar] [CrossRef]
- Huang, X.; Han, S.; Huang, W.; Liu, X. Enhancing solar cell efficiency: The search for luminescent materials as spectral converter. Chem. Soc. Rev. 2013, 42, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yao, Q.; Li, Y. Effects of down-conversion luminescent film in dye-sensitive solar cells. Appl. Phys. Lett. 2006, 88, 173119. [Google Scholar] [CrossRef]
- Griffini, G.; Bella, F.; Nisic, F.; Dragonetti, C.; Roberto, D.; Levi, M.; Bongiovanni, R.; Turri, S. Multifunctional luminescent down-shifting fluoropolymer coatings: A straightforward strategy to improve the UV-light harvesting ability and long-term outdoor stability of organic dye-sensitized solar cells. Adv. Energy Mater. 2015, 5, 1401312. [Google Scholar] [CrossRef]
- McKenna, B.; Evans, R.C. Towards efficient spectral converters through materials design for luminescent solar devices. Adv. Mater. 2017, 29, 1606491. [Google Scholar] [CrossRef]
- Li, Q.; Lin, J.; Wu, J.; Lan, Z.; Wang, Y.; Peng, F.; Huang, M. Improving photovoltaic performance of dye-sensitized solar cell by downshift luminescence and p-doping effect of Gd2O3: Sm3+. J. Lumin. 2013, 134, 59–62. [Google Scholar] [CrossRef]
- Rehman, A.U.; Aslam, M.; Khan, M.; Shahid, I.; Siddiq, A.; Iqbal, M.A.; Ahmed, S. Enhancing the photovoltaic performance of solid-state dye-sensitive solar cells with composite materials and luminescent down-shifting. J. Electron. Mater. 2020, 49, 6292–6299. [Google Scholar] [CrossRef]
- He, W.; Atabaev, T.S.; Kim, H.K.; Hwang, Y.H. Enhanced sunlight harvesting of dye-sensitized solar cells assisted with long persistent phosphor materials. J. Phys. Chem. C 2013, 117, 17894–17900. [Google Scholar] [CrossRef]
- Liang, L.; Yulin, Y.; Mi, Z.; Ruiqing, F.; LeLe, Q.; Xin, W.; Zhang, L.; Zhou, X.; Jianglong, H. Enhanced performance of dye-sensitized solar cells based on TiO2 with NIR-absorption and visible upconversion luminescence. J. Solid State Chem. 2013, 198, 459–465. [Google Scholar] [CrossRef]
- Klampaftis, E.; Ross, D.; McIntosh, K.R.; Richards, B.S. Enhancing the performance of solar cells via luminescent down-shifting of the incident spectrum: A review. Sol. Energy Mater. Sol. Cells 2009, 93, 1182–1194. [Google Scholar] [CrossRef]
- Li, J.; Yin, O.; Zhao, L.; Wang, Z.; Dong, B.; Wan, L.; Wang, S. Enhancing theelectric photoelectric conversion efficiency of dye-sensitized solar cell using the upconversion luminescence materials Y2O3: Er3+ nanorods of TiO2 photoanode. Mater. Lett. 2018, 227, 209–212. [Google Scholar] [CrossRef]
- Bai, J.; Duan, P.; Wang, X.; Han, G.; Wang, M.; Diao, G. Upconversion luminescence enhancement by Fe3+ doping in CeO2: Yb/Er nanomaterials and their application in dye-sensitized solar cells. RSC Adv. 2020, 10, 18868–18874. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, K.; Qu, Y.; Wang, G.; Dai, Q.; Wang, D.; Qin, W. Luminescent material with functionalized carbon nitride as a photovoltaic booster in DSSCs: Enhanced charge separation and transfer. J. Mater. Res. 2019, 34, 616–625. [Google Scholar] [CrossRef]
- Wang, T.H.; Huang, T.W.; Tsai, Y.C.; Chang, Y.W.; Liao, C.S. A photoluminescent layer for improving the performance of dye-sensitized solar cells. Chem. Commun. 2015, 51, 7253–7256. [Google Scholar] [CrossRef]
