The Behavior of Catalytic, Low-Temperature N2O Decomposition (LT-deN2O) in the Presence of Sulfur-Containing Compounds on Nitric Acid Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Activity of the Catalyst
2.2.1. Activity of the Catalyst in the Pilot-Scale Studies
2.2.2. Activity of the Catalyst after Operation in Trials
2.3. Physico-Chemical Characterization of the Catalyst
2.3.1. X-ray Fluorescence (XRF) Spectroscopy
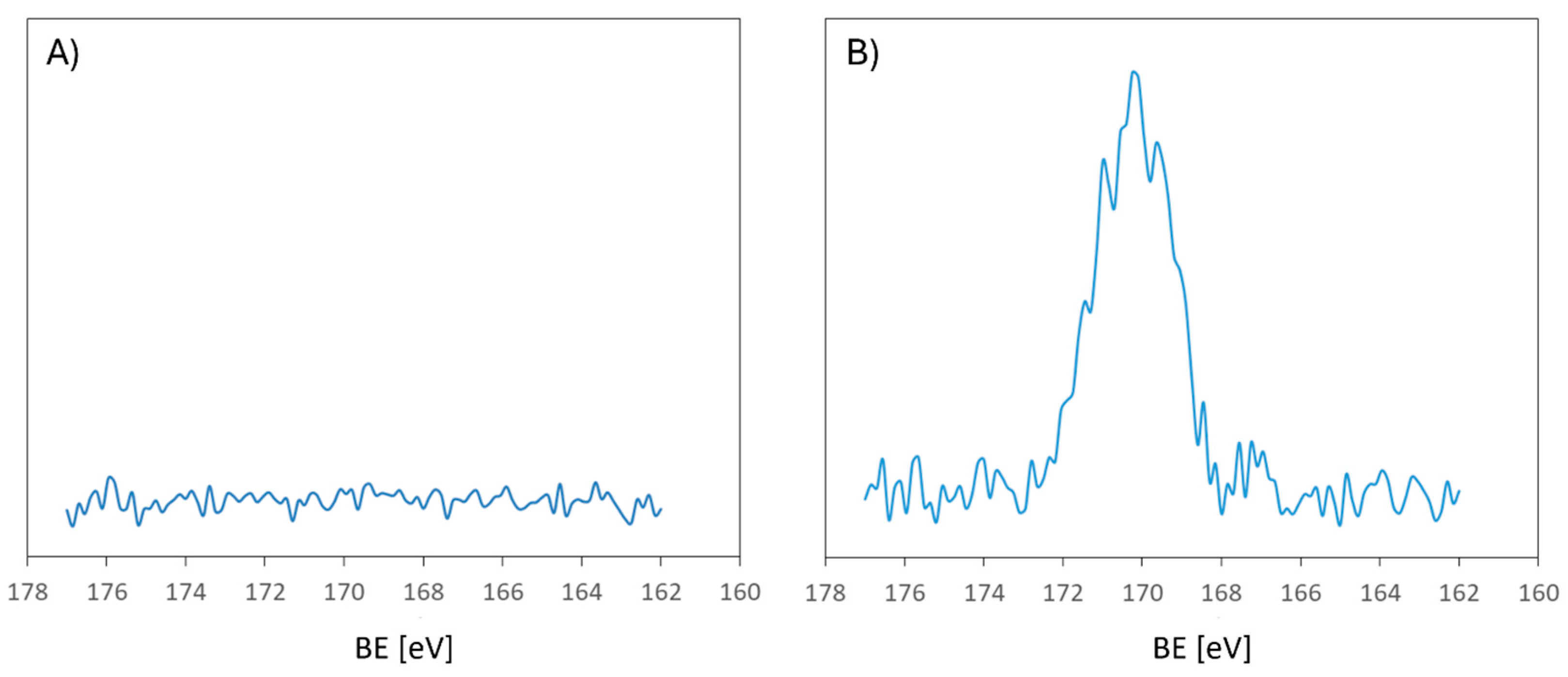
2.3.2. X-ray Photoelectron Spectroscopy (XPS)
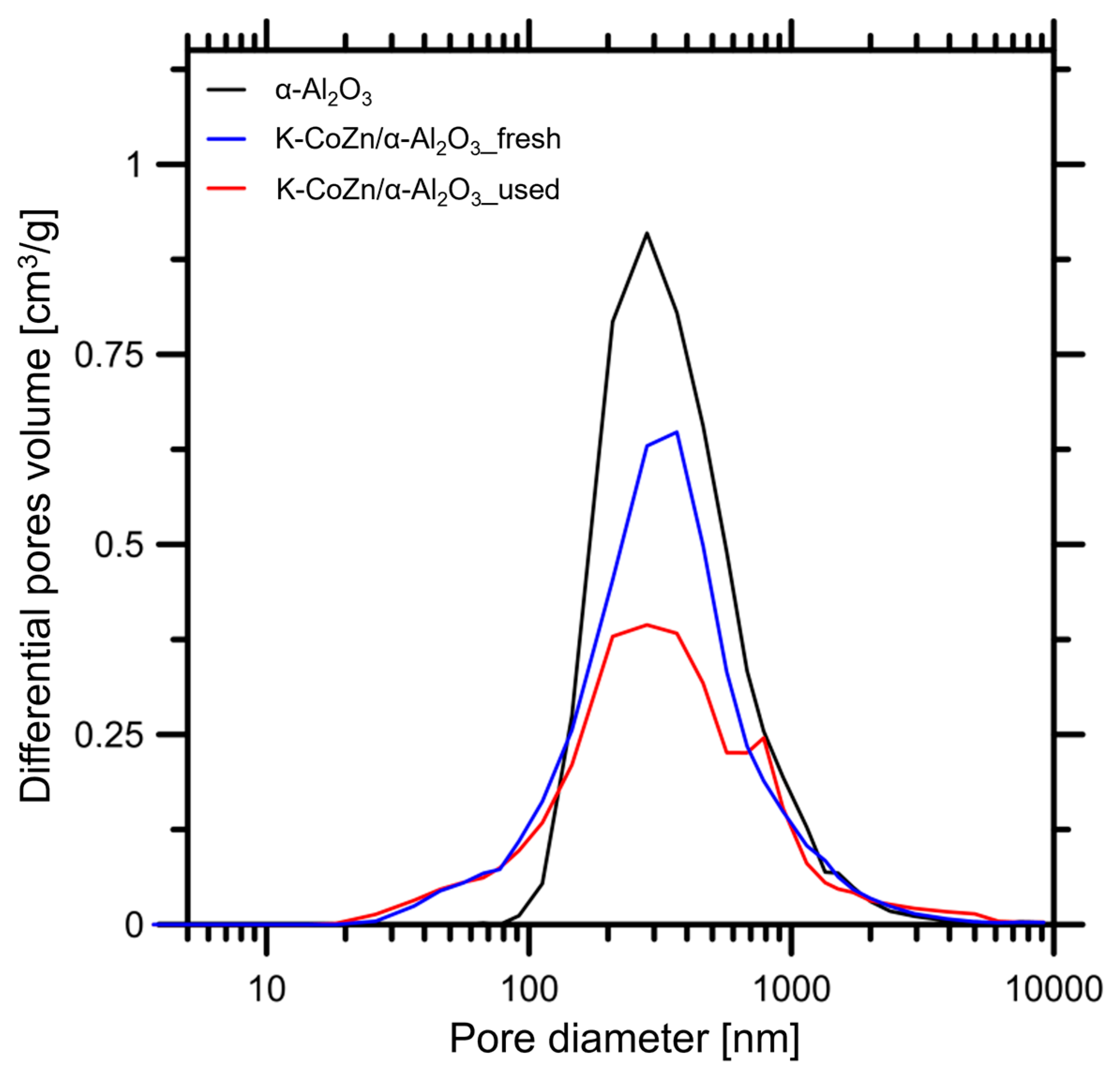
2.3.3. Porosity Measurement
3. Results and Discussion
3.1. Catalytic Activity
3.2. Characterization of the Catalyst
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pérez-Ramırez, J.; Kapteijn, F.; Schöffel, K.; Moulijn, J.A. Formation and Control of N2O in Nitric Acid Production. Appl. Catal. B 2003, 44, 117–151. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, X.; Beaurain, A.; Dujardin, C.; Granger, P. Stoichiometric and Non-Stoichiometric Perovskite-Based Catalysts: Consequences on Surface Properties and on Catalytic Performances in the Decomposition of N2O from Nitric Acid Plants. Appl. Catal. B 2012, 125, 149–157. [Google Scholar] [CrossRef]
- Sádovská, G.; Tabor, E.; Sazama, P.; Lhotka, M.; Bernauer, M.; Sobalík, Z. High Temperature Performance and Stability of Fe-FER Catalyst for N2O Decomposition. Catal. Commun. 2017, 89, 133–137. [Google Scholar] [CrossRef]
- Kuboňová, L.; Fridrichová, D.; Wach, A.; Kuśtrowski, P.; Obalová, L.; Cool, P. Catalytic Activity of Rhodium Grafted on Ordered Mesoporous Silica Materials Modified with Aluminum in N2O Decomposition. Catal. Today 2015, 257, 51–58. [Google Scholar] [CrossRef]
