Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Instruments
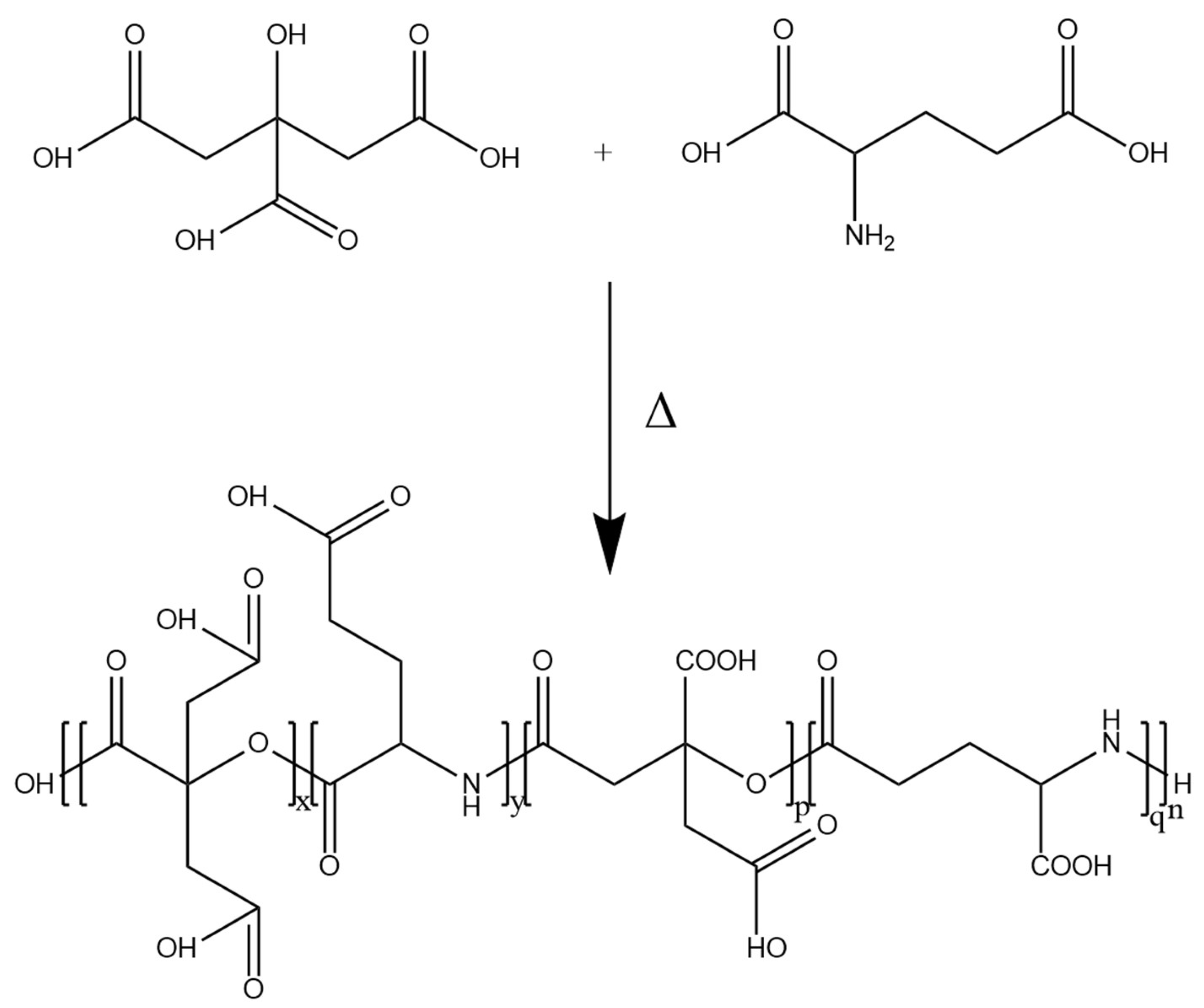
2.2. Synthesis of PCA-GLU Polymer
2.3. Electrochemical Tests
2.4. Theoretical Calculation
3. Results and Discussion
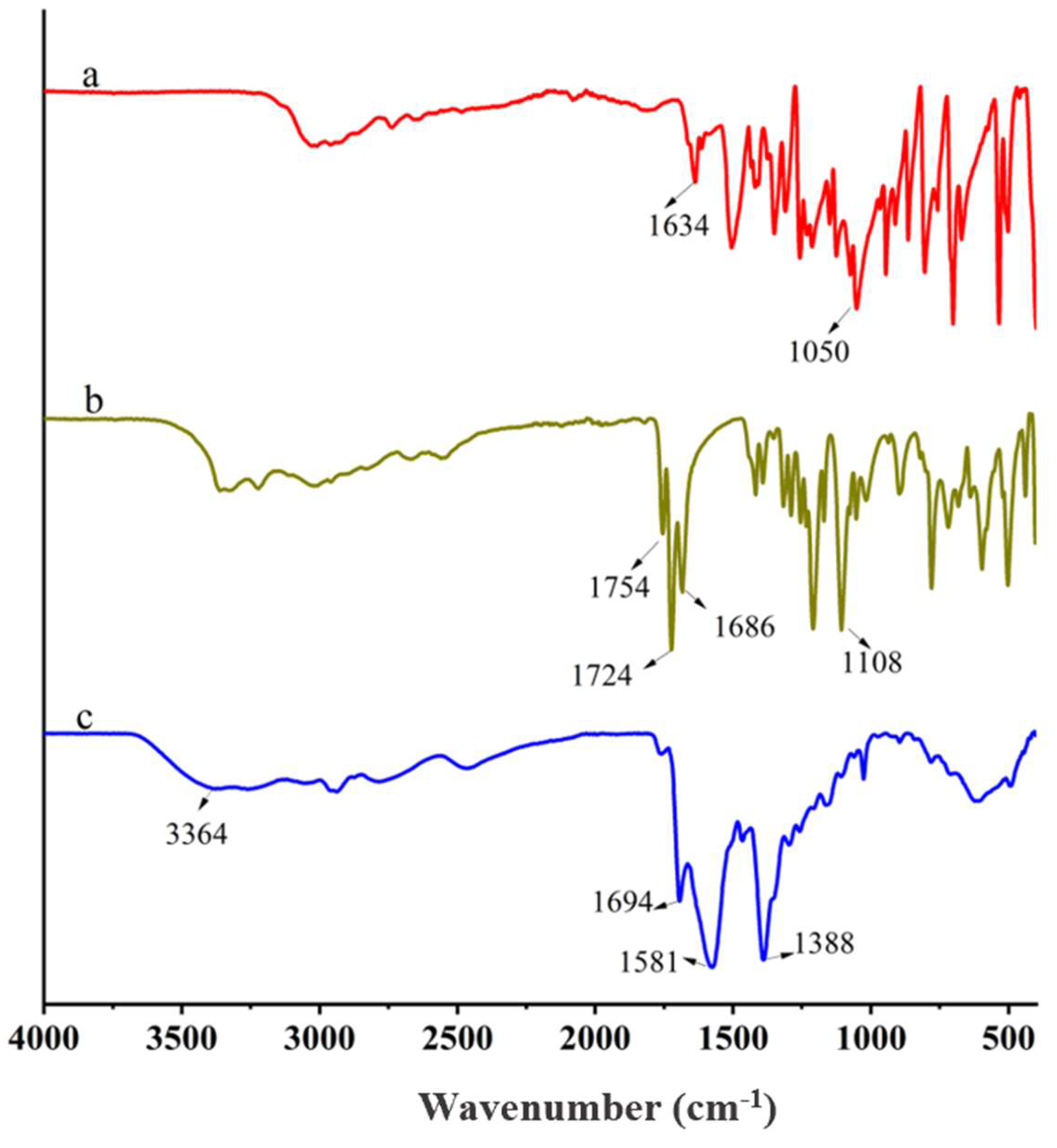
3.1. Synthesis of the PCA-GLU
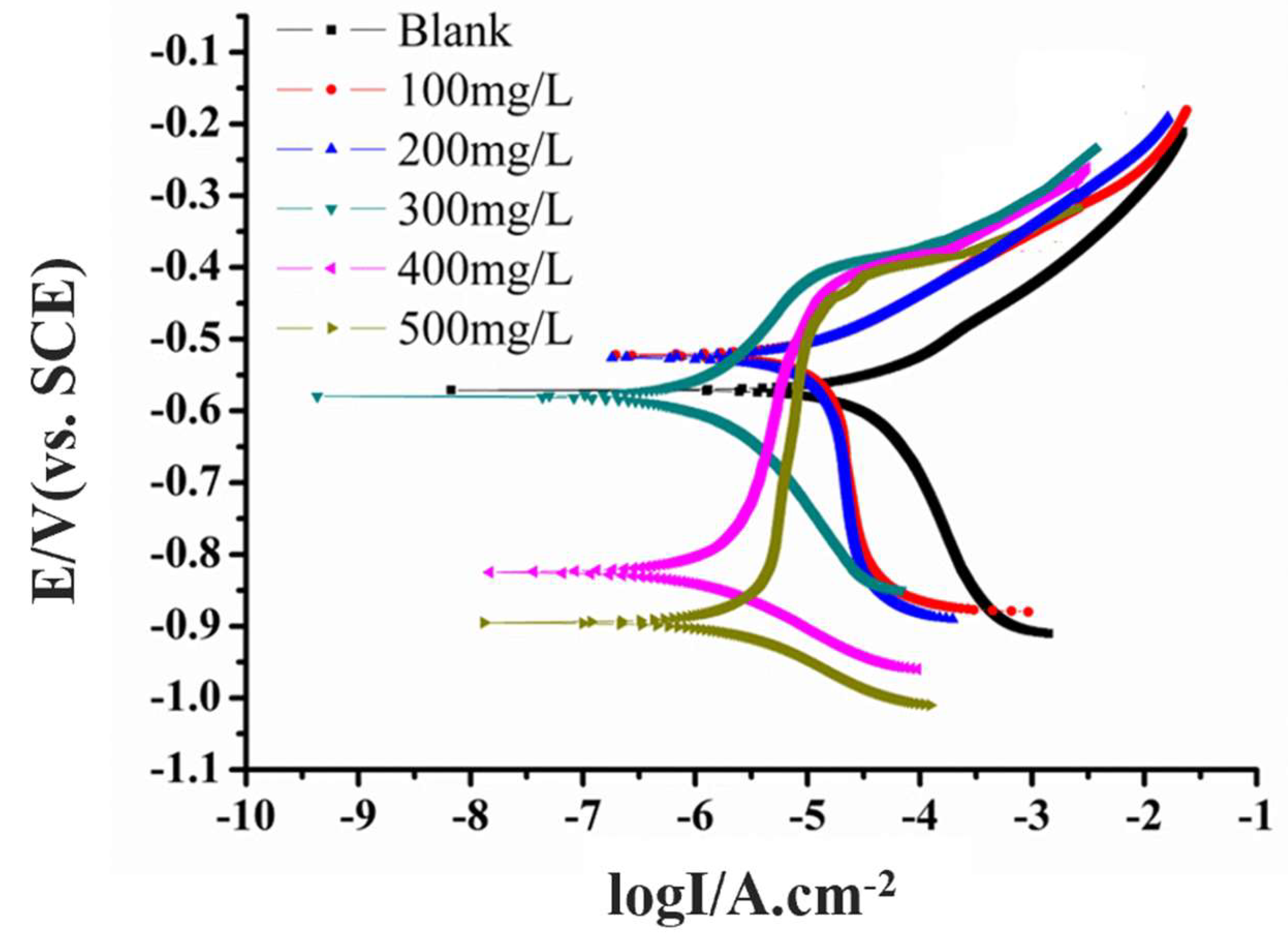
3.2. PDP Measurements
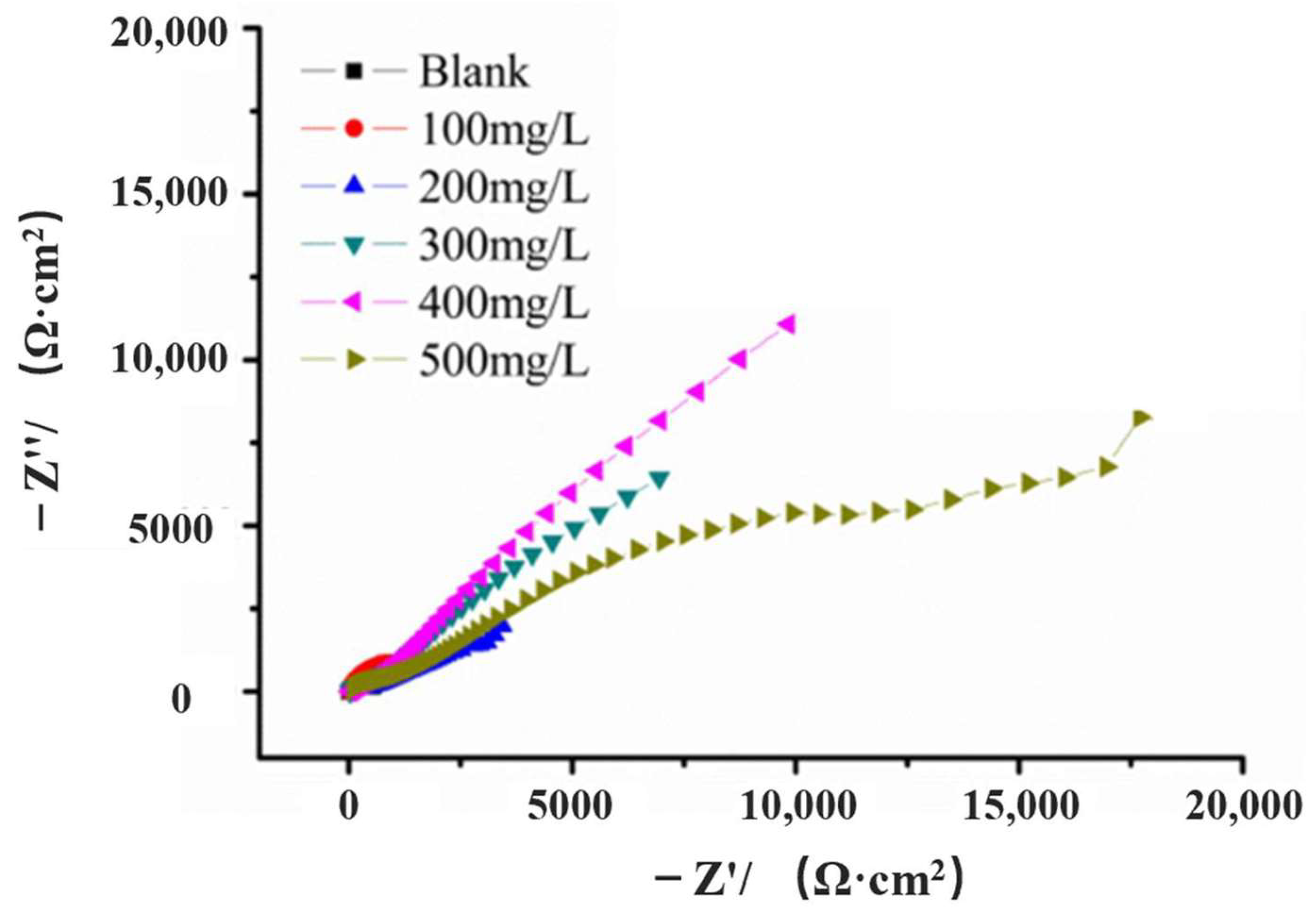
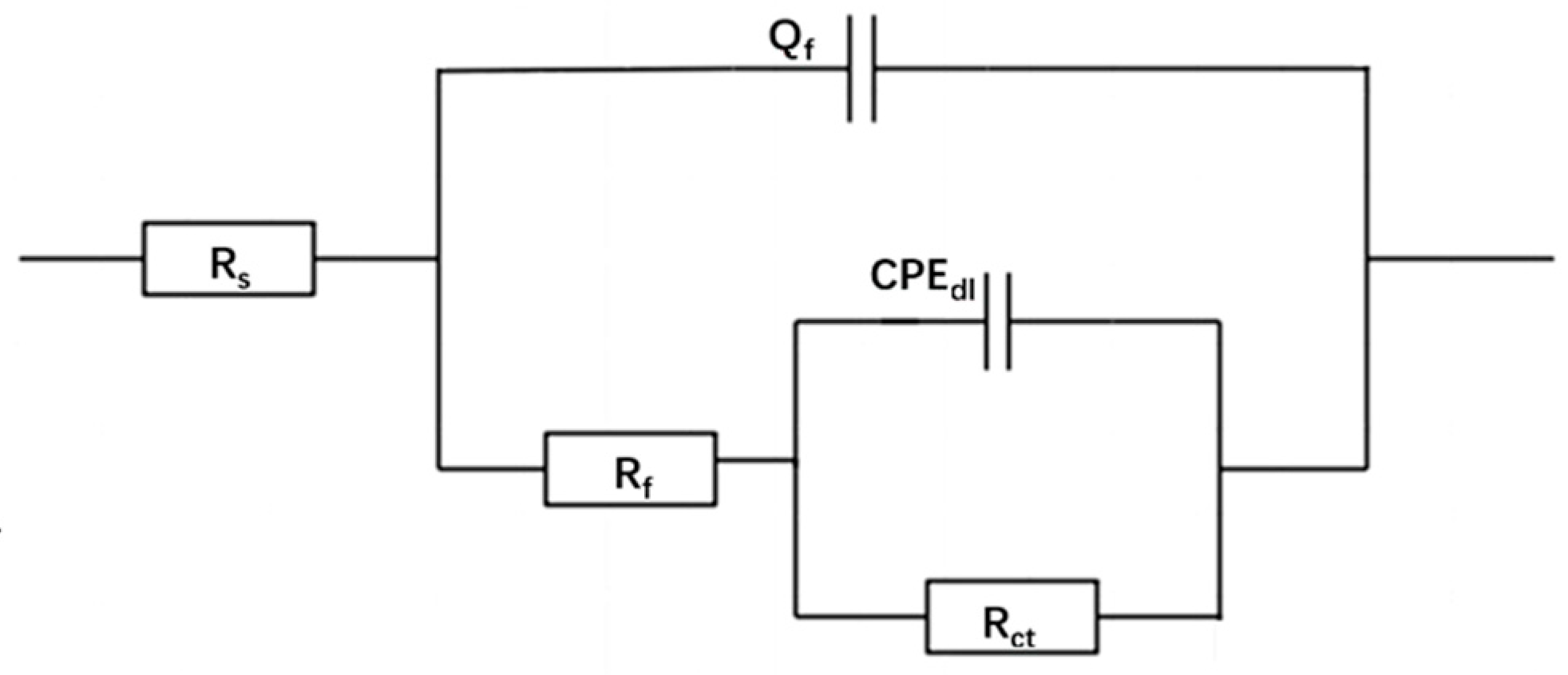
3.3. EIS Measurements
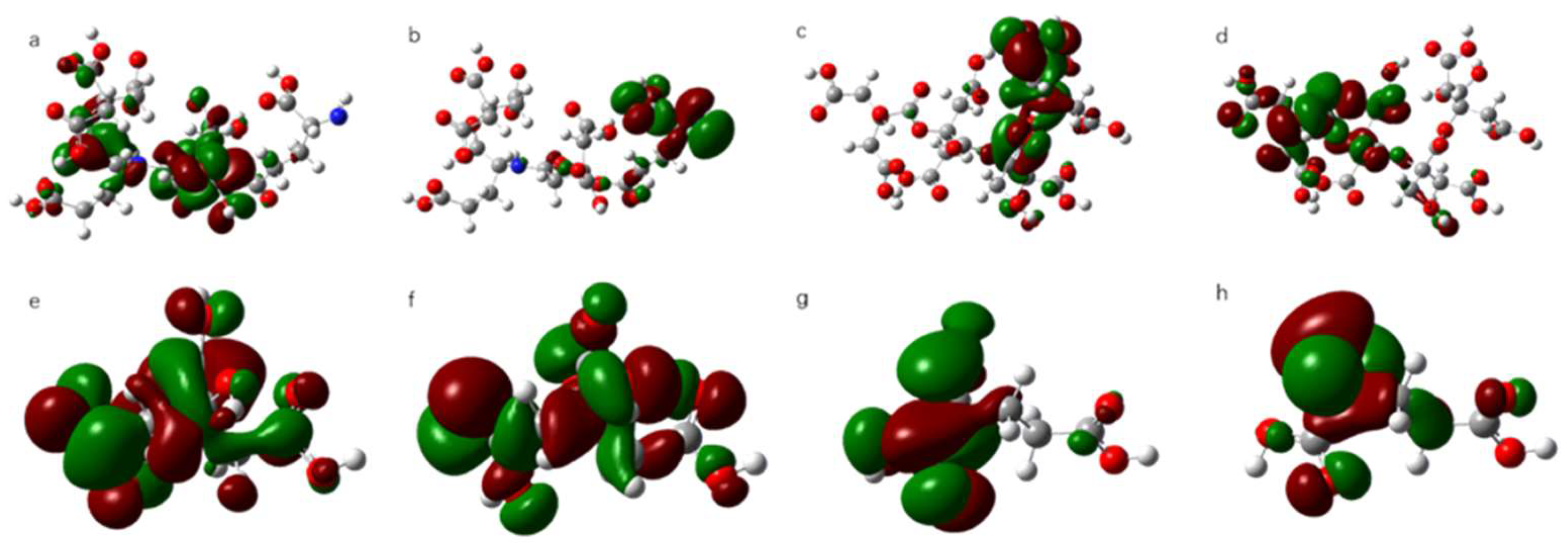
3.4. Quantum Chemical Calculation
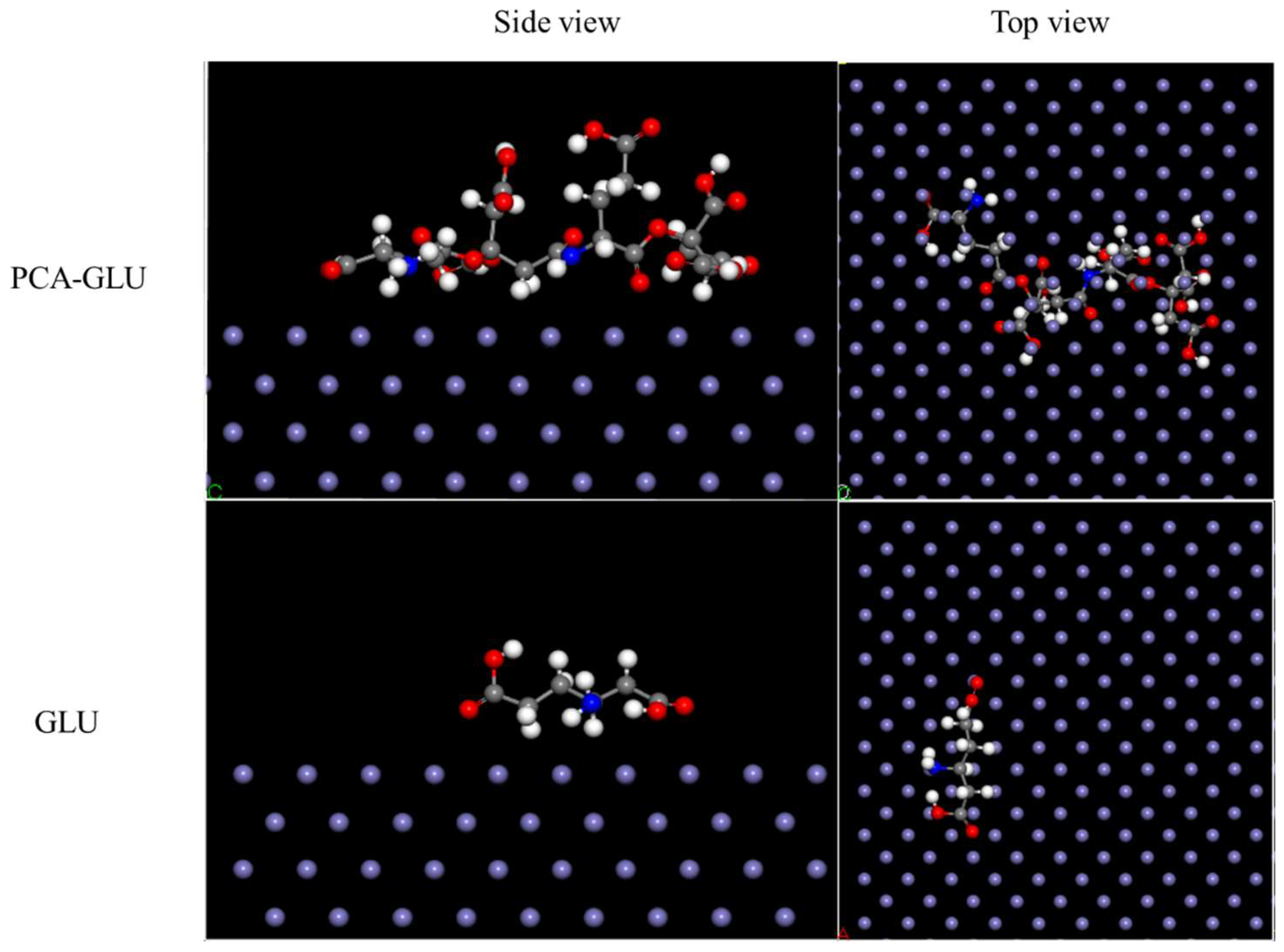
3.5. Interfacial Adsorption
3.5.1. Interaction Energy Calculation
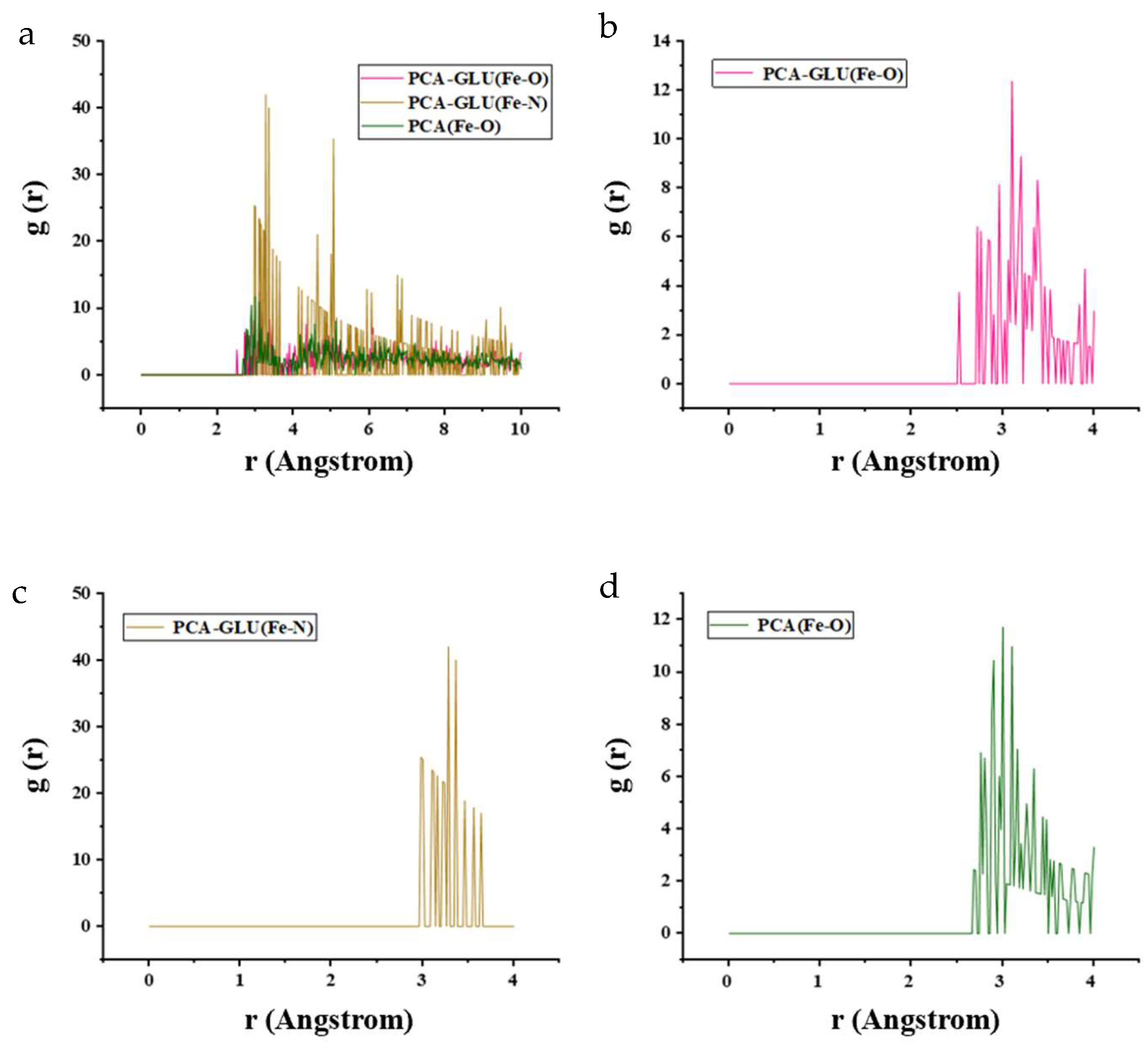
3.5.2. Radial Distribution Function (RDF)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shaban, M.M.; Negm, N.; Farag, R.; Gomaa, A.E.; Farag, A.; Migahed, M. Antiscalant and anti-microbial performance of some synthesized trimeric cationic imidazolium salts in oilfield applications. J. Mol. Liq. 2022, 351, 118610. [Google Scholar] [CrossRef]
- Kamal, R.S.; Migahed, M.A.; El-Sattar, N.E.A.A. Synthesis, characterization, and performance of succinimide derivatives as anti-corrosion and antiscalant in petroleum applications. J. Mol. Liq. 2022, 354, 118869. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, B.; Li, Y.; Zhu, G.; Lei, Y.; Wang, X.; Liu, H.; Zhang, G. Dextran derivatives as highly efficient green corrosion inhibitors for carbon steel in CO2-saturated oilfield produced water: Experimental and theoretical approaches. Chem. Eng. J. 2021, 424, 130519. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Palmeira-Mello, M.V.; Leite, M.C.; Oliveira, J.A.M.; Martins, I.I.; de Sá, R.G.; de Almeida, L.A.; Souza, A.M.; Campos, V.R.; Ponzio, E.A. Corrosion inhibition and ecotoxicological assessment of 1,2,3-triazoles alcohols. Mater. Chem. Phys. 2022, 290, 126508. [Google Scholar] [CrossRef]
- Wang, J.; Liu, D.; Cao, S.; Pan, S.; Luo, H.; Wang, T.; Ding, H.; Mamba, B.B.; Gui, J. Inhibition effect of monomeric/polymerized imidazole zwitterions as corrosion inhibitors for carbon steel in an acid medium. J. Mol. Liq. 2020, 312, 113436. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Zhang, G.; Wang, W.; Dong, S.; Cheng, Y.; Liu, H. Corrosion inhibition of carbon steel in CO2-containing oilfields produced water in the presence of iron-oxidizing bacteria and inhibitors. Corros. Sci. 2016, 105, 149–160. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, T.; Zhang, X.; Asif, M.; Jiang, L.; Dong, S.; Gu, T.; Liu, H. Inhibition effects of benzalkonium chloride on Chlorella vulgaris induced corrosion of carbon steel. J. Mater. Sci. Technol. 2020, 43, 14–20. [Google Scholar] [CrossRef]