- Muthu, S.; Gandhi, M.B.; Meenakshi Flooded, S.P.; Gandhi, S.K.; Sridharan, M.B.; Park, H.J.J. A Review on the Applicability of Luminescent Phosphors and Effective Positioning in Dye-Sensitized Solar Cells for Enhanced Performance and Stability. Sol. RRL 2023, 7, 2300510. [Google Scholar] [CrossRef]
- Kumar, V.; Swami, S.K.; Kumar, A.; Ntwaeaborwa, O.M.; Dutta, V.; Swart, H.C. Eu3+ doped down shifting TiO2 layer for efficient dye-sensitized solar cells. J. Colloid Interface Sci. 2016, 484, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Yuliantini, L.; Nursam, N.M.; Pranoto, L.M.; Hidayat, J.; Sova, R.R.; Rahayu, E.S.; Djamal, M.; Yasaka, P.; Boonin, K.; Kaewkhao, J. Photon up-conversion in Er3+ ion-doped ZnO-Al2O3-BaO-B2O3 glass for enhancing the performance of dye-sensitized solar cells. J. Alloys Compounds 2023, 954, 170163. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Yum, J.H.; Baranoff, E.; Wenger, S.; Nazeeruddin, M.K.; Grätzel, M. Panchromatic engineering for dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 842–857. [Google Scholar] [CrossRef]
- Rho, W.Y.; Jeon, H.; Kim, H.S.; Chun, W.J.; Suh, J.S.; Jun, B.H. Recent progress in dye-sensitive solar cells for improving efficiency: TiO2 nanotube arrays in active layer. J. Nanomater. 2015, 16, 85. [Google Scholar]
- Kusama, H.; Orita, H.; Sugihara, H. TiO2 band shift by nitrogen-containing heterocycles in dye-sensitized solar cells: The periodic density functional theory study. Langmuir 2008, 24, 4411–4419. [Google Scholar] [CrossRef]
- Ansari, A.A.; Nazeeruddin, M.K.; Tavakoli MM, M. Organic-inorganic upconversion nanoparticles hybrid in dye-sensitivezed solar cells. Coord. Chem. Rev. 2021, 436, 213805. [Google Scholar] [CrossRef]
- Li, Q.; Lin, J.; Wu, J.; Lan, Z.; Wang, J.; Wang, Y.; Peng, F.; Huang, M.; Xiao, Y. Preparation of Gd2O3:Eu3+ downconversion luminescent material and its application in dye-sensitized solar cells. Chin. Sci. Bull. 2011, 56, 3114–3118. [Google Scholar] [CrossRef]
- Chebrolu, V.T.; Kim HJ, J. Recent progress in quantum dot sensitized solar cells: An inclusive review of photoanode, sensitive, electrolyte, and the electro-fighter. J. Mater. Chem. C 2019, 7, 4911–4933. [Google Scholar] [CrossRef]
- Shen, J.; Li, Z.; Cheng, R.; Luo, Q.; Luo, Y.; Chen, Y.; Chen, X.; Sun, Z.; Huang, S. Eu3+-doped NaGdF4 nanocrystal down-converting layer for efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2014, 6, 17454–17462. [Google Scholar] [CrossRef]
- Yao, N.; Huang, J.; Fu, K.; Deng, X.; Ding, M.; Shao, M.; Xu, X. Enhanced light harvesting of dye-sensitized solar cells with up/down conversion materials. Electrochim. Acta 2015, 154, 273–277. [Google Scholar] [CrossRef]
- Wang, J.; Du, Z.; Hojamberdiev, M.; Zheng, S.; Xu, Y. Oxalate-assisted morphological effect of NaYF4: Yb3+, Er3+ on photoelectrochemical performance for dye-sensitive solar cells. J. Rare Earths 2018, 36, 353–358. [Google Scholar] [CrossRef]
- Luo, X.; Ahn, J.Y.; Kim, S.H. Aerosol synthesis and luminescent properties of CaAl2O4: Eu2+, Nd3+ down-conversion phosphor particles for enhanced light harvesting of dye-sensitized solar cells. Sol. Energy 2019, 178, 173–180. [Google Scholar] [CrossRef]
- Kamal, R.; Hafez, H. Novel Down-converting single-phased white light Pr3+ doped BaWO4 Nanophosphors material for DSSC applications. Opt. Mater. 2021, 121, 111646. [Google Scholar] [CrossRef]