- Isupova, L.A.; Ivanova, Y.A. Removal of Nitrous Oxide in Nitric Acid Production. Kinet. Catal. 2019, 60, 744–760. [Google Scholar] [CrossRef]
- Kavanaugh, M. Estimates of Future Co, N2O and NOx Emissions from Energy Combustion. Atmos. Environ. (1967) 1987, 21, 463–468. [Google Scholar] [CrossRef]
- Ravishankara, A.R.; Daniel, J.S.; Portmann, R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Revell, L.E.; Bodeker, G.E.; Smale, D.; Lehmann, R.; Huck, P.E.; Williamson, B.E.; Rozanov, E.; Struthers, H.; Bodeker, E.; Smale, D.; et al. The Effectiveness of N2O in Depleting Stratospheric Ozone. Geophys. Res. Lett. 2012, 39, 15806. [Google Scholar] [CrossRef]
- Kondratenko, E.V.; Kondratenko, V.A.; Santiago, M.; Pérez-Ramírez, J. Mechanism and Micro-Kinetics of Direct N2O Decomposition over BaFeAl11O19 Hexaaluminate and Comparison with Fe-MFI Zeolites. Appl. Catal. B 2010, 99, 66–73. [Google Scholar] [CrossRef]
- Houghton, J.T.; Ding, Y.; Griggs, D.J.; Noguer, M.; Van Der Linden, P.J.; Dai, X.; Maskell, K.; Johnson, C.A. Climate Change 2001: The Scientific Basis; Contribution of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change Published for the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- van den Brink, R.W.; Booneveld, S.; Verhaak, M.J.F.M.; de Bruijn, F.A. Selective Catalytic Reduction of N2O and NO in a Single Reactor in the Nitric Acid Industry. Catal. Today 2002, 75, 227–232. [Google Scholar] [CrossRef]
- Inger, M.; Moszowski, B.; Ruszak, M.; Rajewski, J.; Wilk, M. Two-Stage Catalytic Abatement of N2O Emission in Nitric Acid Plants. Catalysts 2020, 10, 987. [Google Scholar] [CrossRef]
