Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Solvents
2.2. Sequential Microwave/Ultrasound-Assisted Extraction
2.3. Total Flavonoid Content (TFC)
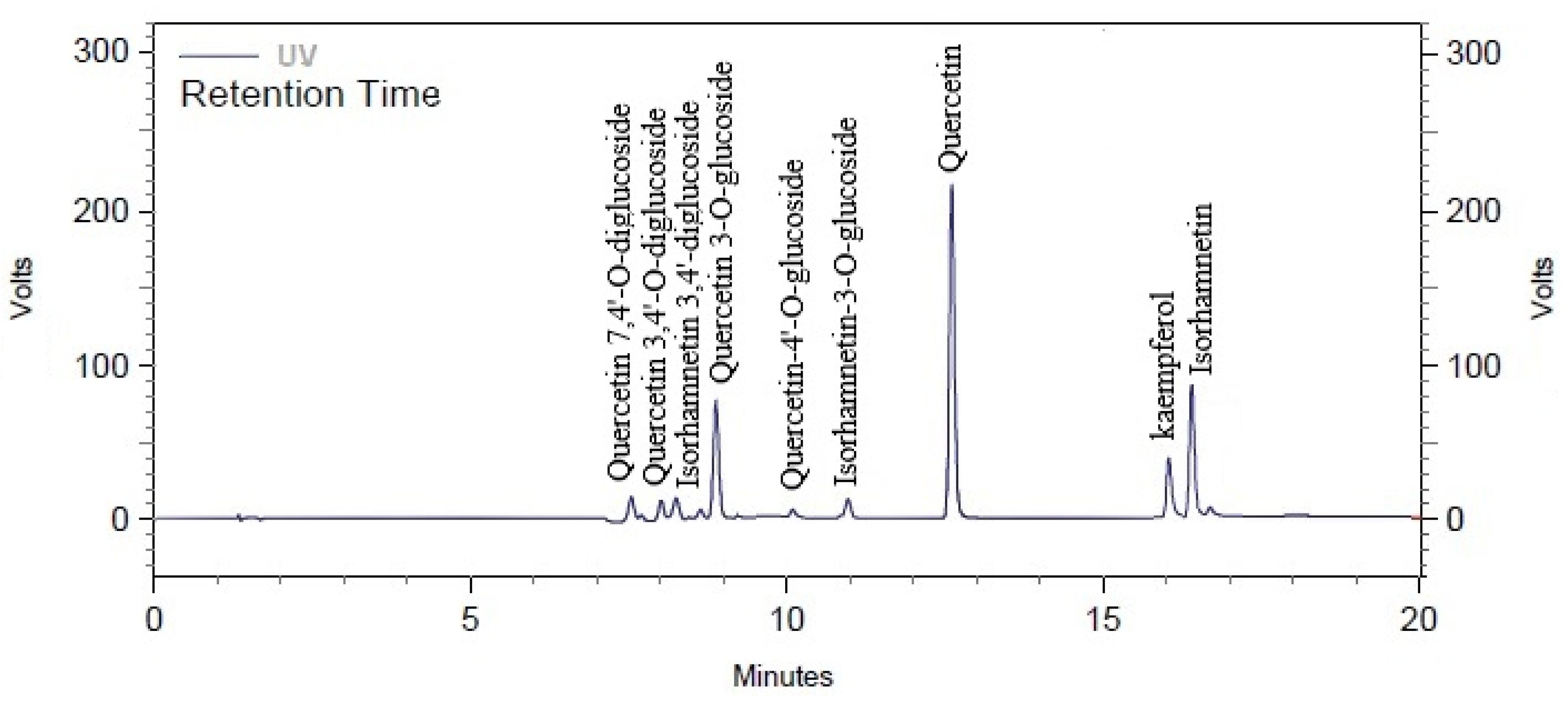
2.4. High-Performance Liquid Chromatography (HPLC)
2.5. Experimental Design for RSM
2.6. Statistical Analysis
3. Results and Discussion
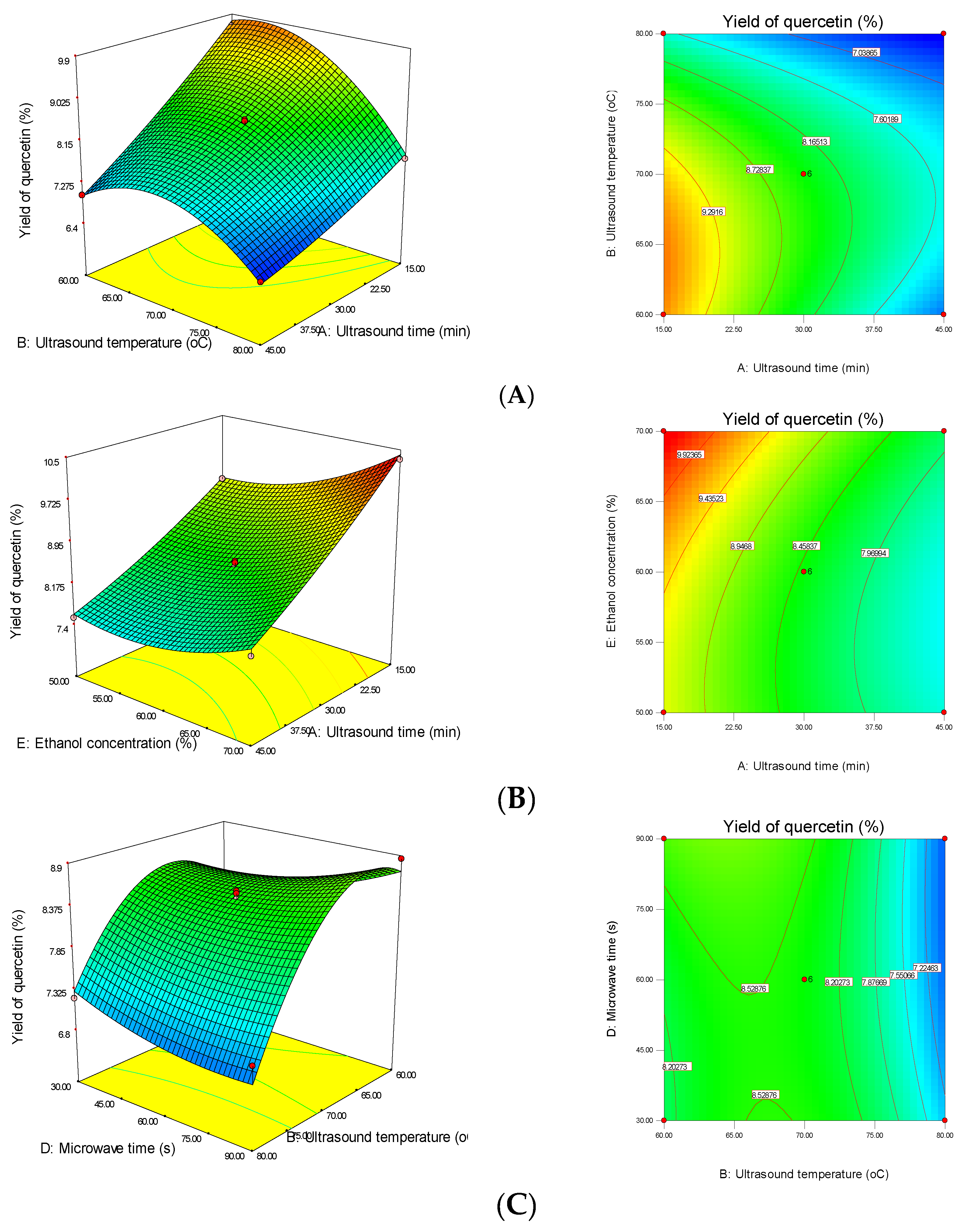
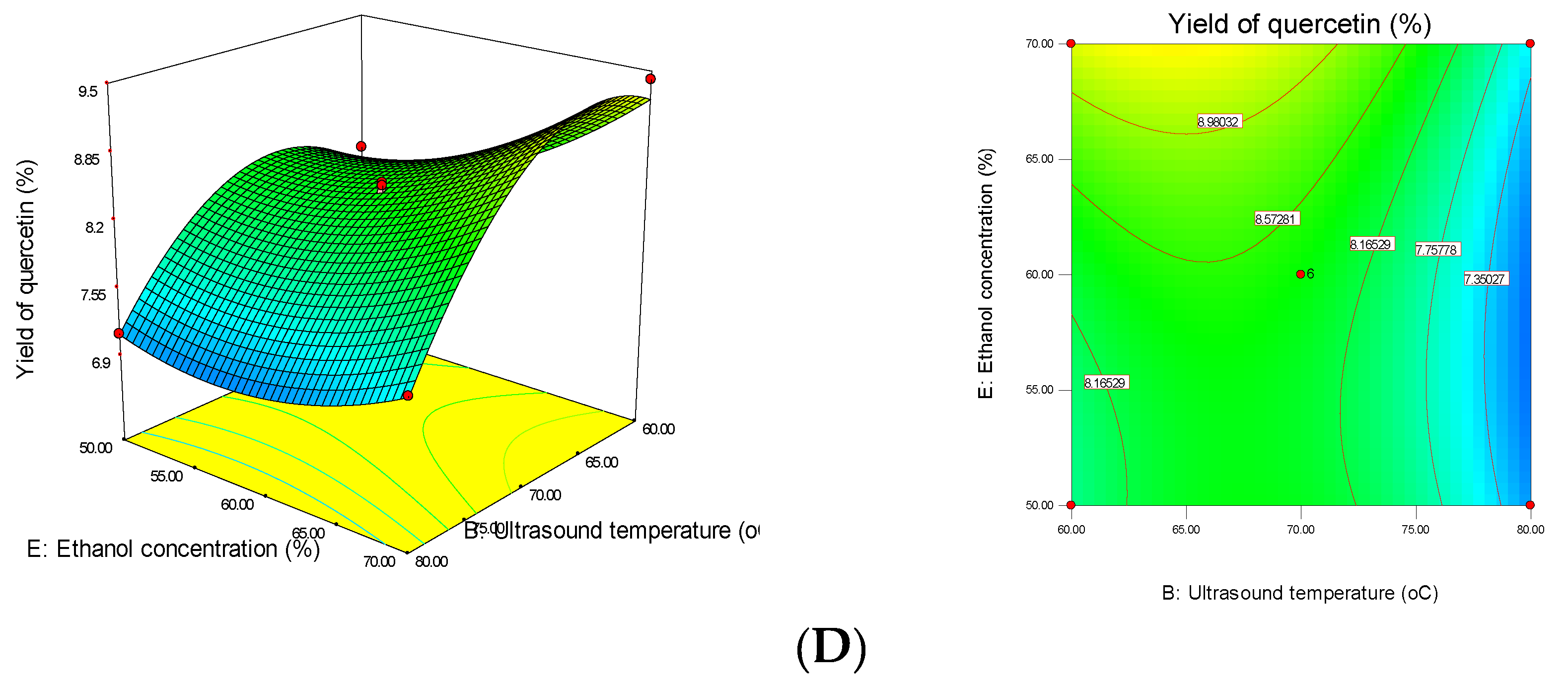
3.1. Effect of MUAE Parameters on the Recovery Yield of Quercetin from DOS
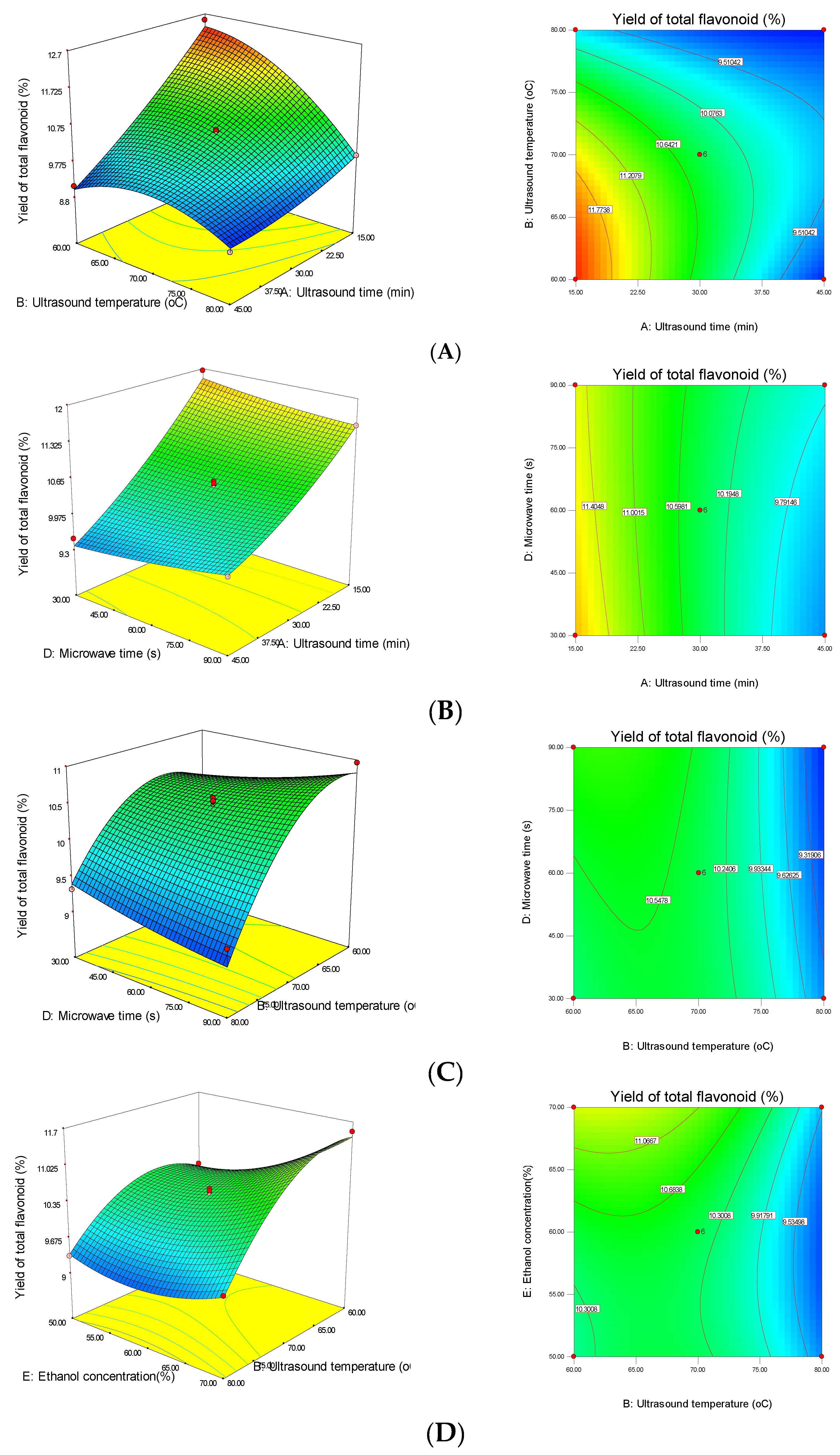
3.2. Effect of MUAE Parameters on the Recovery Yield of Total Flavonoids from DOS
3.3. Interactions between MUAE Factors of Response Surface
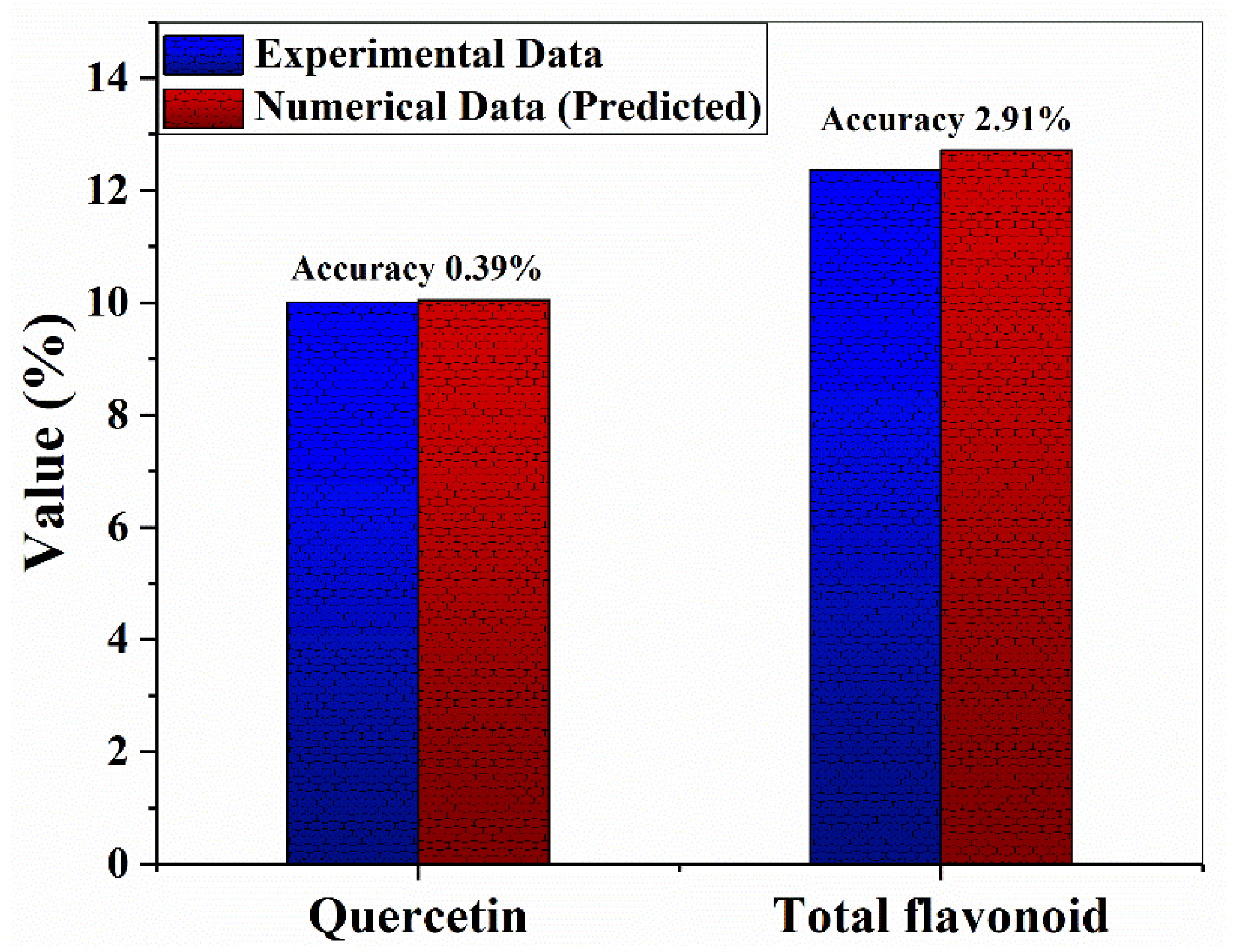
3.4. Validation of the Model and the MUAE Efficiency
3.5. Comparison of the Utilized Extraction Methods in This Study
3.6. Cost Efficiency of Extraction Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nabavi, S.F.; Russo, G.L.; Daglia, M.; Nabavi, S.M. Role of quercetin as an alternative for obesity treatment: You are what you eat! Food Chem. 2015, 179, 305–310. [Google Scholar] [CrossRef] [PubMed]
- HHerranz-López, M.; Olivares-Vicente, M.; Gallego, E.R.; Encinar, J.A.; Pérez-Sánchez, A.; Ruiz-Torres, V.; Joven, J.; Roche, E.; Micol, V. Quercetin metabolites from Hibiscus sabdariffa contribute to alleviate glucolipotoxicity-induced metabolic stress in vitro. Food Chem. Toxicol. 2020, 144, 111606. [Google Scholar] [CrossRef]
- Mohammadinezhad, F.; Talebi, A.; Allahyartorkaman, M.; Nahavandi, R.; Vesal, M.; Khiyavi, A.A. Preparation, characterization and cytotoxic studies of cisplatin-containing nanoliposomes on breast cancer cell lines. Asian Pac. J. Cancer Biol. 2023, 8, 155–159. [Google Scholar] [CrossRef]
- Gorgzadeh, A.; Hheidari, A.; Ghanbarikondori, P.; Arastonejad, M.; Goki, T.G.; Aria, M.; Allahyartorkaman, A.; Moazzam, F. Investigating the properties and cytotoxicity of cisplatin-loaded nano-polybutylcyanoacrylate on breast cancer cells. Asian Pac. J. Cancer Biol. 2023, 8, 345–350. [Google Scholar] [CrossRef]
- Hadisadegh, S.N.; Ghanbarikondori, P.; Sedighi, A.; Afyouni, I.; Javadpour, N.; Ebadi, M. Improving Cancer Therapy: Design, Synthesis, and Evaluation of Carboplatin-Based Nanoliposomes against Breast Cancer Cell Lines. Asian Pac. J. Cancer Biol. 2024, 9, 121–127. [Google Scholar] [CrossRef]