- Mao, X.; Yu, J.; Xu, J.; Wan, L.; Yang, Y.; Lin, H.; Zhou, R. Commercial Upconversion Phosphors with High Light Harvesting: A Superior Candidate for High-Performance Dye-Sensitized Solar Cells. Phys. Status Solidi (a) 2019, 216, 1900382. [Google Scholar] [CrossRef]
- Dutta, J.; Rai VK, K. Upconverting BiYO3 nanophosphors in DSSCs applications. Opt. Laser Technol. 2021, 140, 107087. [Google Scholar] [CrossRef]
- Tadge, P.; Yadav, R.S.; Vishwakarma, P.K.; Rai, S.B.; Chen, T.M.; Sapra, S.; Ray, S. Enhanced photovoltaic performance of Y2O3: Ho3+/Yb3+ upconversion nanophosphor based DSSC and investigation of color tunability in Ho3+/Tm3+/Yb3+ tri-doped Y2O3. J. Alloys Compounds 2020, 821, 153230. [Google Scholar] [CrossRef]
- Fang, X.; Li, M.; Guo, K.; Li, J.; Pan, M.; Bai, L.; Luosha, M.; Zhao, X. Graphene quantum dots optimization of dye-sensitized solar cells. Electrochim. Acta 2014, 137, 634–638. [Google Scholar] [CrossRef]
- Kundu, S.; Sarojinijeeva, P.; Karthick, R.; Anantharaj, G.; Saritha, G.; Bera, R.; Anandan, S.; Patra, A.; Ragupathy, P.; Selvaraj, M.; et al. Enhas thencing the Efficiency of DSSCs by the Modification of TiO2 Photoanodes using N, F and S, co-doped Graphene Quantum Dots. Electrochim. Acta 2017, 242, 337–343. [Google Scholar] [CrossRef]
- Chou, J.C.; Syu, R.H.; Yang, P.H.; Kuo, P.Y.; Nien, Y.H.; Lai, C.H.; Chen, P.F.; Wu, Y.T.; Zhuang, S.W. Graphene quantum dots a co-sensitive with improving light absorption for dye-sensitized solar cells. IEEE Trans. Nanotechnol. 2023, 22, 20–27. [Google Scholar] [CrossRef]
- Rajeswari, R.; Susmitha, K.; Jayasankar, C.K.; Raghavender, M.; Giribabu, L. Enhanced light harvesting with novel photon upconverted Y2CaZnO5: Er3+/Yb3+ nanophosphors for dye sensitized solar cells. Sol. Energy 2017, 157, 956–965. [Google Scholar] [CrossRef]
- Chouryal, Y.N.; Sharma, R.K.; Tomar, N.; Yadav, N.; Kewat, H.L.; Wani, I.A.; Nigam, S.; Surolia, P.K.; Sivakumar, S.; Ghosh, P. Upconverted Nanophosphors for Increasing Efficiency in a Dye-Sensitized Solar Cell. ACS Appl. Energy Mater. 2023, 6, 4934–4941. [Google Scholar] [CrossRef]
- Ambapuram, M.; Ramireddy, R.; Maddala, G.; Godugunuru, S.; Yerva, P.V.S.; Mitty, R. Effective upconverter and light scattering dual function LiYF4: Er3+/Yb3+ assisted photoelectrode for high performance cosensitized dye sensitized solar cells. ACS Appl. Electron. Mater. 2020, 2, 962–970. [Google Scholar] [CrossRef]
- Li, Q.; Lin, J.; Wu, J.; Lan, Z.; Wang, Y.; Peng, F.; Huang, M. Enhancing photovoltaic performance of dye-sensitized solar cell by rare-earth doped oxide of Lu2O3:(Tm3+, Yb3+). Electrochim. Acta 2011, 56, 4980–4984. [Google Scholar] [CrossRef]
- Morassaei MSalehabadi, A.; Akbari, A.; Tavassoli, S.H.; Salavati-Niasari, M. Enhanced dye sensitized solar cells efficiency by utilization of an external layer of CaCe2(MoO4)4: Er3+/Yb3+ nanoparticles. J. Alloys Compounds 2018, 769, 732–739. [Google Scholar] [CrossRef]
- Du, P.; Lim, J.H.; Leem, J.W.; Cha, S.M.; Yu, J.S. Enhanced photovoltaic performance of dye-sensitized solar cells by efficient near-infrared sunlight harvesting using upconverting Y2O3: Er3+/Yb3+ phosphor nanoparticles. Nanoscale Res. Lett. 2015, 10, 321. [Google Scholar] [CrossRef]