- Pinaeva, L.G.; Prosvirin, I.P.; Dovlitova, L.S.; Danilova, I.G.; Sadovskaya, E.M.; Isupova, L.A. MeOx/Al2O3 and MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts for High Temperature N2O Decomposition and NH3 Oxidation. Catal. Sci. Technol. 2016, 6, 2150–2161. [Google Scholar] [CrossRef]
- Kamphus, M. Emission Monitoring in Nitric Acid Plants. Nitrogen+Syngas 2014, 328, 48–53. [Google Scholar]
- Inger, M.; Antoniak-Jurak, K.; Ruszak, M.; Kowalik, P.; Wilk, M. Dobór Nośnika Tlenkowego Dla Katalizatora Do Niskotemperaturowego Rozkładu N2O. CHEMIK 2016, 70, 255–260. [Google Scholar]
- Thiemann, M.; Scheibler, E.; Wiegand, K.W. Nitric Acid, Nitrous Acid, and Nitrogen Oxides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Capała, P.; Ruszak, M.; Rudawska, A.; Inger, M.; Wilk, M. The Technology of Tail Gases Purifying in Nitric Acid Plants and Design of DeN2O and DeNOx Reactors—Review. Appl. Sci. 2023, 13, 7492. [Google Scholar] [CrossRef]
- KOBiZE. Raport z Rynku CO2; KOBiZE: Warsaw, Poland, 2023. [Google Scholar]
- Bańkowska, K. Światowe Porozumienie Klimatyczne a Rozwój Obszarów Wiejskich. Wieś i Rol. 2016, 710, 87–103. [Google Scholar] [CrossRef]
- Luboińska, U. Emisja Gazów Cieplarnianych. Wybrane Zagadnienia Dotyczące Emisji CO2 w Polsce; Kancelaria Senatu: Warsaw, Poland, 2020. [Google Scholar]
- Kim, M.J.; Lee, S.J.; Ryu, I.S.; Jeon, M.W.; Moon, S.H.; Roh, H.S.; Jeon, S.G. Catalytic Decomposition of N2O over Cobalt Based Spinel Oxides: The Role of Additives. Mol. Catal. 2017, 442, 202–207. [Google Scholar] [CrossRef]
- Jabłońska, E.M.; Buselli, L.; Nocuń, E.M.; Palkovits, R. Silver-Doped Cobalt (Magnesium) Aluminum Mixed Metal Oxides as Potential Catalysts for Nitrous Oxide Decomposition. ChemCatChem 2018, 10, 296–304. [Google Scholar] [CrossRef]