- Maghsoudloo, M.; Bagheri Shahzadeh Aliakbari, R.; Jabbari Velisdeh, Z. Pharmaceutical, nutritional, and cosmetic potentials of saponins and their derivatives. Nano Micro Biosyst. 2023, 2, 1–6. [Google Scholar]
- Shineh, G.; Mobaraki, M.; Afzali, E.; Alakija, F.; Velisdeh, Z.J.; Mills, D.K. Antimicrobial Metal and Metal Oxide Nanoparticles in Bone Tissue Repair. Biomed. Mater. Devices 2024, 2, 918–941. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Kaszuba, K.; Piwowarczyk, K.; Świeca, M.; Dziki, D.; Czyż, J. Onion skin—Raw material for the production of supplement that enhances the health-beneficial properties of wheat bread. Food Res. Int. 2015, 73, 97–106. [Google Scholar] [CrossRef]
- Ryu, R.; Jung, U.J.; Seo, Y.-R.; Kim, H.-J.; Moon, B.S.; Bae, J.-S.; Lee, D.G.; Choi, M.-S. Beneficial effect of persimmon leaves and bioactive compounds on thrombosis. Food Sci. Biotechnol. 2015, 24, 233–240. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidative phenolic constituents of skins of onion varieties and their activities. J. Funct. Foods 2013, 5, 1191–1203. [Google Scholar] [CrossRef]
- Beesk, N.; Perner, H.; Schwarz, D.; George, E.; Kroh, L.W.; Rohn, S. Distribution of quercetin-3, 4′-O-diglucoside, quercetin-4′-O-monoglucoside, and quercetin in different parts of the onion bulb (Allium cepa L.) influenced by genotype. Food Chem. 2010, 122, 566–571. [Google Scholar] [CrossRef]
- Sharifi, F.; Sedighi, A.; Rehman, M. Design and simulation of a point-of-care microfluidic device for acoustic blood cell separation. Eng. Proc. 2020, 2, 76. [Google Scholar] [CrossRef]
- Hatami, A.; Saadatmand, M. Extremely Precise Blood–Plasma Separation from Whole Blood on a Centrifugal Microfluidic Disk (Lab-on-a-Disk) Using Separator Gel. Diagnostics 2022, 12, 2873. [Google Scholar] [CrossRef] [PubMed]
- Hatami, A.; Saadatmand, M.; Garshasbi, M. Cell-free fetal DNA (cffDNA) extraction from whole blood by using a fully automatic centrifugal microfluidic device based on displacement of magnetic silica beads. Talanta 2024, 267, 125245. [Google Scholar] [CrossRef]
- Nishad, J.; Saha, S.; Kaur, C. Enzyme-and ultrasound-assisted extractions of polyphenols from Citrus sinensis (cv. Malta) peel: A comparative study. J. Food Process. Preserv. 2019, 43, e14046. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Piccinelli, A.L.; Pagano, I.; Carabetta, S.; Di Sanzo, R.; Russo, M.; Ibañez, E.; Cifuentes, A.; Rastrelli, L. Response surface methodology to optimize supercritical carbon dioxide/co-solvent extraction of brown onion skin by-product as source of nutraceutical compounds. Food Chem. 2018, 269, 495–502. [Google Scholar] [CrossRef]