- Yuan, X.; Dong, S.; Zheng, Q.; Yang, W.; Huang, T. Novel and efficient curcumin-based fluorescent polymer for scale and corrosion inhibition. Chem. Eng. J. 2020, 389, 124296. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, B.; Wang, X.; Lu, Y.; Li, F.; Li, C. The improved corrosion resistance of carbon steel in soft water with dendritic-polymer corrosion inhibitors. Chem. Eng. J. 2023, 452, 139043. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Yuan, X.; Xu, Y.; Xu, D.; Zhang, D.; Feng, D.; Wang, F. Marine Biofilms with Significant Corrosion Inhibition Performance by Secreting Extracellular Polymeric Substances. ACS Appl. Mater. Interfaces 2021, 13, 47272–47282. [Google Scholar] [CrossRef]
- Farahati, R.; Ghaffarinejad, A.; Mousavi-Khoshdel, S.M.; Rezania, J.; Behzadi, H.; Shockravi, A. Synthesis and potential applications of some thiazoles as corrosion inhibitor of copper in 1 M HCl: Experimental and theoretical studies. Prog. Org. Coatings 2019, 132, 417–428. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Qurashi, A.; Chauhan, D.S.; Quraishi, M. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2020, 321, 114385. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2021, 406, 126866. [Google Scholar] [CrossRef]
- Alnajjar, A.O.; El-Lateef, H.M.A.; Khalaf, M.M.; Mohamed, I.M. Steel protection in acidified 3.5 wt% NaCl by a novel hybrid composite of CoCrO3/polyaniline: Chemical fabrication, physicochemical properties, and corrosion inhibition performance. Constr. Build. Mater. 2021, 317, 125918. [Google Scholar] [CrossRef]