- Dutta, J.; Rai, V.K.; Durai, M.M.; Thangavel, R. Development of Y2O3:Ho3+/Yb3+ upconverting nanophosphors for enhancing solar cell efficiency of dye-sensitized solar cells. IEEE J. Photovolt. 2019, 9, 1040–1045. [Google Scholar] [CrossRef]
- Qin, Y.; Hu, Z.; Lim, B.H.; Chang, W.S.; Chong, K.K.; Zhang, P.; Zhang, H. Sol-hydrothermal synthesis of TiO2: Sm3+ nanoparticles and their enhanced photovoltaic properties. J. Alloys Compounds 2016, 686, 803–809. [Google Scholar] [CrossRef]
- Zahedifar, M.; Chamanzadeh, Z.; Madani, M.; Moradi, M.; Sharifpour, N. Synthesis and characterization of GdVO4: Dy 3+ nanosheets as down converter: Application in dye-sensitized solar cells. J. Mater. Sci. Mater. Electron. 2016, 27, 4447–4456. [Google Scholar] [CrossRef]
- Chamanzadeh, Z.; Sharifpour, N.; Zahedifar, M.; Madani, M. Synthesis of CeVO4: Dy3+ Nanoparticles as Down Converter and Its Application for Efficiency Enhancement in Dye-Sensitized Solar Cells. J. Nanostructures 2023, 13, 811–825. [Google Scholar]
- Shen, Z.; Jin, S.; Hao, H.; Hou, H.; Zhang, G.; Bi, J.; Liu, G. Synthesis and characterization of Sm3+-doped barium stannate down-conversion nanocrystals and its application in dye-sensitized solar cells. Mater. Chem. Phys. 2019, 230, 215–220. [Google Scholar] [CrossRef]
- Zahedifar, M.; Chamanzadeh, Z.; Mashkani, S.H. Synthesis of LaVO4: Dy3+ luminescent nanostructure and optimization of its performance as down-converter in dye-sensitized solar cells. J. Lumin. 2013, 135, 66–73. [Google Scholar] [CrossRef]
- Llanos, J.; Brito, I.; Espinoza, D.; Sekar, R.; Manidurai, P. A down-shifting Eu3+-doped Y2WO6/TiO2 photoelectrode for improved light harvesting in dye-sensitized solar cells. R. Soc. Open Sci. 2018, 5, 171054. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.J.; Ko, Y.N.; Kang, Y.C.; Jung KY, Y. Enhancement of light-harvesting efficiency of dye-sensitized solar cells via forming TiO2 composite double layers with down/up converting phosphor dispersion. RSC Adv. 2014, 4, 10039–10042. [Google Scholar] [CrossRef]
- Maaouni, N.; Rosaiah, P.; Kumar, K.G.; Dhanalakshmi, M.A.; Albaqami, M.D.; Ayub, R. Enhanced DSSC efficiency through integration of red-emitting MgAl2O4: Eu3+ phosphor within TiO2 layer. Opt. Mater. 2024, 151, 115349. [Google Scholar] [CrossRef]
- Chen, E.Z.; Gu, X.Y.; Wei, K.; Cheng, Y.; Chen, Z.L.; Sun, G.Z.; Pan, X.J.; Zhou, J.Y.; Xie, E.Q. Role of long persistence phosphors on their enhancement in performances of photoelectric devices: In case of dye-sensitized solar cells. Appl. Surf. Sci. 2020, 507, 145098. [Google Scholar] [CrossRef]
- Que, M.; Que, W.; Yin, X.; Shao, J. Enhanced sunlight harvesting of dye-sensitized solar cells through the insertion of a (Sr, Ba, Eu) 2SiO4-TiO2 composite layer. Mater. Res. Bull. 2016, 83, 19–23. [Google Scholar] [CrossRef]
- Xie, G.; Wu, J.; Lin, J.; Li, Q.; Lan, Z.; Xiao, Y.; Yue, G.; Ye, H.; Huang, M. Improving Photoelectrical Performance of a Dye Sensitized Solar Cell by Doping Rare-earth Oxide Y2O3:(Eu3+, Gd3+). Energy Sources Part A Recovery Util. Environ. Eff. 2012, 34, 1534–1540. [Google Scholar] [CrossRef]
- Teymourinia, H.; Salavati-Niasari, M.; Amiri, O.; Farangi, M. Facile synthesis of graphene quantum dots from corn powder and their application as down-conversion effect in quantum dot-dye-sensitized solar cell. J. Mol. Liq. 2018, 251, 267–272. [Google Scholar] [CrossRef]