- Prechtl, P. Study of N2O Decomposition over Fe-ZSM-5 with Transient Methods. Ph.D. Thesis, EPFL, Lausanne, Switzerland, 2007. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Vazzana, F.; Marella, M.; Tomaselli, M.; Mantegazza, M. Novel Catalysts and Catalytic Technologies for N2O Removal from Industrial Emissions Containing O2, H2O and SO2. Adv. Environ. Res. 2000, 4, 325–338. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, X.; Zheng, C.; Hu, D.; Zhang, J.; Gao, X. Study on Catalytic Soot Oxidation over Spinel Type ACo2O4 (A = Co, Ni, Cu, Zn) Catalysts. Aerosol Air Qual. Res. 2017, 17, 2317–2327. [Google Scholar] [CrossRef]
- Xie, G.; Liu, Z.; Zhu, Z.; Liu, Q.; Ge, J.; Huang, Z. Simultaneous Removal of SO2 and NOx from Flue Gas Using a CuO/Al2O3 Catalyst Sorbent: I. Deactivation of SCR Activity by SO2 at Low Temperatures. J. Catal. 2004, 224, 36–41. [Google Scholar] [CrossRef]
- Liu, Z.; He, F.; Ma, L.; Peng, S. Recent Advances in Catalytic Decomposition of N2O on Noble Metal and Metal Oxide Catalysts. Catal. Surv. Asia 2016, 20, 121–132. [Google Scholar] [CrossRef]
- Komvokis, V.G.; Marnellos, G.E.; Vasalos, I.A.; Triantafyllidis, K.S. Effect of Pretreatment and Regeneration Conditions of Ru/γ-Al2O3 Catalysts for N2O Decomposition and/or Reduction in O2-Rich Atmospheres and in the Presence of NOX, SO2 and H2O. Appl. Catal. B 2009, 89, 627–634. [Google Scholar] [CrossRef]
- Kapteijn, F.; Rodriguez-Mirasol, J.; Moulijn, J.A. Heterogeneous Catalytic Decomposition of Nitrous Oxide. Appl. Catal. B 1996, 9, 25–64. [Google Scholar] [CrossRef]
- Yuan, N.; Gao, C.; Sun, X.; Li, J. Density Functional Theory Study of Mechanism of Reduction of N2O by CO over Fe-ZSM-5 Zeolites. Catalysts 2024, 14, 49. [Google Scholar] [CrossRef]