- Relić, D.; Héberger, K.; Sakan, S.; Škrbić, B.; Popović, A.; Đorđević, D. Ranking and similarity of conventional, microwave and ultrasound element sequential extraction methods. Chemosphere 2018, 198, 103–110. [Google Scholar] [CrossRef]
- Jin, E.Y.; Lim, S.; Kim, S.O.; Park, Y.-S.; Jang, J.K.; Chung, M.-S.; Park, H.; Shim, K.-S.; Choi, Y.J. Optimization of various extraction methods for quercetin from onion skin using response surface methodology. Food Sci. Biotechnol. 2011, 20, 1727–1733. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Ćujić-Nikolić, N.; Janković, T. Optimization of ultrasound-assisted extraction parameters for improving content of acteoside, luteolin-7-O-glucoside, and total polyphenols in extracts of Plantago lanceolata aerial parts. J. Food Process. Preserv. 2021, 45, e15866. [Google Scholar] [CrossRef]
- Ngo, T.V.; Scarlett, C.J.; Bowyer, M.C.; Vuong, Q.V. Isolation and Maximisation of Extraction of Mangiferin from the Root of Salacia chinensis L. Separations 2019, 6, 44. [Google Scholar] [CrossRef]
- Velisdeh, Z.J.; Najafpour, G.D.; Mohammadi, M.; Poureini, F. Optimization of Sequential Microwave-Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Wastes. arXiv 2021, arXiv:210406109. [Google Scholar]
- Rodrigues, L.M.; Romanini, E.B.; Silva, E.; Pilau, E.J.; da Costa, S.C.; Madrona, G.S. Camu-camu bioactive compounds extraction by ecofriendly sequential processes (ultrasound assisted extraction and reverse osmosis). Ultrason. Sonochem. 2020, 64, 105017. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mu, T.; Sun, H.; Fauconnier, M.L. Optimization of ultrasonic–microwave synergistic extraction of flavonoids from sweet potato leaves by response surface methodology. J. Food Process. Preserv. 2019, 43, e13928. [Google Scholar] [CrossRef]
- Zeković, Z.; Pintać, D.; Majkić, T.; Vidović, S.; Mimica-Dukić, N.; Teslić, N.; Versari, A.; Pavlić, B. Utilization of sage by-products as raw material for antioxidants recovery—Ultrasound versus microwave-assisted extraction. Ind. Crops Prod. 2017, 99, 49–59. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, D.; Gao, R.; Gao, L. Experimental and modeling analysis of flavones extraction from Vaccinium bracteatum Thunb. leaves by ultrasound and microwave assisted simultaneously. Chem. Pap. 2019, 73, 783–790. [Google Scholar] [CrossRef]