- Chen, T.; Chen, M.; Pi, J.; Fu, C. Self-assembly anchored film of two modified polyamides as promising cost-effective inhibitor for protecting carbon steel in neutral medium. Mater. Today Commun. 2022, 31, 103790. [Google Scholar] [CrossRef]
- Kumar, A.M.; Rajesh, T.; Obot, I.; Bin Sharfan, I.I.; Abdulhamid, M.A. Water-soluble chitosan salt as ecofriendly corrosion inhibitor for N80 pipeline steel in artificial sea water: Experimental and theoretical approach, International. Int. J. Biol. Macromol. 2023, 254, 127697. [Google Scholar] [CrossRef]
- AT, J.R.; Kuruvilla, M.; Joseph, A. Computational modelling and theoretical calculations on hydroxy citric acid and mangostine and comparing the corrosion inhibition effect of aqueous and alcoholic extracts Garcinia cambogia leaves for mild steel in hydrochloric acid. J. Mol. Struct. 2023, 1274, 134302. [Google Scholar] [CrossRef]
- Rahimi, A.; Abdouss, M.; Farhadian, A.; Guo, L.; Kaya, S.; Neshati, J. Enhancement corrosion resistance of mild steel in 15% HCl solution by a novel bio-based polyurethane for oil well acidizing. J. Ind. Eng. Chem. 2022, 113, 332–347. [Google Scholar] [CrossRef]
- Roy, P.; Saha, S.K.; Banerjee, P.; Dey, S.; Sukul, D. Experimental and theoretical investigation towards anti-corrosive property of glutamic acid and poly-γ-glutamic acid for mild steel in 1 M HCl: Intramolecular synergism due to copolymerization. Res. Chem. Intermed. 2017, 43, 4423–4444. [Google Scholar] [CrossRef]