- Sadovskaya, E.; Pinaeva, L.; Skazka, V.; Prosvirin, I. Kinetics of Oxygen Exchange and N2O Decomposition Reaction over MeOx/CeO2 (Me = Fe, Co, Ni) Catalysts. Materials 2023, 16, 929. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Zasada, F.; Piskorz, W.; Kotarba, A.; Sojka, Z. Computational Spectroscopy and DFT Investigations into Nitrogen and Oxygen Bond Breaking and Bond Making Processes in Model DeNOx and DeN2O Reactions. Catal. Today 2007, 119, 219–227. [Google Scholar] [CrossRef]
- Wilk, M.; Kruk, J. Gołębiowski Andrzej Katalizator Do Rozkładu Podtlenku Azotu, Zwłaszcza w Gazach z Instalacji Kwasu Azotowego. P.378481, 19 December 2005. [Google Scholar]
- Inger, M.; Wilk, M.; Saramok, M.; Grzybek, G.; Grodzka, A.; Stelmachowski, P.; Makowski, W.; Kotarba, A.; Sojka, Z. Cobalt Spinel Catalyst for N2O Abatement in the Pilot Plant Operation-Long-Term Activity and Stability in Tail Gases. Ind. Eng. Chem. Res. 2014, 53, 10335–10342. [Google Scholar] [CrossRef]
- Inger, M.; Kowalik, P.; Saramok, M.; Wilk, M.; Stelmachowski, P.; Maniak, G.; Granger, P.; Kotarba, A.; Sojka, Z. Laboratory and Pilot Scale Synthesis, Characterization and Reactivity of Multicomponent Cobalt Spinel Catalyst for Low Temperature Removal of N2O from Nitric Acid Plant Tail Gases. Catal. Today 2011, 176, 365–368. [Google Scholar] [CrossRef]
- Lichtman, D.; Craig, J.H.; Sailer, V.; Drinkwine, M. AES and XPS Spectra of Sulfur in Sulfur Compounds. Appl. Surf. Sci. 1981, 7, 325–331. [Google Scholar] [CrossRef]

| Specification | Unit | Residual Gas |
|---|---|---|
| N2 | % vol. | ~97.2 |
| O2 | % vol. | 2.5 |
| H2O | % vol. | 0.3 |