- Wizi, J.; Wang, L.; Hou, X.; Tao, Y.; Ma, B.; Yang, Y. Ultrasound-microwave assisted extraction of natural colorants from sorghum husk with different solvents. Ind. Crops Prod. 2018, 120, 203–213. [Google Scholar] [CrossRef]
- Gorgani, L.; Mohammadi, M.; Najafpour, G.D.; Nikzad, M. Sequential microwave-ultrasound-assisted extraction for isolation of Piperine from Black Pepper (Piper nigrum L.). Food Bioproc Technol. 2017, 10, 2199–2207. [Google Scholar] [CrossRef]
- Dorneles, M.S.; Noreña, C.P.Z. Microwave-assisted extraction of bioactive compounds from Araucaria angustifolia bracts followed by encapsulation. J. Food Process. Preserv. 2020, 44, e14484. [Google Scholar]
- Li, C.; Liu, S.; Song, Y.; Nie, K.; Ben, H.; Zhang, Y.; Han, G.; Jiang, W. A facile and eco-friendly method to extract Apocynum venetum fibers using microwave-assisted ultrasonic degumming. Ind. Crops Prod. 2020, 151, 112443. [Google Scholar] [CrossRef]
- De la Lama-Calvente, D.; Fernández-Rodríguez, M.J.; Garrido-Fernández, A.; Borja, R. Process optimization of the extraction of reducing sugars and total phenolic compounds from the invasive alga Rugulopteryx okamurae by response surface methodology (RSM). Algal Res. 2024, 80, 103500. [Google Scholar] [CrossRef]
- Shehata, M.G.; Abd El Aziz, N.M.; Youssef, M.M.; El-Sohaimy, S.A. Optimization conditions of ultrasound-assisted extraction of phenolic compounds from orange peels using response surface methodology. J. Food Process. Preserv. 2021, 45, e15870. [Google Scholar] [CrossRef]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Olszewska, M. Separation of quercetin, sexangularetin, kaempferol and isorhamnetin for simultaneous HPLC determination of flavonoid aglycones in inflorescences, leaves and fruits of three Sorbus species. J. Pharm. Biomed. Anal. 2008, 48, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Huang, W.; Xue, A.; Niu, H.; Jia, Z.; Wang, J. Optimised ultrasonic-assisted extraction of flavonoids from Folium eucommiae and evaluation of antioxidant activity in multi-test systems in vitro. Food Chem. 2009, 114, 1147–1154. [Google Scholar] [CrossRef]
- Mansur, A.R.; Song, N.E.; Jang, H.W.; Lim, T.G.; Yoo, M.; Nam, T.G. Optimizing the ultrasound-assisted deep eutectic solvent extraction of flavonoids in common buckwheat sprouts. Food Chem. 2019, 293, 438–445. [Google Scholar] [CrossRef]