- Zhu, J.-J.; Ni, Q.-Y.; Chen, J.-Q.; Liu, T.-R.; Yang, J.-Y.; Zhao, Y.-Z.; Meng, X.-J.; Ge, H.-H. Copolymer of citric acid and glutamic acid as calcium scale inhibitor. Desalination Water Treat. 2020, 175, 182–188. [Google Scholar] [CrossRef]
- Kumar, P.; Soni, I.; Jayaprakash, G.K.; Kumar, S.; Rao, S.; Flores-Moreno, R.; Sowmyashree, A. Experimental and theoretical studies of hexylmeythylimidazolium tetrafluoroborate ionic liquid as cathodic corrosion inhibitor for mild steel. Inorg. Chem. Commun. 2022, 146, 110110. [Google Scholar] [CrossRef]
- Luo, X.; Chen, B.; Li, J.; Lan, B.; Zhou, C.; Ren, Z.; Ci, C.; Liu, Y. Synthesis of natural glucomannan derivative as a highly-efficient green inhibitor for mild steel in the simulated seawater. J. Ind. Eng. Chem. 2023, 124, 132–146. [Google Scholar] [CrossRef]
- Djama, M.; Benhaddad, L.; Idir, B.; Achoui, N.; Daifallah, H. Synergistic corrosion inhibition effect of copolymer and an amphoteric surfactant on carbon steel in 3.5 NaCl solution: Experimental and theoretical research. J. Solid State Electrochem. 2023, 27, 2139–2162. [Google Scholar] [CrossRef]
- Hou, B.; Xu, N.; Zhang, Q.; Xuan, C.; Liu, H.; Zhang, G. Effect of benzyl substitution at different sites on the inhibition performance of pyrimidine derivatives for mild steel in highly acidic solution. J. Taiwan Inst. Chem. Eng. 2019, 95, 541–554. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.; Abd-El-Nabey, B.; Sidahmed, I.; El-Zayady, A.; Saadawy, M. Sidahmed, Inhibitive action of some plant extracts on the corrosion of steel in acidic media. Corros. Sci. 2006, 48, 2765–2779. [Google Scholar] [CrossRef]
- Hosseini, M.; Mertens, S.F.; Arshadi, M.R. Synergism and antagonism in mild steel corrosion inhibition by sodium dodecylbenzenesulphonate and hexamethylenetetramine. Corros. Sci. 2003, 45, 1473–1489. [Google Scholar] [CrossRef]
- Subbiah, K.; Lee, H.-S.; Al-Hadeethi, M.R.; Park, T.; Lgaz, H. Assessment of the inhibitive performance of a hydrazone derivative for steel rebar in a simulated concrete medium: Establishing the inhibition mechanism at an experimental and theoretical level. Chem. Eng. J. 2023, 458, 141347. [Google Scholar] [CrossRef]
- Han, T.; Guo, J.; Zhao, Q.; Wu, Y.; Zhang, Y. Enhanced corrosion inhibition of carbon steel by pyridyl gemini surfactants with different alkyl chains. Mater. Chem. Phys. 2019, 240, 122156. [Google Scholar] [CrossRef]
- Gece, G. The use of quantum chemical methods in corrosion inhibitor studies. Corros. Sci. 2008, 50, 2981–2992. [Google Scholar] [CrossRef]
- Zhang, G.; Hou, X.; Hou, B.; Liu, H. Benzimidazole derivatives as novel inhibitors for the corrosion of mild steel in acidic solution: Experimental and theoretical studies. J. Mol. Liq. 2019, 278, 413–427. [Google Scholar] [CrossRef]