| NOx | ppm | 65 |
| N2O | ppm | 100 |
| NH3 | ppm | 1–3 |
| p | kPa | 959 |
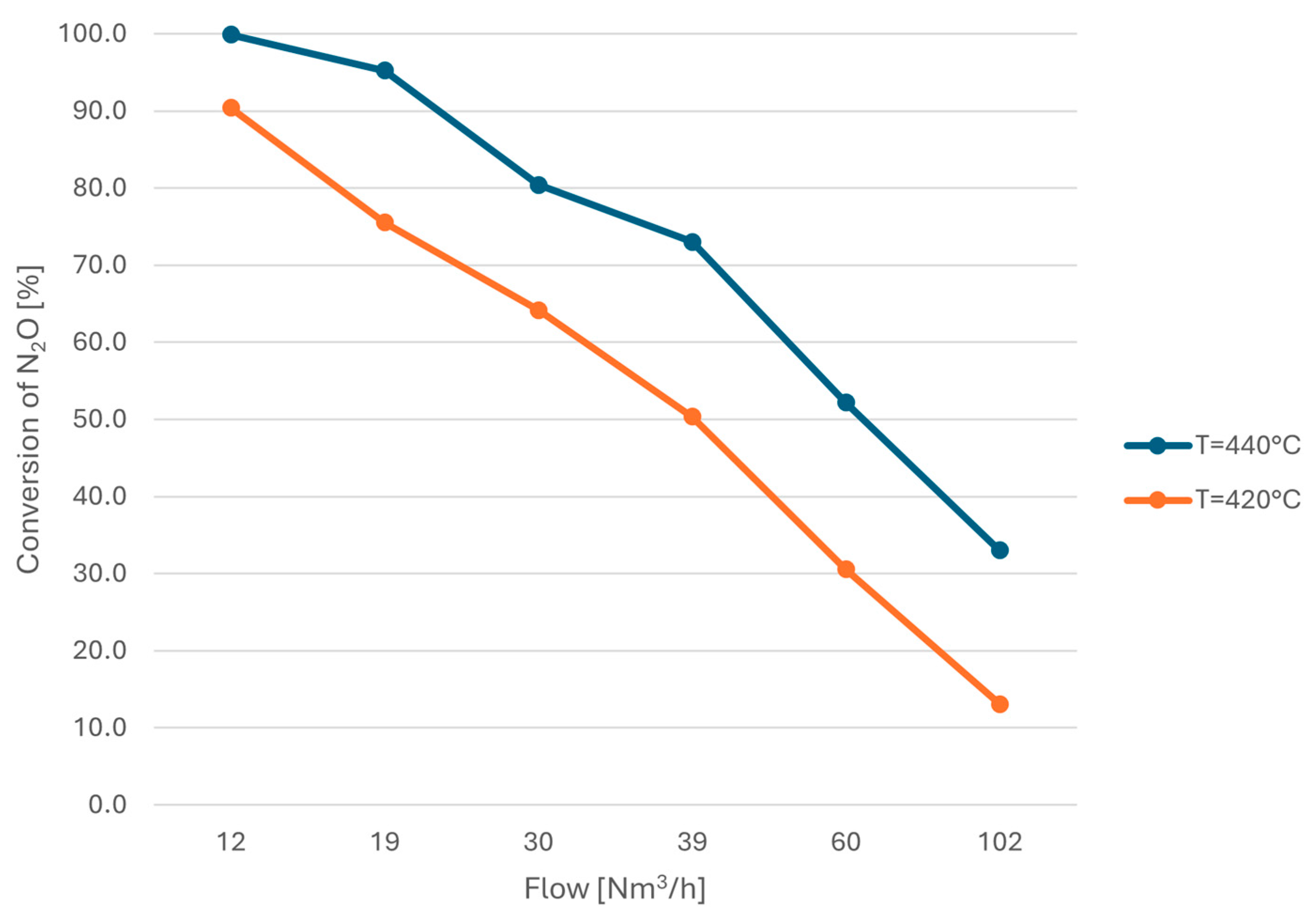
| No. | C0,N2O [ppm] | XN2O [%] | Flow [Nm3/h] | Tinlet [°C] | Toutlet [°C] | p [bar] |
|---|---|---|---|---|---|---|
| 4.1 | 59.5 | 77.9 | 12 | 408.4 | 409.0 | 9.5 |
| 4.2 | 61.5 | 90.4 | 421.5 | 421.7 | 9.6 | |
| 4.3 | 64.7 | 98.6 | 434.3 | 436.8 | 9.6 | |
| 4.4 | 64.2 | 99.9 | 439.6 | 442.7 | 9.6 | |
| 4.5 | 66.0 | 75.5 | 19 | 415.9 | 413.4 | 9.6 |
| 4.6 | 71.2 | 90.5 | 432.3 | 431.0 | 9.5 | |
| 4.7 | 71.0 | 95.2 | 442.4 | 444.6 | 9.6 | |
| 4.8 | 66.6 | 54.1 | 30 | 414.8 | 412.5 | 9.5 |
| 4.9 | 69.0 | 64.1 | 421.3 | 420.7 | 9.6 | |
| 4.10 | 72.6 | 76.6 | 428.3 | 431.1 | 9.6 | |
| 4.11 | 73.0 | 80.4 | 438.6 | 439.2 | 9.6 | |
| 4.12 | 81.1 | 31.7 | 40 | 408.5 | 409.5 | 9.5 |
| 4.13 | 78.1 | 50.3 | 420.4 | 422.4 | 9.5 | |
| 4.14 | 76.3 | 65.5 | 434.3 | 434.7 | 9.6 | |
| 4.15 | 74.9 | 73.0 | 441.6 | 441.9 | 9.6 | |
| 4.16 | 81.4 | 18.6 | 61 | 412.4 | 408.6 | 9.6 |
| 4.17 | 81.6 | 30.5 | 422.5 | 418.7 | 9.6 | |
| 4.18 | 79.8 | 41.9 | 429.8 | 430.0 | 9.6 | |
| 4.19 | 78.0 | 52.2 | 440.6 | 440.4 | 9.6 | |
| 4.20 | 84.4 | 13.1 | 103 | 421.9 | 419.4 | 9.5 |
| 4.21 | 82.4 | 21.9 | 430.7 | 428.5 | 9.5 | |
| 4.22 | 81.8 | 33.0 | 439.6 | 439.5 | 9.5 | |
| 4.23 | 116.8 | −0.71 | 120 | 437.2 | 445.7 | 9.5 |
| 4.24 | 115.4 | −0.72 | 437.0 | 445.8 | 9.5 | |
| 4.25 | 117.1 | −0.69 | 436.7 | 446.3 | 9.5 | |
| 4.26 | 116.2 | −0.67 | 100 | 435.2 | 444.4 | 9.5 |
| 4.27 | 116.5 | −0.66 | 435.9 | 446.6 | 9.5 | |
| 4.28 | 117.5 | −0.63 | 436.4 | 447.9 | 9.5 |
| No. | C0,N2O [ppm] | XN2O [%] | Flow [Nm3/h] | Tinlet [°C] | Toutlet [°C] |
|---|---|---|---|---|---|