- Ben-Ali, S.; Akermi, A.; Mabrouk, M.; Ouederni, A. Optimization of extraction process and chemical characterization of pomegranate peel extract. Chem. Pap. 2018, 72, 2087–2100. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, G.; Khan, M.A.; Yan, Z.; Beta, T. Ultrasonic-assisted enzymatic extraction and identification of anthocyanin components from mulberry wine residues. Food Chem. 2020, 323, 126714. [Google Scholar] [CrossRef]
- Kia, A.G.; Ganjloo, A.; Bimakr, M. A short extraction time of polysaccharides from fenugreek (Trigonella foencem graecum) seed using continuous ultrasound acoustic cavitation: Process optimization, characterization and biological activities. Food Bioprocess Technol. 2018, 11, 2204–2216. [Google Scholar] [CrossRef]
- Mushtaq, A.; Roobab, U.; Denoya, G.I.; Inam-Ur-Raheem, M.; Gullón, B.; Lorenzo, J.M.; Barba, F.J.; Zeng, X.; Wali, A.; Aadil, R.M. Advances in green processing of seed oils using ultrasound-assisted extraction: A review. J. Food Process. Preserv. 2020, 44, e14740. [Google Scholar] [CrossRef]
- Jenča, A.; Mills, D.K.; Ghasemi, H.; Saberian, E.; Forood, A.M.K.; Petrášová, A.; Jenčová, J.; Velisdeh, Z.J.; Zare-Zardini, H.; Ebrahimifar, M. Herbal Therapies for Cancer Treatment: A Review of Phytotherapeutic Efficacy. Biol. Targets Ther. 2024, 18, 229–255. [Google Scholar] [CrossRef]

| Variables | Codes | Units | Levels | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Ultrasound time | X1 | Min | 15 | 30 | 45 |
| Ultrasound temperature | X2 | °C | 60 | 70 | 80 |
| Solvent-to-solid ratio | X3 | mL/g | 20 | 30 | 40 |
| Microwave time | X4 | S | 30 | 60 | 90 |
| Ethanol concentration | X5 | % (v/v, water) | 50 | 60 | 70 |
| Run | Independent Variables | Responses (Dependent Variables) | |||||
|---|---|---|---|---|---|---|---|
| X1: US Time (min) | X2: US Temp (°C) | X3: S/S Ratio (mL/g) | X4: MW Time (min) | X5: Ethanol Conc % (v/v, Water) | Y1: Quercetin Yield (%) | Y2: Flavonoid Yield (%) | |
| 1 | −1 | −1 | 0 | 0 | 0 | 9.63 ± 0.16 | 12.52 ± 0.21 |
| 2 | 0 | −1 | 0 | 0 | 1 | 9.42 ± 0.28 | 11.52 ± 0.06 |
| 3 | 0 | −1 | −1 | 0 | 0 | 8.41 ± 0.09 | 10.41 ± 0.7 |
| 4 | 1 | 0 | 0 | −1 | 0 | 7.64 ± 0.74 | 9.52 ± 0.95 |
| 5 | 0 | 0 | 0 | 0 | 0 | 8.51 ± 0.40 | 10.49 ± 0.8 |
| 6 | 0 | 0 | 1 | −1 | 0 | 8.79 ± 0.47 | 10.74 ± 0.41 |
| 7 | 0 | 0 | 0 | 0 | 0 | 8.35 ± 0.27 | 10.53 ± 0.16 |