- Yuan, H.; Zhao, Y.; Wang, Y.; Duan, J.; He, B.; Tang, Q. Sonochemistry-assisted black/red phosphorus hybrid quantum dots for dye-sensitized solar cells. J. Power Sources 2019, 410, 53–58. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, J.; He, B.; Jiao, Z.; Tang, Q. Improved charge extraction with N-doped carbon quantum dots in dye-sensitized solar cells. Electrochim. Acta 2018, 282282, 255–262. [Google Scholar] [CrossRef]
- Mahalingam, S.; Manap, A.; Rabeya, R.; Lau, K.S.; Chia, C.H.; Abdullah, H.; Amin, N.; Chelvanathan, P. Electron transport of chemically treated graphene quantum dots-based dye-sensitized solar cells. Electrochim. Acta 2023, 439, 141667. [Google Scholar] [CrossRef]
- Dinari, M.; Momeni, M.M.; Goudarzirad, M. Dye-sensitized solar cells based on nanocomposite of polyaniline/graphene quantum dots. J. Mater. Sci. 2016, 51, 2964–2971. [Google Scholar] [CrossRef]
- Shejale, K.P.; Jaiswal, A.; Kumar, A.; Saxena, S.; Shukla, S. Nitrogen doped carbon quantum dots as Co-active materials for highly efficient dye sensitized solar cells. Carbon 2021, 183, 169–175. [Google Scholar] [CrossRef]
- Basham JIMor, G.K.; Grimes, C.A. Forster resonance energy transfer in dye-sensitive solar cells. ACS Nano 2010, 4, 1253–1258. [Google Scholar] [CrossRef]
- Mor, G.K.; Basham JPaulo, M.; Kim, S.; Varghese, O.K.; Vaish, A.; Grimes, C.A. High-efficiency forster resonance energy transfer in solid-state dye sensitized solar cells. Nano Lett. 2010, 10, 2387–2394. [Google Scholar] [CrossRef]
- Gordon, F.; Elcoroaristizabal, S.; Ryder, A.G. Modelling Forster resonance energy transfer (FRET) using anisotropy solved multi-dimensional emission spectroscopy (ARMES). Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129770. [Google Scholar]
- Lakowicz, J.R.R. (Ed.) Principles of Fluorescence Spectroscopy; Springer US: Boston, MA, USA, 2006. [Google Scholar]
- Kamat, P.V. Quantum dot solar cells. The next big thing in photovoltaics. J. Phys. Chem. Lett. 2013, 4, 908–918. [Google Scholar] [CrossRef]
- Yuliantini, L.; Paramudita, I.; Pranoto, L.M.; Shobih, S.; Hidayat, J.; Nursam NM, M. Application of Down-conversion Materials based on Trivalent Europium Ion-doped Fluoroborotellurite Glass for Transparent Dye-sensitive Solar Cell. In Proceedings of the 2022 International Conference on Radar, Antenna, Microwave, Electronics, and Telecommunications (ICRAMET), Bandung, Indonesia, 6–7 December 2022; pp. 114–118. [Google Scholar]
- Agrawal, A.; Siddiqui, S.A.; Soni, A.; Khandelwal, K.; Sharma, G.D. Performance analysis of TiO2 based dye sensitized solar cell prepared by screen printing and doctor blade deposition techniques. Sol. Energy 2021, 226, 9–19. [Google Scholar] [CrossRef]
- Raïssi, M.; Pellegrin, Y.; Lefevre, F.X.; Boujtita, M.; Rousseau, D.; Berthelot, T.; Odobel, F. Digital printing of efficient dye-sensitized solar cells (DSSCs). Sol. Energy 2020, 199, 92–99. [Google Scholar] [CrossRef]
- Yildiz, Z.K.; Atilgan, A.B.D.U.L.L.A.H.; Atli, A.; Özel, K.; Altinkaya, C.; Yildiz, A. Enhancement of efficiency of natural and organic dye sensitized solar cells using thin film TiO2 photoanodes fabricated by spin-coating. J. Photochem. Photobiol. A Chem. 2019, 368, 23–29. [Google Scholar] [CrossRef]