| 4.29 | 120.1 | −0.7 | 120 | 437.4 | 446.2 |
| 4.30 | 116.2 | −0.7 | 100 | 435.2 | 444.4 |
| 4.31 | 111.5 | −0.6 | 60 | 432.8 | 445.2 |
| 4.32 | 112.1 | −0.6 | 39 | 432.1 | 444.8 |
| 4.33 | 118.3 | −0.1 | 19 | 438.4 | 445.1 |
| 4.34 | 88.1 | −5.9 | 11 | 422.9 | 452.1 |
| Sample | SSA [m2/g] | Vt [cm3/g] | P [%] | d [nm] | ρ [g/cm3] |
|---|---|---|---|---|---|
| α-Al2O3 | 7.4 | 0.56 | 68 | 300 | 1.2 |
| K-CoZn/α-Al2O3_fresh | 7.6 | 0.44 | 65 | 235 | 1.4 |
| K-CoZn/α-Al2O3_used | 6.5 | 0.35 | 59 | 210 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moszowski, B.; Mulica-Musiał, M.; Piszko, P.J.; Dobrzyński, M. The Behavior of Catalytic, Low-Temperature N2O Decomposition (LT-deN2O) in the Presence of Sulfur-Containing Compounds on Nitric Acid Plants. Appl. Sci. 2024, 14, 9353. https://doi.org/10.3390/app14209353
Moszowski B, Mulica-Musiał M, Piszko PJ, Dobrzyński M. The Behavior of Catalytic, Low-Temperature N2O Decomposition (LT-deN2O) in the Presence of Sulfur-Containing Compounds on Nitric Acid Plants. Applied Sciences. 2024; 14(20):9353. https://doi.org/10.3390/app14209353
Chicago/Turabian StyleMoszowski, Bartosz, Martyna Mulica-Musiał, Paweł J. Piszko, and Maciej Dobrzyński. 2024. "The Behavior of Catalytic, Low-Temperature N2O Decomposition (LT-deN2O) in the Presence of Sulfur-Containing Compounds on Nitric Acid Plants" Applied Sciences 14, no. 20: 9353. https://doi.org/10.3390/app14209353
APA StyleMoszowski, B., Mulica-Musiał, M., Piszko, P. J., & Dobrzyński, M. (2024). The Behavior of Catalytic, Low-Temperature N2O Decomposition (LT-deN2O) in the Presence of Sulfur-Containing Compounds on Nitric Acid Plants. Applied Sciences, 14(20), 9353. https://doi.org/10.3390/app14209353