| 8 | −1 | 0 | 0 | 0 | −1 | 9.21 ± 0.81 | 11.32 ± 0.43 |
| 9 | −1 | 0 | 0 | 1 | 0 | 9.43 ± 0.62 | 11.50 ± 0.68 |
| 10 | 1 | −1 | 0 | 0 | 0 | 7.01 ± 0.45 | 9.11 ± 0.5 |
| 11 | 0 | 1 | 0 | −1 | 0 | 7.21 ± 0.12 | 9.32 ± 0.35 |
| 12 | 0 | 0 | 1 | 0 | 1 | 9.51 ± 0.6 | 11.74 ± 0.69 |
| 13 | 1 | 0 | 0 | 0 | 1 | 7.96 ± 0.87 | 9.94 ± 0.78 |
| 14 | 0 | 0 | −1 | 1 | 0 | 8.75 ± 0.44 | 10.69 ± 0.08 |
| 15 | 0 | 0 | −1 | −1 | 0 | 8.62 ± 0.7 | 10.53 ± 0.14 |
| 16 | 0 | 1 | 1 | 0 | 0 | 7.27 ± 0.69 | 9.44 ± 0.26 |
| 17 | 1 | 1 | 0 | 0 | 0 | 6.51 ± 0.51 | 8.84 ± 0.82 |
| 18 | 0 | 0 | 0 | 1 | −1 | 8.42 ± 0.42 | 10.30 ± 0.35 |
| 19 | 0 | 0 | 0 | 0 | 0 | 8.40 ± 0.7 | 10.48 ± 0.54 |
| 20 | 0 | 0 | 0 | 1 | 1 | 9.19 ± 0.31 | 11.08 ± 0.3 |
| 21 | −1 | 1 | 0 | 0 | 0 | 7.58 ± 0.19 | 9.71 ± 0.27 |
| 22 | −1 | 0 | 0 | −1 | 0 | 9.92 ± 0.36 | 11.96 ± 0.46 |
| 23 | 0 | 1 | 0 | 1 | 0 | 7.13 ± 0.09 | 9.24 ± 0.79 |
| 24 | 1 | 0 | 0 | 0 | −1 | 7.52 ± 0.55 | 9.78 ± 0.61 |
| 25 | 0 | 0 | 1 | 1 | 0 | 9.16 ± 0.12 | 10.75 ± 0.66 |
| 26 | 0 | 0 | −1 | 0 | 1 | 9.46 ± 0.94 | 11.35 ± 0.29 |
| 27 | 0 | 1 | −1 | 0 | 0 | 7.23 ± 0.8 | 9.40 ± 0.76 |
| 28 | −1 | 0 | 0 | 0 | 1 | 10.32 ± 0.3 | 12.03 ± 0.51 |
| 29 | 1 | 0 | 1 | 0 | 0 | 7.95 ± 0.22 | 9.93 ± 0.37 |
| 30 | 0 | 0 | 1 | 0 | −1 | 8.61 ± 0.71 | 10.62 ± 0.63 |
| 31 | 0 | 0 | 0 | 0 | 0 | 8.41 ± 0.17 | 10.35 ± 0.51 |
| 32 | 0 | −1 | 0 | 1 | 0 | 8.86 ± 0.23 | 10.96 ± 0.78 |
| 33 | −1 | 0 | 1 | 0 | 0 | 10.01 ± 0.35 | 12.01 ± 0.09 |
| 34 | 0 | −1 | 0 | −1 | 0 | 7.95 ± 0.77 | 10.11 ± 0.8 |
| 35 | 0 | 0 | 0 | −1 | 1 | 9.34 ± 0.52 | 11.16 ± 0.74 |
| 36 | −1 | 0 | −1 | 0 | 0 | 9.98 ± 0.64 | 11.91 ± 0.05 |
| 37 | 0 | −1 | 0 | 0 | −1 | 8.11 ± 0.94 | 10.23 ± 0.17 |
| 38 | 1 | 0 | 0 | 1 | 0 | 7.77 ± 0.9 | 9.81 ± 0.39 |
| 39 | 0 | 1 | 0 | 0 | −1 | 7.11 ± 0.18 | 9.33 ± 0.14 |
| 40 | 1 | 0 | −1 | 0 | 0 | 7.84 ± 0.75 | 9.67 ± 0.5 |
| 41 | 0 | −1 | 1 | 0 | 0 | 8.52 ± 0.4 | 10.79 ± 0.25 |
| 42 | 0 | 1 | 0 | 0 | 1 | 7.47 ± 0.05 | 9.57 ± 0.58 |
| 43 | 0 | 0 | 0 | 0 | 0 | 8.48 ± 0.92 | 10.40 ± 0.06 |
| 44 | 0 | 0 | 0 | 0 | 0 | 8.31 ± 0.24 | 10.25 ± 0.28 |
| 45 | 0 | 0 | −1 | 0 | −1 | 8.62 ± 0.30 | 10.73 ± 0.69 |
| 46 | 0 | 0 | 0 | −1 | −1 | 8.43 ± 0.51 | 10.38 ± 0.16 |
| Source | Sum of Squares | df. | Mean Square | F-Value | p-Value | Significance | |
|---|---|---|---|---|---|---|---|
| Model | Y1 | 36.97 | 20 | 1.85 | 85.15 | <0.0001 | ** |
| X1 | Y1 | 15.76 | 1 | 15.76 | 726.04 | <0.0001 | ** |