- Najm, A.S.; Alwash, S.A.; Sulaiman, N.H.; Chowdhury, M.S.; Techato, K. N719 dye as a sensitizer for dye-sensitized solar cells (DSSCs): A review of its functions and certain rudimentary principles. Environ. Prog. Sustain. Energy 2023, 42, e13955. [Google Scholar] [CrossRef]
- Wang, X.; Bolag, A.; Yun, W.; Du, Y.; Eerdun, C.; Zhang, X.; Bao, T.; Ning, J.; Alata, H.; Ojiyed, T. Enhanced performance of dye-sensitized solar cells based on a dual anchored diphenylpyranylidene dye and N719 co-sensitization. J. Mol. Struct. 2020, 1206, 127694. [Google Scholar] [CrossRef]
- Bera, D.; Qian, L.; Tseng, T.K.; Holloway, P.H. Quantum dots and their multimodal applications: A review. Materials 2010, 3, 2260–2345. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, J.; Chen, J.W.; Hsiao, P.T.; Tung, Y.L.; Chang, C.C.; Chen, C.M.M. Efficiency improvement of dye-sensitized solar cells by in situ fluorescence resonance energy transfer. J. Mater. Chem. A 2017, 5, 9081–9089. [Google Scholar] [CrossRef]
- Mohd Nazree, M.A.N. Reducing Re-Absorption Losses in Luminescent Solar Concentrators (LSC’s) by Using Nanomaterials; IRC: Delft, The Netherlands, 2016. [Google Scholar]
- Liu, Q.; Ding, X.; Pang, Y.; Cao, Y.; Lei, J.; Wu, J.; Zhang, T. New insights into the safety assessment of quantum dots: Potential release pathways, environmental transformations, and health risks. Environ. Sci. Nano 2022, 9, 3277–3311. [Google Scholar] [CrossRef]
- Ren, X.; Zhang, X.; Xie, H.; Cai, J.; Wang, C.; Chen, E.; Guo, T. Perovskite quantum dots for emerging displays: Recent progress and perspectives. Nanomaterials 2022, 12, 2243. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Correia, S.F.; Monguzzi, A.; Liu, X.; Meinardi, F. Spectral converters for photovoltaics–What’s ahead. Mater. Today 2020, 33, 105–121. [Google Scholar] [CrossRef]
- Zhou, D.; Song, H. Phosphors for Solar Cells. In Phosphor Handbook; CRC Press: Boca Raton, FL, USA, 2022; pp. 651–688. [Google Scholar]
- Rehman, A.U.; Khan, M.; Khan, A.D.; Raja, A.A.; Aslam, M.; Khan, S.; Imran, M. The effect of plasmonic multilayered photoanode structures on absorption the of dye-sensitivezed solar cells. Jpn. J. Appl. Phys. 2021, 60, 011004. [Google Scholar] [CrossRef]
- Gnida, P.; Amin, M.F.; Pajok, A.K.; Jarzbek, B. Polymers in High-efficiency solar cells: The latest reports. Polymers 2022, 14, 1946. [Google Scholar] [CrossRef]
- Yun, S.; Qin, Y.; Uhl, A.R.; Vlachopoulos, N.; Yin, M.; Li, D.; Hagfeldt, A. New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- Skandalos, N.; Kapsalis, V.; Ma, T.; Karamanis, D. Towards 30% Efficiency by 2030 of Eco-Designed Building Integrated Photovoltaics. Solar 2023, 3, 434–457. [Google Scholar] [CrossRef]
- Yu, G.; Yang, H.; Luo, D.; Cheng, X.; Ansah, M.K. A review on developments and researches of building integrated photovoltaic (BIPV) windows and shading blinds. Renew. Sustain. Energy Rev. 2021, 149, 111355. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y. Design, development and characterisation of a Building Integrated Concentrating Photovoltaic (BICPV) smart window system. Sol. Energy 2021, 220, 722–734. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Use of Building Integrated Photovoltaic (BIPV): A Significant Step toward Green Buildings. In Energy Security and Sustainability; CRC Press: Boca Raton, FL, USA, 2016; pp. 73–110. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).