- Lu, H.; Ji, X.; Ci, X.; Zhu, H.; Wang, Q.; Zong, Y.; Tian, H. Investigation of triazole derivatives as corrosion inhibitors on Q235 steel in NaCl solution: Experimental and theoretical studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131892. [Google Scholar] [CrossRef]
- Khanna, R.; Kalia, V.; Kumar, R.; Kumar, R.; Kumar, P.; Dahiya, H.; Pahuja, P.; Jhaa, G.; Kumar, H. Synergistic experimental and computational approaches for evaluating pyrazole Schiff bases as corrosion inhibitor for mild steel in acidic medium. J. Mol. Struct. 2023, 1297, 136845. [Google Scholar] [CrossRef]

| Black | 100 (mg/L) | 200 (mg/L) | 300 (mg/L) | 400 (mg/L) | 500 (mg/L) | |
|---|---|---|---|---|---|---|
| −Ecorr (mV) | 575 | 533 | 534 | 579 | 828 | 899 |
| Icorr (A) | 3.872 × 10−5 | 1.793 × 10−5 | 1.171 × 10−5 | 1.643 × 10−6 | 1.413 × 10−6 | 2.848 × 10−6 |
| H (%) | - | 55.09 | 69.76 | 95.76 | 96.35 | 92.64 |
| Sample | Rs (Ω·cm2) | CPEdl (S·secn/cm2) | Rf (Ω·cm2) | Rct (Ω·cm2) | η (%) |
|---|---|---|---|---|---|
| blank | 6.659 | 21.5 × 10−4 | 37.55 | 198 | - |
| 100 mg/L | 6.492 | 16.3 × 10−4 | 22.64 | 353 | 51.00 |
| 200 mg/L | 6.791 | 8.75 × 10−4 | 28.52 | 405 | 65.84 |
| 300 mg/L | 17.51 | 2.47 × 10−4 | 38.88 | 1408 | 92.31 |
| 400 mg/L | 16.1 | 2.51 × 10−4 | 147.1 | 2161 | 96.73 |
| 500 mg/L | 64.92 | 9.34 × 10−5 | 61.19 | 1913 | 90.12 |