| X2 | Y1 | 6.76 | 1 | 6.76 | 311.41 | <0.0001 | ** |
| X3 | Y1 | 0.13 | 1 | 0.13 | 3.15 | 0.0542 | |
| X4 | Y1 | 0.10 | 1 | 0.10 | 4.91 | 0.0614 | |
| X5 | Y1 | 2.76 | 1 | 2.76 | 126.94 | <0.0001 | ** |
| X1X2 | Y1 | 0.60 | 1 | 0.60 | 27.67 | <0.0001 | ** |
| X1X4 | Y1 | 0.09 | 1 | 0.096 | 4.43 | 0.0456 | * |
| X1X5 | Y1 | 0.11 | 1 | 0.11 | 5.17 | 0.0318 | * |
| X2X4 | Y1 | 0.25 | 1 | 0.25 | 11.29 | 0.0025 | * |
| X2X5 | Y1 | 0.23 | 1 | 0.23 | 10.39 | 0.0035 | * |
| X12 | Y1 | 0.11 | 1 | 0.11 | 5.11 | 0.0328 | * |
| X22 | Y1 | 5.48 | 1 | 5.48 | 252.36 | <0.0001 | ** |
| X32 | Y1 | 0.88 | 1 | 0.88 | 40.37 | <0.0001 | ** |
| X42 | Y1 | 0.17 | 1 | 0.17 | 7.90 | 0.0095 | * |
| X52 | Y1 | 0.86 | 1 | 0.86 | 39.73 | <0.0001 | ** |
| Residual | Y1 | 0.54 | 25 | 0.022 | |||
| Lack of Fit | Y1 | 0.51 | 20 | 0.026 | 4.49 | 0.0515 | Not significant |
| Pure Error | Y1 | 0.029 | 5 | 5.72 × 10−3 | |||
| Cor Total | Y1 | 37.51 | 45 | ||||
| Std. Dev. | Y1 | 0.15 | |||||
| R2 | Y1 | 0.9855 | |||||
| Adjusted R2 | Y1 | 0.9740 | |||||
| Predicted R2 | Y1 | 0.9441 | |||||
| Mean | Y1 | 8.44 | |||||
| C.V. % | Y1 | 1.75 | |||||
| Source | Sum of Squares | df. | Mean Square | F-Value | p-Value | Significance | |
|---|---|---|---|---|---|---|---|
| Model | Y2 | 34.93 | 20 | 1.75 | 65.18 | <0.0001 | ** |
| X1 | Y2 | 16.73 | 1 | 16.73 | 624.25 | <0.0001 | ** |
| X2 | Y2 | 7.29 | 1 | 7.29 | 272.05 | <0.0001 | ** |
| X3 | Y2 | 0.16 | 1 | 0.16 | 3.96 | 0.0621 | |
| X4 | Y2 | 0.09 | 1 | 0.09 | 4.65 | 0.0592 | |
| X5 | Y2 | 2.03 | 1 | 2.03 | 75.78 | <0.0001 | ** |
| X1X2 | Y2 | 1.61 | 1 | 1.61 | 60.19 | <0.0001 | ** |
| X1X4 | Y2 | 0.14 | 1 | 0.14 | 5.25 | 0.0307 | * |
| X1X5 | Y2 | 0.07 | 1 | 0.076 | 2.82 | 0.1054 | |
| X2X4 | Y2 | 0.22 | 1 | 0.22 | 8.07 | 0.0088 | * |
| X2X5 | Y2 | 0.28 | 1 | 0.28 | 10.29 | 0.0037 | * |
| X12 | Y2 | 0.26 | 1 | 0.26 | 9.71 | 0.0046 | * |
| X22 | Y2 | 2.96 | 1 | 2.96 | 110.43 | <0.0001 | ** |
| X32 | Y2 | 0.63 | 1 | 0.63 | 23.49 | <0.0001 | ** |
| X42 | Y2 | 0.01 | 1 | 0.019 | 0.72 | 0.4056 | |
| X52 | Y2 | 0.78 | 1 | 0.78 | 29.19 | <0.0001 | ** |
| Residual | Y2 | 0.67 | 25 | 0.027 | |||
| Lack of Fit | Y2 | 0.62 | 20 | 0.031 | 2.81 | 0.1276 | Not significant |
| Pure Error | Y2 | 0.055 | 5 | 1.10 × 10−2 | |||
| Cor Total | Y2 | 35.60 | 45 | ||||
| Std. Dev. | Y2 | 0.16 | |||||
| R2 | Y2 | 0.9812 | |||||
| Adjusted R2 | Y2 | 0.9661 | |||||
| Predicted R2 | Y2 | 0.9287 | |||||
| Mean | Y2 | 10.49 | |||||
| C.V. % | Y2 | 1.56 | |||||
| Extraction Method | QE (%) | TFC (%) |
|---|---|---|
| MAC | 2.96 ± 0.03 f | 3.64 ± 0.02 f |
| SOX | 3.09 ± 0.05 e | 5.24 ± 0.03 e |
| MAE | 5.03 ± 0.02 d | 7.91 ± 0.06 d |
| UAE | 5.36 ± 0.0 c | 8.34 ± 0.05 c |
| UMAE | 7.66 ± 0.04 b | 10.18 ± 0.14 b |
| MUAE | 10.32 ± 0.18 a | 12.52 ± 0.20 a |
| Compound | Chemical Formula | Molecular Weight (g/mol) | Retention Time (min) | Concentration (%) |
|---|---|---|---|---|
| Quercetin 7,4′-O-diglucoside | C27H30O17 | 626.5 | 7.10 | 0.65 ± 0.02 c |
| Quercetin 3,4′-O-diglucoside | C27H30O17 | 626.5 | 7.40 | 0.73 ± 0.02 c |
| Isorhamnetin 3,4′-diglucoside | C28H32O17 | 640.5 | 7.60 | ND |
| Unknown | 8.20 | ND | ||
| Quercetin 3-O-glucoside | C21H20O12 | 464.4 | 8.44 | 0.36 ± 0.01 c |
| Quercetin-4′-O-glucoside | C21H20O12 | 464.4 | 10.50 | 3.15 ± 1.20 b |
| Isorhamnetin-3-O-glucoside | C22H22O12 | 478.4 | 12.60 | ND |
| Quercetin | C15H10O7 | 302.23 | 13.60 | 5.43 ± 0.06 a |
| kaempferol | C15H10O6 | 286.24 | 16.50 | 0.46 ± 0.08 c |
| Isorhamnetin | C16H12O7 | 316.26 | 16.80 | ND |
| Unknown | 17.20 | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velisdeh, Z.J.; Najafpour Darzi, G.; Poureini, F.; Mohammadi, M.; Sedighi, A.; Bappy, M.J.P.; Ebrahimifar, M.; Mills, D.K. Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste. Appl. Sci. 2024, 14, 9225. https://doi.org/10.3390/app14209225
Velisdeh ZJ, Najafpour Darzi G, Poureini F, Mohammadi M, Sedighi A, Bappy MJP, Ebrahimifar M, Mills DK. Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste. Applied Sciences. 2024; 14(20):9225. https://doi.org/10.3390/app14209225
Chicago/Turabian StyleVelisdeh, Zeinab Jabbari, Ghasem Najafpour Darzi, Fatemeh Poureini, Maedeh Mohammadi, Armin Sedighi, Mohammad Jabed Perves Bappy, Meysam Ebrahimifar, and David K. Mills. 2024. "Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste" Applied Sciences 14, no. 20: 9225. https://doi.org/10.3390/app14209225
APA StyleVelisdeh, Z. J., Najafpour Darzi, G., Poureini, F., Mohammadi, M., Sedighi, A., Bappy, M. J. P., Ebrahimifar, M., & Mills, D. K. (2024). Turning Waste into Wealth: Optimization of Microwave/Ultrasound-Assisted Extraction for Maximum Recovery of Quercetin and Total Flavonoids from Red Onion (Allium cepa L.) Skin Waste. Applied Sciences, 14(20), 9225. https://doi.org/10.3390/app14209225