| Inhibitors Additive | ELUMO (eV) | EHOMO (eV) | μ | χ | η | σ | ω | ΔN | ΔE |
|---|---|---|---|---|---|---|---|---|---|
| PCA-GLU | −0.798 | −6.832 | −3.815 | 3.815 | 3.017 | 0.331 | 2.412 | 0.528 | 6.034 |
| PCA | −0.628 | −7.138 | −3.883 | 3.883 | 3.255 | 0.310 | 2.316 | 0.476 | 6.510 |
| GLU | −0.070 | −6.801 | −3.436 | 3.436 | 3.366 | 0.297 | 1.769 | 0.529 | 6.731 |
| CA | −0.194 | −7.524 | −3.956 | 3.956 | 3.665 | 0.273 | 2.135 | 0.415 | 7.330 |
| Inhibitors | PCA-GLU | PCA | GLU | CA |
|---|---|---|---|---|
| Interaction (kcal/mol) | −224.23 | −182.01 | −83.15 | −77.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, N.; Liu, K.; Zhao, Y.; Deng, Y.; Ge, H. Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater. Appl. Sci. 2024, 14, 9465. https://doi.org/10.3390/app14209465
Chang N, Liu K, Zhao Y, Deng Y, Ge H. Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater. Applied Sciences. 2024; 14(20):9465. https://doi.org/10.3390/app14209465
Chicago/Turabian StyleChang, Nanxin, Kuaiying Liu, Yuzeng Zhao, Yining Deng, and Honghua Ge. 2024. "Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater" Applied Sciences 14, no. 20: 9465. https://doi.org/10.3390/app14209465
APA StyleChang, N., Liu, K., Zhao, Y., Deng, Y., & Ge, H. (2024). Inhibition Performance and Mechanism of Poly(Citric Acid–Glutamic Acid) on Carbon Steel Corrosion in Simulated Seawater. Applied Sciences, 14(20), 9465. https://doi.org/10.3390/app14209465





